Abstract
BACKGROUND
African Americans smoke fewer cigarettes per day than Whites but experience greater smoking attributable morbidity and mortality. African American-White differences may also exist in cessation but rigorously designed studies have not been conducted to empirically answer this question.
METHODS/DESIGN
Quit2Live is, to our knowledge, the first head-to-head trial designed with the primary aim of examining African American-White disparities in quitting smoking. Secondary aims are to identify mechanisms that mediate and/or moderate the relationship between race and quitting. The study is ongoing. Study aims are accomplished through a 5-year prospective cohort intervention study designed to recruit equal numbers of African Americans (n=224) and Whites (n=224) stratified on age (< 40, ≥ 40) and gender, key factors known to impact cessation, and all within a restricted income range (≤ 400% federal poverty level). All participants will receive 12 weeks of varenicline in combination smoking cessation counseling. The primary outcome is cotinine-verified 7-day point prevalence abstinence from smoking at week 26. Secondary outcomes are cotinine-verified 7-day point prevalence abstinence from smoking at weeks 4 and 12.
DISCUSSION
Findings from Quit2Live will not only address if African American-White disparities in quitting smoking exist but, more importantly, will examine mechanisms underlying the difference. Attention to proximal, modifiable mechanisms (e.g., adherence, response to treatment, depression, stress) maximizes Quit2Live’s potential to inform practice. Findings will provide an empirically-derived approach that will guide researchers and clinicians in identifying specific factors to address to improve cessation outcomes and reduce tobacco-related morbidity and mortality in African American and White smokers.
TRIAL REGISTRATION NUMBER
ClinicalTrials.gov: NCT01836276
Keywords: Smoking cessation, African American, White, disparities, varenicline
Racial and ethnic disparities in smoking-related disease and death are well-documented. African Americans use fewer cigarettes per day than White Americans [1–3], yet they have the highest incidence rates for all cancers combined, higher overall cancer mortality rates, and twice the rate of premature death attributable to cardiovascular disease compared to Whites [4, 5]. African American smokers also have a 43–55% higher relative risk of smoking-attributable lung cancer compared to Whites and are at higher risk for nearly all smoking-related chronic diseases [6–8].
There are many purported reasons for the higher tobacco-related disease burden in African American smokers. On average, African Americans take in 30% more nicotine per cigarette smoked [9] and are exposed to higher levels of select lung carcinogens (e.g., 1-hydroxypyrene) at lower levels of smoking compared to Whites [10]. Greater exposure per cigarette smoked may be due, in part, to the preference for menthol cigarettes among African American smokers. Menthol has a cooling sensation that reduces the irritant quality of cigarette smoke and may facilitate deeper inhalation and greater exposure to nicotine [11]. Another plausible reason for the higher tobacco-related disease burden is that, although African Americans are more likely to attempt to quit smoking in a given year, they are less successful [12, 3].
The decreased likelihood of success for African American smokers has been attributed to the fact that they are less likely than Whites to receive provider advice/assistance to quit [13–16] and to be prescribed smoking cessation pharmacotherapy to aid in their attempts [17, 12, 18]; however disparities in smoking cessation have persisted for African American smokers in clinical trials where pharmacotherapy and quitting assistance have been provided [19–22]. To-date, no known trials have been conducted with the primary aim of prospectively examining African American-White differences in cessation. Existing trials have often enrolled unequal proportions of African American and White smokers and, because examination of racial differences in cessation was not the primary aim, were underpowered to make such comparisons [19, 22]. Others have relied on self-reported abstinence [23, 24], which is prone to misreporting and overestimation of abstinence [25–28], reported abstinence at early (e.g., Weeks 1–4) but not later time points (e.g., Week 26) [29, 30], or been conducted in special treatment settings (e.g., Veterans Affairs patients, smokers in the criminal justice system) which limits generalizability of the findings [23, 31]. Existing studies have also not been stratified by race on age or gender or ensured recruitment of African American and White smokers of comparable socioeconomic status (SES) [20, 21], all key factors known to impact smoking cessation [32, 24, 33–38]. Mechanisms underlying African American-White differences in quitting are also not well understood. Multiple factors, including demographic (e.g., socioeconomic status) [29, 33, 36, 37] and smoking characteristics (e.g., menthol, nicotine intake) [39–42], adherence and/or response to treatment (e.g., reductions in withdrawal, craving) [18, 17, 27, 31, 43], psychosocial (e.g., psychological distress, perceived and contextual disadvantage, stress) [44–46, 24, 47–53], and biological factors linked to nicotine metabolic inactivation (e.g., CYP2A6, 3hydroxycotinine/cotinine) [54–58] have been studied as they relate to cessation in African Americans and Whites, separately, but few studies have explored the relative importance of these factors in explaining African American-White differences in quitting.
The current clinical trial, Quit2Live, is designed explicitly to address these gaps. Quit2Live uses a stratified design to recruit an equal number of African American and White smokers across gender and age, provides the same treatment to all participants (varenicline plus counseling), and will biochemically confirm smoking status at multiple time points. In addition, because the majority of US adult smokers are of lower socioeconomic status [32] and lower socioeconomic status adversely impacts cessation [59], Quit2Live recruits participants within a restricted income range [≤ 400% federal poverty level (FPL)]. This paper describes the study design, enrollment, and baseline characteristics of participants in the trial.
METHODS
Study design
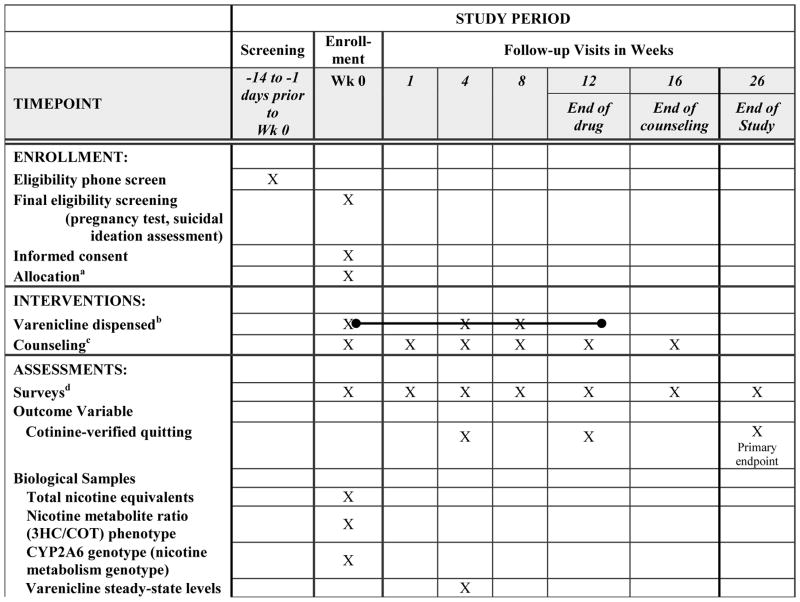
Quit2Live is a 5-year prospective cohort intervention study, stratified on race (African American, White) and, within race, on age (< 40, ≥ 40) and gender, with the primary aim of examining differences in quitting between African American and White smokers and secondary aims of identifying mechanisms (e.g., demographic, smoking, treatment process, psychosocial, and biological factors) that explain the relationship between race and quitting. All participants will receive 12 weeks of varenicline in combination with 6 sessions of smoking cessation counseling. Because women and younger smokers are less likely to quit smoking than their male or older counterparts [34, 35, 38], Quit2Live will stratify on these factors, along with race, to ensure recruitment of African American and White smokers who are comparable on key covariates known to impact cessation. Using the stratified design, 56 participants will be recruited into each of the 2 race by age and gender cohorts. The schedule of enrollment, intervention, and assessment activities is displayed in Table 1. The primary outcome is cotinine-verified 7-day point prevalence smoking abstinence at month 6. All study visits will be completed at Swope Health Central, a Federally Qualified Health Center located in Kansas City, Missouri. Methods of recruitment, screening, enrollment, and retention are identical and do not vary by race. Study procedures are approved and monitored by the University of Kansas Medical Center (KUMC) IRB (#00001602).
Table 1.
Schedule of enrollment, intervention, and assessment activities
All participants were allocated to receive the same treatment
A 4-week supply of varenicline was dispensed at the Week 0, 4, and 8 in-person visits
Counseling sessions were conducted in person at Weeks 0, 4, 8, and 12 and by phone at Weeks 1 and 16
See measures section and Table 2 for a description of each measure and the time point it was administered
Recruitment
Recruitment started in February 2013 and ended in May 2015. Final 6-month follow-up will be completed in November 2015. Participants are recruited through clinic- and community-based efforts, including fliers, physician letters, radio, television, and social media ads, and word-of- mouth referrals from current and former participants.
Eligibility
Eligible participants are non-Hispanic African American or White adults, 18 years of age or older who smoke 3–20 cigarettes per day on 25 days or more during the preceding month, and are interested in quitting smoking, taking varenicline for 3 months, have a functioning telephone, and are willing to complete all study-related requirements. Individuals are excluded if they have a medical contraindication for varenicline, which includes being pregnant or breastfeeding, renal impairment, currently taking the blood thinner warfarin, history of panic or anxiety disorder, psychosis, bipolar disorder, or an eating disorder, being treated for depression in the last year, receiving treatment for alcohol or other drugs in the past year, known allergy or sensitivity to varenicline, being treated for a heart attack or any acute cardiovascular event in the past two months, and/or being diagnosed with angina or arrhythmia in the past two months. Individuals are also excluded if they have used a tobacco product other than cigarettes (e.g., cigars, cigarillos, smokeless tobacco) in the past 30 days, are planning to move from the Kansas City area during the 6 month study period, have used varenicline in the preceding three months, are unwilling to refrain from use of other smoking cessation pharmacotherapies during the study period, have unstable housing (e.g., lived in a shelter, on the street, or in a detoxification center), or another smoker in the household is enrolled in the study. Varenicline carries an FDA black box warning for neuropsychiatric complications (i.e., depressed mood, suicidal behavior), therefore, the Patient Health Questionaire-2, a commonly used depression screener [60, 61], is administered to all individuals. Those scoring 3 or higher are excluded because of concern that varenicline could exacerbate underlying depressive symptoms. Participants are also excluded if the total yearly income for all people in their household places them at > 400% of the FPL [62].
Screening and Consent
Interested individuals contact us by telephone and are screened for eligibility by study staff. Those who are provisionally eligible after the phone screening are scheduled for final, in-person eligibility screening, which consists of pregnancy testing on women who are not post-menopausal or sterilized, assessment of willingness to use birth control to avoid pregnancy while taking varenicline among these same women, and assessment of active suicidal ideation over the preceding two weeks. Individuals who are eligible following final, in-person screening participate in a consenting interview conducted by study staff. Those providing written informed consent are enrolled into the study and immediately participate in baseline (Week 0) activities (described below).
Intervention
The intervention consists of 12 weeks of varenicline, six smoking cessation counseling sessions, and a behaviorally-oriented smoking cessation guide that was designed to be used in conjunction with counseling.
Varenicline
Varenicline was chosen because it is the most effective of the currently approved first-line smoking cessation monotherapies [63]. More importantly, however, is that a very small proportion of participants enrolled in existing varenicline trials have been racial/ethnic minorities and cessation outcomes in these trials have not been reported by race. Our group conducted a small pilot trial of varenicline for cessation in African American moderate to heavy smokers (> 10 cigarettes per day) and found modest quit rates at Week 12 compared to rates found for predominately White smokers in published clinical trials (24% versus 49%) [64–66]. Fully powered clinical trials have been conducted examining the efficacy of other first-line medication for smoking cessation in African American smokers (e.g., bupropion, nicotine gum and patch; [27, 25, 67, 68]) but, to our knowledge, this is the first large-scale study examining varenicline as a cessation aid in African American smokers.
At baseline (Week 0), a research counselor gives each participant a 4-week supply of varenicline and instructions on titrating up to the full dose following standard dosing guidelines (0.5 mg once daily on Days 1–3, 0.5 mg twice daily on Days 4–7, and 1 mg twice daily on Day 8 through Week 12). Participants are encouraged to initiate varenicline the day after their baseline visit (Day 1) and to set a target quit date for one week later (Day 8). Varenicline is dispensed in 30-day pill boxes at Weeks 0, 4, 8 to aid participants in taking their medication as prescribed [66, 69], to assist staff with monitoring medication adherence, and to enhance retention at the Week 4 and 8 study visits. One week of extra medication is included in the pill box to ensure that participants do not run out of varenicline before the refill visit. Varenicline is discontinued and the safety re-evaluated in participants reporting a serious adverse event that is deemed likely or possibly related to the medication (e.g., suicidal ideation or behavior).
Counseling
Participants receive smoking cessation counseling sessions in person at Weeks 0 (baseline), 4, 8, and 12 and by phone at Weeks 1 and 16. Trained counseling staff follow the current Tobacco Use and Dependence Clinical Practice Guidelines and give clear advice to stop smoking, provide assistance with quitting, and arrange follow-up [70]. The baseline counseling session provides participants information on the proper use of varenicline. It also addresses the health risks and benefits of quitting and assists participants in identifying triggers and developing a quit plan. Follow-up counseling sessions are tailored to participant’s smoking status. For those who report quitting, sessions focus on strategies for preventing relapse, including alternatives to smoking and identifying and managing stressors that could lead to relapse. For those who report continued smoking, sessions focus on exploring barriers to quitting, discussing problems that lead to relapse/continued smoking, and reaffirming a quit date and plan. All counseling sessions are recorded for quality control purposes. The baseline session lasts 30 minutes, on average, with follow-up sessions averaging 15 minutes. Counseling fidelity is monitored by a licensed psychologist during biweekly counseling supervision meetings.
Written Materials
Participants receive the Quit2Live Stop Smoking Guide at baseline. The 30-page guide has been designed to go hand-in-hand with counseling and includes information on the health consequences of tobacco use, benefits of quitting, specific strategies to promote abstinence such as making a quit plan, obtaining social support, identifying reasons for quitting and activities that could take the place of smoking, strategies for dealing with urges, managing withdrawal and craving, coping with a lapse, and relapse prevention, instructions on medication use and tips for managing common medication side effects. Versions of the guide have been used successfully in three of our previous smoking cessation studies [25, 27, 66].
Retention
Study staff contact participants one week prior to each study visit via phone, text, email and postcards. For any missed session, participants receive up to 6 additional contacts to facilitate rescheduling. Participants are given a $30 gift card at Weeks 0 and 4, a $20 gift card at Week 8, and a $40 gift card at Week 12, and a $60 gift card at Week 26 in appreciation for their time and participation. Remuneration is based on session attendance and not on smoking status. Participants also receive a study t-shirt and water bottle at Weeks 1 and 16, respectively in appreciation for their time.
Outcome and Study Measures
Baseline demographic, smoking history, treatment process, psychosocial, and biological measures were selected based on those that have been found to be predictive of smoking cessation in African American and White smokers in previous studies and/or those that have been speculated to account for African American-White differences in quitting (e.g., menthol, nicotine dependence, cigarettes per day). Only those baseline measures included in the current paper are summarized below. A full list of the measures by time point is provided in Table 2.
Table 2.
Quit2Live Measures at Each Time Point
| Eligibility | Baseline Week 0 | Week 1 | Week 4 | Week 8 | Week 12 | Week 16 | Week 26 | |
|---|---|---|---|---|---|---|---|---|
| Smoking Abstinence | ||||||||
| Cotinine | x | x | x | |||||
| Demographic Measures | ||||||||
| Demographics | x | x | ||||||
| Perceived health | x | |||||||
| Body mass index | x | x | x | x | ||||
| Waist circumference | x | x | x | x | ||||
| Smoking Measures | ||||||||
| Smoking history | x | |||||||
| Social influences | x | |||||||
| Nicotine dependence | x | |||||||
| Treatment Process Measures | ||||||||
| Withdrawal | x | x | x | x | ||||
| Craving | x | x | x | x | ||||
| Reinforcing effects of nicotine | x | x | x | x | ||||
| Medication-related side effects | x | x | x | x | x | |||
| Medication adherence (self-report) | x | x | x | |||||
| Medication adherence (steady-state) | x | |||||||
| Psychosocial Measures | ||||||||
| Stress | x | x | x | x | ||||
| Depressive symptoms | x | x | x | x | x | |||
| Anxiety symptoms | x | x | x | x | ||||
| Financial strain | x | |||||||
| Discrimination | x | |||||||
| Race consciousness | x | |||||||
| Perceived social status | x | |||||||
| Proneness to psychological distress | x | |||||||
| Distrust of others | x | |||||||
| Satisfaction with life | x | |||||||
| Neighborhood problems | x | |||||||
| Neighborhood cohesion and trust | x | |||||||
| Biological Measures | ||||||||
| Nicotine intake | x | |||||||
| Nicotine metabolite ratio (3HC/COT) | x | |||||||
| Nicotine metabolism genotype (CYP2A6) | x | |||||||
| Varenicline steady-state levels | x |
Smoking Abstinence
The primary endpoint is cotinine-verified 7-day point prevalence smoking abstinence, defined as no cigarettes for the previous 7 days at Week 26. The recommended cut-off of 15ng/ml for salivary cotinine will be used to differentiate smokers from non-smokers [26]. Secondary endpoints will be salivary cotinine verified-7 day point prevalence abstinence at Weeks 4 and 12 (end of drug).
Demographic Measures
Baseline demographic measures include participant age, gender, marital/partner status, employment status, health insurance status, educational level [71], income [72], perceived health [73], height, weight, waist circumference, and body mass index [74].
Smoking Measures
Baseline assessment of smoking history includes number of cigarettes smoked per day (cpd), type of cigarette smoked (menthol versus non-menthol), age when started smoking regularly, length of the longest quit attempt [75], social influences on smoking [76], and time to the first cigarette of the day, a marker of nicotine dependence [77].
Treatment Process Measures
Baseline treatment process measures include nicotine withdrawal [78], craving [79], and the reinforcing effects of nicotine [80].
Psychosocial Measures
Baseline psychosocial measures include perceived stress [81], discrimination [82], race consciousness [83], financial strain [24], symptoms of depression and anxiety [60, 84], proneness to psychological distress [85], distrust of others [86], perceived social status [87], perceived neighborhood problems, perceived neighborhood social cohesion and trust [88, 89], and overall life satisfaction [90].
Data Analysis
Sample Size Justification
The primary outcome is cotinine-verified 7-day point prevalence abstinence from smoking at month 6. Based on data from existing varenicline trials [91, 66], we postulate a 28% cessation rate in White and a 15% cessation rate in African American participants. The current sample size of 224 African American and 224 White participants provides 90% power to detect a difference in cessation with a type I error rate of 0.05 using a two-sample, two-tailed Chi-square test.
Primary Analyses
To accomplish the study’s primary aim of examining differences in smoking cessation between African American and White smokers, we will compare the cotinine verified 7-day point prevalence abstinence rates at Week 26 between African Americans and Whites using the chi-square test. Our research hypothesis is that African Americans will have significantly lower cotinine-verified 7-day abstinence from smoking at Week 26 than Whites. For our primary comparison, those lost to follow-up will be treated as smokers. We also will look at completers only and will utilize multiple imputation techniques to ensure valid comparisons between the two groups if the loss to follow-up does not appear random. Given this is not a randomized study, we will also utilize multiple logistic regression to compare the 7-day point prevalence abstinence rates between African Americans and Whites adjusting for our stratification variables (age, gender) along with baseline level of smoking. We will examine both main effects and pairwise interactions effects and determine if the expected difference between African Americans and Whites still exists in the presence of these other factors. Interactions not significant at the 0.10 level will be dropped from the model. To evaluate secondary endpoints, we will compare cotinine-verified 7-day point prevalence abstinence rates between African Americans and Whites at Week 12 (end of drug) using the same methods as above.
Analysis for the Current Paper
In this paper we provide descriptive summaries of baseline (Week 0) demographic, smoking, treatment process, and psychosocial characteristics using frequencies and percentages for categorical variables and means and standard deviations for quantitative variables. Baseline differences in these factors by race were computed using independent samples t tests for continuous variables and chi-square tests for discrete variables. Time to accrual and eligibility and enrollment rates were also computed within each of the eight strata. Future manuscripts will present findings on primary and secondary outcomes and explanatory mechanistic factors.
RESULTS
Recruitment Flow
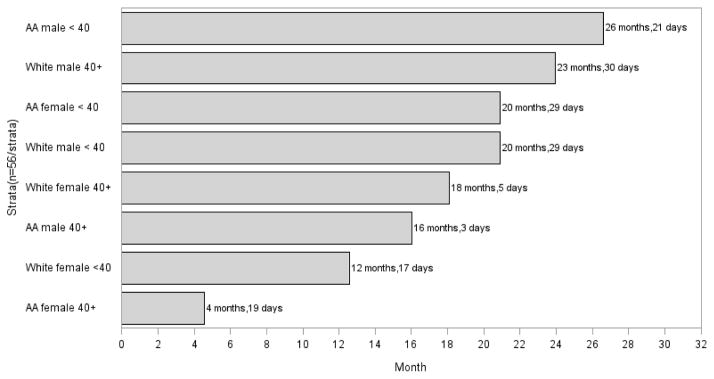
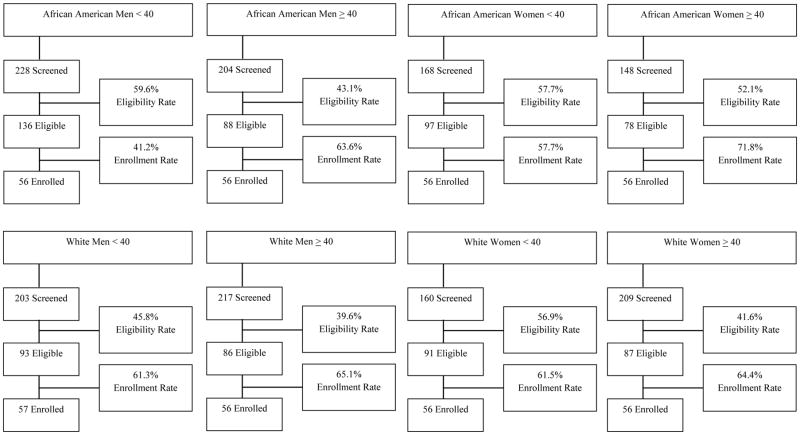
Fifty-six participants have been recruited into seven of the eight race, age, and gender strata. A coding error resulted in an additional White male < 40 being enrolled (n=57), bringing the sample total to 224 African American and 225 White smokers. An overview of accrual into the study and screening, eligibility, and enrollment data for all strata is provided in Figures 1 and 2, respectively. African American women ≥ 40 were the first group to be fully enrolled; all 56 were enrolled in 4 months and 19 days. Despite having only a moderate eligibility rate compared to the other groups (52.1% of those screened were eligible), African American women ≥ 40 had the highest enrollment rates; 2.7 individuals were screened to enroll 1 and 71.8% of those who were eligible kept their baseline appointment and were enrolled, which contributed to their rapid accrual. Conversely, African American men < 40 were the last group to be enrolled, taking 26 months and 21 days. Interestingly, while this group had the highest eligibility rate (59.6% of those screened were eligible), they also had the lowest enrollment rates; 4 individuals were screened to enroll 1 and 58.8% of those who were eligible did not keep their baseline appointment, which contributed to their slow accrual relative to the other stratum. Accrual for the remaining stratum ranged from 12 months, 17 days for White women < 40 to 23 months, 30 days for White men ≥ 40.
Figure 1.
Time to Accrual by Strata*
*Time to accrual was calculated as the time between when the study opened for enrollment through when the last participant was enrolled into each stratum. Recruitment for all groups began on February 4, 2013.
AA=African American
Figure 2.
Participant Flow During Screening and Enrollment by Strata
Eligibility Rate = eligible/screened, Enrollment Rate = enrolled/eligible
The top three reasons for ineligibility, overall, are use of non-cigarette tobacco products (e.g., hand-rolled cigarettes, cigarillos; 20.5%), followed by medical contraindications to varenicline (20.2%) and smoking ≤ 2 or ≥ 21 cpd (14.2%). Other primary reasons for ineligibility include > 400% FPL (7.3%), a pharmacotherapy assisted quit attempt in the past 3 months (5.7%), unstable housing (2.4%), smoking on < 25 days in past 30 (2.1%), refusal to sign informed consent (1.5%), and no phone number to be reached for counseling calls (1.4%).
Baseline Characteristics
Participant baseline characteristics, including differences by race, are presented in Table 3. African American participants have lower educational attainment, are less likely to be employed, have lower overall household incomes and percent federal poverty levels, and are less likely to own their home compared to White. With respect to smoking and treatment process characteristics, African Americans are more likely than White to smoke menthol cigarettes, to smoke fewer cigarettes per day and to start smoking later in life. While African American and White smokers note similar amounts of nicotine dependence and withdrawal and craving for cigarettes, they report differences on two of the five reinforcing effects of nicotine subscales: African American smokers have more aversion to smoking (e.g., dizziness, nausea) and less craving reduction immediately following a cigarette. In terms of psychosocial characteristics, African American smokers have higher perceived stress and depression and lower satisfaction with life compared to White. Despite experiencing an equivalent number of situations of everyday discrimination, African American encounter situations more frequently and they are more likely to think about/be conscious of their race than White. Finally, African American participants are more distrustful of the intentions of others’ and they report more problems in their neighborhood (e.g., noise, vandalism, safety) and less connectedness between neighbors.
Table 3.
Baseline Participant Characteristics
| Range for Scale Scoresa | African American (n=224) | White (n=225) | p-valueb | |
|---|---|---|---|---|
| DEMOGRAPHIC CHARACTERISTICSc
| ||||
| Age, mean(SD) | 42.4 (12.0) | 40.5 (11.2) | 0.0930 | |
| Gender, n (%) | ||||
| Female | 112 (50.0%) | 112 (49.8%) | 0.9624 | |
| Male | 112 (50.0%) | 113 (50.2%) | ||
| Cohabitation Status, n (%) | ||||
| Living alone | 142 (63.4%) | 115 (51.1%) | 0.0085 | |
| Living with a partner | 82 (36.6%) | 110 (48.9%) | ||
| Employment Status, n (%) | ||||
| Employed full-time | 77 (34.4%) | 142 (63.1%) | <0.0001* | |
| Employed part-time | 41 (18.3%) | 29 (12.9%) | ||
| Not currently employed | 75 (33.5%) | 28 (12.4%) | ||
| Retired | 11 (4.9%) | 5 (2.2%) | ||
| Student/Homemaker | 20 (8.9%) | 21 (9.3%) | ||
| Education Level, n (%) | ||||
| Less than high school (HS) graduate | 30 (13.4%) | 14 (6.2%) | 0.0027* | |
| HS graduate or HS equivalent (GED) | 64 (28.6%) | 44 (19.6%) | ||
| Some college or tech school | 90 (40.2%) | 116 (51.6%) | ||
| College graduate or higher | 40 (17.9%) | 51 (22.7%) | ||
| Health Insurance that Pays for Most Medical Care, n (%) | ||||
| No | 107 (47.8%) | 102 (45.3%) | 0.6051 | |
| Yes | 117 (52.2%) | 123 (54.7%) | ||
| Income, mean(SD) | $21,293 ($15,501) | $35,806 ($21,035) | <0.0001* | |
| Number of people in household, including self | 2.7 (1.6) | 3.0 (1.8) | 0.0716 | |
| Poverty level, n (%) | ||||
| ≤100 | 115 (51.3%) | 43 (19.1%) | <0.0001* | |
| 101–200 | 72 (32.1%) | 87 (38.7%) | ||
| 201–250 | 15 (6.7%) | 25 (11.1%) | ||
| 251–300 | 9 (4.0%) | 27 (12.0%) | ||
| 301–400 | 13 (5.8%) | 43 (19.1%) | ||
| Housing, n (%) | ||||
| Own a home | 38 (17.0%) | 77 (34.2%) | <0.0001* | |
| Rent or stay with others | 186 (83.0%) | 148 (65.8%) | ||
| Perceived Health, n (%) | ||||
| Good, Fair, or Poor | 125 (55.8%) | 113 (50.2%) | 0.2361 | |
| Very good/Excellent | 99 (44.2%) | 112 (49.8%) | ||
| Height in inches, mean(SD) | 66.8 (3.7) | 66.8 (3.9) | 0.9984 | |
| Weight in pounds, mean(SD) | 195.3 (51.2) | 182.9 (44.1) | 0.0065 | |
| BMI, mean(SD) | 30.8 (8.1) | 28.9 (7.1) | 0.0074 | |
| Waist circumference in inches, mean(SD) | 37.6 (6.7) | 36.3 (6.2) | 0.0410 | |
|
| ||||
| SMOKING CHARACTERISTICSd
| ||||
| Cigarettes per day, mean (SD) | 12.5 (5.7) | 16.9 (4.6) | <0.0001* | |
| Age when you started smoking regularly, mean(SD) | 18.5 (6.5) | 16.5 (4.8) | <0.0002* | |
| Menthol smoker, n (%) | ||||
| Non-Menthol | 31 (13.8%) | 166 (73.8%) | <0.0001* | |
| Menthol | 193 (86.2%) | 59 (26.2%) | ||
| Longest quit attempt in months, mean(SD) | 24.8 (70.9) | 25.3 (51.7) | 0.9328 | |
| Time to first cigarette, n (%) | ||||
| After 30 minutes | 47 (21.0%) | 57 (25.3%) | 0.2745 | |
| Within 30 minutes | 177 (79.0%) | 168 (74.7%) | ||
| Number of your five best friends smoke, mean(SD) | 0–5 | 2.9 (1.8) | 2.7 (1.7) | 0.2180 |
| Number of smokers in the home(not including self), mean(SD) | 0.6 (1.0) | 0.6 (0.8) | 0.8502 | |
| Partner smoking status, n (%) | ||||
| No partner/spouse | 94 (42.0%) | 78 (34.7%) | 0.1899 | |
| Partner/spouse is a non-smoker | 68 (30.4%) | 69 (30.7%) | ||
| Partner/spouse is a smoker | 62 (27.6%) | 78 (34.7%) | ||
|
| ||||
| TREATMENT PROCESS CHARACTERISTICSe
| ||||
| Withdrawal, mean(SD) | 0–32 | 5.3 (4.3) | 5.2 (3.1) | 0.8090 |
| Craving, mean(SD) | 4–28 | 14.1 (7.9) | 15.3 (6.7) | 0.0890 |
| Reinforcing effects of nicotine, mean (SD) | ||||
| Smoking satisfaction | 3–21 | 12.2 (4.9) | 12.1 (4.5) | 0.9108 |
| Psychological reward | 5–35 | 16.7 (7.2) | 16.6 (7.3) | 0.9034 |
| Aversion | 2–14 | 3.0 (1.9) | 2.4 (1.2) | 0.0004* |
| Enjoyment of respiratory tract sensations | 1–7 | 3.2 (1.9) | 3.0 (1.8) | 0.4651 |
| Craving reduction | 1–7 | 4.8 (2.0) | 5.4 (1.6) | 0.0021* |
|
| ||||
| PSYCHOSOCIAL CHARACTERISTICSf
| ||||
| Perceived stress, mean(SD) | 0–16 | 4.5 (2.7) | 3.6 (2.3) | <0.0001* |
| Depression, mean(SD) | 0–27 | 2.1 (3.4) | 1.2 (1.8) | 0.0006* |
| Anxiety, mean(SD) | 0–21 | 3.0 (3.6) | 2.1 (2.7) | 0.0038 |
| Financial strain, mean(SD) | 8–24 | 15.6 (4.3) | 14.6 (4.1) | 0.0137 |
| Perceived discrimination, mean (SD) | ||||
| # of situations encountered | 0–5 | 2.7 (1.6) | 2.6 (1.6) | 0.3167 |
| Frequency encountered | 0–25 | 6.8 (5.4) | 5.1 (4.1) | 0.0003* |
| Race Consciousness, “I think about my race…”, n (%) | ||||
| Never | 109 (48.7%) | 158 (70.2%) | <0.0001* | |
| Once a year | 25 (11.2%) | 25 (11.1%) | ||
| Once a month | 22 (9.8%) | 18 (8.0%) | ||
| Once a week | 14 (6.3%) | 17 (7.6%) | ||
| At least daily | 54 (24.1%) | 7 (3.1%) | ||
| Perceived social status, mean(SD) | 1–10 | 4.8 (1.5) | 5.0 (1.5) | 0.2444 |
| Satisfaction with life, mean(SD) | 5–35 | 20.1 (6.3) | 23.8 (5.5) | <0.0001* |
| Personality factors, mean (SD) | ||||
| Cynicism/distrust of others’ intentions | 0–8 | 5.7 (1.6) | 4.8 (1.7) | <0.0001* |
| Neuroticism/proneness to psychological distress | 0–48 | 12.6 (7.1) | 12.5 (6.3) | 0.9396 |
| Neighborhood disadvantage (self-reported), mean (SD) | ||||
| Social cohesion and trust | 5–25 | 16.2 (4.7) | 17.8 (4.5) | 0.0003* |
| Neighborhood problems | 10–30 | 15.5 (4.7) | 13.5 (3.4) | <0.0001* |
Higher scores on each scale indicate more of the factor /trait – e.g., more withdrawal, more craving, greater reinforcing effects of nicotine, etc.
Bonferroni corrections were applied to minimize the likelihood of a Type I error due to multiple testing. Variables with a p-value below the corrected Type I error rate for the category are considered significant and noted with an asterik.
The Bonferroni correction for Demographic Characteristics sets the significance cut-off at p < 0.0033 (0.05/15)
The Bonferroni correction for Smoking Characteristics sets the significance cut-off at p < 0.0063 (0.05/8)
The Bonferroni correction for Treatment Process Characteristics sets the significance cut-off at p < 0.0071 (0.05/7)
The Bonferroni correction for Psychosocial Characteristics sets the significance cut-off at p < 0.0038 (0.05/13)
DISCUSSION
Quit2Live is the first known prospective head-to-head trial, stratified on race, gender, and age, to explicitly examine whether disparities in cessation exist between African American and White smokers while concurrently exploring demographic, smoking, treatment process, psychosocial, and biological mechanisms to explain the expected disparity. Quit2Live will also be the first fully powered trial to report cessation outcomes for African Americans treated with varenicline and will answer important questions regarding the efficacy of varenicline for this understudied but prevalent subgroup of smokers.
Baseline differences in smoking [42, 3], socioeconomic [92, 93], and psychosocial characteristics [94, 82, 95] are largely consistent with differences identified between African Americans and Whites in previous studies. What is not known, and what will be addressed through the study’s secondary aims, is how the factors in this study independently and jointly moderate and/or mediate the relationship between race and cessation. The selection of variables across demographic, smoking, treatment process, psychosocial, and biological domains offers a comprehensive approach that is strongly grounded in the literature. Our analytic plan will allow us to model each domain independently (e.g., race → demographic factors → cessation) and, if multiple mediators and/or moderators are identified, to examine the interrelationship of factors across demographic, smoking, treatment process, psychosocial, and biological domains. In addition, we are conducting assessments at multiple time points, which will allow for the examination of how factors differ at baseline and change over time for African American and White and how these differences are related to cessation. Few longitudinal comparisons have been conducted to provide evidence of differences in factors that facilitate quitting for African American and White [29, 30].
In summary, Quit2Live addresses an important public health problem, health disparities in relation to smoking. This prospective stratified cohort design will move the field beyond descriptive, post-hoc analyses. Findings from this study will not only examine if African American- White disparities in quitting exist but, more importantly, will identify mechanisms underlying the difference, including interrelationships of factors across domains. Attention to many proximal, modifiable factors (e.g., adherence, response to treatment, depression, stress, peer/family smoking norms) further enhances the potential of our findings to inform practice by moving the field away from a generic focus on race toward an empirically derived approach that will guide researchers in identifying specific factors to address to improve cessation outcomes and reduce tobacco-related morbidity and mortality in future studies with African American.
Acknowledgments
Research reported in this publication was supported by R01-DA031815 (N.L. Nollen) from the NIH, National Institute on Drug Abuse, Frontiers: The Heartland Institute for Clinical and Translational Research which is supported by a CTSA grant to the University of Kansas Medical Center from the NIH National Center for Advancing Translational Science (NCATS; grant # UL1TR000001), and by the National Cancer Institute Cancer Center Support Grant P30 CA168524 and used the Biospecimen Repository. The work was also supported by P50CA180890 (N.L. Benowitz) from the National Cancer Institute and the FDA Center for Tobacco Products, P30DA012393 (N.L. Benowitz) from the National Institute on Drug Abuse, and with instrumentation and analytical chemistry support from the National Institutes of Health, S10 RR026437. We acknowledge the support of the Endowed Chair in Addictions for the Department of Psychiatry (R.F. Tyndale), and CIHR grant TMH-109787 (R. F. Tyndale).
Footnotes
TRIAL STATUS
The final participant was enrolled in May 2015. The active treatment and follow-up phases are ongoing, with the final week 26 follow-up visit scheduled for November 2015. Outcomes data have not been examined and will not be available until June 2016.
COMPETING INTERESTS
Pfizer Global Pharmaceuticals provided study medication but played no role in the design or conduct of the study or in interpretation and analysis of the data (WS953655). Dr. Benowitz has served as a paid consultant to Pfizer as a member of its smoking cessation medication advisory board and also as an unpaid scientific advisor to Pfizer regarding a multi-site international clinical trial that has been conducted on the safety of varenicline for smoking cessation. Dr. Tyndale has served as a paid consultant to Apotex.
AUTHORS’ CONTRIBUTIONS:
Conception and design: NN, LC, NB, RT, MM, JA
Acquisition of data: NN, LC, EE, TS, NB, RT, MM
Analysis and interpretation of data: NN, MM, QY
Writing, review, and revision of the manuscript: All authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicole L. Nollen, Email: nnollen@kumc.edu, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas
Lisa Sanderson Cox, Email: lcox@kumc.edu, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas.
Qing Yu, Email: qyu@kumc.edu, Department of Biostatistics, University of Kansas School of Medicine, Kansas City, Kansas.
Edward F. Ellerbeck, Email: eellerbe@kumc.edu, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas
Taneisha S. Scheuermann, Email: tscheuermann@kumc.edu, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas
Neal L. Benowitz, Email: neal.benowitz@ucsf.edu, Division of Clinical Pharmacology and Experimental Therapeutics, Departments of Medicine, Bioengineering, & Therapeutic Sciences, University of California, San Francisco, San Francisco, California
Rachel F. Tyndale, Email: r.tyndale@utoronto.ca, Center for Addiction and Mental Health and Departments of Psychiatry and Pharmacology, University of Toronto, Toronto, Ontario
Matthew S. Mayo, Email: mmayo@kumc.edu, Department of Biostatistics, University of Kansas School of Medicine, Kansas City, Kansas
Jasjit S. Ahluwalia, Email: j.ahluwalia@rutgers.edu, Department of Epidemiology, Rutgers School of Public Health, Piscataway, New Jersey
References
- 1.Centers for Disease Control and Prevention. Tobacco Control State Highlights, 2010. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 2.Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11(2):203–10. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. doi: 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures for African Americans 2013–2014. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Y, McGee DL, Cooper RS. Prediction of coronary heart disease mortality in blacks and whites: pooled data from two national cohorts. Am J Cardiol. 1999;84(1):31–6. doi: 10.1016/s0002-9149(99)00187-3. S0002-9149(99)00187-3 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg L, Palmer JR, Rao RS, Adams-Campbell LL. Risk factors for coronary heart disease in African American women. Am J Epidemiol. 1999;150(9):904–9. doi: 10.1093/oxfordjournals.aje.a010098. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–6. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial Differences in the Relationship Between Number of Cigarettes Smoked and Nicotine and Carcinogen Exposure. Nicotine Tob Res. 2011 doi: 10.1093/ntr/ntr072. ntr072 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark PI, Gautam S, Gerson LW. EFfect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110(5):1194–8. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- 12.Fu SS, Kodl MM, Joseph AM, Hatsukami DK, Johnson EO, Breslau N, et al. Racial/Ethnic Disparities in the Use of Nicotine Replacement Therapy and Quit Ratios in Lifetime Smokers Ages 25 to 44 Years. Cancer Epidemiology Biomarkers & Prevention. 2008;17(7):1640–7. doi: 10.1158/1055-9965.epi-07-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doescher MP, Saver BG. Physicians’ advice to quit smoking. The glass remains half empty. J Fam Pract. 2000;49(6):543–7. [PubMed] [Google Scholar]
- 14.Hymowitz N, Jackson J, Carter R, Eckholdt H. Past quit smoking assistance and doctors’ advice for white and African-American smokers. J Natl Med Assoc. 1996;88(4):249–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Quintero C, Crum RM, Neumark YD. Racial/ethnic disparities in report of physician-provided smoking cessation advice: analysis of the 2000 National Health Interview Survey. Am J Public Health. 2006;96(12):2235–9. doi: 10.2105/AJPH.2005.071035. AJPH.2005.071035 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S, Melcer T, Sun J, Rosbrook B, Pierce JP. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med. 2000;18(4):305–11. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 17.Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34(5):404–12. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Fu SS, Sherman SE, Yano EM, van Ryn M, Lanto AB, Joseph AM. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20(2):108–16. doi: 10.4278/0890-1171-20.2.108. [DOI] [PubMed] [Google Scholar]
- 19.Croghan IT, Hurt RD, Ebbert JO, Croghan GA, Polk OD, Stella PJ, et al. Racial differences in smoking abstinence rates in a multicenter, randomized, open-label trial in the United States. J Public Health. 2010;18(1):59–68. doi: 10.1007/s10389-009-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cropsey KL, Weaver MF, Eldridge GD, Villalobos GC, Best AM, Stitzer ML. Differential success rates in racial groups: results of a clinical trial of smoking cessation among female prisoners. Nicotine Tob Res. 2009;11(6):690–7. doi: 10.1093/ntr/ntp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. J Subst Abuse Treat. 2009;37(3):247–55. doi: 10.1016/j.jsat.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray RP, Connett JE, Buist AS, Gerald LB, Eichenhorn MS. Experience of Black participants in the Lung Health Study smoking cessation intervention program. Nicotine Tob Res. 2001;3(4):375–82. doi: 10.1080/14622200110081435. [DOI] [PubMed] [Google Scholar]
- 23.Burgess DJ, van Ryn M, Noorbaloochi S, Clothier B, Taylor BC, Sherman S, et al. Smoking cessation among African American and white smokers in the Veterans Affairs health care system. Am J Public Health. 2014;104(Suppl 4):S580–7. doi: 10.2105/AJPH.2014.302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Cofta-Woerpel LM, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health. 2010;100(4):702–6. doi: 10.2105/AJPH.2009.172676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction. 2006;101(6):883–91. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz N, Jacob P, Ahijevych K, Jarvis M, Hall S, Hansson A, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 27.Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst. 2012;104(4):290–8. doi: 10.1093/jnci/djr513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenknecht LE, Burke GL, Perkins LL, Haley NJ, Friedman GD. Misclassification of smoking status in the CARDIA study: a comparison of self-report with serum cotinine levels. Am J Public Health. 1992;82(1):33–6. doi: 10.2105/ajph.82.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29(3):262–73. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro Y, Cano MA, Businelle MS, Correa-Fernandez V, Heppner WL, Mazas CA, et al. A cross-lagged path analysis of five intrapersonal determinants of smoking cessation. Drug Alcohol Depend. 2014;137:98–105. doi: 10.1016/j.drugalcdep.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cropsey KL, Clark CB, Zhang XN, Hendricks PS, Jardin BF, Lahti AC. Race and Medication Adherence Moderate Cessation Outcomes in Criminal Justice Smokers. Am J Prev Med. 2015 doi: 10.1016/j.amepre.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agaku IT, King BA, Dube SR Centers for Disease C and Prevention. Current cigarette smoking among adults - United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 33.Kotz D, West R. Explaining the social gradient in smoking cessation: it’s not in the trying, but in the succeeding. Tob Control. 2009;18(1):43–6. doi: 10.1136/tc.2008.025981. [DOI] [PubMed] [Google Scholar]
- 34.Pierce JP, White MM, Messer K. Changing age-specific patterns of cigarette consumption in the United States, 1992–2002: association with smoke-free homes and state-level tobacco control activity. Nicotine Tob Res. 2009;11(2):171–7. doi: 10.1093/ntr/ntp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–57. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: results from a prospective study of an Australian national sample. Addiction. 2006;101(1):121–7. doi: 10.1111/j.1360-0443.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 37.Siahpush M, Yong HH, Borland R, Reid JL, Hammond D. Smokers with financial stress are more likely to want to quit but less likely to try or succeed: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104(8):1382–90. doi: 10.1111/j.1360-0443.2009.02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–62. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 39.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD, Richter PA, Strauss WJ, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA. 1998;280(2):135–9. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63(3):360–7. doi: 10.1111/j.1742-1241.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 41.Gundersen DA, Delnevo CD, Wackowski O. Exploring the relationship between race/ethnicity, menthol smoking, and cessation, in a nationally representative sample of adults. Prev Med. 2009;49(6):553–7. doi: 10.1016/j.ypmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–6. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 43.Nollen NL, Mayo MS, Ahluwalia JS, Tyndale RF, Benowitz NL, Faseru B, et al. Factors associated with discontinuation of bupropion and counseling among African American light smokers in a randomized clinical trial. Ann Behav Med. 2013;46(3):336–48. doi: 10.1007/s12160-013-9510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catley D, Ahluwalia JS, Resnicow K, Nazir N. Depressive symptoms and smoking cessation among inner-city African Americans using the nicotine patch. Nicotine Tob Res. 2003;5(1):61–8. CDLTHMGVLB7C9AYV [pii] [PubMed] [Google Scholar]
- 45.Catley D, Harris KJ, Okuyemi KS, Mayo MS, Pankey E, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine Tob Res. 2005;7(6):859–70. doi: 10.1080/14622200500330118. HX31847128412012 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. Am J Prev Med. 1996;12(5):378–87. [PubMed] [Google Scholar]
- 47.Kendzor DE, Businelle MS, Reitzel LR, Rios DM, Scheuermann TS, Pulvers K, et al. Everyday discrimination is associated with nicotine dependence among African American, Latino, and White smokers. Nicotine Tob Res. 2014;16(6):633–40. doi: 10.1093/ntr/ntt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kendzor DE, Reitzel LR, Mazas CA, Cofta-Woerpel LM, Cao Y, Ji L, et al. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Soc Sci Med. 2012;74(9):1394–401. doi: 10.1016/j.socscimed.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH. Associations between smoking cessation and anxiety and depression among U.S. adults. Addict Behav. 2009;34(6–7):491–7. doi: 10.1016/j.addbeh.2009.01.005. S0306-4603(09)00026-4 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Reitzel LR, Businelle MS, Kendzor DE, Li Y, Cao Y, Castro Y, et al. Subjective social status predicts long-term smoking abstinence. BMC Public Health. 2011;11:135. doi: 10.1186/1471-2458-11-135. 1471-2458-11-135 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reitzel LR, Mazas CA, Cofta-Woerpel L, Li Y, Cao Y, Businelle MS, et al. Subjective social status affects smoking abstinence during acute withdrawal through affective mediators. Addiction. 2010;105(5):928–36. doi: 10.1111/j.1360-0443.2009.02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reitzel LR, Vidrine JI, Businelle MS, Kendzor DE, Cao Y, Mazas CA, et al. Neighborhood perceptions are associated with tobacco dependence among African American smokers. Nicotine Tob Res. 2012;14(7):786–93. doi: 10.1093/ntr/ntr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–88. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 54.Al Koudsi N, Ahluwalia JS, Lin SK, Sellers EM, Tyndale RF. A novel CYP2A6 allele (CYP2A6*35) resulting in an amino-acid substitution (Asn438Tyr) is associated with lower CYP2A6 activity in vivo. Pharmacogenomics J. 2009;9(4):274–82. doi: 10.1038/tpj.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho MK, Mwenifumbo JC, Zhao B, Gillam EM, Tyndale RF. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black-African descent. Pharmacogenet Genomics. 2008;18(1):67–75. doi: 10.1097/FPC.0b013e3282f3606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lerman C, Schnoll RA, Hawk LW, Jr, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. The Lancet Respiratory medicine. 2015 doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mwenifumbo JC, Al Koudsi N, Ho MK, Zhou Q, Hoffmann EB, Sellers EM, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum Mutat. 2008;29(5):679–88. doi: 10.1002/humu.20698. [DOI] [PubMed] [Google Scholar]
- 58.Mwenifumbo JC, Zhou Q, Benowitz NL, Sellers EM, Tyndale RF. New CYP2A6 gene deletion and conversion variants in a population of Black African descent. Pharmacogenomics. 11(2):189–98. doi: 10.2217/pgs.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CDC. Cigarette smoking among adults and trends in smoking cessation -- United States, 2008. MMWR. 2009;58(44):1227–32. [PubMed] [Google Scholar]
- 60.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 61.Lowe B, Kroenke K, Grafe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J Psychosom Res. 2005;58(2):163–71. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Department of Health and Human Services, Office of the Secretary. Annual Update of the HHS Poverty Guidelines, 42. U.S.C. 9902 (2) Fed Regist. 2012;77(17):4034–5. [Google Scholar]
- 63.Fiore M, et al. Treating Tobacco Use and Dependence: Clinical Practice Guideline 2008 Update. US Department of Health and Human Services Public Health Service; 2008. [Google Scholar]
- 64.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 65.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 66.Nollen NL, Cox LS, Nazir N, Ellerbeck EF, Owen A, Pankey S, et al. A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine Tob Res. 2011;13(9):868–73. doi: 10.1093/ntr/ntr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahluwalia JS, McNagny SE, Clark WS. Smoking cessation among inner-city African Americans using the nicotine transdermal patch.[see comment] J Gen Intern Med. 1998;13(1):1–8. doi: 10.1046/j.1525-1497.1998.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–74. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- 69.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45(7):908–15. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiore M, Jaen C, Baker T, Bailey W, Benowitz N, Curry S, et al. Treating Tobacco Use and Dependence Clinical Practice Guideline: 2008 Update. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 71.Dorsey R, Graham G. New HHS data standards for race, ethnicity, sex, primary language, and disability status. JAMA. 2011;306(21):2378–9. doi: 10.1001/jama.2011.1789. [DOI] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 73.Idler EL, Russell LB, Davis D. Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. First National Health and Nutrition Examination Survey. Am J Epidemiol. 2000;152(9):874–83. doi: 10.1093/aje/152.9.874. [DOI] [PubMed] [Google Scholar]
- 74.Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinectic Books; 1988. [Google Scholar]
- 75.Al-Delaimy WK, Edland S, Pierce JP, Mills AL, MMW . Technical Report on Analytic Methods and Approaches Used in the 2008 California Tobacco Survey Analysis. Vol 1: Data Collection Methodology. La Jolla, CA: University of California: San Diego; 2009. [Google Scholar]
- 76.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 77.Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14(12):1467–73. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 78.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 79.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–23. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 81.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park, CA: Sage; 1988. pp. 31–68. [Google Scholar]
- 82.Sternthal MJ, Slopen N, Williams DR. Racial Disparities in Health. Du Bois Review: Social Science Research on Race. 2011;8(01):95–113. doi: 10.1017/s1742058x11000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. Reactions to Race. [Google Scholar]
- 84.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 85.Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005;30(1):11–7. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–8. [Google Scholar]
- 87.Adler N. Social Status Ladder. John D and Catherine T MacArthur Research Network on Socioeconomic Status and Health; [Accessed 2014 September 16]. http://www.macses.ucsf.edu/research/psychosocial/subjective.php. [Google Scholar]
- 88.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–24. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 89.Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med. 2001;23(3):177–85. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- 90.Diener E, Emmons R, Larsen RA, Griffin S. The Satisfaction with Life Scale. J Pers Assess. 1985;49(1) doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 91.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther. 2009;31(3):463–91. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 92.Bureau of Labor Statistics, U.S. Department of Labor. The Economics Daily. 2015. Educational attainment, employment, and unemployment among African Americans. [Google Scholar]
- 93.Macartney S, Bishaw A, Fontenot K. Poverty rates for selected detailed race and Hispanic groups by state and place: 2007–2011. American Community Survey Briefs. 2013 [Google Scholar]
- 94.Brewer LC, Carson KA, Williams DR, Allen A, Jones CP, Cooper LA. Association of Race Consciousness With the Patient-Physician Relationship, Medication Adherence, and Blood Pressure in Urban Primary Care Patients. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–16. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]