Abstract
Background
Clinical studies have suggested the prognostic value of galectin-3, a marker of fibrosis, in chronic heart failure. However, the specific role of galectin-3, compared with established biomarkers, remains uncertain.
Methods and Results
The Penn Heart Failure Study was an ambulatory heart failure cohort that included 1385 participants with reduced (1141), preserved (106), and recovered (138) left ventricular ejection fraction (LVEF). Cox regression models determined the association between galectin-3 and risk of all-cause mortality, cardiac transplantation, or placement of a ventricular assist device. Receiver operating characteristic curves compared the prognostic accuracy of galectin-3, high-sensitivity soluble Toll-like receptor 2 (ST2), troponin I, and B-type natriuretic peptide (BNP) at 1 and 5 years. Higher galectin-3 levels were associated with an increased risk of adverse events (adjusted hazard ratio of 1.96 for each doubling in galectin-3; P < .001). This association was most pronounced among participants with preserved LVEF (adjusted hazard ratio 3.30; P < .001). At 5 years, galectin-3 was the most accurate discriminator of risk among participants with preserved LVEF (area under the curve 0.782; P = .81 vs high-sensitivity ST2; P = .029 vs troponin I; P = .35 vs BNP). BNP was most accurate among participants with reduced and recovered LVEF (areas under the curves 0.716 and 0.728, respectively).
Conclusions
Galectin-3 could have prognostic value for long-term events among patients with heart failure and preserved ejection fraction.
Keywords: Biomarker, ejection fraction, risk stratification, ventricular function
Chronic heart failure imparts a major health burden and financial cost to an aging population.1,2 Clinical research in heart failure has had a major focus on biomarkers to quantify the complex biologic processes implicated in the pathogenesis of the cardiomyopathy and the ability of those biomarkers to stratify heart failure patients regarding their risk of adverse outcomes. Accurate risk stratification can guide the clinical management of heart failure patients and enable clinicians to identify high-risk patients that might require initiation of advanced therapies. Established biomarkers of heart failure include troponin, a marker of myocyte injury, and B-type natriuretic peptide (BNP), a marker of neurohormonal activation; these biomarkers are widely used in the clinical care and management of heart failure patients.3,4 Newer biomarkers include high-sensitivity soluble Toll-like receptor 2 (ST2), a marker of myocyte stress.5
Recently, galectin-3 has emerged as a biomarker of inflammation and fibrosis in heart failure. Galectin-3 is a soluble β-galactoside–binding lectin released by activated cardiac macrophages that binds to and activates fibroblasts, forming collagen and resulting in fibrosis.6–8 Tissue fibrosis is a central pathway in the progression of heart failure. Cardiac fibrosis impairs ventricular function and contributes to both systolic and diastolic dysfunction. Animal studies have shown that galectin-3 plays a key role in tissue fibrosis and ventricular remodeling.8–10 In particular, galectin-3 is expressed at higher levels in cardiac fibroblasts.11 Human studies have demonstrated the prognostic utility of galectin-3 in heart failure.12 Several cohort studies have exhibited the ability of galectin-3 to predict major events in heart failure, such as death and hospitalization.13–15
Results have been inconsistent regarding the relative prognostic utility of galectin-3 compared with established markers (particularly BNP and ST2).12 Although several studies have shown that galectin-3 is independently associated with outcomes after adjustment for BNP, others have shown that galectin-3 does not improve prognostic accuracy when combined with BNP.16–20 In addition, only 1 small study with limited follow-up compared the prognostic value of galectin-3 between patients with reduced and preserved left ventricular ejection fraction (LVEF).21 Therefore, it is unknown how the prognostic utility of galectin-3 compares with established biomarkers, as well as whether the prognostic value of galectin-3 differs among heart failure patients with reduced, preserved, or recovered ejection fraction.
We therefore sought to characterize the independent determinants of galectin-3 levels, determine the association of galectin-3 levels with the risk of major adverse events in heart failure, and compare the ability of galectin-3 to predict major adverse events with that of established biomarkers (high-sensitivity ST2, troponin I, and BNP). Our primary analysis focused on a large cohort of ambulatory heart failure patients with a broad spectrum of disease. Secondary analyses focused on patient subgroups with different “phenotypes” of heart failure defined by their LVEF and subgroups with different cardiomyopathy etiologies.
Materials and Methods
Study Population
The Penn Heart Failure Study, sponsored by the National Heart, Lung, and Blood Institute, was a prospective cohort study of outpatients with chronic heart failure recruited from referral centers at the University of Pennsylvania (Philadelphia, Pennsylvania), Case Western Reserve University (Cleveland, Ohio), and the University of Wisconsin (Madison, Wisconsin). The primary inclusion criterion was a clinical diagnosis of heart failure as determined by a heart failure specialist. Participants were excluded if they had a noncardiac condition resulting in an expected mortality of <6 months as judged by the treating physician or if they were unable to provide informed consent. The resulting cohort spanned a full spectrum of heart failure severity, ranging from mild disease to severe disease requiring advanced therapies. Every participant provided written informed consent. Participating Institutional Review Boards approved the study protocol.
Data Collection
At the time of study entry, detailed clinical data were obtained with the use of standardized questionnaires administered to the participant and physician, with verification through medical records. Subsequent adverse events—all-cause mortality, cardiac transplantation, and placement of a ventricular assist device (VAD)—were ascertained every 6 months by means of direct contact with participants and verified through death certificates, medical records, or family members. Outcomes were collected through September 2013. Participants with a VAD placement before study entry were excluded from the analysis. We previously classified participants into 3 “phenotypes” of heart failure based on LVEF: reduced, preserved, and recovered LVEF.22 Participants were classified as “reduced” if echocardiography at study entry showed an LVEF <50%. Participants with normal left ventricular function as defined by an LVEF ≥50%, were further classified as either “preserved” or “recovered” based on retrospective chart review of earlier echocardiograms. Participants who had previously documented LVEF of only ≥50% were classified as “preserved;” those who had a documented history of LVEF <50% were classified as “recovered.”
Biomarker Assays
Biomarker measurement was performed for participants recruited from October 2003 to May 2009. Blood samples were obtained at the time of study entry, processed, and stored at −80°C until the time of assay. Galectin-3 chemiluminescent microparticle immunoassays were performed on the Architect i2000SR instrument (Abbott Laboratories, Wiesbaden, Germany).23 The total coefficient of variation was <8%. The limit of detection was ≤1.7 ng/mL; the linear measurement range was 5.5–103.1 ng/mL. BNP, troponin I, and creatinine were measured with the use of standard Architect immunoassays (Abbott Laboratories, Abbott Park, Illinois). High-sensitivity ST2 was measured with the use of a sandwich monoclonal immunoassay (Presage ST2 Assay; Critical Diagnostics, San Diego, California). The satisfactory performance of these assays was previously described.24 Biomarker values lower than the limit of detection were replaced with the lower limit (eg, 0.005 ng/mL for troponin I).
Statistical Methods
Standard descriptive statistics were used to summarize participant characteristics at study entry. Independent determinants of galectin-3 levels (log transformed) were ascertained from a multivariable linear regression model. Candidate variables were: age, sex, race (white, black, other), cardiomyopathy etiology (ischemic, nonischemic), New York Heart Association (NYHA) functional classification, LVEF, heart failure phenotype (reduced, preserved, recovered LVEF), cardiac resynchronization therapy, defibrillator use, body mass index, and creatinine. For continuous variables (eg, age), linearity was evaluated with the use of scatterplot smoothers. The inclusion of variables was based on a stepwise model-selection procedure to choose the subset of variables that minimized the Akaike information criterion.
Cox regression models were used to estimate the association between galectin-3 and risk of the composite outcome of all-cause death, cardiac transplantation, or VAD placement. Participants who were alive and free of cardiac transplantation or VAD placement at the end of follow-up were censored. Galectin-3 was transformed with the use of a log (base 2) transformation such that the hazard ratio compared 2 populations whose galectin-3 level differed by a multiplicative factor of 2. Adjustment variables were specified a priori based on clinical rationale: age, sex, race, etiology, cardiac resynchronization therapy, defibrillator use, creatinine, and study site. Creatinine was included instead of the estimated glomerular filtration rate (GFR) because the model also included the variables in the estimated GFR equation (ie, age, sex, and race). Adjustment for study site was achieved by stratifying the baseline hazard function. Age exhibited nonproportional hazards and was therefore adjusted for with a time-varying covariate, which was obtained by multiplying age by a linear term for time. NYHA functional classification, LVEF, and heart failure phenotype were not adjusted for, because of the potential for these measures of heart failure severity to be in the causal pathway between galectin-3 and risk. We considered the adjusted analysis to be the primary analysis, for which P < .05 indicated statistical significance. Owing to potential underlying biologic differences, prespecified interaction analyses were performed according to cardiomyopathy etiology and heart failure phenotype.
Time-dependent receiver operating characteristic (ROC) curves were used to compare the ability of galectin-3, high-sensitivity ST2, troponin I, and BNP (on their original untransformed scales) to classify participants regarding occurrence of death, cardiac transplantation, or VAD placement within 1 and 5 years.25 Stratified analyses were performed according to phenotype, for which the analysis focused on events within 5 years owing to limited sample sizes and events within strata. Confidence intervals for the areas under the time-dependent ROC curves (AUCs) were obtained from 1000 bootstrap samples. AUCs were compared with the use of Wald tests based on the empirical standard deviation. All hypothesis tests were 2 sided. All analyses were completed with the use of R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria), including the MASS, survival, and survival ROC extension packages.
Results
Across the cohort of 1385 participants, the median age at study entry was 57 years; the majority of study participants were male (66.5%) and white (74.3%; Table 1). Nonischemic heart failure was more common than ischemic (68.9% vs 31.1%). The median LVEF was 30%, with 82.4%, 7.7%, and 10.0% of participants defined as having reduced, preserved, and recovered LVEF, respectively. The median galectin-3 level was 17.6 ng/mL (interquartile range [IQR] 13.4–23.5 ng/mL). Galectin-3 levels exhibited modest correlation with high-sensitivity ST2 (0.31), troponin I (0.34), and BNP (0.37). The magnitude of correlation of galectin-3 levels with creatinine (0.58) was similar to that of estimated GFR (−0.61). Summary statistics stratified by heart failure phenotype are provided in Supplementary Table 1.
Table 1.
Characteristics of Study Participants at Study Entry (n = 1385)
| Demographic characteristics | |
| Age, y | 57 (48–66) |
| Male sex | 921 (66.5) |
| Race | |
| White | 1029 (74.3) |
| Black | 306 (22.1) |
| Other | 50 (3.6) |
| Medical history and risk factors | |
| History of diabetes | 408 (29.5) |
| History of hypertension | 817 (59.0) |
| History of hyperlipidemia | 880 (63.5) |
| Tobacco use | |
| Current | 133 (9.6) |
| Former | 744 (54.0) |
| Never | 502 (36.4) |
| Heart failure characteristics | |
| Heart failure phenotype | |
| Reduced LVEF | 1141 (82.4) |
| Preserved LVEF | 106 (7.7) |
| Recovered LVEF | 138 (10.0) |
| NYHA functional classification | |
| I | 229 (16.5) |
| II | 644 (46.5) |
| III | 409 (29.5) |
| IV | 103 (7.4) |
| Ischemic cardiomyopathy etiology | 431 (31.1) |
| Cardiac resynchronization therapy | 364 (26.3) |
| Defibrillator use | 597 (43.1) |
| Clinical measures | |
| LVEF, % | 30 (20–43) |
| Body mass index, kg/m2 | 29 (25–33) |
| Systolic blood pressure, mm Hg | 112 (100–126) |
| Diastolic blood pressure, mm Hg | 70 (60–78) |
| Creatinine, mg/dL | 0.92 (0.77–1.26) |
| Estimated GFR, mL min−1 1.73 m−2 | 67 (50–84) |
| Medication use | |
| ACE inhibitors or ARBs | 1215 (88) |
| Aldosterone antagonists | 472 (34) |
| Beta-blockers | 1230 (89) |
| Digoxin | 550 (40) |
| Hydralazine or nitrates | 238 (17) |
| Biomarkers | |
| Galectin-3, ng/mL | 17.6 (13.4–23.5) |
| High-sensitivity ST2, ng/mL | 27.1 (19.9–39.8) |
| Troponin I | |
| Detectable | 902 (65.1) |
| Troponin I,* ng/mL | 0.02 (0.01–0.04) |
| BNP, pg/mL | 175 (49–578) |
Value are presented as n (%) or median (interquartile range). LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; GFR, glomerular filtration rate; ACE; angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ST2, soluble Toll-like receptor 2; BNP, B-type natriuretic peptide.
Among those participants with detectable troponin I levels.
Increased age, female sex, nonwhite race, NYHA functional classification, ischemic etiology, presence of an internal cardiac defibrillator, increased body mass index, and increased creatinine levels were independently associated with increased galectin-3 levels at study entry (Table 2). Interestingly, there were no differences in galectin-3 levels across participants with reduced (median 18.0 ng/mL, IQR 13.4–23.6 ng/mL), preserved (median 17.2 ng/mL, IQR 13.6–24.3 ng/mL), or recovered (median 16.0 ng/mL, IQR 13.4–20.8 ng/mL) LVEF (P = .12). Elevated galectin-3 levels, particularly among participants with preserved LVEF, were consistent with the severity of heart failure and renal dysfunction among these participants.22
Table 2.
Independent Determinants of Galectin-3 Levels at Study Entry
| Parameter | Difference* | 95% CI | P Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (10-y difference) | 8.6% | 7.2%–10.1% | <.001 |
| Female sex (vs male) | 13.8% | 10.4%–17.1% | <.001 |
| Race | .008 | ||
| Black (vs white) | 5.0% | 0.4%–9.9% | |
| Other (vs white) | 11.6% | 1.6%–22.7% | |
| Heart failure characteristics | |||
| NYHA functional classification | <.001 | ||
| II (vs I) | 6.7% | 1.4%–12.3% | |
| III (vs I) | 19.2% | 12.7%–26.0% | |
| IV (vs I) | 34.5% | 24.3%–45.5% | |
| Ischemic etiology (vs nonischemic) | 7.1% | 2.6%–11.7% | .002 |
| Defibrillator use (vs no) | 6.0% | 2.1%–10.1% | .002 |
| Clinical measures | |||
| Body mass index (5-kg/m2 difference) | 2.2% | 0.9%–3.5% | .001 |
| Creatinine (0.5-mg/dL difference) | 15.7% | 14.5%–17.0% | <.001 |
CI, confidence interval; other abbreviations as in Table 1.
Exponentiated regression coefficient from a multivariable linear regression model for natural log–transformed galectin-3, representing the percentage difference in galectin-3 between each contrast.
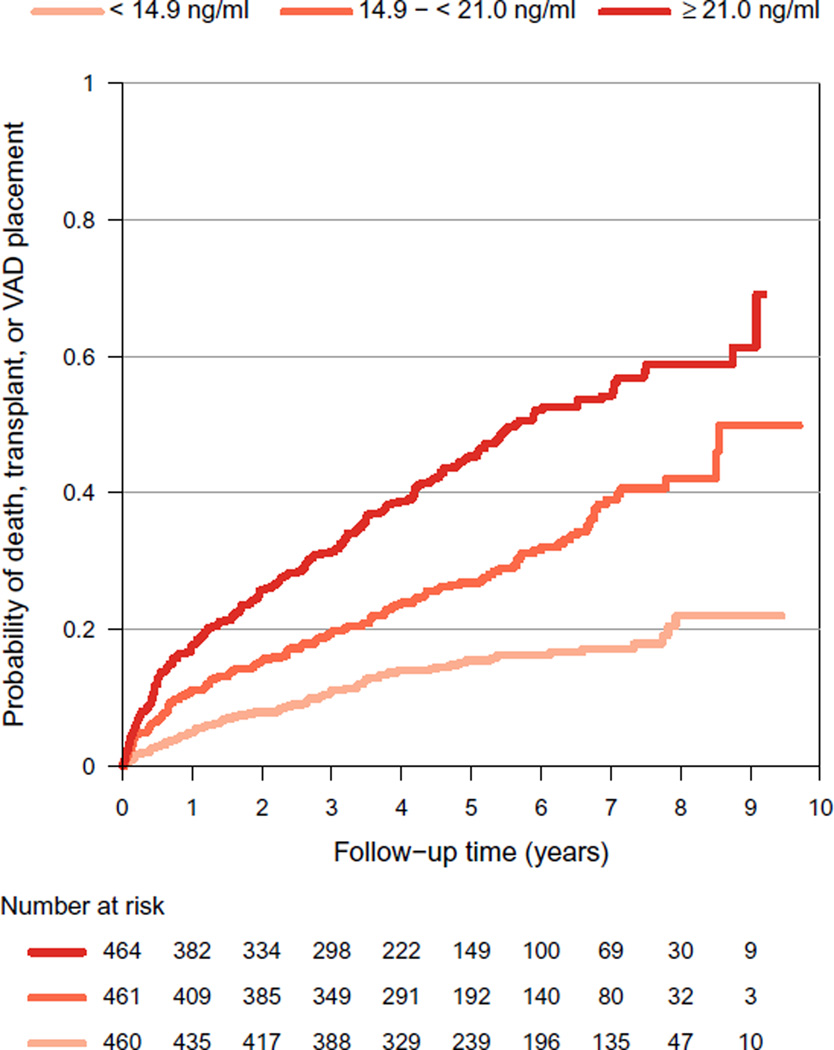
Over a median follow-up of 4.6 years (maximum 9.7 y), there were 298 deaths, 116 cardiac transplants, and 30 VAD placements. Higher galectin-3 levels were associated with a significantly increased risk of these events in unadjusted and adjusted models (Fig. 1; Table 3). Among all participants, a doubling in galectin-3 level was associated with a 96% increase in the hazard of all-cause mortality, cardiac transplantation, or VAD placement (P < .001). There were no appreciable differences in the results when stratified by ischemic or nonischemic cardiomyopathy etiology (P = .13; Table 3). However, there were important differences in the results when stratified by phenotype (Table 3). In particular, galectin-3 levels were strongly associated with risk among participants with preserved LVEF (adjusted hazard ratio 3.30; P < .001). Interaction analyses indicated that the association among participants with preserved LVEF was significantly different from that among participants with reduced LVEF (P = .031) but not recovered LVEF (P = .23). Note that the numbers of deaths, cardiac transplants, and VAD placements were, respectively, 262, 111, and 30 among participants with reduced LVEF; 19, 0, and 0 among participants with preserved LVEF; and 17, 5, and 0 among participants with recovered LVEF.
Fig. 1.
Probability of all-cause mortality, cardiac transplantation, or placement of a ventricular assist device (VAD) according to galectin-3 levels.
Table 3.
Association Between Galectin-3 and Risk of All-Cause Mortality, Cardiac Transplantation, or Placement of a Ventricular Assist Device, Among All Participants and Stratified by Cardiomyopathy Etiology (Ischemic or Nonischemic) and Heart Failure Phenotype (Reduced, Preserved, or Recovered LVEF)
| Unadjusted Model | Adjusted Model* | ||||
|---|---|---|---|---|---|
| Group | n | HR† (95% CI) | P Value | HR† (95% CI) | P Value |
| All participants | 1385 | 2.14 (1.89–2.42) | <.001 | 1.96 (1.64–2.33) | <.001 |
| Cardiomyopathy etiology | .13‡ | ||||
| Ischemic | 431 | 1.80 (1.47–2.21) | <.001 | 1.75 (1.39–2.20) | <.001 |
| Nonischemic | 954 | 2.19 (1.86–2.57) | <.001 | 2.16 (1.74–2.70) | <.001 |
| Heart failure phenotype | .15‡ | ||||
| Reduced LVEF | 1141 | 2.08 (1.83–2.37) | <.001 | 1.90 (1.57–2.29) | <.001 |
| Preserved LVEF | 106 | 3.37 (1.99–5.69) | <.001 | 3.30 (1.89–5.77) | <.001 |
| Recovered LVEF | 138 | 1.83 (0.98–3.42) | .059 | 1.86 (0.96–3.62) | .068 |
Adjusted for age, sex, race, cardiomyopathy etiology, cardiac resynchronization therapy, defibrillator use, creatinine, and site.
Hazard ratio compares the hazard of all-cause mortality, cardiac transplantation, and placement of a ventricular assist device between 2 populations whose galectin-3 level differed by a multiplicative factor of 2.
Interaction P value evaluates equality of hazard ratios across groups.
Galectin-3 was the least accurate discriminator of adverse events through 1 year of follow-up (AUC 0.627), although only the prediction accuracy of BNP (AUC 0.723) was significantly greater than that of galectin-3 (P < .001; Table 4). BNP was also the most accurate discriminator of adverse events through 5 years of follow-up (AUC 0.722; Table 4). However, when the cohort was stratified according to heart failure phenotype, galectin-3 was the most accurate discriminator of adverse events through 5 years of follow-up among participants with preserved LVEF (AUC 0.782; Table 5). In comparison, all other markers had a lower AUC than galectin-3 (P = .81 vs high-sensitivity ST2; P = .029 vs troponin I; P = .35 vs BNP; Table 5). BNP was the most accurate discriminator of adverse events through 5 years of follow-up among participants with reduced LVEF (AUC 0.716; P < .001 vs galectin-3) and among participants with recovered LVEF (AUC 0.728; P = .42 vs galectin-3).
Table 4.
Area Under the Time-Dependent Receiver Operating Characteristic Curve (AUC) to Quantify the Ability of Galectin-3, High-Sensitivity Soluble Toll-Like Receptor 2 (ST2), Troponin I, and B-Type Natriuretic Peptide (BNP) to Discriminate Major Adverse Events Through 1 and 5 Years of Follow-Up Among All Participants
| 1 Year* | 5 Years† | |||
|---|---|---|---|---|
| Marker | AUC (95% CI) | P Value‡ | AUC (95% CI) | P Value‡ |
| Galectin-3 | 0.627 (0.582–0.672) | — | 0.644 (0.611–0.677) | — |
| High-sensitivity ST2 | 0.639 (0.588–0.690) | .70 | 0.627 (0.594–0.660) | .43 |
| Troponin I | 0.662 (0.626–0.698) | .19 | 0.682 (0.655–0.709) | .062 |
| BNP | 0.723 (0.683–0.763) | <.001 | 0.722 (0.691–0.753) | <.001 |
CI, confidence interval;.
Kaplan-Meier survival at 1 year: 0.889.
Kaplan-Meier survival at 5 years: 0.708.
P values evaluate equality of each biomarker’s AUC with that of galectin-3.
Table 5.
AUC to Quantify the Ability of Galectin-3, High-Sensitivity ST2, troponin I, and BNP to Discriminate Major Adverse Events Through 5 Years of Follow-Up Among Participants With Reduced, Preserved, and Recovered LVEF
| Reduced LVEF (n = 1141)* | Preserved LVEF (n = 106)† | Recovered LVEF (n = 138)‡ | ||||
|---|---|---|---|---|---|---|
| Marker | AUC (95% CI) | P Value§ | AUC (95% CI) | P Value§ | AUC (95% CI) | P Value§ |
| Galectin-3 | 0.637 (0.602–0.672) | — | 0.782 (0.640–0.923) | — | 0.634 (0.459–0.809) | — |
| High-sensitivity ST2 | 0.630 (0.595–0.665) | .75 | 0.756 (0.598–0.914) | .81 | 0.652 (0.466–0.838) | .89 |
| Troponin I | 0.677 (0.649–0.705) | .050 | 0.589 (0.464–0.714) | .029 | 0.651 (0.528–0.774) | .89 |
| BNP | 0.716 (0.685–0.747) | <.001 | 0.683 (0.528–0.837) | .35 | 0.728 (0.575–0.881) | .42 |
Discussion
In a large ambulatory cohort of 1385 heart failure patients, we found that older age, more severe symptoms of heart failure, and reduced renal function were independently associated with elevated levels of galectin-3. Participants with elevated galectin-3 levels had a substantially increased risk of all-cause mortality, cardiac transplantation, or VAD placement. These results are consistent with earlier studies that demonstrated significant associations between galectin-3 levels and risk of adverse outcomes.13–15 We also determined that although baseline levels of galectin-3 did not differ between participants with reduced, preserved, and recovered LVEF,26 the association between galectin-3 and risk of major adverse events was strongest among participants with preserved LVEF.
When used to assess risk among all participants, galectin-3 exhibited modest accuracy to predict major adverse events in both the short and the long term, and it was less accurate than established biomarkers such as BNP. However, among the subgroup of participants with preserved LVEF, galectin-3 exhibited the highest prediction accuracy in the long term. Galectin-3 had superior accuracy compared with established biomarkers such as troponin I. Altogether, these data suggest the potential utility of galectin-3 in the growing population of patients with heart failure and preserved ejection fraction.
Our results expand on earlier observations of an interaction between galectin-3 levels and LVEF on adverse clinical outcomes and suggest a potential role for galectin-3 assessment in heart failure with preserved LVEF.18,21 Importantly, we have developed earlier results by clarifying and carefully defining important differences among heart failure phenotypes and demonstrating a specific role for galectin-3 in patients with preserved LVEF and assessment of long-term prognosis. In the Aldo-DHF trial, baseline galectin-3 levels were not associated with clinical outcomes over a maximum follow-up time of 12 months.27 Our study demonstrates a significant association with outcomes and prognostic utility over an extended follow-up time. Of note, the growing population of patients with preserved LVEF has been a challenging population to manage clinically owing to the lack of specific and sensitive prognostic markers and therapeutic strategies. With further validation of our results, galectin-3 has a potential to fulfill this unmet need.
Galectin-3 is hypothesized to be a mediator of fibrosis and involved in myofibroblast activation and matrix production and turnover.12 Nonhuman animal studies have demonstrated an association between galectin-3 and histologic evidence of fibrosis and increased expression in hypertrophied hearts.8–10 Interestingly, recently published studies of carefully phenotyped postmortem hearts from patients with heart failure and preserved LVEF demonstrated significant increases in myocardial fibrosis compared with control subjects.28 However, the pathobiologic basis for our results require additional study.
Strengths of the present study include the diversity of heart failure severity among study participants, the duration of follow-up, our focus on biomarkers that quantify different biologic pathways in heart failure, our comprehensive definition of subgroups based on LVEF, and the use of statistical methods that facilitate estimation of prediction accuracy in the presence of censored survival outcomes. However, this study has limitations. First, we considered clinically relevant biomarkers in heart failure, but comparisons with other biomarkers could also have revealed important differences. Second, we were unable to evaluate serial changes in biomarker levels over time, which have been shown to predict adverse outcomes.29,30 Third, our results could have been affected by the potential for heart failure subgroups to be misclassified based on the availability and accuracy of LVEF measurements, but we do not expect that such misclassification would be differential based on galectin-3 levels and therefore expect such bias toward the null. Fourth, this referral cohort represents the characteristics of those patients cared for at specialty centers. In particular, patients with preserved LVEF are underrepresented and could have more severe disease compared with community-based cohorts. Therefore, our results might not generalize to the population of heart failure patients seen early in the course of their disease or at initial presentation.31,32 Further research is needed to determine the role of galectin-3 among these patients.
In summary, we found that among the entire cohort of 1385 patients with chronic heart failure, although galectin-3 was strongly associated with the risk of major adverse events, its prediction accuracy was less than that of established biomarkers. However, galectin-3 was highly useful for risk stratification among heart failure patients with preserved LVEF, with greater prognostic accuracy compared with patients with reduced or recovered LVEF, and with superior accuracy compared with established markers of myocyte injury, neurohormonal activation, and myocyte stress. Taken together, our results suggest that galectin-3 could have an important role in defining prognosis among heart failure patients with preserved ventricular function.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health (R01 HL088577 to T.P.C.). Assays were performed by Abbott Diagnostics and Critical Diagnostics. None of these institutions had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Disclosures
None.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.cardfail.2015.10.022.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell JB. The economic burden of heart failure. Clin Cardiol. 2000;23:III-6–III-10. doi: 10.1002/clc.4960231503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perna ER, Macin SM, Canella JP, Augier N, Stival JL, Cialzeta JR, et al. Ongoing myocardial injury in stable severe heart failure: value of cardiac troponin T monitoring for high-risk patient identification. Circulation. 2004;110:2376–2382. doi: 10.1161/01.CIR.0000145158.33801.F3. [DOI] [PubMed] [Google Scholar]
- 4.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-Type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 7.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH, d’Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, et al. N-Acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296:H404–H412. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010;7:1–8. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 12.Coburn E, Frishman W. Comprehensive review of the prognostic value of galectin-3 in heart failure. Cardiol Rev. 2014;22:171–175. doi: 10.1097/CRD.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 13.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108:385–390. doi: 10.1016/j.amjcard.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueland T, Aukrust P, Broch K, Aakhus S, Skardal R, Muntendam P, et al. Galectin-3 in heart failure: high levels are associated with all-cause mortality. Int J Cardiol. 2011;150:361–364. doi: 10.1016/j.ijcard.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Andrès N, Rossignol P, Iraqi W, Fay R, Nuée J, Ghio S, et al. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail. 2012;14:74–81. doi: 10.1093/eurjhf/hfr151. [DOI] [PubMed] [Google Scholar]
- 16.Lok DJ, van der Meer P, de la Porte PW, Lipsic E, van Wijngaarden J, Hillege HL, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the Deal-HF study. Clin Res Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, et al. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail. 2012;5:72–88. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasco-Sánchez FJ, Aramburu-Bodas O, Salamanca-Bautista P, Morales-Rull JL, Galisteo-Almeda L, Páez-Rubio MI, et al. Predictive value of serum galectin-3 levels in patients with acute heart failure with preserved ejection fraction. Int J Cardiol. 2013;169:177–182. doi: 10.1016/j.ijcard.2013.08.081. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad T, Fiuzat M, Neely B, Neely ML, Pencina MJ, Kraus WE, et al. Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart Fail. 2014;2:260–268. doi: 10.1016/j.jchf.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayes-Genis A, de Antonio M, Vila J, Peñafiel J, Galán A, Barallat J, et al. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol. 2014;63:158–166. doi: 10.1016/j.jacc.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 21.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. doi: 10.1161/CIRCULATIONAHA.113.006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaze DC, Prante C, Dreier J, Knabbe C, Collet C, Launay JM, et al. Analytical evaluation of the automated galectin-3 assay on the Abbott Architect immunoassay instruments. Clin Chem Lab Med. 2014;52:919–926. doi: 10.1515/cclm-2013-0942. [DOI] [PubMed] [Google Scholar]
- 24.Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, et al. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–190. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 26.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher-Krainer E, et al. Galectin-3 in patients with heart failure with preserved ejection fraction: results from the Aldo-DHF trial. Eur J Heart Fail. 2015;17:214–223. doi: 10.1002/ejhf.203. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 30.Miller WL, Hartman KA, Burritt MF, Grill DE, Jaffe AS. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2009;54:1715–1721. doi: 10.1016/j.jacc.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Psaty BM, Shah SJ, Gottdiener J. Letter by Psaty et al regarding article, “Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes”. Circulation. 2015;131:e343. doi: 10.1161/circulationaha.114.012243. [DOI] [PubMed] [Google Scholar]
- 32.Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer N, et al. Response to letter regarding article, “Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2015;131:e344. doi: 10.1161/circulationaha.114.012888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.