Abstract
Introduction
To describe the psychometric properties of the Penn Parkinson’s Daily Activities Questionnaire-15 (PDAQ-15), a 15-item measure of cognitive instrumental activities of daily living for Parkinson’s disease (PD) patients derived from the original 50-item PDAQ.
Methods
PDAQ-15 items were chosen by expert consensus. Knowledgeable informants of PD participants (n=161) completed the PDAQ-15. Knowledgeable informants were defined as an individual having regular contact with the PD participant. PD participants were assigned a diagnosis of normal cognition, mild cognitive impairment, or dementia based on expert consensus.
Results
PDAQ-15 scores correlated strongly with global cognition (Dementia Rating Scale-2, r=0.71, p<0.001) and a performance-based functional measure (Direct Assessment of Functional Status, r=0.83; p<0.001). PDAQ-15 scores accurately discriminated between non-demented PD participants (normal cognition/mild cognitive impairment) and PD with dementia (ROC curve area=0.91), with and without any cognitive impairment (normal cognition versus mild cognitive impairment/dementia, ROC curve area=0.85) and between participants with mild cognitive impairment and dementia (ROC curve area=0.84).
Conclusions
The PDAQ-15 shows good discriminant validity across cognitive stages, correlates highly with global cognitive performance, and appears suitable to assess daily cognitive functioning in PD.
Introduction
Cognitive impairment in Parkinson’s disease (PD) is common and detrimental [1,2]. Cognitive deficits in PD patients with mild cognitive impairment (PD-MCI) impact the ability to perform instrumental activities of daily living (IADLs) [3,4], and impairments in PD dementia (PDD) have profound functional consequences [5–7]. Cognitive impairment in PD is a target of therapeutic interventions, and treatment benefits should reflect improvement in cognition and function, as required by the Food and Drug Administration (FDA) for new Alzheimer’s disease (AD) treatments [8].
The Penn Parkinson’s Daily Activities Questionnaire (PDAQ) [9] is an item-response theory (IRT)-based questionnaire designed to assess cognitive IADLs in PD patients across the cognitive spectrum. The PDAQ is a 50-item questionnaire completed by a knowledgeable informant (KI) of a PD patient, such as a spouse, child, or other individual close to the patient (e.g., paid caregiver). Initial psychometric testing of the PDAQ demonstrated strong test-retest reliability, construct validity, and was sensitive and specific to cognitive impairment in PD. The 50-item PDAQ takes approximately 10–15 minutes to complete, so an abbreviated version of the PDAQ would be useful as a brief instrument of IADL function for use in research and clinical care. We describe the psychometric properties of the PDAQ-15, a brief version of the PDAQ consisting of 15 of the 50 original items.
Methods
Item selection
Of the original 50 PDAQ items, 15 were chosen for inclusion in the PDAQ-15. Items were chosen by three study team members (A.S., J.R. and D.W.) based on 1) face validity for relevance to PDD, 2) diversity of content, and 3) range of difficulty of the activity derived from the original psychometric testing of the PDAQ. The items chosen can be found in supplementary materials available online. Both the KI version and a version for self-report by PD patients are included. In the present validation study, items were scored based on KI rating of the PD patient’s difficulty in performing each IADL on the following scale: “none,” “a little,” “somewhat,” “a lot,” “cannot do.” Each item is scored 0–4 (total score range= 0–60) with higher scores indicating better IADL function.
Psychometric testing
Agreement between the 50-item and 15-item versions of the PDAQ regarding ability estimates and additive scores (i.e., sum of individual item scores) were confirmed in the 50-item PDAQ development cohort. Subsequently, the PDAQ-15 was validated in the independent cohort described here. KIs completed the PDAQ-15 as part of the annual assessment process for PD patients enrolled in the University of Pennsylvania Morris K. Udall Center. Responses were obtained via paper administration. KIs were defined as an individual having regular contact with the PD patient. PD patients in the Udall Center undergo annual clinical evaluations performed by trained research staff. The University of Pennsylvania Institutional Review Board approved the study. Informed consent was obtained from all participants.
Motor examinations included Part III of the Unified Parkinson’s Disease Rating Scale (UPDRS) [10] and Hoehn and Yahr [11] staging. The Mattis Dementia Rating Scale-2 (MDRS-2) [12] was used to assess global cognition. Depression was assessed with the short form of the Geriatric Depression Scale (GDS-15) [13]. Regarding ADL assessment, a well-validated questionnaire developed for AD and commonly used in PD studies (Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory; ADCS-ADL) [14] was completed by KIs. Additionally, the Direct Assessment of Functional Status (DAFS) [15,16] was administered as a direct measure of everyday functioning in a subset of PD patients. The DAFS is a performance-based assessment of daily functioning administered in a structured format using props (e.g., checkbook, pillbox). Seven activities are assessed, including time orientation, communication, finances, shopping, grooming, eating, and medication management. The DAFS has demonstrated evidence of construct validity relative to other functional measures in older adults as well as excellent test-retest reliability. All PD patient evaluations were performed while in “on” state. PD participants were assigned a diagnosis of normal cognition, mild cognitive impairment or dementia based on agreement of two experts as part of diagnosis consensus process following the International Parkinson’s and Movement Disorder Society guidelines for PD-MCI and PDD [17,18]. Experts involved in the consensus diagnosis process were blinded to PDAQ-15 scores as well as DAFS scores. The consensus diagnostic process has been described in detail in previous publication using the Penn Morris K. Udall cohort [19].
Statistical Analysis
Internal consistency among the PDAQ-15 items was assessed using Cronbach’s alpha and item-total correlation analyses. Association between PDAQ-15 scores, 50-item PDAQ-scores and clinical measures were assessed using linear regression and partial correlation analysis using Pearson’s coefficient. Correlations between both ability estimates and additive scores were performed for the PDAQ-15 and 50-item PDAQ. Ability estimates are derived from item-response theory and indicate a respondent’s location on an underlying, latent trait (here, instrumental activities of daily living with cognitive demands). Additive or observed scores are based on classic test theory, and are simply the sum of a respondent’s scored responses. Although the PDAQ-15 is an abbreviated assessment which utilizes additive scoring, it was derived from the 50-item PDAQ which was developed using IRT. Therefore, we provide three indicators of the appropriate use of the shortened version. First, we correlated the ability estimates of both scales, and the additive scores of the scales. We then correlated the ability estimate of the 50-item PDAQ and additive score of the PDAQ-15. This final correlation was performed to determine if the primary 50-item PDAQ outcome was highly correlated with the primary PDAQ-15 outcome.
Regression and partial correlation were utilized to examine association between cognition and directly observed ADL function and the PDAQ-15 adjusting for age, gender, education and measures of motor function (i.e., UPDRS Part III). These analyses were performed to support construct validity and convergent validity of the PDAQ-15 regarding the scale’s ability to assess cognitive IADLs relative to established measures of function (ADCS-ADL, DAFS) and cognition (MDRS-2). Additionally, discriminant validity was assessed through examining differences in the strength of correlation among the ADCS-ADL, PDAQ-15 and UPDRS-III motor score. As the ADCS-ADL includes many basic ADLs dependent on motor function, the PDAQ-15 focuses on instrumental ADLs with a cognitive demand; therefore, these analyses aimed to provide support that the PDAQ-15 may be less affected by motor function than the ADCS-ADL. Receiver operating characteristic (ROC) analyses were performed to measure the ability of the PDAQ-15 to distinguish between subjects with and without dementia (normal cognition/MCI), as well as subjects with MCI versus dementia. Optimal cut-offs were defined as the greatest combined sensitivity and specificity, with sensitivity greater than 80%. All analyses were conducted without adjustment for multiple comparisons at a two-sided alpha = 0.05 significance level. Analyses were carried out using SPSS version 22.
Results
Participant Characteristics
A total of 161 PD patient and KI dyads participated in the study. Table 1 presents demographic data and clinical characteristics of the full sample as well as by cognitive diagnosis (i.e., normal cognition, MCI, or dementia). Consensus diagnosis of cognitive impairment indicated normal cognition in 43% of PD patients (n=69), 29% PD-MCI (n=47), and 28% dementia (n=45). KIs were 75.8% spouses/partners (n=122), 13.7% a child of the PD patient (n=22), and 10.6% (n=17) other. A subset of PD patients (n=62) completed the DAFS.
Table 1.
Sample Characteristics and Clinical Assessment Data by Consensus Diagnosis
| Diagnosis | Age (years) |
Education (years) |
% Male |
Age of onset (years) |
Disease Duration (years) |
LEDD | % on DA |
UPDRS- III |
H&Y | GDS-- 15 |
MDRS-- 2 |
ADCS-- ADL |
DAFS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Normal Cognition (n=69) |
70.81 (7.15) |
16.69 (2.16) |
71.0% | 63.57 (8.26) |
7.25 (5.08) |
708.82 (411.05) |
49.3% | 25.17 (11.58) |
2.00 (2.00– 3.00) |
2.64 (3.11) |
139.75 (3.20) |
72.75 (9.545) |
50.58 (2.24) *n=19 |
|
PD-MCI (n=47) |
72.72 (8.35) |
15.09 (2.35) |
70.2% | 63.15 (10.18) |
9.57 (5.90) |
871.21 (495.77) |
59.6% | 36.24 (12.99) |
2.00 (2.00– 3.00) |
3.64 (3.28) |
132.51 (5.81) |
66.48 (12.61) |
44.54 (7.59) *n=13 |
|
Dementia (n=45) |
77.04 (7.36) |
15.49 (2.81) |
80.0% | 66.51 (9.00) |
10.53 (5.47) |
859.51 (439.86) |
33.3% | 43.64 (14.52) |
3.00 (3.00– 4.25) |
5.22 (3.88) |
106.42 (18.45) |
40.82 (18.26) |
24.23 (12.57) *n=30 |
|
Total (n=161) |
73.11 (7.96) |
15.99 (2.54) |
73.3% | 64.27 (9.11) |
8.84 (5.59) |
798.34 (449.16) |
47.8% | 33.55 (1.19) |
3.00 (2.00– 3.00) |
3.60 (3.50) |
128.32 (17.44) |
62.46 (1.53) |
36.56 (15.46) *n=62 |
A subset of participants completed the DAFS. Sample sizes for each diagnostic group are provided.
Age, education, age of onset, disease duration, LEDD, UPDRS-III, GDS-15, MDRS-2, ADCS-ADL, and the DAFS all report: means (standard deviations). Gender (“% Male”) and percentage of patients on dopamine agonist therapy (“% on DA”) are represented as percentages. The H&Y results report median (interquartile range).
LEDD= Total levodopa equivalent daily dose; DA=dopamine agonist; UPDRS-III=Unified Parkinson’s Disease Rating Scale-Part III; H&Y=Hoehn & Yahr; GDS-15=Geriatric Depression Scale-15; MDRS-2=Mattis Dementia Rating Scale-2; ADCS-ADL=Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory; DAFS=Direct Assessment of Functional Status
Reliability
Items included in the PDAQ-15 demonstrated strong internal consistency (Cronbach’s alpha=0.97; item-total correlation coefficients ranged from 0.76–0.89). Association between the 50-item PDAQ ability estimates and PDAQ-15 ability estimates was very high (r=0.99, p<0.001). Association between the 50-item PDAQ additive scores and PDAQ-15 additive scores (r=0.99, p<0.001) was also very high. Association between the 50-item PDAQ ability estimates and the PDAQ-15 additive scores was also high (r=0.96, p<0.001).
Association with motor function, cognition, and direct assessment of daily function
Descriptive statistics for outcome measures for the full sample and by cognitive diagnosis are presented in Table 1, and additional information regarding the outcome measures can be found in supplementary materials available online. Correlations between the total PDAQ-15 and MDRS-2 scores were high (r=0.71, p<0.001). The PDAQ-15 was significantly correlated with UPDRS motor score (r=0.54, p <0.001); however, in a linear regression model controlling for age, gender, education, MDRS-2 score and UPDRS motor score, MDRS-2 score was a much stronger predictor of PDAQ-15 score than was UPDRS motor score (β=0.61, SE=0.06, p<0.001 vs. β= −0.26, SE=0.07, p=0.02).
The PDAQ-15 demonstrated greater specificity for cognitive performance compared to the ADCS-ADL. The ADCS-ADL was more strongly correlated with UPDRS part III in bivariate analysis (r=0.64; p<0.001) compared with the PDAQ-15 (r=0.54, p <0.001). The ADCS-ADL remained more strongly correlated with UPDRS part III after adjustment for MDRS-2 (r=0.43; p<0.001) compare with the PDAQ-15 (r=0.28, p<0.001). The lower correlation between the PDAQ-15 and UPDRS part III compared to the ADCS-ADL suggests the PDAQ-15 is less affected by motor demands.
The PDAQ-15 was strongly associated with directly observed daily function (i.e., DAFS score; r=0.83; p<0.001). After controlling for UPDRS motor score the PDAQ retained its strong correlation with the DAFS score (r=0.78; p<0.001), indicating that the association was not confounded by motor disability.
Discrimination between subjects by cognitive categorization
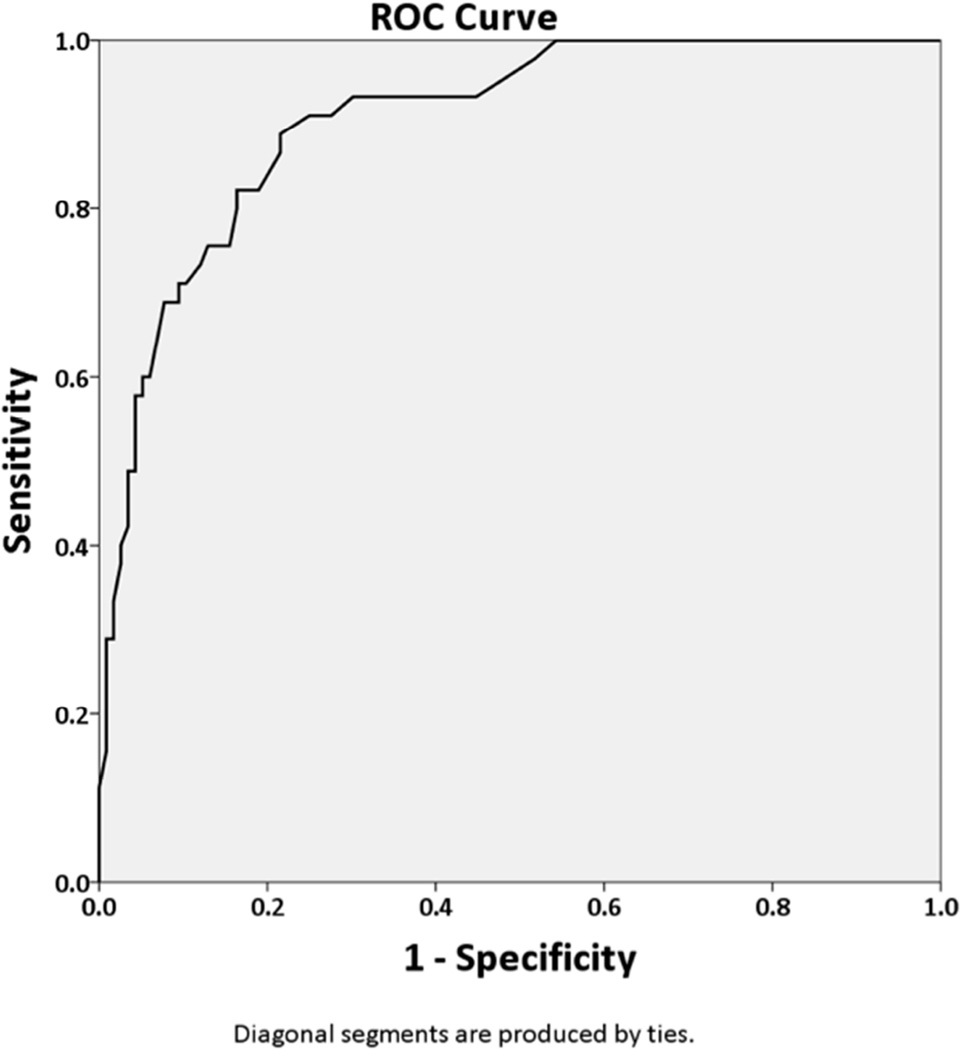
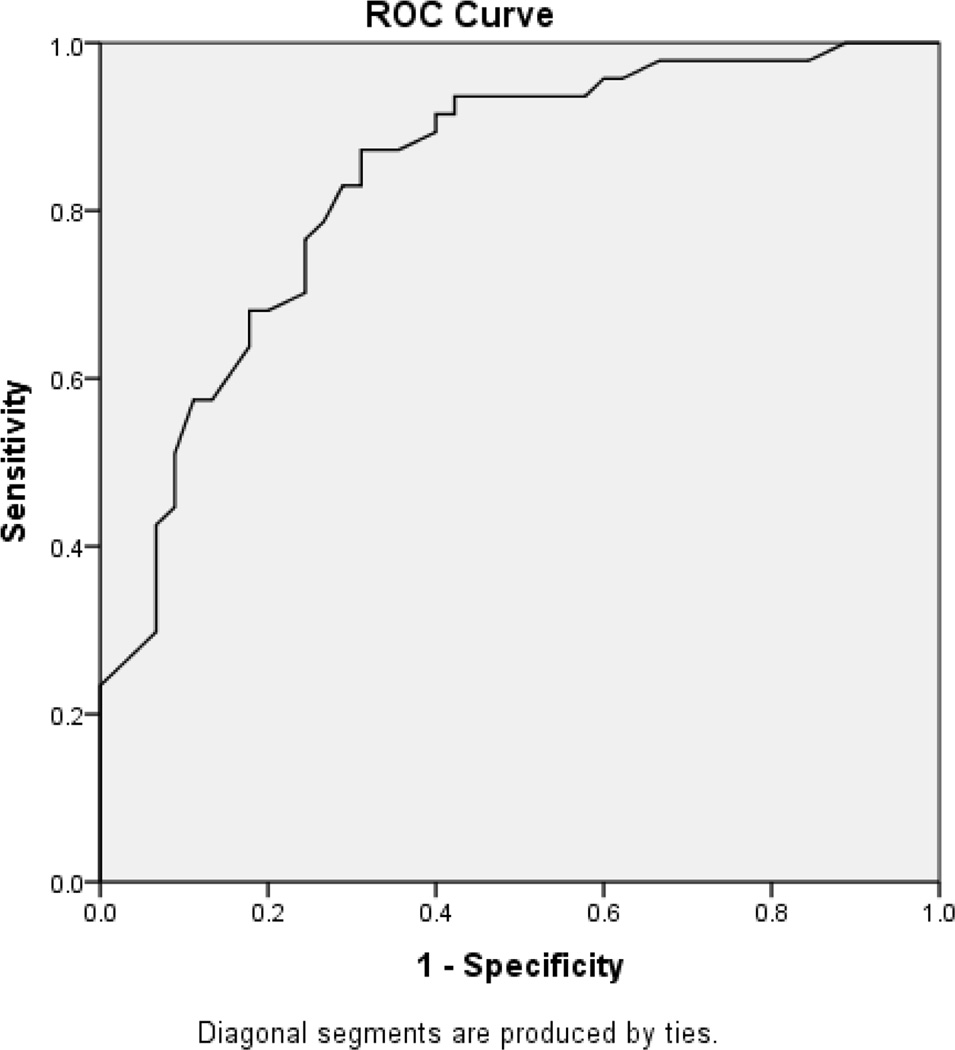
PDAQ-15 scores for PD patients with normal cognition, MCI, and dementia are presented in Table 1. Differences in scores across diagnostic groups were significant (F(2, 158)=80.79, p<0.001, η2=0.51). The cut-off that gave the best overall accuracy for the distinction between non-demented (normal cognition/MCI) and demented subjects was a score of 43 (sensitivity 82%, specificity 84%). Using a cutoff of 43, positive predictive value (PPV) was 0.66 (95% CI = 0.54, 0.78), and negative predicitive value (NPV) was 0.92 (95% CI= 0.87, 0.97). The optimal cutoff between MCI and dementia was a score of 37 (sensitivity 83%, specificity 71%). Using a cutoff of 37, PPV was 0.80 (95% CI = 0.68, 0.92) and NPV was 0.75 (95% CI = 0.63, 0.87).” Receiver operating characteristic (ROC) analysis for discrimination between intact, MCI and demented subjects are shown in Figure 1.
Figure 1. ROC curves for the distinction between subjects without (normal cognition/MCI) or with dementia (1A); and for the distinction between MCI subjects and dementia subjects (1B).
The area under the ROC curves were:
(A) For the distinction between subjects without (normal cognition/MCI) or with dementia was 0.91 (95% CI 0.86–0.95).
(B) For the distinction between MCI and dementia was 0.84 (95% CI 0.76–0.92).
Discussion
The PDAQ-15 demonstrates strong psychometric properties across the spectrum of cognitive impairment in PD. There is strong construct validity of the PDAQ-15 relative to global cognition, an existing measure of ADL function, and directly observed ADL function in PD patients, and good discriminant validity across the stages of cognitive impairment in PD. Global cognition was a stronger predictor of the PDAQ-15 score than motor symptom severity. Thus, the PDAQ-15 is potentially valuable for PD studies that seek to separate the impact of cognition from motor function on IADLs. This is particularly relevant for treatment studies that have the potential to improve motor performance, cognition, and function. It is also consistent with the FDA’s position for AD, in that therapeutic intervention studies should include improvements in both cognition and function [8].
In studies that include PDD patients, it is recommended that KIs complete the PDAQ-15. Research demonstrates that as patients progress into moderate or severe stages of dementia, they cannot provide reliable estimates of ADL function and health-related quality of life (HRQL) [20,21]. Additionally, greater cognitive impairment is associated with overestimation of ability to perform ADLs in PD [22]. This is a particularly important consideration for longitudinal studies, as a high percentage of PD patients eventually transition to dementia. As self-report of IADL function may be of interest in studies of PD patients with normal cognition or PD-MCI, future studies will compare KI-administration and self-report administration of the PDAQ-15.
Over 75 different ADL instruments have been published [23], however, many scales contain activities dependent on motor skills. Additionally, a smaller number of ADL scales have been tested in PD specifically [9,24,25]. To our knowledge, the only existing PD-specific IADL scale aside from the PDAQ is the Parkinson’s Disease Cognitive Functional Rating Scale (PD-CFRS) [26], a 12-item questionnaire administered in KI interview format. Initial validation of the PD-CFRS demonstrated adequate reliability and discriminant validity across stages of cognitive impairment in PD. Advantages of the PDAQ-15 include derivation from the original 50-item PDAQ, which was developed utilizing item-response theory, and the strong psychometric properties of the original PDAQ in a large sample of patients. The abbreviated assessment (i.e., 15 vs. 50 items) allows clinicians and researchers to quickly assess the severity of IADL impairment in clinical settings.
Although the PD-CFRS is a psychometrically sound available option, there are several reasons why the PDAQ-15 is a useful addition to the literature. From a psychometric perspective, it is important to have several measures that are intended to measure the same construct. The existence of different scales is a means to determine other psychometric properties of each test, such as convergent or divergent validity. Additionally, data collection for the original PDAQ project began in 2007 with funding as a Morris K. Udall Center of Excellence, which precedes the publication of the PD-CFRS (2013). This in part made possible our large sample size (n=161), which is nearly three times the size of the PD-CFRS validation sample (n=53). Also from a psychometric perspective, the PD-CFRS utilizes a 3-point Likert scale, while the PDAQ-15 using a 5-point Likert scale, allowing for a wider range of ability to be estimated by KIs. There are also important differences between the PD-CFRS and the PDAQ-15 regarding cultural factors. Some items of the PD-CFRS, such as use of “public transport,” are uncommon in the majority of the United States where personal automobile transportation is dominant. This also highlights why it is beneficial to have options have assessing a specified construct, as cultural factors may impact the population being examined.
This study has several limitations. Studies demonstrating the PDAQ-15 is sensitive to treatment effects and change over time are needed. With regard to cognitive assessment, in the present study we used a measure of global cognition. Global cognitive measures may be susceptible to ceiling effects in PD, as primary deficits in executive functioning may not be fully captured. Additionally, all clinical assessments were performed in the “on” state, and results may have been impacted if PD patients were assessed in the “off” state. Regarding reliability, test-retest and inter-rater reliability of the PDAQ-15 should be examined in future research.
The PDAQ-15 demonstrates strong psychometric properties as an abbreviated version of the 50-item PDAQ. Future research is needed to replicate these results and examine the ability of the PDAQ-15 to assess responsiveness to therapeutic interventions as well as ability to assess changes in IADL function over time.
Supplementary Material
Table 2.
PDAQ-15 scores by cognitive diagnosis
| N | Mean | SD | 95% CI for Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|
| Lower Bound |
Upper Bound |
||||||
| Normal cognition | 69 | 54.35 | 7.88 | 53.45 | 56.24 | 15 | 60 |
| MCI | 47 | 45.96 | 11.89 | 42.47 | 49.45 | 8 | 60 |
| Dementia | 45 | 26.16 | 15.58 | 21.47 | 30.84 | 0 | 55 |
| Total | 161 | 44.02 | 16.45 | 41.46 | 46.58 | 0 | 60 |
HIGHLIGHTS.
The PDAQ-15 is a brief measure of daily function dependent on cognition.
It was derived from the original 50-item PDAQ.
It correlates highly with measures of global cognitive abilities.
It correlates highly with other existing measures of daily function.
It has good discriminant validity across cognitive stages in Parkinson’s disease.
Acknowledgments
The PDAQ is copyright-protected by the University of Pennsylvania. Use of the PDAQ in investigational studies sponsored in whole or part by for-profit entities or for commercial purposes by any entities is prohibited without express written consent of the University of Pennsylvania.
Study Funding
This study was funded by a Morris K. Udall Parkinson’s Disease Research Center of Excellence grant from NINDS (NS-053488) and by SAP4100027296, a health research grant awarded by the Department of Health of the Commonwealth of Pennsylvania from the Tobacco Master Settlement Agreement under Act 2001-77.
Andrew Siderowf is a full time employee of Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Co.
Jacqueline Rick is supported by a Morris K. Udall Parkinson’s Disease Research Center of Excellence grant from NINDS (NS-053488).
Nabila Dahodwala receives research support from the NIH (K23AG034236), the National Parkinson Foundation and the Parkinson Council.
John E. Duda receives research support from the Department of Veterans Affairs, the National Institutes of Health, and the Michael J. Fox Foundation for Parkinson’s Research.
Howard Hurtig receives salary support from NINDS P50 NS053488 and royalties from UpToDate.
Matthew Stern serves as a consultant for Adamas, Civitas, Merz, Teva and is an officer of the International Parkinson and Movement Disorder Society.
Sharon X. Xie is supported by a Morris K. Udall Parkinson’s Disease Research Center of Excellence grant from NINDS (NS-053488) and by NIH grant # AG10124.
Lior Rennert is supported by NIH Mental Health Training Grant T32MH065218.
Jason Karlawish is on the Professional Advisory Board for Magellan Health and is a co-holder of a license of an Integrated NeuroDegenerative Disease Database developed at the University of Pennsylvania. He receives royalties for "Do We Have a Pill for That: Treating Dementia," Johns Hopkins University Press. He is supported by NIH grants AG10124, AG038440, AG10483, AG025152; CDC grant U48DP005053, and an investigator initiated grant from the Alzheimers Association.
John Q. Trojanowski serves as an Associate Editor of Alzheimer's & Dementia. He may accrue revenue on patents submitted by the University of Pennsylvania wherein he is inventor including and he is co-inventor on patents submitted the University of Pennsylvania wherein he is inventor that have generated income he has received from the sale of Avid to Eli Lily. Finally, he receives research support from the NIH (AG 10124, AG 17586, AG-19724AG 024904, NS053488, AG029213), Janssen, GSK, Cure PSP and the Michael J. Fox Foundation.
Daniel Weintraub has received research support from Boehringer Ingelheim, National Institutes of Health, and Penn Center for Excellence in Research on Neurodegenerative Diseases (CERND). He has received consulting fees or honoraria from: Acadia Pharmaceuticals, Boehringer Ingelheim, General Electric, Merck Serono, Novartis Pharmaceuticals, Pfizer, Sanofi Aventis, Johnson and Johnson, and Solvay. supported by a Morris K. Udall Parkinson’s Disease Research Center of Excellence grant from NINDS (NS-053488) and by NIH grant # NS065087, and by the Michael J. Fox Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work performed while at American Institute of Certified Public Accountants
Statistical Analysis
The statistical analysis was conducted by Daniel Weintraub, Laura Brennan, and Sharon Xie. Affiliations are listed above.
Disclosures
Laura Brennan has no disclosures.
Jonathan D. Rubright has no disclosures.
Judy Shea has no disclosures.
Author Contributions
Laura Brennan: Drafting and revisions of manuscript for content, study concept, interpretation of data.
Andrew Siderowf: Drafting of manuscript, study concept or design, analysis and interpretation of data, statistical analysis, study supervision.
Jonathan D. Rubright Revising the manuscript for content, analysis and interpretation of data, statistical analysis.
Jacqueline Rick: Study design and concept, analysis and interpretation of data, critical review of manuscript.
Daniel Weintraub: Revising the manuscript for content, study concept or design, analysis and interpretation of data.
Nabila Dahodwala: Revising the manuscript for content, analysis and interpretation of data.
Howard Hurtig: Revising the manuscript for content, analysis and interpretation of data.
Matthew Stern: Revising the manuscript for content, analysis and interpretation of data.
John E. Duda: Revising the manuscript for content, analysis and interpretation of data.
Sharon X. Xie: Statistical analysis, and revising the manuscript for content, analysis and interpretation of data.
Lior Rennert: Statistical analysis, and revising the manuscript for content, analysis and interpretation of data.
Jason Karlawish: Revising the manuscript for content, analysis and interpretation of data, study concept and design, study supervision or coordination
John Q. Trojanowski: Revising the manuscript for content, analysis and interpretation of data.
Judy Shea: Revising the manuscript for content, analysis and interpretation of data, study concept and design, study supervision or coordination
References
- 1.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J. Neurol. Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Bronnick K, Williams-Gray C. Mild cognitive impairment in Parkinson disease A multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin RC, Triebel KL, Kennedy RE, Nicholas AP, Watts RL, Stover NP, et al. Impaired financial abilities in Parkinson’s disease patients with mild cognitive impairment and dementia. Parkinsonism Relat. Disord. 2013;19:986–990. doi: 10.1016/j.parkreldis.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirogovsky E, Schiehser DM, Obtera KM, Burke MM, Lessig SL, Song DD, et al. Instrumental activities of daily living are impaired in Parkinson’s disease patients with mild cognitive impairment. Neuropsychology. 2014;28:229–237. doi: 10.1037/neu0000045. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov. Disord. 2010;25:1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronnick K, Ehrt U, Emre M, De Deyn PP, Wesnes K, Tekin S, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2006;77:1136–1142. doi: 10.1136/jnnp.2006.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahn D, Sullivan E, Shear P, Pfefferbaum A, Heit G, Silverberg G. Differential Contributions of Cognitive and Motor Component Processes to Physical and Instrumental Activities of Daily Living in Parkinson’s Disease. Arch. Clin. Neuropsychol. 1998;13:575–583. [PubMed] [Google Scholar]
- 8.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N. Engl. J. Med. 2013;368:1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 9.Brennan L, Siderowf A, Rubright J, Rick J, Dahodwala N, Duda JE, et al. Development and initial testing of The Penn Parkinson’s Daily Activities Questionnaire. Mov. Disord. 2015 doi: 10.1002/mds.26339. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahn S, Elton R. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent Dev. Park. Dis. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 11.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 12.Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 13.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink T, editor. Clin. Gerontol. A Guid. to Assess. Interv. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 14.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer’s Dis. Assoc. Disord. 1997 [PubMed] [Google Scholar]
- 15.Loewenstein DA, Amigo E, Duara R, Guterman A, Hurwitz D, Berkowitz N, et al. A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. J. Gerontol. 1989;44:P114–P121. doi: 10.1093/geronj/44.4.p114. [DOI] [PubMed] [Google Scholar]
- 16.Loewenstein DA, Bates CB. The direct assessment of functional status revised (DAFSR). Manual for administration and scoring. Neuropsychological Laboratories and the Wien Center for Alzheimer’s Disease and Memory Disorders, Mount Sinai Medical Center. 2006 [Google Scholar]
- 17.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, a Broe G, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov. Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 18.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pigott K, Rick J, Xie SX, Hurtig H, Chen-plotkin A, Duda JE, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85:1276–1282. doi: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coucill W, Bryan S, Bentham P, Buckley A. A. Laight, EQ-5D in patients with dementia: an investigation of inter-rater agreement. Med. Care. 2001;39:760–771. doi: 10.1097/00005650-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Boyer F, Novella JL, Morrone I, Jolly D, Blanchard F. Agreement between dementia patient report and proxy reports using the Nottingham Health Profile. Int. J. Geriatr. Psychiatry. 2004;19:1026–1034. doi: 10.1002/gps.1191. [DOI] [PubMed] [Google Scholar]
- 22.Shulman LM, Pretzer-Aboff I, Anderson KE, Stevenson R, Vaughan CG, Gruber-Baldini AL, et al. Subjective report versus objective measurement of activities of daily living in Parkinson’s disease. Mov. Disord. 2006;21:794–799. doi: 10.1002/mds.20803. [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Cohen AS. Equating health status measures with item response theory: illustrations with functional status items. Med. Care. 2000;38:II43–II59. doi: 10.1097/00005650-200009002-00008. [DOI] [PubMed] [Google Scholar]
- 24.Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov. Disord. 2002;17:867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 25.Marras C, Tröster AI, Kulisevsky J, Stebbins GT. The tools of the trade: a state of the art “How to Assess Cognition” in the patient with Parkinson’s disease. Mov. Disord. 2014;29:584–596. doi: 10.1002/mds.25874. [DOI] [PubMed] [Google Scholar]
- 26.Kulisevsky J, Fernández de Bobadilla R, Pagonabarraga J, Martínez-Horta S, Campolongo A, García-Sánchez C, et al. Measuring functional impact of cognitive impairment: validation of the Parkinson’s disease cognitive functional rating scale. Parkinsonism Relat. Disord. 2013;19:812–817. doi: 10.1016/j.parkreldis.2013.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.