Abstract
Laboratory and observational research studies suggest that vitamin D and marine omega-3 fatty acids may reduce risk for pneumonia, acute exacerbations of respiratory diseases including chronic obstructive lung disease (COPD) or asthma, and decline of lung function, but prevention trials with adequate dosing, adequate power, and adequate time to follow-up are lacking. The ongoing Lung VITAL study is taking advantage of a large clinical trial—the VITamin D and OmegA-3 TriaL (VITAL)—to conduct the first major evaluation of the influences of vitamin D and marine omega-3 fatty acid supplementation on pneumonia risk, respiratory exacerbation episodes, asthma control and lung function in adults. VITAL is a 5-year U.S.-wide randomized, double-blind, placebo-controlled, 2×2 factorial trial of supplementation with vitamin D3 ([cholecalciferol], 2000 IU/day) and marine omega-3 FA (Omacor® fish oil, eicosapentaenoic acid [EPA] +docosahexaenoic acid [DHA], 1 g/day) for primary prevention of CVD and cancer among men and women, at baseline aged ≥50 and ≥55, respectively, with 5107 African Americans. In a subset of 1973 participants from 11 urban U.S. centers, lung function is measured before and two years after randomization. Yearly follow-up questionnaires assess incident pneumonia in the entire randomized population, and exacerbations of respiratory disease, asthma control and dyspnea in a subpopulation of 4314 randomized participants enriched, as shown in presentation of baseline characteristics, for respiratory disease, respiratory symptoms, and history of cigarette smoking. Self-reported pneumonia hospitalization will be confirmed by medical record review, and exacerbations will be confirmed by Center for Medicare and Medicaid Services data review.
Keywords: Vitamin D, Omega-3 Fatty Acids, Respiratory Symptoms, COPD, Lung Function, Randomized Clinical Trial
Introduction
Acute exacerbations of chronic respiratory diseases including chronic obstructive lung disease (COPD) or asthma;1 pneumonia (www.cdc.gov/nchs); and decline of lung function2 are major risk factors for lost disability-adjusted life-years, and increased morbidity and mortality in adults world-wide. 3–8 Vitamin D and fish oil/marine omega-fatty acid supplementation have been postulated to help reduce these risk factors. While there have been significant advances in understanding their potential role in improving respiratory health in adults, significant gaps remain. In its 2011 report, the IOM comprehensively reviewed the scientific literature on vitamin D and health, concluding that although there is clear evidence that vitamin D confers bone benefits, available research is insufficient to determine whether vitamin D is beneficial for nonskeletal diseases, stating that adequately powered randomized clinical trials were needed to assess whether supplementation should be recommended for other disorders.9–11
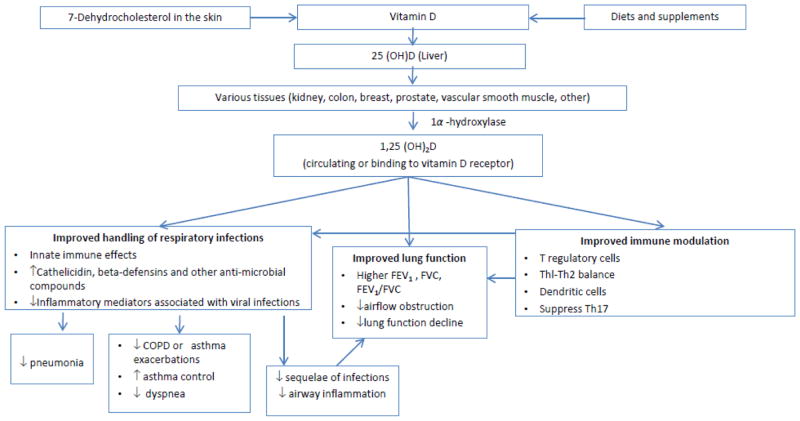
Vitamin D is both a nutrient and a prohormone (Figure 1), and blood levels are dependent on dietary intake and exposure to sunlight. The National Health and Nutrition Examination Surveys estimate that a third of Americans have insufficient levels of vitamin D (defined as a 25-hydroxivitamin D[25((OH)D] level of <20 ng/ml). Aging, obesity, black race/ethnicity, and COPD are predictors of lower vitamin D levels.12 Viral and bacterial infections are the most common precipitants of exacerbations of chronic respiratory disease. Vitamin D has beneficial effects on antimicrobial, oxidant,13 and immune pathways relevant to lung infection, airway inflammation and airway remodeling.14 In laboratory studies vitamin D upregulates antimicrobial peptides3, 15, 16 and reduces inflammatory and allergic responses through modulating innate, T regulatory and adaptive immunity.17 Mechanistic studies also support a role for vitamin D supplementation in the improvement of the many co-morbidities associated with COPD progression and ultimately, death. These co-morbidities include osteopenia, osteoporosis and fractures, respiratory infections, myopathy and weakness, diabetes, cancer, deep venous thrombosis, and cardiovascular diseases.12 We and others have published observational studies of associations of higher vitamin D levels with reduction of serious infection,18–20 lower lung function decline,21 and improvement in asthma control in adults,22 but clinical trials in adults are conflicting,23 often limited by low power, suboptimal follow-up time or dosing schedule.24 Fish contain omega-3 fatty acids (eicosapentaenoic acid [EPA]+docosahexaenoic acid [DHA]), which modulate the innate inflammatory response by a pathway distinct from Vitamin D (Figure 2). Laboratory studies suggest that omega-3 FAs have beneficial anti-inflammatory effects on arachidonic acid metabolic pathways and the downstream balance of eicosanoids, including prostaglandins and leukotrienes25 that influence neutrophil recruitment and bronchoconstriction.26 Particularly in children, fish intake has been associated with reduced wheeze and asthma in many (but not all) epidemiologic cohort studies. Despite compelling biologic data suggesting that marine omega-3 FA beneficially influences pathways relevant to airflow obstruction or asthma, prior trials of omega-3 FAs for adults are few (10 for asthma reported in the 2014 Wendell review 25) and underpowered (16 to 46 subjects), though 9 of the 10 suggested potential benefits in inflammatory, lung function or symptom outcomes. It is important to clarify these relationships in larger randomized clinical trials.
Fig. 1.
Mechanisms by which vitamin D may improve handling of respiratory infections, lung function, or immune modulation, with downstream influences on incident pneumonia, respiratory symptoms and disease progression. Adapted from Manson [27] and Litonjua [60]. 56, 60, 61
Fig. 2.
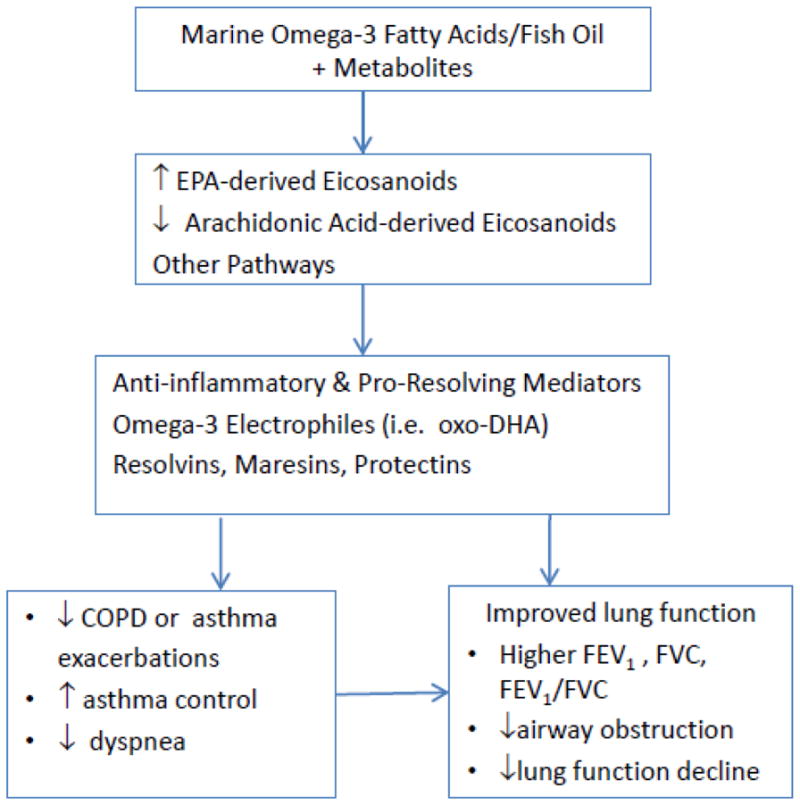
Mechanisms by which marine omega-3 fatty acids may reduce inflammation, improve lung function, and reduce exacerbations of asthma or COPD. Adapted from Manson [27] and Wendell [25].
2. Materials and Methods
2.1. Overview of study design and aims
The Lung VITAL study has taken advantage of a large clinical trial—the VITamin D and OmegA-3 TriaL (VITAL)—to conduct the first major evaluation of the influences of vitamin D and marine omega-3 fatty acid supplementation on obstructive and infectious lung diseases in adults. VITAL is a cost effective randomized, double-blind, placebo-controlled, 2×2 factorial trial of vitamin D (in the form of vitamin D3 [cholecalciferol], 2000 IU/day) and marine omega-3 fatty acid (Omacor® fish oil, eicosapentaenoic acid [EPA]+docosahexaenoic acid [DHA], 1 g/day) supplements in the primary prevention of cancer and CVD among men and women, aged ≥50 and ≥55, respectively, with an oversampling of African Americans. The rationale and study design for VITAL have been described in detail in 2011, with an update in 2015.27, 28
In the VITAL cohort of 25,874 participants, randomized equally to vitamin D and/or omega-3 fatty acids or placebo (four study arms), we are conducting Lung VITAL, an ancillary respiratory study to determine the efficacy of daily supplemental vitamin D in prevention of exacerbations of respiratory disease in a sub-cohort enriched for active, symptomatic respiratory disease (including doctor-diagnosed COPD or asthma) at baseline (Table 1)(N=4314); in reduction of airflow obstruction and decline of lung function (forced expiratory flow in one second [FEV1) among a sub-cohort (baseline N=1974 lung function tests performed;1924 of acceptable quality by ATS criteria); in prevention of pneumonia (N=25,874--i.e., the entire randomized population); and, secondarily, in improvement of asthma control in those with doctor-diagnosed asthma (N=1840). A tertiary aim is to explore whether the effects of vitamin D3 or EPA+DHA supplementation on exacerbations of respiratory disease, FEV1, incident pneumonia or asthma control vary by baseline dietary intake of these nutrients, by achieved 25 (OH) D or plasma levels of EPA+DHA; or by race/skin pigmentation or sunlight exposure (for vitamin D3).
Table 1.
Respiratory outcomes in randomized participants.
| Primary Outcomes | Indices | Data Source | Population Subset | # Repeated Measures |
|---|---|---|---|---|
| Pneumonia | Incident report in past year | VITAL questionnaire | 25,874 | Annually x5 |
| Respiratory exacerbation episodes | Count of episodes in past year treated with antibiotics or steroid pills or injections | Respiratory questionnaire | 4314 (COPD: 2561a) (Asthma: 1840) (COPD or Asthma: 3289) | Annually x5 |
| Lung function | FEV1, FVC, FEV1/FVC level and change in level | Spirometry (EasyOne) | 1973 (1924 b) | 2x:(baseline & 2 yr post randomization) |
|
| ||||
| Secondary Outcomes | ||||
|
| ||||
| Asthma control | Juniper Asthma Control Score | Respiratory questionnaire | 1840 of 4314 | Annually x5 |
| Acute respiratory events | Hospitalization/ER visits | CMS (Medicare/Medicaid data) | ~23,207c | Annually x5 |
| Dyspnea | MMRC dyspnea score | Respiratory questionnaire | 4314 | 2x:(baseline & 2 yr post randomization) |
COPD defined as either doctor or other health care professional’s diagnosis of COPD/emphysema/chronic bronchitis), or chronic cough/chronic phlegm by ATS-DLD criteria.
2027 participants from 11 continental U.S. locations performed pre-randomization lung function testing by spirometry. Of these 2027 participants, 1973 were randomized and 1924 had lung function tests of acceptable quality.
Number of randomized subjects who will be or turn 65 years or older during the trial
2.2. Sponsor
This ancillary study, entitled Lung VITamin D and OmegA-3 TriaL (Lung VITAL)(R01HL101932) is supported by the National Heart Lung and Blood Institute (NHLBI). The study was approved by the Partners Human Research Committee, the Institutional Review Board of Brigham and Women’s Hospital (BWH). It is registered with clinicaltrials.gov (2010-P-000622). A study website for the parent trial is maintained at www.vitalstudy.org.
2.3. Summary of eligibility, recruitment, enrollment and randomization (Table 1)
The eligibility criteria for the VITAL trial have been previously published.27 In brief, women aged 55 years and older, and men aged 50 years and older were eligible if they had no prior history of cancer or cardiovascular disease at baseline, and if they met safety criteria (no allergy to fish, renal failure, history of hypercalcemia, hypo- or hyperparathyroidism, severe liver disease, granulomatous disease or other serious illness). Prior to randomization, all participants signed a detailed informed consent form and were required to complete a 3-month, placebo run-in to demonstrate good pill-taking compliance, defined as taking ≥ 2/3 of the study pills. Study enrollment and randomization began in November 2011 and ended in March 2014. The trial will be completed in 2018, with subsequent follow-up.
Enrollment for pneumonia and questionnaire-based respiratory outcome assessment
All 25,874 enrolled VITAL participants were eligible to be followed for incident pneumonia. All VITAL participants who reported symptoms of wheeze, usual cough or phlegm; or doctor-diagnosed asthma, COPD, emphysema, or chronic bronchitis on the VITAL screening questionnaire and consented were sent a more detailed baseline respiratory questionnaire. For those who answered the baseline respiratory questionnaire (N=6533), selection criteria for annual post-randomization follow-up included (1) pre-randomization report of exacerbations of respiratory disease/flare up of chest trouble in the past year, ascertained via standard (COPDGene)29 questions,30 (2) lack of asthma control in past week, (3) wheezing or whistling in the chest more than once a week or most days and nights in the last 12 months (ATS-DLD31), (4) dyspnea/shortness of breath, or (5) chronic bronchitis by American Thoracic Society-DLD definition32 (usually coughing or bringing up phlegm on most days, for 3 consecutive months or more during the year) with history of current smoking or past smoking at least 10 pack-years or currently working at a dusty job. VITAL participants who did not meet criteria 1–5 but on lung function testing had reduced FEV1 and/or airflow obstruction (Table 4) with Preserved Ratio Impaired Spirometry[PRISm],33 or GOLD spirometry grades I through IV were also enrolled for annual detailed respiratory questionnaire follow-up, as airflow obstruction itself is a risk factor for future respiratory episodes, subsequent to baseline questionnaire and spirometry administration. Thus a subcohort of 4314 VITAL participants enriched for active respiratory morbidity were selected to be followed annually by Lung VITAL by detailed respiratory questionnaire for exacerbation of respiratory disease/flare-up of chest trouble, asthma control, dyspnea, and relevant environmental exposure history.
Table 4.
Respiratory disease and symptom rates for all randomized subjects and for those selected for respiratory questionnaire follow-up.
| Randomized (n=25874) | Respiratory Questionnaire follow-up cohort | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| All (4314) | COPD (N=2561) | Asthma (N=1840) | ||||||
|
| ||||||||
| N | N (%) | N | N (%) | N | N (%) | N | N (%) | |
| COPD dxa | 25486 | 2258 (8.9) | 4307 | 1637 (38.0) | ||||
| COPD dx or symptomsb | - | - | 4308 | 2561 (59.5) | ||||
| Asthma dx | 25743 | 2955 (11.5) | 4311 | 1840 (42.7) | ||||
| COPD or Asthma | 25785 | 5007 (19.4) | 4313 | 3289 (76.3) | ||||
| Pneumonia in the past year | 25718 | 341 (1.3) | 4289 | 162 (3.8) | 2546 | 118 (4.6) | 1831 | 77 (4.2) |
| Dyspnea score of at least 1c | 3938 | 2124 (53.9) | 2499 | 1428 (57.1) | 1778 | 981 (55.2) | ||
| ER/hospital for lung problems | 4024 | 269 (6.7) | 2559 | 217 (8.5) | 1822 | 163 (9.0) | ||
| Chest/lung exacerbations past year | 4024 | 1824 (45.3) | 2559 | 1164 (45.5) | 1822 | 893 (49.0) | ||
| Antibiotics | 4022 | 1408 (35.0) | 2558 | 954 (37.3) | 1821 | 700 (38.4) | ||
| Steroids | 4022 | 1008 (25.1) | 2559 | 650 (25.4) | 1821 | 562 (30.9) | ||
|
| ||||||||
| N | Mean (SD) [range] | N | Mean (SD) [range] | N | Mean (SD) [range] | |||
|
| ||||||||
| Chest/lung exacerbations in past year requiring antibiotics or steroidsd | 4023 | 0.91 (1.67) [0–11] | 2558 | 0.97 (1.76) [0–11] | 1821 | 1.04 1.78 [0–11] | ||
| Flare-up episodes requiring an increase in antibiotics or steroids, or hospitalizatione | 3993 | 0.28 (0.58) [0–6] | 2534 | 0.33 (0.61) [0–6] | 1809 | 0.38 (0.66) [0–6] | ||
| Dyspnea scorec | 3938 | 1.07 (1.22) [0–4] | 2499 | 1.20 (1.28) [0–4] | 1778 | 1.14 (1.25) [0–4] | ||
| Juniper score (for those with asthma, with or without COPD) | 1041 | 0.84 (0.86) [0–4.5] | 1703 | 0.67 (0.78) [0–4.5] | ||||
Doctor or other health care professional’s diagnosis of COPD/emphysema/chronic bronchitis by questionnaire
Doctor or other health care professional’s diagnosis of COPD/emphysema/chronic bronchitis, or chronic cough/chronic phlegm by questionnaire
Derived from MMRC questions (supplementary file)
Mean (SD) of the count/person of exacerbations with either antibiotics or steroid pills or injections (supplementary file).
Mean (SD) of the count/person (supplementary file)
Enrollment for lung function assessment at baseline (during the run-in)
A subcohort of 2027 participants from 11 continental U.S. locations performed pre-randomization lung function testing by spirometry. At those locations, baseline lung function testing was performed in those demonstrating good pill-taking compliance after two of the three months of the run-in period, who were still eligible and interested in participating in the trial. Additional criteria for baseline lung function testing included ability to stand for height and weight measurements; not having acute wheezing/an acute asthma or COPD exacerbation at the time of the visit; absence of history of radiation to the chest for a disorder other than breast cancer; a pacemaker or defibrillator; chest or abdominal surgery, detached retina or eye surgery or a heart attack in the last three months; and absence of history of hospitalization for any heart problem in the past month.
Of the 2027 participants who performed lung function testing, 1973 were randomized and 1924 had lung function tests of acceptable quality. Of those 1973 randomized participants who performed baseline lung function maneuvers, 977(949 with acceptable quality tests) were from the sub group of 1054 VITAL participants from the New England region who consented to detailed, in-person assessment at Harvard Catalyst, the NIH-sponsored Clinical and Translational Science Center (CTSC) in Boston. The additional 996 randomized participants performed lung function testing (975/996 of acceptable quality) through home visits by technicians from EMSI (Examination Management Services, Inc.) centers in Baltimore, Chicago, Dallas, Detroit, Minneapolis/St. Paul, Philadelphia, Phoenix, Los Angeles, St. Louis, and Washington, D.C. Initially, beginning in October 2011 EMSI out-of-Boston inclusion criteria for conducting baseline spirometry were more restrictive than those for the Boston CTSC, including only VITAL participants in the area of the local EMSI office who were at risk for airflow obstruction because of COPD, asthma or smoking histories. To increase the number with lung function tests and hence the power to test the effects of the supplements on lung function, and in acknowledgement of the fact that lung function decline occurs in all adults, and that airflow obstruction occurs in a significant proportion of asymptomatic non-smoking adults, these criteria were expanded in April 2012 to include all consenting VITAL/Lung VITAL participants within each of the 10 out-of-Boston EMSI catchment areas.
2.4. Intervention
As described previously, the doses of vitamin D3 and marine omega-3 FA and their formulation were chosen after comprehensive testing and review of the available literature to achieve optimal efficacy and safety. VITAL is testing the following interventions: vitamin D3 ([cholecalciferol], 2000 IU/day) and marine ω-3 FA (Omacor® fish oil, eicosapentaenoic acid [EPA]+docosahexaenoic acid [DHA], 1 g/day) and matching inert placebos. Participants are permitted up to 800 IU/d of additional vitamin D supplementation and up to 1200 mg/d of elemental calcium from all sources. All participants also agreed not to consume non-study fish-oil supplements during the trial.
2.5. Blood Collection
During the run-in, prior to randomization, blood samples for the parent VITAL study and its ancillary studies, including Lung VITAL, were collected during run-in from a subset of 16,956 participants. Of 16,956 of these baseline bloods, 1,054 were collected at the Boston Harvard Catalyst CTSC. During the run-in, for consenting participants who had not yet contributed a baseline blood sample, blood collection for VITAL was included as part of the EMSI spirometry home visit protocol (see 2.7). Among this subset, follow-up samples are being collected after randomization from approximately 6000 participants, including those examined during the 2-year outside-of-Boston Metropolitan area Lung VITAL EMSI spirometry follow-up and the 2-year comprehensive (including spirometry) VITAL CTSC visit by New England participants. Blood samples will confirm intervention compliance, monitor changes in background intake in the study population over time (placebo group), and determine whether treatment effects are modified by baseline and post-randomization blood levels.
2.6 Study Assessments: questionnaire and record-based respiratory outcomes
2.6.1. Pneumonia
For each of the 5 post-randomization years, annual VITAL follow-up questionnaires assess the endpoint of a report of diagnosis of pneumonia in the past year (incident pneumonia) in all 25,874 randomized participants. Lung VITAL adjudicates self-report of pneumonia by hospital record and CMS review and documents, using ICD-9 codes, concurrent diagnoses for each hospitalization.
2.6.2. COPD and asthma
An annual respiratory questionnaire provides more detailed data on respiratory diagnoses, outcomes and medication use in the subset of 4314 randomized at-risk participants selected from the U.S.-wide VITAL cohort (Section 2.3). Self-reported COPD34 will be defined as self-report of doctor- or other health professional- diagnosed emphysema, chronic bronchitis or chronic obstructive pulmonary disease/COPD.35 For those with spirometry (Table 3), COPD and PRISm33 will be defined using the GOLD criteria (www.goldcopd.com). Active (current) asthma will be defined as ever doctor’s diagnosis of asthma with any wheeze or asthma medication use in the past 12 months.
Table 3.
Baseline characteristics of the Lung VITAL population.
| Randomized (n=25874) | Respiratory questionnaire follow-up cohort (n=4314) | |
|---|---|---|
|
| ||
| Mean (IQR) | Mean (IQR) | |
| Age at randomization (years) | 67.1 (62.5–71.2) | 67.6 (62.9 – 71.7) |
| Body Mass Index (kg/m2) | 28.2 (24.4–30.9) | 29.9 (25.1–33.3) |
|
| ||
| N (%) | N (%) | |
|
| ||
| Gender | ||
| Female | 13081 (50.6) | 2463 (57.1) |
| Male | 12793 (49.4) | 1851 (42.9) |
| Race/Ethnicity | ||
| African American | 5107 (20.0) | 875 (20.3) |
| Non-Hispanic White | 18047 (69.8) | 2951 (68.4) |
| Hispanic | 1013 (3.9) | 169 (3.9) |
| Asian | 389 (1.5) | 57 (1.3) |
| Other/Mixed | 228 (0.9) | 50 (1.2) |
| Unknown | 1090 (4.2) | 212 (4.9) |
| Smoking Status | ||
| Current | 1728 (6.7) | 555 (12.9) |
| Former | 10371 (40.1) | 2036 (47.2) |
| Never | 13191 (51.0) | 1720 (39.9) |
| unknown | 584 (2.3) | 3 (0.1) |
| Pack-years | ||
| Zeroa | 13191 (51.0) | 1720 (39.9) |
| ≤ 10 | 5705 (22.1) | 904 (21.0) |
| 10–30 | 3903 (15.1) | 851 (19.7) |
| >30 | 2169 (8.4) | 820 (19.0) |
| unknown | 906 (3.5) | 19 (0.4) |
“No” to the question: In your lifetime, have you smoked 100 cigarettes or more?
2.6.3. Respiratory exacerbation episodes
Respiratory exacerbation episodes and their severity are ascertained using questions from the COPDGene study (www.copdgene.org). They are defined as the number of episodes in the past year [0 to >10(assigned the number 11 for analytic purposes in Table 4)] when the participant reports being treated for antibiotics for a chest illness, or being treated with steroid pills or injections. Severity of exacerbations in the past year is ascertained (a) by a count of times the participant has been to the emergency room or hospitalized for lung problems; and (b) by asking “Have you had a flare-up of chest trouble in the last 12 months”, with a follow-up question, for each episode, regarding how it was treated (please see supplementary file: Respiratory Questionnaire).36 For the 4314 randomized at-risk participants, respiratory exacerbations episodes will be evaluated annually by respiratory questionnaire. Secondarily, for those 65 years or older, they will be ascertained by review of Medicare data on urgent care/emergency room visits, hospitalization, and prescription records.
2.6.4. Asthma control
Asthma control is ascertained as a score using the Juniper Asthma Control Questionnaire (ACQ), 37 which has been validated to measure the goals of asthma management as defined by international guidelines (minimization of day- and night-time symptoms, activity limitation, beta(2)-agonist use and bronchoconstriction). This tool consists of five to seven items (symptoms, rescue bronchodilator use and FEV1% predicted), and can be used without loss of validity or change in interpretation in short form.38 Juniper Score is calculated as the mean over 6 asthma symptom scores (each on a scale of 0 to 6). Juniper Asthma Control Questionnaire (ACQ). 37
2.6.5. Dyspnea
Dyspnea level is ranked using a Modified Medical Research Council (MMRC) dyspnea scale39. Patients who are able to walk are scored from grades 0 through 4: (1) shortness of breath when hurrying on the level or up a slight hill; (2) walking slower than people of the same age on the level because of breathlessness or having to stop for breath when walking at your own pace on the level; or (3) stopping for breath after walking 100 yards or after a few minutes on the level; or (4) too breathless to leave my house.40 The MMRC tool has been demonstrated to be a simple and valid method of categorizing disability in patients with COPD, is correlated with the St. George’s Respiratory Questionnaire (SGRQ),39 and is used as part of the BODE index.40
2.7. Lung function measures at baseline (during run-in) and 2-years post-randomization
A trainer with more than 30 years of experience in coaching/performing and teaching spirometry received supplementary instruction in standardized methods for lung function testing with the EasyOne spirometer (http://www.nddmed.com) with supporting software from Dr. John Hankinson (http://hankconsulting.com), director of the spirometry reading center. He then trained technicians at the Harvard Catalyst CTSC and at each of the EMSI 10 sites in spirometry and (for the EMSI sites) in the home visit protocol. Requirements for certification of technicians to conduct spirometry exams include: (1) completion of the on-site spirometry course; (2) completion of the web-based spirometry training course (http://spirotrain.com); (3) passing of 50-question spirometry on-line exam; and (4) submission of 10 practice spirometry exams. The spirometry reading center has provided centralized evaluation of spirometry quality, and centralized reading of our pulmonary function tests, which are uploaded to a secure website (http://hankconsulting.com/QCSummary.htm).
For EMSI home visits for spirometry, to confirm that the inclusion and exclusion criteria are met for lung function testing, the central EMSI call center makes home visit appointments that are confirmed by the local technician. At home visits, after written consent, a brief questionnaire regarding medication use and recent wheeze or respiratory illness is administered, followed by height, weight, blood pressure and spirometric lung function measurements. Lung function tests are sent over a secure network for review by Dr. Hankinson, who sends readings to the Boston data center, with annotated spirograms as feedback for technicians regarding any concerns about reproducibility and technique. Technicians also perform phlebotomy for consenting participants who have not yet had a blood draw.
2.8. Medicare Hospital, Emergency Room and Prescription Data
For all participants, when they are 65 or older, we will review Medicare data for urgent care/emergency room and hospital visits for COPD, asthma, pneumonia, and co-morbid diagnoses linked to COPD. Additional co-morbidities known to worsen COPD prognosis may be considered as tertiary endpoints include bone density/fractures, cancer, cardiac failure, and deep venous thrombosis, which will be ascertained by questionnaire as well as by by administrative Medicare record review. Many of these co-morbidities are primary outcomes in the parent grant VITAL or in other proposed VITAL ancillary studies.
Annually, we will obtain Identifiable (Medicare) Data Files through ResDAC (www.resdac.unm.edu/Index.asp), which is a Centers for Medicare and Medicaid Services (CMS) contractor. Medicare Part A inpatient hospital data will be obtained from the MedPAR files, which are based on final action stay records. Emergency room visits with hospital admissions will be obtained from MedPAR and from the Standard Analytical Files (SAFs) if there was no admission. SAF records are based on inpatient and outpatient institutional claims. We will ascertain HMO enrollment status, since it may be more difficult to ascertain medical data for adults 65 and older who are HMO members or participate in other Medicare Advantage programs (28% of those on Medicare41). Annually we will also ascertain COPD and asthma medication use by questionnaire and compare this report to the Medicare prescription data base (PDE). The PDE is complex as there can be multiple claims per visit as well as multiple prescriptions. Thus prescription use will be considered a secondary source of data. In past studies we examined COPD[International Classification of Disease 9th revision (ICD-9): 490–496, except 493], pneumonia (ICD-9:480–487), diabetes mellitus (ICD-9: 250), congestive heart failure (CHF, ICD-9: 428), and myocardial infarction (MI), (ICD-9: 410).42
2.9. Data Management
For Lung VITAL, data management and coordination with VITAL were designed for effective follow-up with protection of human subjects and data security. Data management includes development and maintenance of the Manuals of Operations, all protocols and data collection instruments; the tracking of acceptable time-windows for visits, specimen collection, and technician feed-back; coordination with the centralized spirometry reading centers, and the EMSI and CTSC clinical centers; and the obtaining and secure archiving of all participant-related Medicare data. We continuously run quality assurance checks for all data, track data streams and archive all data, starting with the original (raw data) to the final and validated SAS data sets.
2.10. Analytic Plan
Randomization is completed, and the trial includes 25,874 participants nationally. The first analyses will compare baseline characteristics by randomized treatment assignment to ensure that balance was achieved by the randomization. This has been done for the parent trial and balance was achieved for key variables related to CVD and/or cancer. Characteristics to be examined include known risk factors for COPD, asthma or pneumonia (age, gender, race/ethnicity, and smoking); and baseline vitamin D and omega-3 fatty acid levels as assessed by dietary questionnaire in all participants and blood assays in a subsample. We expect balance by age, gender, race/ethnicity and smoking to occur in this ancillary study, but for Aims not including the entire randomized population of 25,874 (Table 1), we will take care of any minor imbalance through adjustment for these variables in our analyses.
This randomized, multicenter, double-blinded, placebo-controlled clinical trial will be analyzed according to the intent-to-treat paradigm, meaning that outcomes on subjects will be analyzed on the basis of the assigned treatment. The primary objective is population-level inference on the effects of the treatment on the stated outcomes. Our primary outcomes include: respiratory exacerbation episodes; lung function (change in FEV1 (ml/yr) from baseline to end of observation, FEV1/FVC (forced vital capacity), and pneumonia. Secondary outcomes include asthma control and dyspnea. Each prespecified specific aim will be tested at a significance level of 0.05. Exploratory analyses of non-prespecified hypotheses will be reported with cautionary caveats and emphasis on confidence intervals for effects as opposed to hypothesis tests. This study employs a 2×2 factorial design so that a single population can be used to evaluate the effects both of vitamin D supplementation and also the effects of fish oil supplementation on lung disease. In primary analyses these treatments are expected/assumed to operate independently, without interaction for the lung disease outcomes, so that the test of vitamin D effect employs 2 × 12937 subjects, and the test of fish oil effects likewise for the outcome of pneumonia. To explore potential interactions and nonlinear relationships, in secondary analyses we will use product and polynomial terms in generalized linear models for effects of key factors.
Because the treatments are equally unobtrusive, it is reasonable to expect that any dropout that occurs would be nondifferential across arms, leading only to loss of power. Under the intent-to-treat paradigm, all available information will be used to make inference on treatments on the basis of the assignments.
Effects of dietary supplements on respiratory exacerbation episodes
The primary analyses will treat number of respiratory exacerbation episodes as distinct outcomes. Number of episodes and change in number of episodes will be treated as a count. Primary analyses will include only those with diagnosis of COPD by questionnaire, those with COPD by spirometry, or those with chronic bronchitis by American Thoracic Society-DLD definition (usually coughing or bringing up phlegm on most days, for 3 consecutive months or more during the year)32 with history of current smoking or past smoking at least 10 pack-years or currently working at a dusty job (Table 3). Because of the overlap between COPD, asthma, and respiratory symptoms of chronic bronchitis and wheeze at this age, and because of potential gender bias in diagnostic labeling, secondary analyses will include everyone in the primary analyses plus those with active asthma by questionnaire who were not included in the primary analyses.43 The number of episodes and their time of occurrence on study, and the total follow-up time for each individual will be employed to fit a generalized linear model with negative binomial response (R 3.2 package MASS, function glm.nb), as in Calverley et al.36 Specifically, the log mean count of events n(i) for subject i has form:
where c(i) is the vitamin treatment indicator [e.g., 1 if subject i received vitamin D, 0 if placebo], f(i) is the fish oil treatment indicator, α, β, and φ are parameters to be estimated by maximum likelihood, t(i) is the observation time for subject I, and Q>0 is an overdispersion parameter. β (φ) has the interpretation of a log rate ratio comparing vitamin (fish oil) treatment to placebo. If necessary, additional variables can be added to the linear predictor component of the generalized linear model above, to address, for example, an imbalance between arms in use of controller medications, current smoking or smoking cessation.
Effects of dietary supplements on level and decline of pulmonary function
These aims are assessed in the individuals who receive pulmonary function tests. The primary representation of pulmonary function decline is the change in FEV1 (ml/yr) from baseline to end of observation, FEV1/FVC (forced vital capacity), and secondarily by level of forced expiratory flow between 25 and 75 % of FVC (FEF25-75). Because people with COPD are sometimes unable to reach a plateau of full FVC, we will also measure FEV6 in addition to FVC as an outcome. For those individuals unable to read a plateau of full FVC, this also enables us to calculate the secondary measure of airflow obstruction FEV1/FEV6. FEV1 will be expressed as percent predicted, using both internal adjustment for age and height stratifying by sex, and by using external standards.44–48 Change in PFTs between baseline and follow-up will be calculated and expressed as a continuous outcome. To normalize data, PFTs will be log-transformed where appropriate.
The basic inferential tool is the linear model
where δ(i) is the observed change in FEV1 for subject i, c(i) is a treatment indicator as above, and e(i) is a Gaussian zero mean disturbance with fixed variance. β (φ) is the average effect of vitamin (fish oil) supplement exposure on change in pulmonary function.
Effects of dietary supplements on pneumonia risk
The rate of pneumonia events per year will be modeled with standard Poisson regression. Alternatively, if there is overdispersion we will use the negative binomial model, as was done in a recent study of pneumoccal vaccination rates on people with COPD and asthma.49 Analyses will be conducted for the population as a whole, and (secondarily) with an indicator variable for baseline COPD included and an interaction between baseline self-reported COPD status or COPD by spirometry and treatment.
Beyond our primary analyses, asthma control and dyspnea will be considered secondary outcomes. To normalize data, the asthma control will be log-transformed if appropriate. We will examine both proportional odds models for the ordinal dyspnea score and logistic regression for MMRC score 1 or greater vs 0. For primary Lung VITAL outcomes, we will also explore whether the effects of vitamin D3 or EPA+DHA supplementation on COPD exacerbations, asthma control, FEV1 decline or pneumonia vary by baseline dietary intake of these nutrients; achieved 25 (OH) D or RBC levels of EPA+DHA levels; or race/skin pigmentation or sunlight exposure (for vitamin D3). These analyses will be a variant of the primary analyses, only this time with an interaction term as well as the main effects.
Co-morbidity outcomes related to COPD progression
While Lung VITAL will evaluate pneumonia as an outcome, the parent grant VITAL and other ancillary grants aim to examine other co-morbidities known to be associated with COPD and COPD progression (e.g., bone density/fractures, cancer, cardiac failure, deep venous thrombosis) as their primary outcomes. This will give us a unique insight into the potential for Vitamin D to reduce COPD morbidity through reducing the risk of co-morbidities. We will also explore supplement effects on the more prevalent of these co-morbidities in our subgroup with COPD.
2.11. Statistical Power (Table 2)
Table 2.
Statistical models and power for respiratory outcomes (Section 2.10 and 2.11)
| Outcome | Model | Assumptions | Detectable Effect (80% Power) |
|---|---|---|---|
| Pneumonia | Poisson regression | Incidence 2/100 person-years; 10,000 per arm | 1.3/100 person-yrs |
| Respiratory exacerbation episodes | Generalized linear model with negative binomial response | Exacerbation rate ~ 1/year; 1200 per arm with COPD | 0.062 (rate ratio of 95%, treated to untreated) |
| Change in percent-predicted pulmonary function (e.g., FEV1) between baseline and follow-up | Linear regression | Standard deviation of 230 ml for a 3 year change; 962 per arm | 29 ml |
| Asthma control scorea | Generalized linear model with negative binomial response | Standard deviation of change over 6 months of 0.87; 340 per arm | 0.19 in score |
Secondary outcome.
Unless otherwise noted, power calculations were performed with the STPLAN system distributed by MD Anderson Cancer Center (http://biostatistics.mdanderson.org). Power calculations are based on simple two-sample tests, which are reasonable proxies for assessing operating characteristics of inferences on main effects in the factorial analysis.
Effects of dietary supplements on COPD and asthma exacerbations
COPD exacerbation rates of approximately 1/year were reported by Calverley et al in TORCH in individuals with COPD.36 While moderate to severe exacerbations may be lower in our cohort, we anticipate that the rate of any exacerbation will be similar. Power estimates for respiratory exacerbation episodes as an outcome were based on expecting to annually follow 1720 individuals with symptomatic COPD at baseline [N enrolled=2561(Table 1)] and 2400 individuals with either COPD or asthma (N enrolled =3289). Assuming 1200/arm with COPD, with average follow-up of five years, we have 80% power to detect a difference of 0.062 (e.g., rate ratio of 95%, treated to untreated). Focusing on the 2400 individuals who were expected to have symptomatic COPD or asthma at baseline, assuming 1200/arm with average follow-up of five years, we have 80% power to detect a difference of 0.05 (e.g., rate ratio of 95%, treated to untreated) and 90% power to detect an effect of 0.06, at a 5% type I error rate, using the Poisson model (no overdispersion). Overdispersion and adjustments for center effects or other types of accidental imbalance will likely increase the size of the minimal detectable effect by a small amount. Given enrollment exceeded that anticipated, these power estimates are conservative.
Effects of dietary supplements on airflow obstruction and decline of pulmonary function
The standard deviation of three-year change in FEV1 is not reported by Calverley36 but can be approximated using simulation to generate a 2-sample 95% confidence interval with N=1500 subjects per arm having the same width as Calverley’s, which leads to an underlying standard deviation of approximately 230 ml for a 3 year change. This standard deviation is used for the following power calculations: with 1035 per arm, a level 0.05 2-sided t test can detect a difference in change between arms of 28 ml with power 80% or 33 ml with power 90%. While we achieved slightly lower numbers of acceptable quality lung function tests at baseline, the difference in power is minor: with 962 per arm, a level 0.05 2-sided t test can detect a difference in change between arms of 29.4 ml with power 80% or 34 ml with power 90%. In general populations, these differences would be equivalent to one-third the effect of never smoking compared to smoking,50 or equivalent to the effect of quitting smoking vs continuing smoking.50
Effects of dietary supplements on pneumonia risk
The overall incidence of pneumonia in this age group (any new pneumonia event) is approximately 2/100 person-years. 51, 52 We therefore expect 10 events per 100 individuals in the five year follow-up and, assuming 10000 individuals/arm, can detect a difference between arms of 1.3/100 person years with 80% power, or a difference of 1.5/100 person years with 90% power. Focusing attention on the 1720 individuals assumed to have COPD, for which the baseline pneumonia incidence is closer to 7/100 person-years, we have 80% power to detect a difference of 1.7 events per 100 per year, or 90% power to detect a difference of 2 events per 100 per year. As the total number of randomized individuals/arm (12,937) and the total number being followed for COPD (2561) exceeds the number assumed for these power calculations, our calculations are conservative.
Effect of dietary supplements on asthma control and exacerbations
In the shortened Juniper questionnaire ascertaining symptoms alone, the mean score (SD) at baseline was 1.48(0.93) with a change in score over 28 weeks of −0.48 (0.87).37, 38 We assumed that approximately 3.4% of the population (680 subjects) would have active asthma and answer the Juniper asthma control questionnaire. Assuming Juniper’s findings of a SD of change over 6 months of 0.87 and 340/arm we estimated 80% power to detect an effect of size 0.19 in the asthma control score. These power calculations are likely to be conservative in that we are following 1840 individuals with asthma annually.
3. Results
3.1 Baseline Characteristics
Compared to the entire randomized population, a slightly higher proportion of women (51% vs. 57.1%) is represented in the population followed by detailed respiratory questionnaire (Table 3). The proportion of African American participants is similar (20%). By intention, compared to the entire randomized population, the proportion of those with doctor-diagnosed COPD (e.g., doctor-diagnosed COPD, emphysema or chronic bronchitis) (8.9 % vs 38.0%) and asthma (11.5% vs 42.7%) was higher for those being followed annually for respiratory exacerbations, asthma control, and dyspnea (Table 3). Pneumonia rates in the past year were also higher at baseline for those selected for respiratory questionnaire follow-up: 1.3% of the entire randomized population had pneumonia in the past year at baseline, compared to 3.8% of all those being followed annually by respiratory questionnaire, overall, and 4.6% for those with COPD. More of those selected for respiratory questionnaire follow-up had ever smoked, and amongst the smokers, there was more current smoking (6.7% vs 12.9%) and there was a heavier pack-year history of smoking (Table 3).
In Table 4 we show the count at baseline of all exacerbations in the last year, as ascertained through COPDGene questions (Supplemental File, 11–13), and the count of flare-ups of chest trouble in the last 12 months for which the participant took additional antibiotics or steroid medication and/or was admitted to hospital, a measure of exacerbation severity used by COPDGene (Supplemental File,14). Overall, at baseline, forty-five % of this subpopulation reported an exacerbation of lung problems/chest illness in the last year that was treated with antibiotics or steroid pills/injections (Table 4). The mean of the maximum count per person of exacerbations in the last year treated with either antibiotics or steroids was approximately 1, with a wide range (0 to 11).
3.2 Smoking, Respiratory Symptoms, and Lung Function
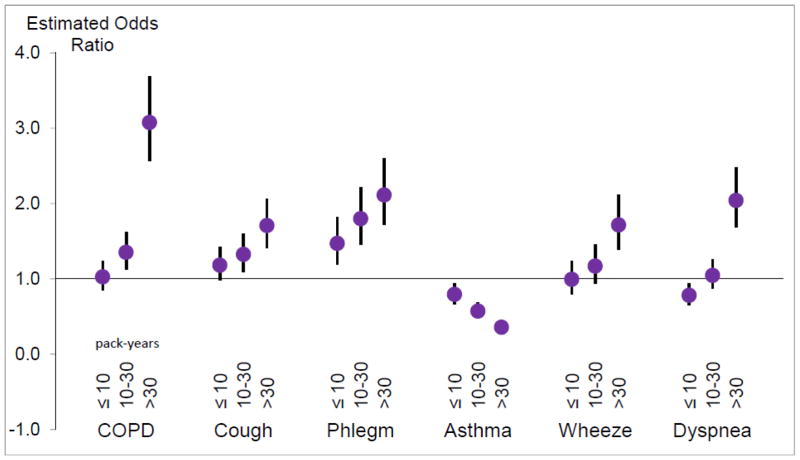
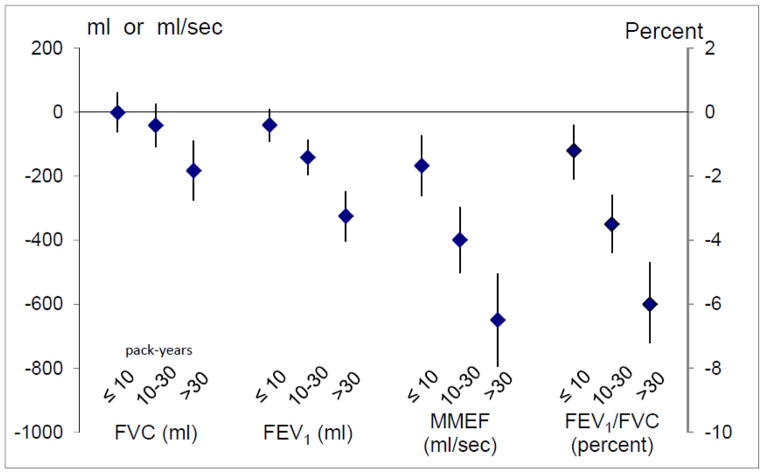
History of smoking was highly prevalent in our subpopulation being followed by respiratory questionnaire: 60.1% had a history of smoking, and 12.9% were current smokers. Current or former smoking was associated with higher rates of wheeze, chronic cough, chronic phlegm, and COPD diagnosis. A higher proportion of those with asthma were never smokers, but 25% of those with asthma were still smoking. With the referent being never smoking, we found a dose-response relationship between pack-year history of smoking and prevalence of COPD, chronic cough, chronic phlegm, wheeze greater than once a week or most days and nights (prevalence=23%), and dyspnea (Figure 3). Diagnosis of asthma decreased with increased smoking history, despite the fact that wheeze increased. In the subset of participants who performed spirometry, pack-year history of smoking was associated with reduced level of lung function (Figure 4).
Fig. 3.
Odds of symptoms (95% CI) for increasing pack-year history of smoking, compared to never smoking. Models are adjusted for age, height, body-mass index (kg/m2), gender, and race/ethnicity.
Fig. 4.
Level of lung function (95% CI) for increasing pack-year history of smoking, compared to never smoking. Models are adjusted for age, height, body-mass index (kg/m2), gender, and race/ethnicity.
3.3 COPD
The subset of randomized participants with baseline lung function testing was enriched for airflow obstruction; 27.3 % of those with lung function testing had COPD according to GOLD criteria and 5.9 % had PRISm (Table 5). Most COPD fell within the mild or moderate GOLD COPD stages. In general, the proportion of individuals with doctor/health professional-diagnosed COPD (or asthma) increased with worsening COPD spirometry grade. However, a significant proportion of those with COPD by lung function testing did not have a doctor/health professional’s diagnosis of COPD or asthma.
Table 5.
Questionnaire report of COPD and asthma diagnosis by spirometry grade for 1924 subjects with acceptable spirometry.
| Spirometry Grade | Definition by pulmonary function | N (1) | % | Percent of (1) with COPD dx | Percent of (1) with COPD dx or asthma dx |
|---|---|---|---|---|---|
| NO COPD | FEV1/FVC ≥ 0.70 | 1285 | 66.8% | 6.8% | 18.3% |
| PRISm | FEV1 < 80% normal | 113 | 5.9% | 7.1% | 23.9% |
| I Mild | FEV1/FVC < 0.70 and FEV1 ≥ 80% normal | 329 | 17.1% | 8.9% | 28.1% |
| II Moderate | FEV1/FVC < 0.70 and FEV1 50–79% normal | 172 | 8.9% | 30.2% | 55.8% |
| III Severe | FEV1/FVC < 0.70 and FEV1 30–49% normal | 22 | 1.1% | 50.0% | 63.6% |
| IV Very Severe | FEV1/FVC < 0.70 and FEV1 <30% normal | 3 | 0.2% | 100% | 100% |
|
| |||||
| 1924 | 100% | N=1905 | N=1919 | ||
4 Discussion
Lung VITAL has many strengths. Pneumonia and acute respiratory exacerbations of chronic respiratory disease, including COPD and asthma, are among the leading causes of morbidity in adults 60 years and older. 3–8 Lower lung function and steeper decline of lung function in adults are associated with increased morbidity and mortality in adults2. Current modalities for treatment of COPD and asthma are limited, and the prevalence of vitamin D insufficiency is high. VITAL,27, 28 the parent study of Lung VITAL, is the largest randomized trial of vitamin D in U.S. adults, and is the only large trial worldwide (one of four with 10,000 or more participants) with an appreciable number of black participants, who are at high risk for vitamin D deficiency, and suffer from higher rates of asthma morbidity. It addresses the need, as stated in the 2011 IOM review, for adequately powered randomized clinical trials to assess whether supplementation should be recommended to reduce incidence or improve control of disorders other than bone disease, including pneumonia and chronic obstructive respiratory diseases.9–11 Simultaneously, our study answers the need, as summarized in the 2014 Wendell review, 25 for an adequately powered trial in adults to clarify whether fish oil/marine omega-3 FA improves airflow obstruction and asthma. Baseline blood collections in most participants will allow assessment of the potential modifying effects of prerandomization blood levels of 25(OH)D and/or EPA/DHA. A recent Belgian study reported that vitamin D supplementation was associated with fewer COPD exacerbations only in a post-hoc analysis focusing on those with low baseline vitamin D levels, but it was small (182 patients), with only one year of follow-up and with intermittent bolus dosing.24, 53 The recent VIDA trial that assessed the effect of vitamin D supplementation to asthmatics with vitamin D deficiency did not find an effect of vitamin D supplementation on the primary outcome of time to first asthma exacerbation.54 However the trial was underpowered, and when analyses were limited to those that achieved a sufficient level of vitamin D (30 ng/mL or greater), an effect was seen for decreasing the overall number of asthma exacerbations.
The dose of vitamin D being tested by VITAL (2000 IU), as well as the dosing schedule (once per day, rather than intermittent large boluses), may provide an optimal balance of efficacy and safety based on current evidence the RDAs and tolerable upper limit set by the IOM. Some investigators have questioned the efficacy or safety of intermittent dosing of large boluses of vitamin D,55, 56 as done in two recent trials of vitamin D and respiratory tract infections in adults, which suggested no benefit from vitamin D with intermittent dosing.57, 58 A daily dose of vitamin D may supply sufficient amounts of the parent compound (vitamin D that is made in the skin on exposure to sunlight and absorbed from the gut, prior to hydroxylation to 25OHD in the liver), which is now thought to have independent effects, apart from 25OHD on the tissues.
While we believe that we have addressed many of the concerns regarding dose-response issues, and the need to base study design on biologic understanding of the supplement and the outcomes discussed by Lappe and Heaney,59 Lung VITAL also has some limitations. While pneumonia is ascertained in the entire population, acute respiratory episodes and asthma control are assessed in a subset (4314 of 25,874), but they are the highest at-risk subjects, and it would be less informative to be annually following participants at very low risk for our outcomes. Pneumonia and symptoms are ascertained by self-report, but pneumonia diagnosis for participants reporting hospitalization is adjudicated by medical record review. For those on Medicare or Medicaid, emergency room/hospital visits for pneumonia and other acute respiratory episodes, as well as antibiotic and steroid prescription are validated through review of CMS data. Our pulmonary function measures are also performed on subsets of the population. However, those subsets are distributed over 11 large U.S. urban communities, representing a diverse population large enough to provide adequate power to evaluate effects of the supplements on lung function. We acknowledge that a significant proportion of our population has COPD defined by doctor’s diagnosis and not by lung function testing. However for those with COPD or asthma diagnosis, we can ascertain the effects of the supplements on measures of morbidity not only by ascertainment of self-reported symptoms but also, in a significant subset, by validation of CMS record review. Because the trial population is older, the results may not be generalizable to younger men and women. However, older populations have the highest disease rates, allowing the trial to be completed in a shorter time period, at greater cost efficiency.27, 28, 60
Thus, to conduct a major evaluation of the influences of vitamin D and marine omega-3 fatty acid supplementation on obstructive and infectious lung diseases in adults, we have partnered with VITAL, a large clinical trial with 5-year follow-up of 25,874 participants aged 50 or older, including 5107 African Americans. This approach is innovative and cost-effective and will help answer the question of whether vitamin D and fish oil can reduce pneumonia risk and improve the health of people with chronic respiratory symptoms, COPD, and asthma.
Acknowledgments
We are indebted to the participants in Lung VITAL and VITAL for their dedicated and conscientious collaboration, and also to the entire staff of Lung VITAL and VITAL. Lung VITAL is supported by grant National Heart, Lung and Blood Institute R01 HL101932. VITAL is supported by grant U01 CA13962, which includes support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Alternative Medicine. Dr. Buring’s spouse is on the Scientific Advisory Board of Pharmavite LLC. Lung VITAL has been approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health. VITAL is registered at clinicaltrials.gov (NCT01728571).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diane R Gold, Email: diane.gold@channing.harvard.edu.
Augusto A. Litonjua, Email: reaal@channing.harvard.edu.
Vincent J. Carey, Email: vincent.carey@channing.harvard.edu.
JoAnn E. Manson, Email: jmanson@rics.bwh.harvard.edu.
Julie E Buring, Email: jburing@rics.bwh.harvard.edu.
I-Min Lee, Email: ilee@rics.bwh.harvard.edu.
David Gordon, Email: dgordon@rics.bwh.harvard.edu.
Joseph Walter, Email: JWALTER@research.bwh.harvard.edu.
Georgina Friedenberg, Email: gfriedenberg@rics.bwh.harvard.edu.
John L Hankinson, Email: john@occspiro.com.
Trisha Copeland, Email: PCOPELAND2@partners.org.
Heike Luttmann-Gibson, Email: hgibson@hsph.harvard.edu.
References
- 1.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224–1238. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 2.Peto R, Speizer FE, Cochrane AL, Moore F, Fletcher CM, Tinker CM, Higgins IT, Gray RG, Richards SM, Gilliland J, Norman-Smith B. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis. 1983;128(3):491–500. doi: 10.1164/arrd.1983.128.3.491. [DOI] [PubMed] [Google Scholar]
- 3.Zasloff M. Inducing endogenous antimicrobial peptides to battle infections. Proc Natl Acad Sci U S A. 2006;103(24):8913–8914. doi: 10.1073/pnas.0603508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiemstra PS. Defensins and cathelicidins in inflammatory lung disease: beyond antimicrobial activity. Biochem Soc Trans. 2006;34(Pt 2):276–278. doi: 10.1042/BST20060276. [DOI] [PubMed] [Google Scholar]
- 5.Wordley J, Walters S, Ayres JG. Short term variations in hospital admissions and mortality and particulate air pollution. Occup Environ Med. 1997;54(2):108–116. doi: 10.1136/oem.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest. 2000;117(2 Suppl):1S–4S. doi: 10.1378/chest.117.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 8.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. [Google Scholar]
- 10.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. The Journal of Clinical Endocrinology and Metabolism. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. 2012;337(6101):1476–1478. doi: 10.1126/science.337.6101.1476. [DOI] [PubMed] [Google Scholar]
- 12.LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL) Contemp Clin Trials. 2015;41:259–268. doi: 10.1016/j.cct.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Macias H, Romieu I. Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J Allergy Clin Immunol. 2014;133(5):1237–1244. doi: 10.1016/j.jaci.2014.03.020. quiz 1245. [DOI] [PubMed] [Google Scholar]
- 14.Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11(4):221–229. [PubMed] [Google Scholar]
- 15.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12(4):388–390. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 16.Zasloff M. Sunlight, vitamin D, and the innate immune defenses of the human skin. J Invest Dermatol. 2005;125(5):xvi–xvii. doi: 10.1111/j.0022-202X.2005.23924.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 18.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42(1):97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 19.Quraishi SA, Litonjua AA, Moromizato T, Gibbons FK, Camargo CA, Jr, Giovannucci E, Christopher KB. Association between prehospital vitamin D status and hospital-acquired Clostridium difficile infections. JPEN J Parenter Enteral Nutr. 2015;39(1):47–55. doi: 10.1177/0148607113511991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quraishi SA, Litonjua AA, Moromizato T, Gibbons FK, Camargo CA, Jr, Giovannucci E, Christopher KB. Association between prehospital vitamin D status and hospital-acquired bloodstream infections. Am J Clin Nutr. 2013;98(4):952–959. doi: 10.3945/ajcn.113.058909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange NE, Sparrow D, Vokonas P, Litonjua AA. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med. 2012;186(7):616–621. doi: 10.1164/rccm.201110-1868OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Confino-Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, Asthma Prevalence and Asthma exacerbations: A large adult population-based study. Allergy. 2014 doi: 10.1111/all.12508. [DOI] [PubMed] [Google Scholar]
- 23.Camargo CA, Jr, Manson JE. Vitamin D supplementation and risk of infectious disease: no easy answers. Am J Clin Nutr. 2014;99(1):3–4. doi: 10.3945/ajcn.113.078329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold DR, Manson JE. Severe vitamin D deficiency: a prerequisite for COPD responsiveness to vitamin D supplementation? Ann Intern Med. 2012;156(2):156–157. doi: 10.7326/0003-4819-156-2-201201170-00013. [DOI] [PubMed] [Google Scholar]
- 25.Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J Allergy Clin Immunol. 2014;133(5):1255–1264. doi: 10.1016/j.jaci.2013.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baraldi E, Carraro S, Alinovi R, Pesci A, Ghiro L, Bodini A, Piacentini G, Zacchello F, Zanconato S. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58(6):505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradhan AD, Manson JE. Update on the Vitamin D and OmegA-3 trial (VITAL) J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowler RP, Kim V, Regan E, Williams A, Santorico SA, Make BJ, Lynch DA, Hokanson JE, Washko GR, Bercz P, Soler X, Marchetti N, Criner GJ, Ramsdell J, Han MK, Demeo D, Anzueto A, Comellas A, Crapo J, Dransfield M, Wells JM, Hersh CP, MacIntyre N, Martinez F, Nath H, Niewoehner D, Sciurba F, Sharafkhaneh A, Silverman EK, van Beek EJ, Wilson C, Wendt C, Wise RA. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014 doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. 1993;104(2):600–608. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 32.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 33.Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, Beaty TH, Han MK, Curtis JL, Curran-Everett D, Lynch DA, DeMeo DL, Crapo JD, Silverman EK. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance--United States, 1971–2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 35.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 36.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 37.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 41.Linehan K. Medicare Advantage update: benefits, enrollment, and payments after the ACA. Issue Brief Natl Health Policy Forum. 2013;(850):1–12. [PubMed] [Google Scholar]
- 42.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113(8):978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes PJ, Drazen JM, Rennard SI, Thomson NC. Asthma and COPD: Basic Mechanisms and Clinical Management. 2. San Diego: Academic Press; 2009. [Google Scholar]
- 44.Hankinson JL, Crapo RO, Jensen RL. Spirometric reference values for the 6-s FVC maneuver. Chest. 2003;124(5):1805–1811. doi: 10.1378/chest.124.5.1805. [DOI] [PubMed] [Google Scholar]
- 45.Cole TJ, Stanojevic S, Stocks J, Coates AL, Hankinson JL, Wade AM. Age- and size-related reference ranges: a case study of spirometry through childhood and adulthood. Stat Med. 2009;28(5):880–898. doi: 10.1002/sim.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 47.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 48.Stanojevic S, Wade A, Stocks J, Hankinson J, Coates AL, Pan H, Rosenthal M, Corey M, Lebecque P, Cole TJ. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177(3):253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med. 2007;22(1):62–67. doi: 10.1007/s11606-007-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U.S. Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Atlanta: Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 51.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294(21):2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 52.Venables WN, Ripley BD, editors. Modern Applied Statistics with S. Fourth Edition. New York: Springer; 2002. [Google Scholar]
- 53.Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, Decallonne B, Bouillon R, Decramer M, Janssens W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 54.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, Kazani SD, Moore WC, Moy J, Sorkness CA, Avila P, Bacharier LB, Bleecker E, Boushey HA, Chmiel J, Fitzpatrick AM, Gentile D, Hundal M, Israel E, Kraft M, Krishnan JA, LaForce C, Lazarus SC, Lemanske R, Lugogo N, Martin RJ, Mauger DT, Naureckas E, Peters SP, Phipatanakul W, Que LG, Sheshadri A, Smith L, Solway J, Sullivan-Vedder L, Sumino K, Wechsler ME, Wenzel S, White SR, Sutherland ER. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311(20):2083–2091. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–4628. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss ST, Litonjua AA. Vitamin D dosing for infectious and immune disorders. Thorax. 2015;70(10):919–920. doi: 10.1136/thoraxjnl-2015-207334. [DOI] [PubMed] [Google Scholar]
- 57.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, Florkowski CM, Livesey JH, Camargo CA, Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 58.Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, Kilpin K, McLaughlin D, Fletcher G, Mein CA, Hoti M, Walton R, Grigg J, Timms PM, Rajakulasingam RK, Bhowmik A, Rowe M, Venton TR, Choudhury AB, Simcock DE, Sadique Z, Monteiro WR, Corrigan CJ, Hawrylowicz CM, Griffiths CJ. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs) Thorax. 2015;70(5):451–457. doi: 10.1136/thoraxjnl-2014-206449. [DOI] [PubMed] [Google Scholar]
- 59.Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol. 2012;4(2):95–100. doi: 10.4161/derm.19833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313(13):1311–1312. doi: 10.1001/jama.2015.1353. [DOI] [PubMed] [Google Scholar]
- 61.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, O’Connor GT, Sandel M, Strunk RC, Bacharier LB, Zeiger RS, Schatz M, Hollis BW, Weiss ST. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38(1):37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]