Abstract
Although substantial evidence has demonstrated that parity and 17β-estradiol (E2) reduce mammary carcinogenesis, it is not clear how this protection is conferred. Thus, we examined the effects of parity and E2 treatment in the mammary glands of ovariectomized 15 week-old virgin mice, 15 week-old primiparous mice, and 9 month-old retired breeders. E2 treatment significantly increased lipid peroxidation, protein carbonylation, and protein nitrosylation in the virgin mice, but not in the age-matched primiparous mice or retired breeders. Mammary gland expression of the oxidative stress response protein Cu/Zn superoxide dismutase was consistently reduced in all of the E2-treated mice regardless of parity. Expression of the oxidative stress and DNA repair protein apurinic endonuclease (Ape1) was significantly increased only in the mammary glands of the E2-treated retired breeders. These findings suggest that E2 and parity help to reduce mammary oncogenesis by maintaining the structure and function of proteins, lipids, and DNA.
Keywords: 17β-estradiol, estrogen receptor α, superoxide dismutase, apurinic endonuclease, oxidative stress, mammary gland
1. Introduction
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancerrelated deaths of women in the United States (DeSantis et al 2015). This disease annually affects ~1.7 million women globally (Torre et al 2015). Thus, breast cancer represents a substantial and formidable worldwide health concern.
Epidemiological studies have demonstrated that a woman’s risk of developing breast cancer is related to her reproductive history and that women who have had a child have a reduced risk of developing this disease (MacMahon et al 1970, Russo et al 2005). This protective effect of pregnancy has been recapitulated in rodents where parity significantly reduces chemically-induced mammary carcinogenesis (Medina and Smith 1999, Thordarson et al 1995, Yang et al 1999, Blakely et al 2006). Furthermore, pregnancy-associated lactation is also associated with a reduced incidence of breast cancer and longer periods of lactation are associated with prolonged periods of disease free survival (Kwan et al 2015, Collaborative Group on Hormonal Factors in Breast Cancer 2002).
It has been proposed that pregnancy-induced changes in the hormonal milieu cause persistent changes in mammary gland gene expression that may help to explain the basis of parity-induced protection (Blakely et al 2006, Sivaraman et al 1998, Ginger et al 2001). Treatment of rodents with the steroid hormones progesterone and 17β-estradiol (E2), which rise dramatically during pregnancy, reduces the incidence of mammary cancer in rodents that have been exposed to a chemical carcinogen (Blakely et al 2006, Sivaraman et al 1998, Ginger et al 2001). In fact, E2 alone significantly reduces the incidence of chemically-induced mammary cancer (Rajkumar et al 2001). Moreover, recent analysis of the Women’s Health Initiative findings demonstrate that women who begin estrogen replacement therapy at the time of menopause have a significantly reduced risk of breast cancer (LaCroix et al 2011). Taken together, these studies suggest that parity and E2 confer protection in the mammary glands of both humans and rodents.
E2 mediates its genomic effects by binding to estrogen receptors α (ERα) and β (ERβ) and interacting with estrogen response elements (EREs) in DNA to modulate gene expression (Klinge 2001). ERα plays a critical role in mediating the effects of E2 in the mammary gland and is required for ductal elongation and mammary gland development (Bocchinfuso and Korach 1997). A clear indication of the importance of this receptor in mammary gland development is the failure of mammary ducts to elongate in ERα-null mice (Hewitt and Korach 2003).
Reactive oxygen species (ROS) are normal by-products of cellular metabolism and, as cellular proliferation increases, so too does ROS production. Accumulation of ROS can damage proteins, lipids, and DNA and has been linked to age-related degeneration and disease (Finkel and Holbrook 2000, Klaunig et al 2010, Curtis et al 2010). We previously showed that the oxidative stress response proteins Cu/Zn superoxide dismutase 1 (SOD1) and apurinic endonuclease (Ape1) interact with endogenously-expressed ERα in MCF-7 breast cancer cells, enhance the association of ERα with EREs in native DNA, and alter estrogen-responsive gene expression (Rao et al 2008, Curtis et al 2009, Schultz-Norton et al 2011). In addition, both SOD1 and Ape1 are overexpressed in invasive breast cancer cells compared to normal mammary tissue (Curtis et al 2010). To understand how pregnancy and exposure to E2 influence mammary gland biology, we examined expression of SOD1 and Ape1 and markers of protein and lipid damage in the mammary glands of ovariectomized virgin mice, primiparous mice, and retired breeders that had or had not been exposed to E2.
2. Materials and Methods
2.1 Mice
The C57BL/6J mice utilized included 15 week-old virgin female mice, 15 week-old female mice that had completed one full-term pregnancy, gone through 3 weeks of lactation and 10 days of involution, and 9 month-old retired breeders. All mice were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained on a 12h light/dark schedule with access to water and food ad libitum.
2.2 Ovariectomy and silastic tubing implantation
Mice were anesthetized by inhalation of 4% isoflurane, bilaterally ovariectomized, and implanted subcutaneously with silastic tubing (0.062 in/0.125 in, inner/outer diameter,2.7 cm in length; Dow Corning, Midland, MI) that was plugged at both ends with medical adhesive (Dow Corning). Tubing contained 35 µl cottonseed oil (vehicle) or 35 µl of cottonseed oil with 180 µg/ml E2, which produces a low physiological level of circulating E2 (~25 pg/ml) and is equivalent to estrous levels of this hormone in mice (Dubal et al 2001, Wise et al 1981, Nelson et al 1992, Couse et al 1995). Seven days after ovariectomy and implantation of silastic tubing, oil- and E2-treated mice were anesthetized with isoflurane, euthanized by decapitation, and mammary glands were harvested for Western blot analysis or immunofluorescent imaging. Ovariectomized mice were maintained on phytoestrogen-free chow. Tissue was harvested from mice in accordance with guidelines of the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee and Division of Animal Resources.
2.3 Immunofluorescent microscopy imaging
Mammary glands were fixed overnight at room temperature in 4% paraformaldehyde, processed, embedded in paraffin, and sectioned into 7 µm slices. Paraffin was removed and sections were rehydrated and incubated in antigen retrieval buffer (10 mM citric acid, pH 6) for 10 min at 95°C. Sections remained in the antigen retrieval buffer for 2 h as the solution cooled and were then washed 3× with PBS. Nonspecific binding was blocked by incubating sections in blocking buffer (PBS with 5% normal donkey serum and 0.05% Tween-20) for 10 min at room temperature. Sections were then incubated in blocking buffer with a PR- (1:100, RM-9102, Thermo Scientific, Pittsburgh, PA), SOD1- (1:100, sc11407, Santa Cruz Biotechnologies), or Ape1- (1:100, sc9919, Biotechnologies, Santa Cruz, CA) specific antibody overnight at 4°C. Tissue sections were then washed 3× with PBS-T (PBS containing 0.1% Tween-20) and incubated with DyLight 488-, or 549- conjugated anti-rabbit or anti-mouse IgG (1:500, Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 1 h in the dark at room temperature, washed 3× with PBS-T, incubated with DAPI nucleic acid stain for 10 min at room temperature, washed 3× with PBS, and mounted with Pro-Long Gold antifade reagent (Life Technologies, Grand Island, NY). DAPI co-staining was included for each treatment to identify nuclei and ensure that similar numbers of cells were present. Control slices, which had not been exposed to primary antibody, were processed in parallel.
2.4 Immunohistochemistry
Paraffin-embedded blocks were sectioned and mounted on charged slides. The 5–7 µm sections were deparaffinized in xylene and rehydrated through a series of graded alcohols. Slides underwent antigen retrieval and were washed with 1× PBS. Endogenous peroxidases were blocked with 3% hydrogen peroxide in 1× PBS for 20 min at 25°C. After three 5 min washes in 1× PBS, slides were incubated in blocking solution (1× PBS with 0.05% Tween-20 and 5% normal donkey serum) for 10 min at 25°C. Control (no primary antibody) and experimental slides were incubated overnight at 4°C, respectively, in blocking solution alone or blocking solution with an ERα- (1:600, ab31312, Abcam Inc., Cambridge, MA) or Ki67- (1:100, #9449, Cell Signaling Technology, Danvers, MA) specific antibody. Biotin-conjugated secondary antibody (1:200; Jackson ImmunoResearch, West Grove PA) was added and slides were incubated at 25°C for 30 min and then washed three times with 1× PBS. The ABC Peroxidase Staining kit (32020; Thermo Scientific, Rockford IL) was applied at 25°C for 30 min. After 3 washes with 1× PBS, staining was visualized with peroxidase-sensitive Sigmafast 3,3’-Diaminobenzidine tablets (DAB; Sigma, St. Louis MO). Exposure times were synchronized so that all tissues samples within an antibody group were exposed to DAB for the exact same time. All slides were counterstained with 0.1% methyl green (Sigma, St. Louis MO) for 3 min at 60°C, dehydrated in ethanol, cleared in xylene and mounted with Permount (Fisher Scientific, Pittsburgh PA).
2.5 Image collection
For immunofluorescence, all images were obtained with a 40× oil-immersion objective using a Leica DMI4000 B confocal microscope and Leica TCS SPE system and Application Suite Advanced Fluorescence software (Leica Microsystems, Inc., Bannockburn, IL). Detector gain and offset, laser power, and bandwidth of emission collection were kept constant for all treatments in each experiment and adjusted so that images had a full range of pixel intensities (0–255) and saturation was minimized.
For immunohistochemistry, images were obtained at 40× using a Leica DMI4000B confocal microscope with the Retiga 2000R digital camera. Exposure times were kept constant for all samples.
2.6 Preparation of mammary gland extracts and Western blot analysis
Each mammary gland was combined with 500 µl RIPA buffer (Thermo Scientific) and 1× Protease Inhibitor Cocktail (Sigma, St. Louis, MO) and homogenized for 10 seconds at low speed with a Pro Homogenizer (ProScientific Inc., Oxford, CT). The buffer was adjusted to 400 mM NaCl with 5 M NaCl, placed on ice, and vortexed every 5 min for 15 min. The lysates were centrifuged at 14,000 × g for 20 minutes at 4°C and the protein concentration of each supernatant was determined using the bicinchoninic acid (BCA) assay (Thermo Scientific) with bovine serum albumin (BSA) as a standard. 15 µg lysate was loaded onto each lane of denaturing 4–20% gradient gels and fractionated. Proteins were transferred to a nitrocellulose membrane and blots were probed with an SOD1- (sc11407, Santa Cruz Biotechnologies), Ape1- (ab194, Abcam Inc.), or β-actin (#3700, Cell Signaling Technology, Danvers, MA) specific antibody. Western blots were imaged and quantitated with a Licor Odyssey Infrared Imaging System.
2.7 Lipid peroxidation assay
Mammary glands (~30 mg tissue) were homogenized on ice in 1 ml of 3’4-methylenedioxyamphetamine (MDA) Lysis Buffer (Abcam Inc.) with 3 µl butylated hydroxytoluene (BHT, 100×, Abcam Inc.) and centrifuged at 13,000×g for 10 min at 4° C to remove insoluble material. 200 µl supernatant or standards containing 0, 4, 8 12, 16 or 20 nmol MDA were combined with 600 µl of thiobarbituric acid solution composed of 375 µl 20% acetic acid solution (pH 3.5) and 225 µl of 1.33% thiobarbituric acid and incubated at 95°C for 60 min. After incubation, 200 µl of each sample or standard (0, 4, 8, 12, 16, 20 nmol MDA) was transferred to a 96 well plate in triplicate and read at 532 nm by a SpectraMax 340 PC Microplate Reader.
2.8 Protein carbonylation assay
Protein carbonylation was quantified using the OxiSelect™ Protein Carbonyl ELISA Kit (STA-310, Cell Biolabs, San Diego, CA). Briefly, BSA standards or whole cell extracts (10 µg/ml) were adsorbed onto a 96-well plate for 2 hrs at 37°C. The protein carbonyls present in whole cell extracts or standards were derivatized to DNP hydrazone and probed with an anti-DNP antibody, followed by an HRP conjugated secondary antibody. The protein carbonyl content in the unknown sample was determined by comparing with a standard curve that was prepared from predetermined reduced and oxidized BSA standards.
2.9 Nitrosylation assay
Protein samples (20 µl containing 40 µg protein) were fractionated on denaturing gels, electroblotted onto nitrocellulose membranes, incubated with 2,4-dinitrophenylhydrazine (DNPH), and the derivatized proteins were detected with an anti-nitrotyrosine antibody (06-284, EMD Millipore Corporation, Billerica, MA). In addition, a replicate gel was run in parallel and subjected to Western blot analysis with a β-actin specific antibody.
2.10 Statistics
Data from the indicated number of independent experiments were combined and are expressed as the mean ± SEM. Statistical analysis was performed using unpaired t tests where a p value of < 0.05 was considered statistically significant.
3. Results
3.1 Effect of E2 on mammary gland expression of ERα and progesterone receptor (PR)
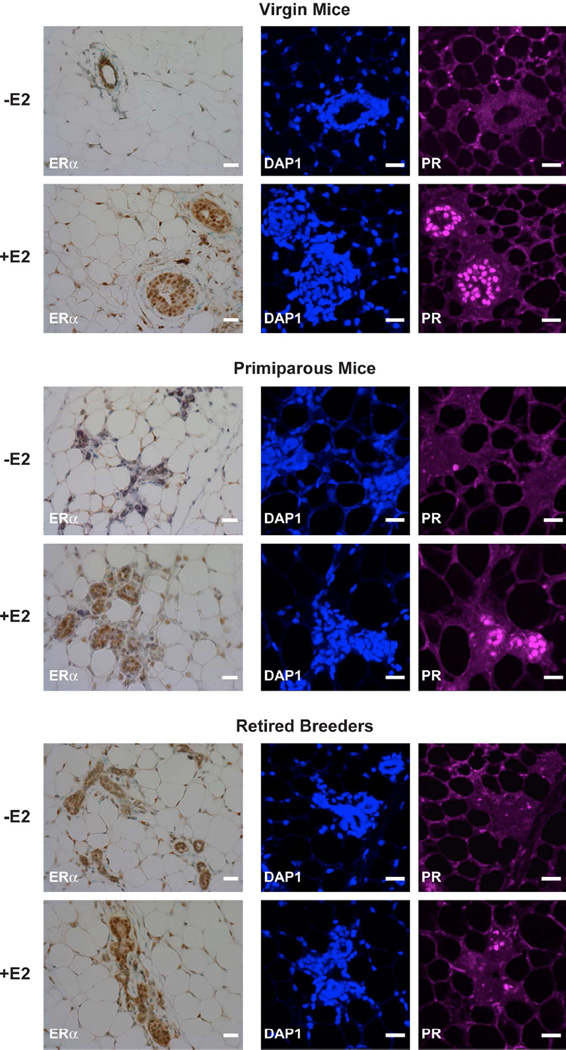
Because of its profound effects on the mammary gland, we first examined the expression of ERα in 15 week-old virgin mice, 15 week-old primiparous mice, and 9 month-old retired breeders. All of the mice were ovariectomized and implanted with silastic tubing containing oil or oil with E2. After 7 days, the mammary glands of the oil- and E2-treated mice were subjected to immunohistochemical analysis. ERαprotein expression was most prominent in the mammary epithelial cells (Fig. 1). When the animals were exposed to E2 for 7 days, ERα protein expression was significantly increased.
Fig. 1. E2 treatment increases PR expression in the mammary gland.
Ovariectomized female mice were treated with oil or E2 for 7 days. ERα and PR expression was examined in mammary gland sections of 15 week-old virgin mice, 15 week-old primiparous mice, and 9 month-old retired breeders using immunohistochemistry and immunofluorescent microscopy. Methyl green or DAPI counter staining was included to identify cell nuclei for immunohistochemistry and immunofluorescence, respectively. Scale bars indicate 25 µm. Images from 6 mice were collected in each group. Representative images are shown.
To determine whether our E2 treatment was effective in enhancing estrogen-responsive gene expression, the level of PR expression was examined. Little PR staining was observed in mammary glands of the oil-treated mice. However, when virgin and age-matched primiparous mice were treated with E2, PR expression was substantially increased. When retired breeders were treated with E2, more modest increases were observed in PR expression in these animals. Thus, although retired breeders expressed PR, the ability of E2 to induce PR expression was diminished compared to virgin and primiparous mice. It is also worth noting that while the virgin mice had rounded glandular structures, the glands of the primiparous and retired breeders were more collapsed in appearance. These findings are consistent with previous reports of ovariectomized, oil- and E2-treated animals (Thordarson et al 1995, Shyamala et al 2002) and studies of ERα knock out mice, which demonstrated that PR expression is dependent on ERα (Kurita et al 2001).
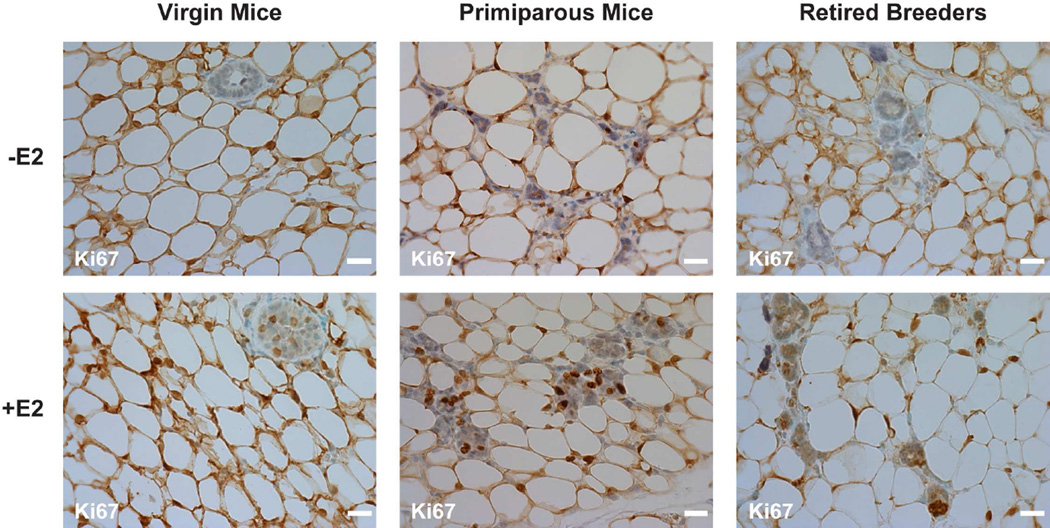
3.2 Effect of E2 on proliferation
To determine whether E2 altered cell proliferation, immunohistochemistry was used to stain mammary gland sections with an antibody to Ki67, a common marker of proliferating cells. Ki67 staining increased in the mammary glands of all of the mice that had been treated with E2 (Fig. 2), but had no effect on the percent of Ki67-stained cells in the lymph nodes of these same animals (Data not shown). Although proliferation was observed in epithelial cells in the mammary glands of the E2-treated animals, no apparent differences were detected in the overall size of the mammary glands of oil- and E2-treated mice (Data not shown). This apparent discrepancy could be due to the fact that the mammary gland is mainly comprised of adipose tissue and that a change in epithelial cell proliferation may be difficult to discern when observing the overall size of the mammary gland.
Fig. 2. E2 treatment increases Ki67 expression in the mammary gland.
Ovariectomized female mice were treated with oil or E2 for 7 days. The expression of Ki67 was examined in virgin mice, primiparous mice, and retired breeders using immunohistochemistry. Methyl green counter staining was included to identify cell nuclei. Scale bars indicate 25 µm. Images from 7–10 mice were collected in each group. Representative images are shown.
3.3 Oxidative stress response in oil- and E2- treated ovariectomized mice
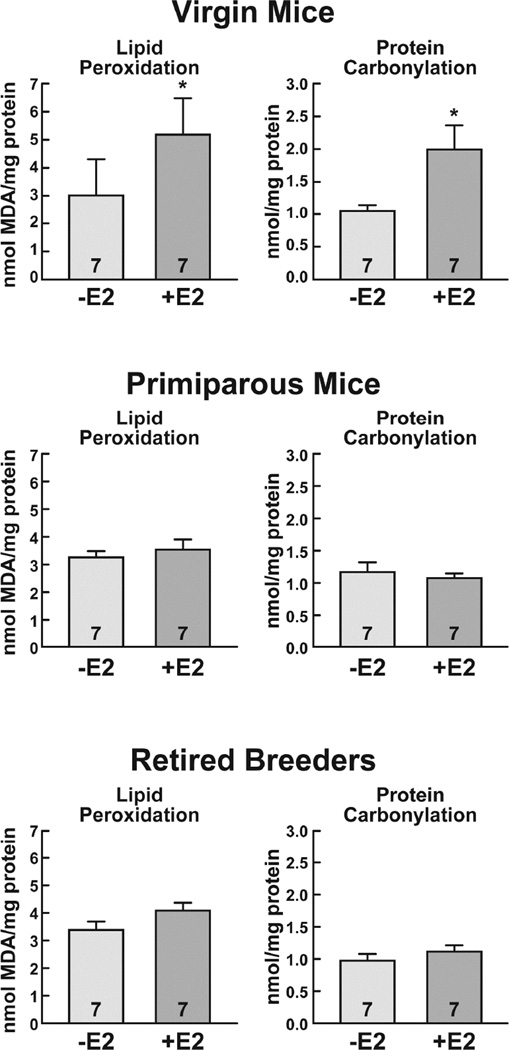
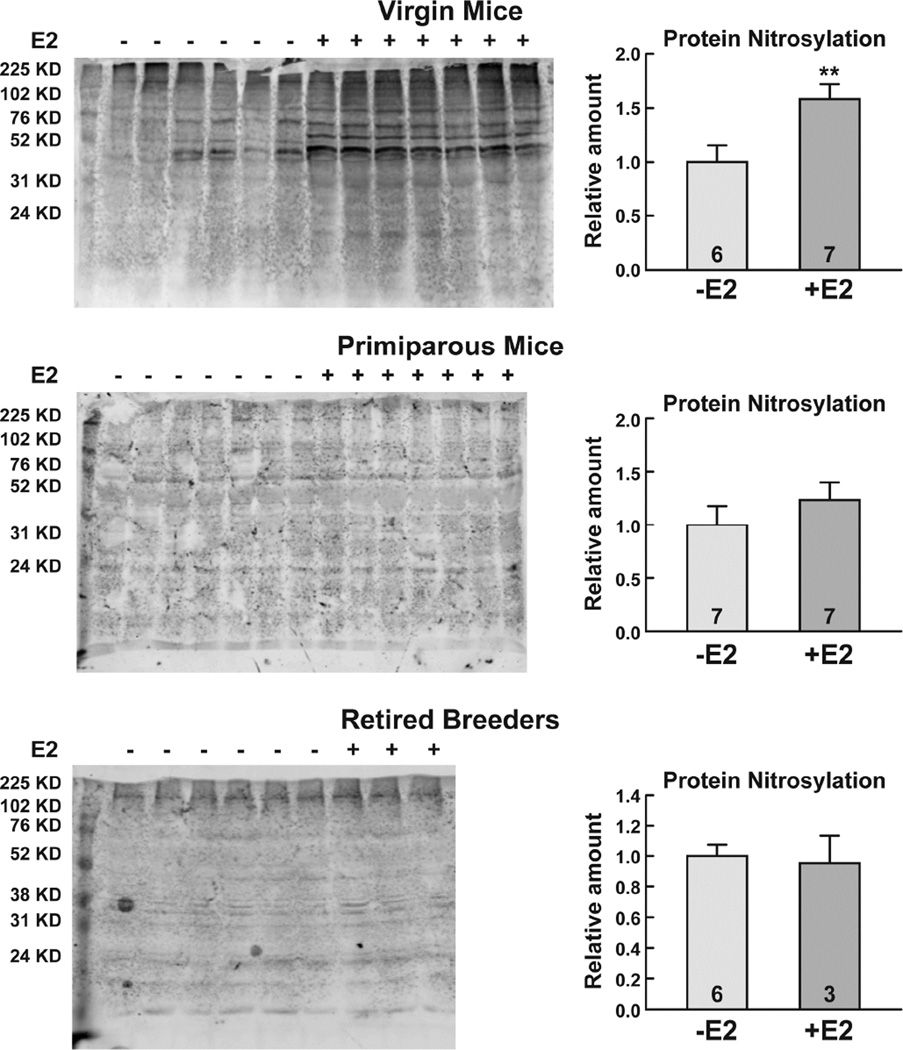
To determine whether the E2-mediated epithelial cell proliferation was associated with an increase in damage to cellular macromolecules, quantitative analyses of lipid peroxidation and protein carbonylation were performed. As seen in Fig. 3, E2 increased lipid peroxidation 1.7-fold (*, p<0.05) and protein carbonylation 1.9-fold (*, p<0.05) in the mammary glands of virgin mice. In contrast, there were no significant differences in lipid peroxidation or protein carbonylation in the mammary glands of oil- and E2-treated primiparous mice or retired breeders. Further analysis of mammary gland extracts using Western blotting demonstrated that E2 treatment elicited a 1.7-fold (**, p<0.01) increase in protein nitrosylation in virgin mice, but had no effect on protein nitrosylation in primiparous mice or retired breeders (Fig. 4). Thus, analysis of three independent damage markers, lipid peroxidation, protein carbonylation, and protein nitrosylation indicated that E2 had profound effects on the integrity of cellular macromolecules in the mammary gland of virgin mice, but not in primiparous mice or retired breeders.
Fig. 3. E2 treatment increases lipid peroxidation and protein carbonylation in the virgin mouse mammary gland.
Ovariectomized female mice were treated with oil or E2 for 7 days. Lipid peroxidation and protein carbonylation were assessed in virgin mice, primiparous mice, and retired breeders. The number of mice included in the oil- and E2-treated groups is indicated at the base of each treatment group bar. Significant differences in oil- and E2-treated mice are indicated by an asterisk (*, p<0.05).
Fig. 4. E2 treatment increases protein nitrosylation in the virgin mouse mammary gland.
Ovariectomized female mice were treated with oil or E2 for 7 days. Whole cell extracts were prepared and subjected to Western blot analysis and the spectrum of nitrosylated proteins was quantitated. Data were combined and the relative amounts of nitrosylation are shown. The number of mice included in the oil- and E2-treated groups is indicated at the base of each treatment group bar. A significant difference in the oil- and E2-treated mice is indicated by asterisks (**, p<0.01).
3.4 Mammary gland expression of oxidative stress response proteins in oil- and E2- treated ovariectomized mice
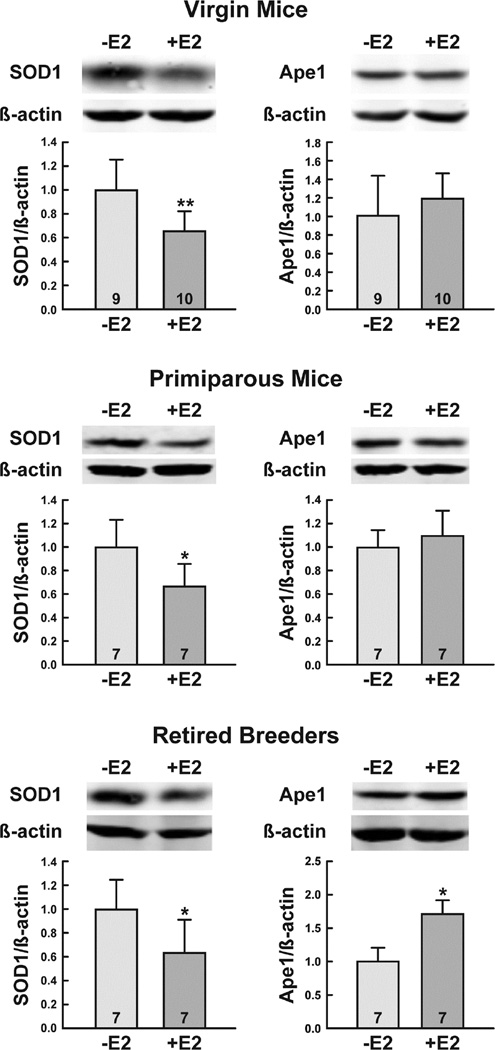
We previously demonstrated that E2 treatment decreases SOD1 and Ape1 levels in the mouse uterus and proposed that this decrease in oxidative stress response protein expression might be linked to the increased incidence of endometrial cancer observed in postmenopausal women who are maintained on long-term estrogen replacement therapy (Yuan et al 2014). In light of these findings, we assessed the levels of SOD1 and Ape1 in the mammary glands of ovariectomized virgin mice, primiparous mice, and retired breeders that had been treated with oil or E2 to determine whether the expression of these proteins was similarly regulated in the mammary gland. Western blot analysis was used to quantitatively determine the levels of SOD1 and Ape1 protein in whole cell mammary gland extracts and the expression of each protein was normalized to β-actin to compensate for any differences in loading. As seen in Fig. 5, E2 decreased the protein levels of SOD1 in all of the mice that were examined. Although it had no effect on Ape1 expression in the mammary glands of virgin mice or age-matched primiparous mice, E2 did significantly increase Ape1 protein levels in the mammary glands of retired breeders.
Fig. 5. E2 treatment differentially alters SOD1and Ape1 protein levels.
Ovariectomized female mice were treated with oil or E2 for 7 days. Whole cell extracts were prepared and subjected to quantitative Western blot analysis. Expression of each protein was normalized to the amount of β-actin present. The number of mice included in the oil- and E2-treated groups is indicated at the base of each treatment group bar. Significant differences in oil- and E2-treated mice are indicated by one (*, p<0.05) or two (**, p<0.01) asterisks.
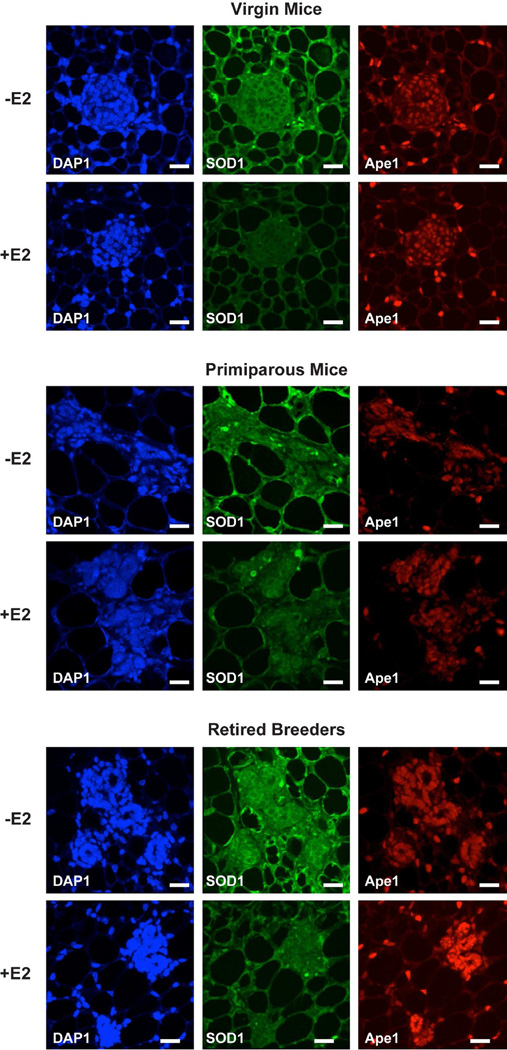
In agreement with our Western blot analysis, immunofluorescent microscopy demonstrated that SOD1 was highly expressed in oil-treated mice, but was globally decreased when mice had been treated with E2 (Fig. 6). When virgin and primiparous mice were treated with E2, Ape1 expression was unaffected. However, Ape1 was more highly expressed in the E2-treated retired breeders than in the virgin or primiparous mice that had been treated with E2.
Fig. 6. E2 influences SOD1 and Ape1 protein expression.
Ovariectomized female mice were treated with oil or E2 for 7 days. Expression of SOD1 and Ape1 was examined using immunofluorescent microscopy. DAPI counter staining was included to identify cell nuclei. Scale bars indicate 25 µm. Images from 7–10 mice were collected in each group. Representative images are shown.
4. Discussion
A number of studies have demonstrated that rodents provide useful models to better understand oncogenesis and breast cancer in humans (Thordarson et al 1995, Yang et al 1999). From studies in rodents, we know that parity (Thordarson et al 1995, Yang et al 1999), hormones of pregnancy (Blakely et al 2006, Sivaraman et al 1998, Ginger et al 2001), or E2 alone (Rajkumar et al 2001)) elicit persistent changes in gene expression (Thordarson et al 1995) and reduce mammary gland oncogenesis. Likewise, it is clear from studies in humans that parity decreases the incidence of breast cancer (Russo et al 2005). However, the mechanisms involved in conferring the protective effects of parity have not been defined. We now show that there is a profound reduction in damage to cellular macromolecules in parous mice compared to virgin mice and that expression of the oxidative stress response and DNA repair protein Ape1 is significantly increased in E2 treated retired breeders (Table 1).
TABLE 1.
Effects of E2 treatment
| Virgin mice | Primiparous mice | Retired breeders | |
|---|---|---|---|
| ERα | ↑ | ↑ | ↑ |
| PR | ↑ | ↑ | ↑ |
| Ki67 | ↑ | ↑ | ↑ |
| SOD1 | ↓ | ↓ | ↓ |
| Ape1 | – | – | ↑ |
| Lipid peroxidation | ↑ | – | – |
| Protein carbonylation | ↑ | – | – |
| Protein nitrosylation | ↑ | – | – |
4.1 E2-mediated regulation of Ape1 and SOD1
Oxidative stress response proteins play critical roles in sustaining cell viability and avoiding cellular damage that can occur if ROS are not appropriately dissipated. The first line of defense against ROS-induced damage is the conversion of superoxide to hydrogen peroxide, which is catalyzed by the antioxidant enzyme SOD. While one might anticipate that E2 would increase SOD1 expression to minimize ROS accumulation, E2 consistently reduced SOD1 expression in the mammary glands of all of the animals we examined regardless of age or parity.
Ape1 plays a critical role in the base excision repair pathway through its recognition of apurinic sites in DNA and cleavage of the phosphodiester backbone (Hegde et al 2008). In addition, Ape1 plays a crucial role in reducing and thereby activating transcription factors involved in multiple cellular processes (Hirota et al 1997, Jayaraman et al 1997, Evans et al 2000, Webster et al 2001, Sweasy et al 2006). In fact, because it plays such a critical role in reducing transcription factors and enhancing their binding to sequence-specific recognition sites in DNA, Ape1 is also referred to as redox factor-1. Thus, the increased expression of Ape1 in the retired breeders may help in maintaining genomic integrity and transcriptional efficiency.
4.2 Tissue-specific regulation of Ape1 by E2
Previous studies from our laboratory demonstrated that E2 treatment significantly reduces SOD1 and Ape1 expression in the uteri of ovariectomized female mice (Yuan et al 2014). These findings led us to hypothesize that decreased Ape1 and SOD1 expression might in part contribute to the increased endometrial cancer observed in menopausal women who are exposed to long-term estrogen replacement therapy. In the present study we found that E2 decreased SOD1 expression in the mammary gland just as it does in the uterus. Unlike the E2-mediated decline in uterine Ape1 levels, E2 had no effect on Ape1 expression in the mammary glands of the 15 week-old virgin or primiparous mice, but substantially increased Ape1 expression in the mammary glands of the 9 month-old retired breeders. Taken together, these studies demonstrate that E2 differentially regulates expression of Ape1, but not SOD1, in the uterus and mammary gland.
4.3 E2 responsive gene expression
Although E2 increased PR expression in all of the mice we examined, the increase in PR was substantially attenuated in the retired breeders compared to the younger animals. One interpretation of these findings might be that the older animals were less sensitive to E2 than the younger mice. However, the E2-mediated increase in Ape1 expression in the retired breeders was greater than observed in the younger mice suggesting that there is not a simple decline in E2 responsiveness with age, but rather a gene-specific effect. Alternatively, since E2 significantly increases association of Ape1 with the PR gene and reduces PR gene expression (Curtis et al 2009), the decline of PR expression in the retired breeders may in part be attributed to the increased repression of the PR gene by Ape1.
4.4 Biological significance
Because it drives the proliferation of normal mammary epithelial cells and breast cancer cells, E2 has been implicated in increasing the incidence and progression of breast cancer (Clemons and Goss 2001). Surprisingly, analysis of the Women’s Health Initiative demonstrated that rather than increasing the incidence of breast cancer in postmenopausal women, estrogen replacement therapy decreases the incidence of breast cancer (LaCroix et al 2011).
From our studies in mice, we have considered 2 ways in which parity and E2 treatment may reduce mammary gland oncogenesis. First, the most obvious difference elicited by E2 was the profound reduction in protein and lipid damage, which occurred in primiparous mice, but not in the age-matched virgin mice. Even at an advanced age, all three markers of cellular damage were significantly less than was observed in the younger, virgin animals supporting the idea that parity causes persistent changes that are protective in rodents (Thordarson et al 1995, Yang et al 1999, Yang et al 1999).
A second way that parity and E2 may reduce oncogenesis is through the enhanced expression of Ape1. Because it plays such a critical role in DNA repair (Hegde et al 2008, Sancar and Sancar 1988, Wilson and Kunkel 2000) and is recruited to the DNA-bound ERα in endogenous, estrogen-responsive genes (Curtis et al 2009, Schultz-Norton et al 2011), it seems plausible that Ape1 could survey DNA at the time of transcription and help to repair DNA lesions. In fact, the enhanced repair of transcriptionally active genes and the preferential repair of the DNA coding strand provide evidence that transcription and DNA repair are functionally coupled (Svejstrup 2002, Mellon et al 1986, Mellon et al 1987). Stabilization of the transcription complex may be instrumental in allowing DNA repair and transcription to occur simultaneously. The capacity of both Ape1 and SOD1 to enhance the ERα-ERE interaction (Curtis et al 2009, Rao et al 2008) could contribute to this stabilization. Furthermore, since chromatin remodeling is essential in making DNA accessible to transcription factors and DNA repair proteins, coupling of transcription and DNA repair would appear to be beneficial (Morrison and Shen 2006, Wuebbles and Jones 2004). Thus, the nuclear localization of Ape1, which is dramatically increased in the E2-treated retired breeders may not only facilitate DNA repair, but also foster transcriptional competence by reducing and thereby activating transcription factors.
HIGHLIGHTS.
Parity protects the mammary gland from E2-mediated protein and lipid damage.
E2 decreases expression of superoxide dismutase in the mouse mammary gland regardless of parity.
E2 increases apurinic endonuclease expression in the mammary glands of retired breeders.
E2 and parity help protect the mammary gland by reducing damage to cellular macromolecules.
Acknowledgments
This work was supported by NIH Grant DK 053884 (to AMN). AKD was supported by a predoctoral fellowship from the NIEHS Reproductive Toxicology Training Grant T32 ES007326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
L.Y., A.D., Y.Z., and A.N. have nothing to declare.
REFERENCES
- Blakely CM, Stoddard AJ, Belka GK, Dugan KD, Notarfrancesco KL, Moody SE, D'Cruz CM, Chodosh LA. Hormone-induced protection against mammary tumorigenesis is conserved in multiple rat strains and identifies a core gene expression signature induced by pregnancy. Cancer Res. 2006;66:6421–6431. doi: 10.1158/0008-5472.CAN-05-4235. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- Clemons M, Goss P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Curtis CD, Thorngren DL, Nardulli AM. Immunohistochemical analysis of oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues. BMC Cancer. 2010;10:9. doi: 10.1186/1471-2407-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CD, Thorngren DL, Ziegler YS, Sarkeshik A, Yates JR, Nardulli AM. Apurinic/apyrimidinic endonuclease 1 alters estrogen receptor activity and estrogen-responsive gene expression. Mol. Endocrinol. 2009;23:1346–1359. doi: 10.1210/me.2009-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015:Convergence of incidence rates between black and white women. CA Cancer. J. Clin. 2015 doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat. Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Ginger MR, Gonzalez-Rimbau MF, Gay JP, Rosen JM. Persistent changes in gene expression induced by estrogen and progesterone in the rat mammary gland. Mol. Endocrinol. 2001;15:1993–2009. doi: 10.1210/mend.15.11.0724. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125:143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Makela S, Gustafsson JA, Dahiya R, Cunha GR. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol. Reprod. 2001;64:272–283. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- Kwan ML, Bernard PS, Kroenke CH, Factor RE, Habel LA, Weltzien EK, Castillo A, Gunderson EP, Maxfield KS, Stijleman IJ, Langholz BM, Quesenberry CP, Jr, Kushi LH, Sweeney C, Caan BJ. Breastfeeding, PAM50 tumor subtype, and breast cancer prognosis and survival. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv087. 10.1093/jnci/djv087. Print 2015 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA: The Journal of the American Medical Association. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S. Age at first birth and breast cancer risk. Bull. World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- Medina D, Smith GH. Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J. Natl. Cancer Inst. 1999;91:967–969. doi: 10.1093/jnci/91.11.967. [DOI] [PubMed] [Google Scholar]
- Mellon I, Bohr VA, Smith CA, Hanawalt PC. Preferential DNA repair of an active gene in human cells. Proc. Natl. Acad. Sci. U. S. A. 1986;83:8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I, Spivak G, Hanawalt PC. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987;51:241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Morrison AJ, Shen X. Chromatin modifications in DNA repair. Results Probl. Cell Differ. 2006;41:109–125. doi: 10.1007/400_008. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Rajkumar L, Guzman RC, Yang J, Thordarson G, Talamantes F, Nandi S. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11755–11759. doi: 10.1073/pnas.201393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol. Endocrinol. 2008;22:1113–1124. doi: 10.1210/me.2007-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005;7:131–142. doi: 10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Sancar GB. DNA repair enzymes. Annu. Rev. Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Ziegler YS, Nardulli AM. ERalpha-associated protein networks. Trends Endocrinol. Metab. 2011;22:124–129. doi: 10.1016/j.tem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J. Steroid Biochem. Mol. Biol. 2002;80:137–148. doi: 10.1016/s0960-0760(01)00182-0. [DOI] [PubMed] [Google Scholar]
- Sivaraman L, Stephens LC, Markaverich BM, Clark JA, Krnacik S, Conneely OM, O'Malley BW, Medina D. Hormone-induced refractoriness to mammary carcinogenesis in Wistar-Furth rats. Carcinogenesis. 1998;19:1573–1581. doi: 10.1093/carcin/19.9.1573. [DOI] [PubMed] [Google Scholar]
- Svejstrup JQ. Mechanisms of transcription-coupled DNA repair. Nat. Rev. Mol. Cell Biol. 2002;3:21–29. doi: 10.1038/nrm703. [DOI] [PubMed] [Google Scholar]
- Sweasy JB, Lang T, DiMaio D. Is base excision repair a tumor suppressor mechanism? Cell Cycle. 2006;5:250–259. doi: 10.4161/cc.5.3.2414. [DOI] [PubMed] [Google Scholar]
- Thordarson G, Jin E, Guzman RC, Swanson SM, Nandi S, Talamantes F. Refractoriness to mammary tumorigenesis in parous rats: is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis. 1995;16:2847–2853. doi: 10.1093/carcin/16.11.2847. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer. J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid. Redox Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- Wilson SH, Kunkel TA. Passing the baton in base excision repair. Nat. Struct. Biol. 2000;7:176–178. doi: 10.1038/73260. [DOI] [PubMed] [Google Scholar]
- Wise PM, Camp-Grossman P, Barraclough CA. Effects of estradiol and progesterone on plasma gonadotropins, prolactin, and LHRH in specific brain areas of ovariectomized rats. Biol. Reprod. 1981;24:820–830. doi: 10.1095/biolreprod24.4.820. [DOI] [PubMed] [Google Scholar]
- Wuebbles RD, Jones PL. DNA repair in a chromatin environment. Cell Mol. Life Sci. 2004;61:2148–2153. doi: 10.1007/s00018-004-4175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yoshizawa K, Nandi S, Tsubura A. Protective effects of pregnancy and lactation against N-methyl-N-nitrosourea-induced mammary carcinomas in female Lewis rats. Carcinogenesis. 1999;20:623–628. doi: 10.1093/carcin/20.4.623. [DOI] [PubMed] [Google Scholar]
- Yuan L, Dietrich AK, Nardulli AM. 17beta-Estradiol alters oxidative stress response protein expression and oxidative damage in the uterus. Mol. Cell. Endocrinol. 2014;382:218–226. doi: 10.1016/j.mce.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]