Abstract
Background/Aims
Patients with head and neck squamous cell carcinoma (HNSCC) are at risk for second primary malignancies (SPMs). The prevalence, distribution and survival of head and neck versus non-head and neck SPMs are not fully elucidated. The objective of this study was to quantify the rate of second primary malignancies in patients with HNSCC.
Methods
Population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) database. Prevalence and location of SPM, and survival data were analyzed.
Results
There were 58,363 HNSCC patients, and the prevalence of HNSCC and non-HNSCC SPMs was 3.0% (1,746) and 8.8% (5,109), respectively. Overall survival (OS) was higher in patients with a HNSCC SPM compared to non-HNSCC SPM (p<0.001), with no difference in disease-specific survival(DSS). Patients with SPM in the lung and esophagus had worse OS (p<0.001), and patients with SPM in the prostate and breast had better OS(p<0.001).
Conclusion
In HNSCC patients who develop SPM, nearly 75% are non-HNSCC SPM. Patients with non-HNSCC SPMs have lower OS. Future clinical practice guidelines should take the risks and locations of SPM development into consideration for screening.
Keywords: Head and neck squamous cell carcinoma (HNSCC), second primary, survival, SEER
INTRODUCTION
The risk of developing a second primary malignancy (SPM) is of particular concern for patients with head and neck squamous cell carcinoma (HNSCC), as SPMs are the second-leading cause of death in these patients [1]. Patients are considered at risk for SPMs in the head and neck due to field cancerization, or the propensity of cancerous squamous epithelial lesions to be associated with surrounding premalignant histologic changes [2]. Field cancerization is likely related to broad exposure to carcinogens such as tobacco smoke and alcohol. Thus, this process may contribute to the multifocal growth of epithelial tumors such as HNSCC.
In addition, patients with HNSCC are at theoretical risk for malignancies at sites other than the head and neck. Tobacco and alcohol abuse are prevalent in this population, predisposing them to cancers of the lung, liver, pancreas, bladder, kidney, female reproductive system, colon and breast [3,4]. The prevalence of SPMs in HNSCC patients in these organ systems versus in the head and neck, and their effect on survival, has not been fully explored. Protocols for follow-up and screening for SPM vary greatly [5]. In addition, guidelines for adequate screening for second cancers based on primary HNSCC subsite have not been established [6].
The objectives of the current study were to categorize the prevalence, distribution and survival patterns of SPM in the head and neck and in other sites in HNSCC patients in the United States using a national oncology registry. The results of this study are intended to inform the development of clinical practice guidelines for additional screening in HNSCC patient diagnostic evaluation and monitoring.
METHODS
For this analysis we used the Surveillance, Epidemiology, and End Results (SEER) database, which began collecting data on cancer patients from a mixture of academic and community hospitals across the United States in 1973. The database includes information on approximately 28% of the U.S. population, with nearly universal follow-up data available. The accuracy and comprehensiveness of data collection, particularly for demographics, tumor characteristics, surgical and radiotherapeutic intervention, and survival statistics is assured by an internal SEER quality control program [7].
The current study used SEER data from 1973 to 2008. The study cohort was defined using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes for squamous cell carcinoma and histologically similar cancers (8010, 8011, 8020, 8051, 8070-8076, 8078, and 8081-8084) from major and minor subsites of the upper aerodigestive tract (nasal cavity, nasopharynx, sinuses and middle/inner ear, oral cavity, oropharynx, hypopharynx, larynx, and trachea). We also collected routine demographic information on age, sex, and race. Index tumor staging was defined as in situ/localized (N0, M0), regional (N+, M0), and distant (any N, M1).
We defined SPMs as first described by Warren and Gates [8]. These were invasive tumors occurring chronologically after the index HNSCC. Tumors were coded as SPMs if the tumor was either not of squamous cell origin or if it developed in a different location from the index tumor. Specific SPM sites in the head and neck and in other organ systems were recorded.
We compiled descriptive statistics on patient and tumor characteristics, including demographic information, and tumor stage. Chi-squared or Student’s t-tests were used to compare characteristics of patients with and without a diagnosed SPM, and with head and neck or non-head and neck SPM. Kaplan-Meier survival curves were constructed to analyze univariate predictors of survival in head and neck and non head and neck SPM. A multivariable Cox regression was performed to control for confounding variables that were significant in univariate analysis. Statistical analyses were conducted using SPSS version 22 (IBM; Armonk, NY). All statistical tests of significance were two-sided (p<0.05).
RESULTS
There were 58,363 HNSCC patients identified in the cohort. Demographic information and tumor characteristics are provided in Table 1. Among all patients in the cohort, 6,855 (11.7%) developed a SPM. The mean time to SPM development was 32.8 months. There were 1746 (3.0%) patients who developed a head and neck SPM, while 5,109 (8.8%) patients developed a non-head and neck SPM.
Table I.
Demographics and Tumor Characteristics of HNSCC Patients. Data from all patients, patients with and without a SPM, and further stratification into patients with non-head and neck SPM and head and neck SPM were collected.
| Total | No SPM | SPM | HN SPM | Non-HN SPM | |
|---|---|---|---|---|---|
|
| |||||
| N | 58363 | 51508 | 6855 | 1746 | 5109 |
|
| |||||
| Age (mean) | 63 | 63 | 65 | 62 | 66 |
|
| |||||
| Sex | |||||
| Male | 42794 (73.3%) | 37706 (73.2%) | 5088 (74.2%) | 1231 (70.5%) | 3857 (75.5%) |
| Female | 15569 (26.7%) | 13802 (26.8%) | 1767 (25.8%) | 515 (29.5%) | 1252 (24.5%) |
|
| |||||
| Race | |||||
| White | 48027 (82.3%) | 42141 (81.8%) | 5886 (85.9%) | 1492 (85.5%) | 4394 (86.0%) |
| Black | 6093 (10.4%) | 5446 (10.6%) | 647 (9.4%) | 153 (8.8%) | 494 (9.7%) |
| Asian/Pacific Islander | 3579 (6.1%) | 3268 (6.3%) | 311 (4.5%) | 92 (5.3%) | 219 (4.3%) |
| Other/Unknown | 664 (1.1%) | 653 (1.3%) | 11 (0.2%) | 9 (0.5%) | 2 (0.0%) |
|
| |||||
| Ethnicity | |||||
| Hispanic | 5990 (10.3%) | 5487 (10.7%) | 503 (7.3%) | 150 (8.6%) | 353 (6.9%) |
| Non-Hispanic | 52373 (89.7%) | 46021 (89.3%) | 6352 (92.7%) | 1596 (91.4%) | 4756 (93.1%) |
|
| |||||
| Index Tumor Stage | |||||
| Local | 17483 (30.0%) | 14420 (28.0%) | 3063 (44.7%) | 935 (53.6%) | 2128 (41.7%) |
| Regional | 24810 (42.5%) | 22251 (43.2%) | 2559 (37.3%) | 572 (32.8%) | 1987 (38.9%) |
| Distant | 5285 (9.1%) | 4961 (9.6%) | 324 (4.7%) | 60 (3.4%) | 264 (5.2%) |
| Missing | 10785 (18.5%) | 9876 (19.2%) | 909 (13.3%) | 179 (10.2%) | 730 (14.3%) |
|
| |||||
| Index Tumor Subsite | |||||
| Oral Cavity | 24432 (41.9%) | 21490 (41.7%) | 2942 (42.9%) | 967 (55.4%) | 1975 (38.7%) |
| Oropharynx | 9478 (16.2%) | 8539 (16.6%) | 939 (13.7%) | 281 (16.1%) | 658 (12.9%) |
| Nasopharynx | 3083 (5.3%) | 2913 (5.7%) | 170 (2.5%) | 33 (1.9%) | 137 (2.7%) |
| Hypopharynx | 2235 (3.8%) | 1962 (3.8%) | 273 (4.0%) | 76 (4.4%) | 197 (3.9%) |
| Larynx/Trachea | 17317 (29.7%) | 14954 (29.0%) | 2363 (34.5%) | 353 (20.2%) | 2010 (39.3%) |
| Other | 1818 (3.1%) | 1650 (3.2%) | 168 (2.5%) | 36 (2.1%) | 132 (2.6%) |
We calculated the rate and distribution of SPM by HNSCC primary subsite. Overall, patients with laryngeal primaries had the highest rate of SPM (13.6%), with the majority of these being non-head and neck SPMs (Table 2). Patients with oral cavity primaries had the highest rate of head and neck SPM (4.0%). Among head and neck SPM, oral cavity SPMs were the most frequent (58.4% of head and neck SPMs), followed by larynx (18.2%) and oropharynx (13.3%) (Table 3). Oral cavity SPMs were most frequently found in patients with oral cavity, oropharyngeal, and hypopharyngeal primaries.
Table 2.
Rate of SPM by Primary HNSCC Subsite.
| Total | Total SPM | HN SPM | Non-HN SPM | |
|---|---|---|---|---|
| Oral Cavity | 24432 | 2942 (12.0%) | 967 (4.0%) | 1975 (8.1%) |
| Oropharynx | 9478 | 939 (9.9%) | 281 (3.0%) | 658 (6.9%) |
| Nasopharynx | 3083 | 170 (5.5%) | 33 (1.0%) | 137 (4.4%) |
| Hypopharynx | 2235 | 273 (12.2%) | 76 (3.4%) | 197 (8.8%) |
| Larynx/trachea | 17317 | 2363 (13.6%) | 353 (2.0%) | 2010 (11.6%) |
| Other | 1818 | 168 (9.2%) | 36 (2.0%) | 132 (7.3%) |
| Total | 58363 | 6855 (11.7%) | 1746 (3.0%) | 5109 (8.8%) |
Table 3.
Most common Subsites for Head and Neck SPMs by HNSCC Primary Site. Oral cavity was the most common overall site for head and neck SPMs, followed by larynx.
| Primary site | Most common head and neck SPM subsite (%) | 2nd most common head and neck SPM subsite (%) | 3rd most common head and neck SPM subsite (%) |
|---|---|---|---|
| Oral Cavity | Oral Cavity (70.0%) | Oropharynx (12.2%) | Larynx(10.8%) |
| Oropharynx | Oral Cavity (55.5%) | Oropharynx (18.5%) | Larynx (14.2%) |
| Nasopharynx | Oral Cavity (39.4%) | Oropharynx (12.1%) | Larynx (9.1%) |
| Hypopharynx | Oral Cavity (57.9%) | Larynx (17.1%) | Oropharynx (15.8%) |
| Larynx | Larynx (42.8%) | Oral Cavity (34.3%) | Oropharynx (12.5%) |
| Other | Oral Cavity (22.2%) | Larynx (16.7%) | Nasopharynx (13.9%) |
| Total | Oral Cavity (58.4%) | Larynx (18.2%) | Oropharynx (13.3%) |
We next examined non-head and neck SPM distributions based on index tumor site (Table 4). Lung cancer was the most frequent non-head and neck SPM, regardless of primary HNSCC tumor site (38.3%), followed by prostate (12.4%), and colorectal tract (9.0%). Laryngeal and hypopharyngeal tumors were associated with a higher rate of lung SPM when compared to other index sites (p<0.001). Hypopharyngeal tumors also were associated with a significantly higher rate of esophageal SPM when compared to the other head and neck index sites (p<0.001).
Table 4.
Non-head and neck SPM distribution by primary site. Hypopharynx and larynx cancers had a higher percentage of lung SPM. Hypopharynx cancers had a higher percentage of esophageal SPM.
| Primary Site | Most common non-head and neck SPM subsite (%) | 2nd most common non-head and neck SPM subsite (%) | 3rd most common non-head and neck SPM subsite (%) |
|---|---|---|---|
| Oral Cavity | Lung (32.8%) | Prostate (12.2%) | Colorectal (8.9%) |
| Oropharynx | Lung (37.5%) | Prostate (13.2%) | Esophagus (7.3%) |
| Nasopharynx | Lung (35.8%) | Colorectal (10.9%) | Prostate (10.9%) |
| Hypopharynx | Lung (44.7%) | Esophagus (14.7%) | Colorectal (13.2%) |
| Larynx | Lung (43.3%) | Prostate (13.3%) | Colorectal (9.2%) |
| Other | Lung (39.4%) | Prostate (11.4%) | Colorectal (9.8%) |
| Total | Lung (38.3%) | Prostate (12.4%) | Colorectal (9.0%) |
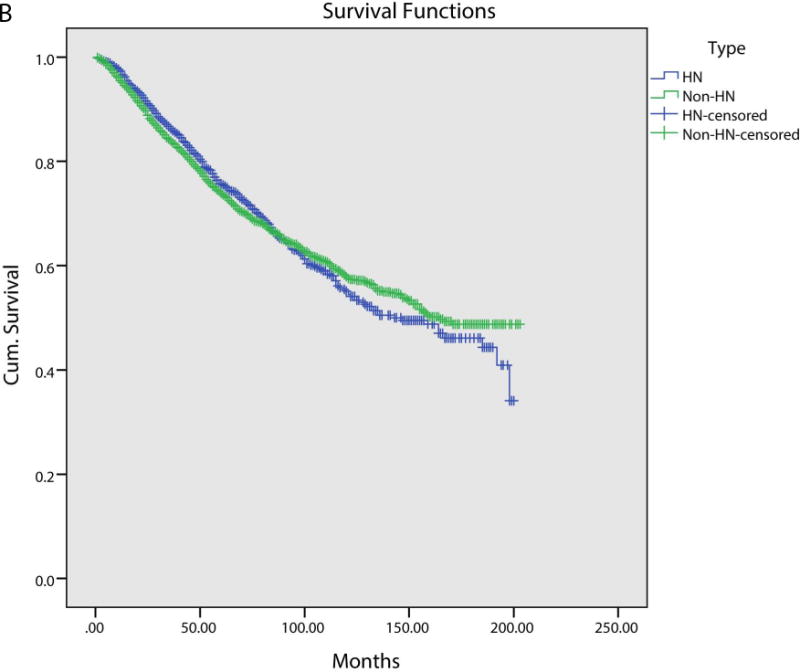
We next assessed for overall survival (OS) and disease specific survival (DSS), stratified between head and neck and non-head and neck SPMs. Patients with non-head and neck SPMs had significantly worse OS on a Kaplan-Meier analysis (Figure 1A). Median OS for head and neck SPMs was 84.0 months, compared to 60.0 months for non-head and neck SPMs (p<0.001). DSS did not show any difference between head and neck and non-head and neck SPM (Figure 1B). As patients with non-head and neck SPMS had worse survival, we performed a multivariate analysis to control for confounding variables. Variables included in this model were predictive in univariate analysis and included age, head and neck subsite and stage. On multivariate analysis, non-head and neck SPMs had worse survival (HR 1.21; 95% CI: 1.17 – 1.25).
Figure 1A.
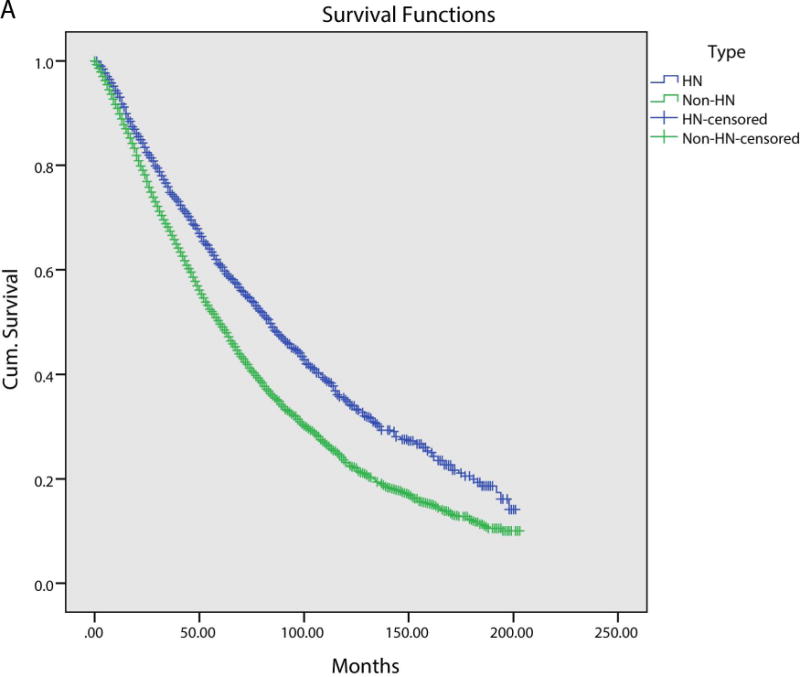
Figure 1B.
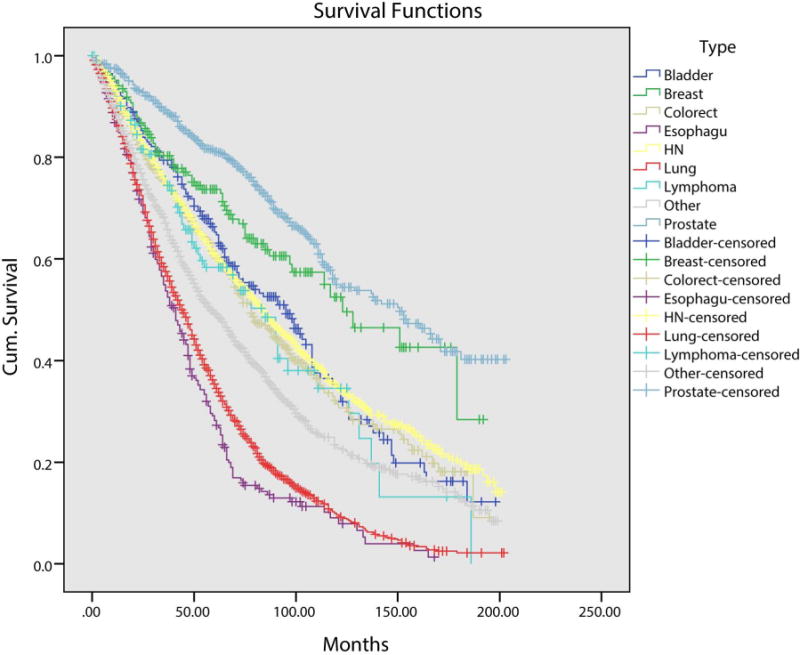
Given the difference in OS between head and neck and non-head and neck SPMs, we next calculated OS stratified by SPM site (Figure 2). Overall mean survival was 65 months. Patients with a lung (median OS 44 months) or esophageal SPM (median OS 41 months) had significantly worse OS when compared to patients with a head and neck SPM (median OS 84 months; p<0.001). Patients with a breast (median OS 123 months) or prostate SPM (median OS 151 months), conversely, had better OS when compared to head and neck SPM patients (p<0.001).
Figure 2.
DISCUSSION
In HNSCC patients who develop SPMs, nearly 75% are non-head and neck SPMs. Patients with non-head and neck SPMs have a lower OS, particularly those with esophageal and lung SPMs. Distribution of SPMs is highly variable, depending on the index tumor subsite. Future clinical practice guidelines should take the risks and locations of SPM development into consideration for screening.
Screening for SPMs is a critical step in HNSCC management both at the initial encounter and in subsequent cancer surveillance. Identification of a SPM at the time of the primary tumor work-up significantly changes management. Subsequent early identification of SPMs is important to provide timely treatment options in these high-risk patient populations. Currently, the standard initial work-up for primary HNSCC includes a complete head and neck exam, chest imaging, endoscopy, and possible positron emission tomography (PET), if indicated [6]. Panendoscopy at the time of primary HNSCC work-up is important for accurate staging and determination of appropriate treatment. While endoscopy also affords an opportunity to detect SPM in the head and neck, the benefit of panendoscopy for screening for SPMs has been a debated topic, with recommendations for and against endoscopy [9–13].
Follow-up screening guidelines for SPMs in patients with HNSCC are not explicit [5]. Current NCCN guidelines discuss screening intervals and suggest practitioners may use endoscopy, chest imaging, or PET scans for follow-up screening [6]. However, there are no explicit instructions on indications for follow-up endoscopy and screening for other tumors. Moreover, no subsite-specific recommendations have been established.
Previous research has explored the relationship of SPMs in head and neck cancers. A pooled multinational analysis of 99,527 head and neck cancer patients found 10,826 SPMs, with increased risk of head and neck and lung SPMs [14]. However, this study did not stratify SPMs by index tumor subsite. A single-institution study compared SPMs in oral cavity versus larynx squamous cell carcinomas, identifying more frequent head and neck SPMs in the oral cavity group and lung SPMs in the larynx group [15]. This study, however, did not examine patients with other HNSCC primary sites. A meta-analysis of 40,487 patients in 1992 found that the overall prevalence of SPMs was 14.2% in patients with HNSCC, including an approximate 4% rate in the head and neck [9]. According to an earlier analysis of the SEER database from 1975–2006, the most common location of SPMs in HNSCC was the lung and bronchus, followed by the oral cavity and the colorectal tract [16]. Hypopharyngeal cancer patients appeared to be at highest excess risk of solid tumor SPM development [17]. Depending on head and neck subsite, estimated five-year survival rates for HNSCC patients with a SPM ranged from 0–67%, compared to 36–46% for those without a SPM [18–22]. The difference became more drastic at 10 years, when patients diagnosed with a SPM had a 19–22% survival rate, compared to 55% for those without a SPM.
In this current study, the prevalence of a SPM in HNSCC was 11.7%, with the majority of SPMs found in non-head and neck sites. Second primary malignancy in non-head and neck sites was also associated with a worse OS. Particularly, SPMs in the lung and esophagus carried a significantly worse prognosis. Although patients with non-head and neck SPMs had a worse OS in our study, further analysis into the contributions of SPMs versus other potential factors, including comorbidities and smoking status, should be considered. Further analysis and prospectively maintained cohorts with robust follow-up after management for SPMs may aid in further delineating causes of death for patients with different SPMs.
Rate and distribution of SPMs in HNSCC is variable based on the primary HNSCC subsite. In our study, we found that laryngeal and hypopharyngeal primary SCCs had a significantly higher rate of lung SPMs than other HNSCCs, and that hypopharyngeal primary SCCs had a higher rate of esophageal SPMs. This is consistent with previous studies, some of which have noted higher rates of lung SPMs after laryngeal primary diagnosis, as well as the potential benefit of esophagoscopy for SPM in hypopharyngeal cancers [11,17].
As our data shows, there is a significantly higher proportion of non-head and neck SPM. On initial workup and subsequent monitoring of the patients, attention should be directed towards other potential sites for SPM. Cancers of the lung, prostate, and colorectal tract compose 38.3%, 12.4%, and 9.0% of non-head and neck SPMs in HNSCC patients. Moreover, identification of these non-head and neck SPMs is of particular importance as they may portend a worse prognosis (particularly lung and esophageal tumors).
Given the high prevalence of non-head and neck tumors in head and neck cancer patients, the head and neck surgeon should reaffirm with patients that they are undergoing adequate screening for these tumors, and should establish proper follow-up and care if a patient’s screening has been lacking. Specifically, lung cancer screening with annual chest x-ray or chest CT should be performed. Guidelines from the NCCN recommend annual CT chest in head and neck cancer patients with 20 or more pack-years of smoking [6]. This is of increased importance in patients with an index hypopharyngeal or laryngeal tumor, as they have higher rates of lung SPM. Strong consideration for esophagoscopy should be made in patients with a hypopharyngeal primary HNSCC given the high rates of esophageal SPM in these patients. Any concerning signs or symptoms could prompt either a transnasal esophagoscopy in clinic or a formal direct laryngoscopy and esophagoscopy in the operating room. Additionally, patients should follow the American Cancer Society guidelines for colorectal cancer screening, including flexible sigmoidoscopy every five years or colonoscopy every 10 years for patients 50 and over. Discussions for prostate cancer screening should be encouraged between the patient and his primary care provider. Overall, standardized screening policies for SPMs should be tailored to the primary HNSCC subsite given differences in SPM distribution and outcomes.
CONCLUSIONS
SPMs are identified in a high number of patients with HNSCC. The majority of these malignancies are of non-HNSCC etiology. Therefore, the head and neck surgeon must be acutely aware of these SPMs and discuss potential symptomatology and screening with patients. Chest imaging should be performed at regular intervals given the high incidence of SPMs in the lung, particularly in laryngeal and hypopharyngeal cancer patients. Future clinical practice guidelines should take the risks and locations of SPM development into consideration for screening.
Acknowledgments
The authors would like to acknowledge Brian E. Spector, MS, for statistical consultation.
Acknowledgment of grant support: Andrew C. Birkeland, M.D. and Steven B. Chinn, M.D., M.P.H. are research fellows funded on a T-32 training grant (T32 DC005356)
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to disclose
Conflict of interest: None
Presentation: This study was presented as a poster at the 2013 American Academy of Otolaryngology-Head and Neck Surgery Annual Meeting
References
- 1.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–13. doi: 10.1002/cncr.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–3. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 4.Cancer facts and figures 2014 American Cancer Society 2014
- 5.Manikantan K, Khode S, Dwivedi RC, Palav R, Nutting CM, Rhys-Evans P, Harrington KJ, Kazi R. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35:744–53. doi: 10.1016/j.ctrv.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 6.NCCN clinical practice guidelines in oncology. National Comprehensive Cancer Network; 2014. [DOI] [PubMed] [Google Scholar]
- 7.Harlan LC, Hankey BF. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21:2232–3. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 8.Warren S, G O. Multiple malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;51:1358–1414. [Google Scholar]
- 9.Haughey BH, Gates GA, Arfken CL, Harvey J. Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol. 1992;101:105–12. doi: 10.1177/000348949210100201. [DOI] [PubMed] [Google Scholar]
- 10.Davidson J, Gilbert R, Irish J, Witterick I, Brown D, Birt D, Freeman J, Gullane P. The role of panendoscopy in the management of mucosal head and neck malignancy-a prospective evaluation. Head Neck. 22:449–54. doi: 10.1002/1097-0347(200008)22:5<449::aid-hed1>3.0.co;2-l. discussion 454–5, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Guardiola E, Pivot X, Dassonville O, Poissonnet G, Marcy PY, Otto J, Poudenx M, Francois E, Bensadoun RJ, Thyss A, Demard F, Schneider M. Is routine triple endoscopy for head and neck carcinoma patients necessary in light of a negative chest computed tomography scan? Cancer. 2004;101:2028–33. doi: 10.1002/cncr.20623. [DOI] [PubMed] [Google Scholar]
- 12.Sun GH, Aliu O, Moloci NM, Mondschein JK, Burke JF, Hayward RA. Association between hospital case volume and the use of bronchoscopy and esophagoscopy during head and neck cancer diagnostic evaluation. Cancer. 2014;120:61–7. doi: 10.1002/cncr.28379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deleyiannis FW, Weymuller EA, Jr, Garcia I, Potosky AL. Geographic variation in the utilization of esophagoscopy and bronchoscopy in head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123:1203–10. doi: 10.1001/archotol.1997.01900110057008. [DOI] [PubMed] [Google Scholar]
- 14.Chuang SC, Scelo G, Tonita JM, Tamaro S, Jonasson JG, Kliewer EV, Hemminki K, Weiderpass E, Pukkala E, Tracey E, Friis S, Pompe-Kirn V, Brewster DH, Martos C, Chia KS, Boffetta P, Brennan P, Hashibe M. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer. 2008;123:2390–6. doi: 10.1002/ijc.23798. [DOI] [PubMed] [Google Scholar]
- 15.Lin K, Patel SG, Chu PY, Matsuo JM, Singh B, Wong RJ, Kraus DH, Shaha AR, Shah JP, Boyle JO. Second primary malignancy of the aerodigestive tract in patients treated for cancer of the oral cavity and larynx. Head Neck. 2005;27:1042–8. doi: 10.1002/hed.20272. [DOI] [PubMed] [Google Scholar]
- 16.Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22:671–9. doi: 10.1007/s10552-011-9739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29:739–46. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya N, Nayak VK. Survival outcomes for second primary head and neck cancer: a matched analysis. Otolaryngol Head Neck Surg. 2005;132:63–8. doi: 10.1016/j.otohns.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JS, Pajak TF, Rubin P, Tupchong L, Brady LW, Leibel SA, Laramore GE, Marcial VA, Davis LW, Cox JD. Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989;17:449–56. doi: 10.1016/0360-3016(89)90094-1. [DOI] [PubMed] [Google Scholar]
- 20.Erkal HS, Serin M, Amdur RJ, Villaret DB, Stringer SP, Mendenhall WM. Synchronous and metachronous squamous cell carcinomas of the head and neck mucosal sites. J Clin Oncol. 2001;19:1358–62. doi: 10.1200/JCO.2001.19.5.1358. [DOI] [PubMed] [Google Scholar]
- 21.Panosetti E, Luboinski B, Mamelle G, Richard JM. Multiple synchronous and metachronous cancers of the upper aerodigestive tract: a nine-year study. Laryngoscope. 1989;99:1267–73. doi: 10.1288/00005537-198912000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Vaamonde P, Martín C, del Río M, LaBella T. Second primary malignancies in patients with cancer of the head and neck. Otolaryngol Head Neck Surg. 2003;129:65–70. doi: 10.1016/S0194-59980300476-5. [DOI] [PubMed] [Google Scholar]


