Abstract
Background
Clinical trials in spinal cord injury (SCI) primarily rely on simplified outcome metrics (i.e. speed, distance) to obtain a global surrogate for the complex alterations of gait control. However, these assessments lack sufficient sensitivity to identify specific patterns of underlying impairment and to target more specific treatment interventions.
Objective
To disentangle the differential control of walking gait patterns following SCI beyond measures of time and distance.
Methods
The gait of 22 motor incomplete SCI subjects and 21 healthy controls was assessed using a high-resolution three-dimensional motion tracking system and complemented by clinical and electrophysiological evaluations applying unbiased multivariate analysis.
Results
Motor incomplete SCI patients showed varying degrees of spinal cord integrity (spinal conductivity) with severe limitations in walking speed and altered gait patterns. Principal component analysis (PCA) applied on all the collected data uncovered robust coherence between parameters related to walking speed, distortion of intralimb coordination and spinal cord integrity explaining 45% of outcome variance (PC 1). Distinct from the first PC, the modulation of gait-cycle variables (step length, gait-cycle phases, cadence; PC 2) remained normal with respect to regained walking speed while hip and knee ranges of motion were distinctly altered with respect to walking speed (PC 3).
Conclusions
In motor incomplete SCI distinct clusters of discretely-controlled gait parameters can be discerned that refine the evaluation of gait impairment beyond outcomes of walking speed and distance. These findings are specifically different from other neurological disorders (stroke, Parkinson) and are more discrete at targeting and disentangling the complex effects of interventions to improve walking outcome following motor incomplete SCI.
Keywords: spinal cord injury, human, gait, motor control
Introduction
More than half of spinal cord injured (SCI) patients exhibit incomplete injuries leading to varying degrees of functional impairment of the affected limbs.1 The provision of patient-customized interventions2 for motor incomplete spinal cord injury (iSCI) is limited by the rather crude clinical tools currently used for assessing locomotion (e.g. motor scores, speed, distance). Detailed analysis of locomotion in iSCI patients serves two important aims. Firstly, the ability to score locomotor function allows the determination of changes in walking capacity to measure the effect of therapeutic interventions.3–6 Secondly, comprehensive assessment of walking beyond unrefined measures of capacity may provide insights into mechanisms underlying changes of neural control.7, 8
The majority of studies on gait recovery after iSCI focus on quantitative measures of time and distance (10-meter walk test, 6-minute walk test), functional scores (e.g. walking index for spinal cord injury (WISCI) and the mobility sub-score of the spinal cord independence measure (SCIM III))9–11 or a subjective rating of gait quality.12 These investigations mainly assessed the temporal profiles of ambulatory capacity13 and the impact of rehabilitation interventions.4, 14 Few studies have used more detailed kinematic methodologies (e.g. quality of walking) to uncover complex alterations of iSCI walking.15–18 The objective of the present study was to establish a comprehensive assessment framework in iSCI applying multivariate data analysis of motor function to: i) reveal the influence of spinal cord integrity on walking outcomes and ii) provide a new approach for disentangling different domains of locomotor control in human SCI.
Materials and Methods
Subjects
Inclusion criteria
iSCI patients aged 18 years and older, who were at least able to stand and walk without the assistance of another person.
Exclusion criteria
Patients suffering from any other neurological disorder or pre-existing gait impairment were excluded. The study was approved by the Zurich Cantonal ethics committee in accordance with the Declaration of Helsinki. Participants provided written informed consent.
The data of 22 iSCI patients (15 males, 7 females; 48.3 ± 15.6 years, weight: 74.6 ± 13.4 kg, height: 171.6 ± 8.4 cm) and 21 healthy controls (8 males, 13 females; 38.4 ± 14.3 years, weight: 66.7 ± 12.1 kg, height: 172.2 ± 8.2 cm) were included in this study (Table 1).
Table 1.
Demographic and clinical characteristics of patients
| ID | Age, years | Sex | Cause of injury | Level of SCI | LEMS Assistive device | |
|---|---|---|---|---|---|---|
| 01 | 73 | m | traumatic | C5 | 24.5 | wheeler |
| 02 | 24 | m | traumatic | C3 | 24.5 | - |
| 03 | 36 | f | disc prolapse | T7/8 | 16.0 | crutches |
| 04 | 47 | f | spinal ischemia | T9/10 | 24.0 | - |
| 05 | 30 | m | traumatic | L2 | 22.5 | - |
| 08 | 60 | f | spinal ischemia | T5 | 23.0 | wheeler |
| 09 | 48 | f | traumatic | T7 | 15.0 | one cane |
| 10 | 39 | f | spinal myelitis | C, T | 19.0 | wheeler |
| 12 | 65 | m | hematoma | C6 | 24.0 | - |
| 13 | 23 | m | traumatic | C7 | 9.5 | wheeler |
| 15 | 78 | m | diverse | C3/4 | 22.5 | wheeler |
| 16 | 60 | m | spinal canal stenosis | T9/10 | 25.0 | - |
| 17 | 55 | m | cervical myelopathy | C5 | 19.5 | - |
| 18 | 63 | f | epidural abscess | T4 | 23.0 | crutches |
| 19 | 43 | m | traumatic | C2 | 24.0 | two canes |
| 20 | 61 | m | disc prolapse | C2 | 25.0 | - |
| 21 | 40 | m | traumatic | C5 | 22.5 | - |
| 22 | 64 | m | spondylitis, abscess | T4 | 23.5 | - |
| 23 | 32 | m | traumatic | T11 | 8.0 | crutches |
| 24 | 36 | m | traumatic | L4/5 | 23.5 | - |
| 25 | 41 | m | traumatic | C7 | 24.0 | - |
| 26 | 48 | f | disc prolapse | T10 | 24.0 | two sticks |
ID = patient identification number; SCI = spinal cord injury; LEMS = lower extremity motor score (mean value of left and right; max = 25); C = cervical, T = thoracic, L = lumbar.
Experimental setup
Subjects walked barefooted overground along a straight 8 m walkway and on a treadmill at different walking speeds. Patients were allowed to use an assistive device for overground walking if needed and were allowed to hold handrails on the treadmill. Subjects first walked overground to assess their preferred walking speed. This value was used to subsequently determine the relative walking speeds on the treadmill: 50% (slow), 100% (preferred), and 150% (fast) of preferred speed. Additionally, the subjects walked at predefined treadmill speeds, starting at a slow speed (0.5 km/h), with increasing speeds in steps of 0.5 km/h until 150% of preferred speed (fast) was reached. If a patient could not reach this speed, the maximal walking speed they were able to walk was recorded instead.
For lower body kinematics, 8 infrared cameras (T10, Vicon motion systems Ltd., Oxford, UK) at 200 Hz and two synchronized digital high-speed video cameras (pilot series, Basler AG, Ahrensburg, D) at 100 Hz were used. Pressure sensors underneath the treadmill belt (Zebris FDM-T, zebris Medical GmbH, Isny im Allgäu, D) recorded force distributions at 120 Hz. 16 reflective markers (16 mm diameter) were placed on bony landmarks according to the Vicon Plug-in Gait lower-body model.
Outcome measures
Time-distance parameters
The following time-distance parameters were determined: preferred overground walking speed (preferred speed) [km/h], step length [cm], cadence [strides/min], stance phase [%], and single support phase [%] during treadmill walking. Initial foot contact defined gait cycle onset. One gait cycle ranged from initial contact of one foot to the subsequent initial contact of the same foot. Time-distance parameters were calculated by the Zebris WinFDM-T software.
Kinematic data
Kinematic data were first processed offline using the Vicon Nexus Software (1.5.2) to fill data gaps and apply the Vicon PlugIn Gait kinematic model. Data were exported for further analysis using custom-written Matlab scripts (Matlab R2010b/R2013a, Mathworks Inc., Massachusetts, USA): Continuous data from ~20 consecutive gait cycles was cut into individual gait cycles and time-normalized using linear interpolation. The maximal range of motion (ROM) of the hip- and knee angles, averaged over all gait cycles, and the resultant velocity vector (angular change per unit time) of these two angles at toe-off (angular velocity at toe-off) were calculated. To assess whether angular velocity at toe-off limits walking speed in patients, their data was compared to controls at patients’ maximal speed by interpolating data of controls using a 3rd degree polynomial least squares fit. Variable Δ values reflect the difference between slow and preferred speed, providing a measure for the modulatory capacity of different features of gait with a doubling of walking speed (i.e. from 50% to 100% of preferred speed). To evaluate multisegmental intralimb coordination, combined hip-knee angle-angle plots, called cyclograms, were studied. The angular component of coefficient of correspondence (ACC)19 was calculated to quantify the cycle-to-cycle cyclogram shape consistency. To quantify cyclogram quality, the shape difference between patients’ cyclograms and a norm cyclogram of healthy individuals at preferred speed was calculated using the square root of sum of squared distances (SSD):20
where cyclogram j (patient) is compared to cyclogram k (norm cyclogram) after uniform scaling and translation of the centroid to the origin while α and β represent hip and knee angles, respectively.
Clinical and electrophysiological data
Lower-limb voluntary muscle strength was measured by trained examiners using the American Spinal Injury Association (ASIA) lower extremity motor score (LEMS). The mean of both legs was evaluated (max. 25). Spinal cord integrity was assessed via electrophysiological recordings according to clinical routine by motor evoked potentials (MEPs) of the tibialis anterior and abductor hallucis muscles and somatosensory evoked potentials (SSEPs) were elicited from the tibialis posterior nerve. MEPs were elicited by single-pulse transcranial magnetic stimulation of respective contralateral motor cortex areas just next to the midline via a figure-of-eight coil connected to a magnetic stimulator (The Magstim Company Ltd., Wales, UK). SSEPs were elicited dorsal of the malleolus medialis and the recording electrodes were placed in the Cz’-Fz configuration according to the international 10/20 system. P40/N50 peaks were chosen for SSEP evaluation. SSEPs and MEPs were evaluated by taking into account both response latency and amplitude as reported previously for SSEPs21, resulting in a 5-point impairment scale.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 19 (IBM Corp., New York, USA) and Matlab.
Principal component analysis
In order to account for the multidimensionality of data, a categorical principal component analysis (PCA) was performed including 23 parameters of all control and iSCI subjects in order to discern clusters of interrelated parameters of walking leveraging the extensive ensemble of gait-related variables.22 Principal components (PCs) were retained according to the Scree plot23 and PC over-determination (at least 2 |factor loadings| ≥ 0.6). Variables with high loadings on the same PC indicate a strong interrelationship. In the context of kinematic outcomes, PCs have been interpreted to reflect neurobiological “motor primitives” or discretely-organized “motor modules” of complex movements.24 To examine the validity and robustness of the PC clusters, a bootstrapping procedure was used with 10’000 iterations on a random subset of 80% of subjects and the factor loadings were cross validated using consensus from Pearson’s R, root mean square (RMS), coefficient of congruence (CC) and Cattell’s S. Monte Carlos studies have indicated that consensus of R, CC, RMS and S together indicate robust converging support for stability of multidimensional patterns, independent of the N used. Here, we use these statistics in our bootstrapping algorithms to reveal that the PC loading patterns are highly robust, despite the limited N available for SCI clinical populations 25, 26.
Pairwise comparisons
Spearman’s rank correlation coefficient ρ (rho) was used to correlate a pair of single outcome measures.
The comparison of two groups was performed by a two-tailed t-test for normally distributed data, and a Mann-Whitney U test or Wilcoxon signed-rank test for non-normally distributed independent or dependent samples, as appropriate. Bonferroni correction was applied (0.05/n).
Results
The gait pattern of healthy controls and iSCI patients was studied both qualitatively and quantitatively considering specific characteristics of lower-limb coordination. Gait pattern features were analyzed at two different walking speeds: 0.5km/h and preferred speed. In order to compare the gait pattern at a matching speed, patients at their preferred speed (mean 2.0 km/h) were additionally compared to controls walking at 2.0 km/h.
Multivariate analysis
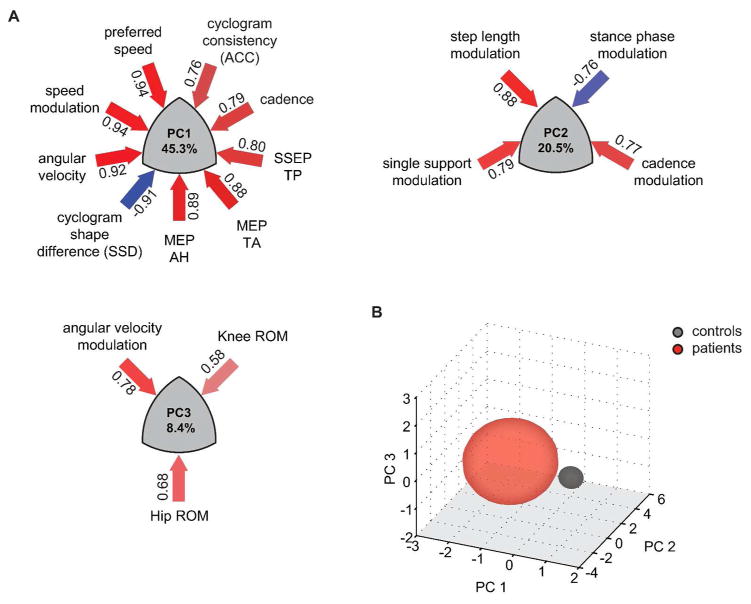
PCA of the 23 variables revealed 3 robustly reproducible PCs (r ≥ 0.98, RMS ≤ 0.01, CC ≥ 0.97, Cattell’s S ≥ 0.5) that together accounted for 74.2% of variance (Figure 1A). The overall variance was mainly influenced by the neurological condition (i.e. healthy vs. SCI) (Figure 1B). PC1 (45.3% of variance) was characterized by variables of walking dynamics (e.g. walking speed, angular velocity) as well as spinal cord integrity (i.e. MEP, SSEP) and quantified metrics of the intralimb coordination (shape difference (SSD), cycle-to-cycle consistency (ACC)). The orthogonal PC2 assembled step length and variables associated with the timing and reciprocal bilateral coordination of the gait cycle. The third PC was predominantly defined by hip- and knee ROMs.
Figure 1. Multivariate analysis of gait-related parameters.
Principal components (PCs) 1–3 explain 74.2% of the total variance. PCs show clustered variance of multiple parameters with high loadings on the corresponding PC. Variable loadings are depicted in numbers next to the arrows whose color reflect magnitude and relationship of loading (positive relationship = red, negative = blue) (A). Transformed data is plotted in the 3D space determined by the orthogonal PCs 1 to 3 and grouped according to neurological condition (B). AH = abductor hallucis, MEP = motor evoked potential, ROM = range of motion, SSEP = somatosensory evoked potential, TA = tibialis anterior, TP = tibialis posterior.
Gait control impairments
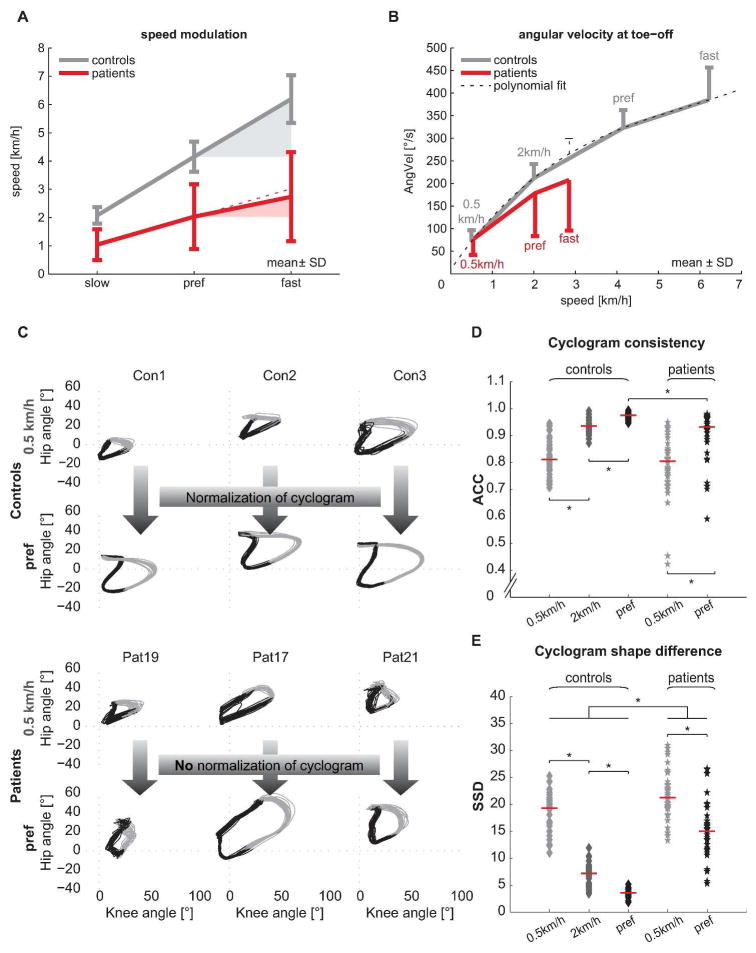
ANOVA and post-hoc Tukey’s test revealed that the two groups (iSCI and control group) significantly differed along PC1, but no group distinction was seen with respect to PC2 or PC3. Measures representing spinal cord integrity (MEP, SSEP) as well as walking speed assembled on PC1 (Figure 1A) and were significantly affected in iSCI subjects. Clinically, patients were limited in preferred and maximal walking speeds (Figure 2A): while all controls were able to increase their speed to 150% of preferred speed (6.18 ± 0.79 km/h) 12 of 19 patients showed a restricted ability to increase their walking speed beyond 100% (2.85 ± 1.55 km/h). Also among the best walkers (preferred speed ≥ 3 km/h) 3 of 5 patients could not attain their relative maximal walking speed. Even more pronounced than speed impediments, patients showed a remarkably limited capacity to modulate angular velocity at toe-off (Figure 2B). At the 0.5 km/h speed the two groups showed comparable angular velocities, while iSCI subjects diverged from control data with increasing walking speed. The increase in angular velocity was significant from 0.5 km/h to preferred as well as from preferred to maximal walking speed in control subjects (paired t-test: P < 0.001 and P = 0.003). Patients could only increase the angular velocity between the 0.5 km/h and preferred speed but not between preferred and maximal speed (paired t-test: P < 0.001 and P = 0.355). At patients’ maximal speed (2.85 km/h) the angular velocity was significantly lower compared to the interpolated values at the respective speed in control subjects (t-test: P = 0.026).
Figure 2. Speed modulation of parameters contributing to PC1.
(A) Absolute speed and speed modulation is limited in iSCI patients. Theoretical linear increase is indicated by the red dotted line. Error bars indicate ± 1SD. (B) The diagram illustrates the modulation of the hip-knee angular velocity at toe-off. A 3rd degree polynomial fit (thin dotted line) was used to interpolate control subjects’ data to obtain their angular velocity at patients’ fast speed. Error bars indicate 1SD. (C) Hip-knee cyclograms of control subjects (top 2 rows) and patients (bottom 2 rows) both at 0.5 km/h and at preferred (pref) walking speed are shown. 20 gait cycles are plotted, black color indicates stance phase, grey color indicates swing phase. (D) The cycle-to-cycle reproducibility of the hip-knee cyclogram (ACC) improved with increasing speed in both groups but was lower in patients at preferred speed. (E) The shape difference of the cyclogram compared to normal (SSD) improved in patients at preferred speed but remained different from controls at all speeds. ACC = angular component of coefficient of correspondence, AngVel = angular velocity at toe-off, SD = standard deviation, SSD = square root of sum of squared distances (cyclogram shape difference). * p < 0.05.
The quality of intralimb coordination (shape difference to normal) showed a striking convergence when increasing speed from 0.5 km/h to preferred in healthy control subjects while iSCI patients with a deteriorated pattern were unable to normalize it (Figure 2C). Metrics quantifying hip-knee cyclogram characteristics showed the sensitivity of the intralimb coordination to changes in speed: the cycle-to-cycle consistency (ACC) increased from 0.5 km/h to preferred speed in both groups (Wilcoxon signed rank test: P < 0.001) but was still higher in control subjects compared to patients at preferred speed (Mann-Whitney U test: P < 0.001; Figure 2D). However, at 0.5 km/h and at the matching speed (2 km/h) the ACC was indistinguishable between groups (Mann-Whitney U test: P = 0.394, P = 0.666). The shape difference to normal of the cyclogram (SSD) improved at preferred speed compared to the 0.5 km/h walking in both groups (paired t-test: P < 0.001). However, it remained different from normal in iSCI patients both at a matching speed (2 km/h) and when comparing preferred speeds (t-test: P < 0.001; Figure 2E), emphasizing the diminished normalization capacity.
Preserved control of gait-cycle parameters
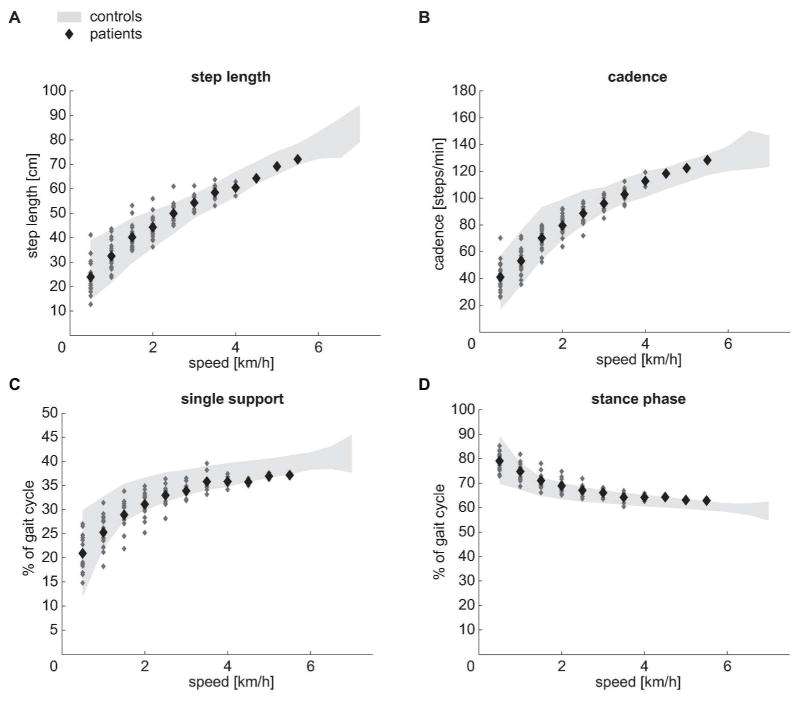
In contrast to the variables determining PC1, PC2 was loaded by gait-cycle parameters that remained well modulated according to walking speed (Figures 3A through D). The values of step length, cadence, single support- and stance phase were well within the range of control subjects.
Figure 3. Speed modulation of parameters contributing to PC2.
(A through D) Modulation of step length, cadence, single support and stance phase of control subjects (grey area, ± 2SD interval) and patients (large dots = group mean values, small dots = individual patient’s data) at different walking speeds (0.5 km/h to 7.0 km/h in controls, 0.5 km/h to 5.5 km/h in patients). The two groups did not differ.
Range of motion
Hip ROM and knee ROM represent the maximal sagittal excursion of single joint angles during a gait cycle. At 0.5 km/h and at the matching speed (2 km/h) iSCI subjects showed a greater hip ROM compared to control subjects while the knee ROM did not differ between groups (t-test: P = 0.005 and P = 0.961 at 0.5 km/h; P < 0.001 and P = 0.613 at matching speed). In contrast, the hip ROM showed no difference between groups at preferred speed while the knee ROM was reduced in iSCI subjects (t-test: P = 0.317 and P = 0.001).
Speed dependence
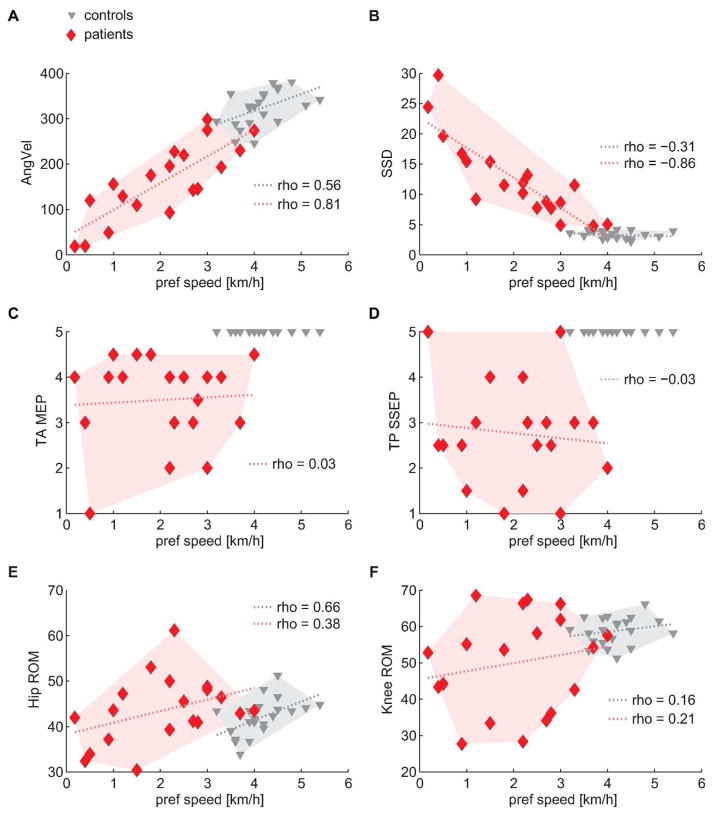
Some gait variables, e.g. angular velocity at toe-off, were linearly related to preferred speed (Figure 4A), where patients attained lower values than control subjects in accordance with their reduced walking speed. By contrast, the cyclogram shape did not reflect preferred walking speed in healthy subjects, given that the SSD remained within a narrow range (~2 to 5) in the control group irrespective of speed (Figure 4B). Patients, on the other hand, showed a high relation between the cyclogram shape and preferred speed. Interestingly, spinal cord integrity (i.e. spinal cord conductivity as measured by evoked potentials) did not determine the speed capacity of patients (Figure 4C and D). The ROMs showed rather weak relations to preferred speed especially in iSCI subjects but also in healthy individuals (Figures 4E and F).
Figure 4. Relation of gait-related parameters to preferred speed.
(A and B) Angular velocity at toe-off and SSD (shape difference to normal) showed linear relations to walking capacity and separated the two groups. (C and D) The degree of spinal cord integrity did not correlate with walking capacity but clearly distinguished the two groups. (E and F) Ranges of motion showed weak to moderate relation to walking capacity and does not distinguish between groups. A linear fit was added to the graphs (dotted lines) to illustrate trends although Spearman’s correlation does not assume linearity of data. AngVel = angular velocity at toe-off, MEP = motor evoked potential, ROM = range of motion, SSD = sum of squared distances (cyclogram shape difference), SSEP = somatosensory evoked potential, TA = tibialis anterior, TP = tibialis posterior.
Discussion
The present study, analyzing combined clinical and kinematic assessments, provides specific findings of the complexity of gait alterations and locomotor control in walking following iSCI. Through statistical data-integration across multiple outcome modalities specific clusters of coherent gait parameters were distinguishable that were related to spinal cord integrity and discretely controlled after iSCI. The findings are specifically different from other neurological disorders (e.g. stroke and Parkinson). The effectiveness of any treatment intervention (training, drugs) in iSCI to improve walking outcome may be evaluated against these distinguishable clusters of outcome.27
Gait control impairments
Clinical (ASIA protocol) and electrophysiological measures (i.e. MEPs and SSEPs) have been shown to be sensitive to assess spinal cord impairment following SCI and are predictive for functional outcome.5, 28–30 While these measures are sensitive at predicting levels of walking outcome (no-, therapeutic-, functional-, and full locomotor capacity31, 32) they may not be able to predict more detailed gait characteristics or continuous measures such as walking speed. This is the first study to show that SCI specifically affects certain clusters of parameters while others remain well-controlled. Different levels of gait-pattern distortion could be quantified by the measure of shape difference to normal (SSD), which was strongly related to speed capacity in iSCI subjects. In addition, while healthy subjects showed a striking convergence towards a uniform pattern at preferred speed, patients could voluntarily change speed up to a certain limit but failed to improve the shape difference when increasing their walking speed. These findings indicate that speed and kinematic features respond differently to changes in supraspinal input. A similar behavior could be observed in stroke patients who showed improved gait symmetry at faster speeds but no changes in compensatory movements.33 Despite a high responsiveness to supraspinal input at the stance to swing transition34 speed increases were not proportionally paralleled by increased angular velocity at toe-off, suggesting that compensatory mechanisms (e.g. increased hip ROM) might instead enable speed increments. The observed behaviors imply that additional factors may play a role: faster walking biomechanically induces less movement variability (i.e. parallel increase of ACC and speed)35 and spasticity probably impedes the translation of increased speed to higher angular velocities.
Preserved control mechanisms
Despite a clear reduction in the capacity to walk beyond the preferred walking speed (i.e., maximal speed)15 specific gait parameters remained unaltered in iSCI subjects, suggesting less dependence on spinal cord integrity. PC2 variables represented intact interlimb coordination to produce temporally (gait-cycle phases) and spatially (step length) adequate steps and, interestingly, across the range of walking speeds that iSCI subjects could attain, these parameters remained adequately modulated. This is in contrast to distinct impairments in the control of gait-cycle parameters observed in neurological disorders affecting supraspinal motor centers. In Parkinsonian patients step length is significantly reduced while cadence can be increased to values even higher than in controls (shuffling gait).36 Stroke patients typically show an asymmetric slow gait with decreased cadence37 and limited stride length.38, 39 In line with these observations iSCI subjects showed preserved temporal accuracy of skilled ankle movements despite diminished absolute active ROM and movement velocity.40 By contrast, stroke patients showed decreased movement accuracy both in the paretic and even the unaffected leg.41 These findings indicate that muscle weakness and movement timing are unrelated and the required control for temporal movement accuracy (within the boundaries of preserved muscle strength/speed) remains largely intact in iSCI.
Domains of locomotor control
The clear distinction between healthy and altered gait, which is congruent with normal and impaired spinal cord integrity assessed by spinal tract conductivity, suggests that human walking is highly dependent on unimpaired supraspinal input (cortical and subcortical).42 The non-linear relation of measures of spinal conductivity to single gait parameters may partly be attributable to the complex interactions of supraspinal inputs that are fine-tuned by spinal networks simultaneously integrating sensory feedback from the periphery. In addition to an immediately diminished drive from supraspinal centers, spinal networks reorganize after injury and functionally change over time,43, 44 which again may differently affect gait parameters. For these reasons it remains difficult to attribute the control of specific gait parameters to particular areas of the sensorimotor system. However, one may assume that the control of distinct clusters of parameters differently depend on spinal cord integrity. Given that PC1 and 2 are orthogonal and therefore unrelated, one may conclude that PC1, comprising spinal cord integrity and speed, more strongly relies on an intact spinal cord. The parameters related to the control of reciprocal limb activation (PC2) were less dependent on spinal cord integrity and may rely more on spinal circuits (e.g. central pattern generators) as shown in previous studies.45–49
Implications for interventions
Through the advancement over the currently simplified outcome evaluation (walking speed and distance) using more comprehensive assessments combined with unbiased multivariate analysis that enables the handling of the complexity of data, patients may receive therapeutic interventions specifically tailored to their impairment.27 The identification of distinct outcome domains may allow elucidating the role of spinal cord integrity in gait control and to reveal information on underlying mechanisms involved in recovery.
Limitations
The number of subjects from this inherently heterogeneous group of patients was rather small and therefore a generalization of results may be limited. Animal studies have revealed that voluntary changes of gait parameters, although immediately responding to supraspinal commands, are still influenced by sub-hierarchical (i.e. spinal) effects such as facilitation or inhibition.7, 50 Thus, discerning supraspinal and spinal neural control of locomotion especially in humans remains very challenging. A major factor influencing walking pattern is spasticity, which was not assessed in this study. In addition, the effects of medication (i.e. anti-spasticity) and walking aids on these outcomes as well as the fact that subjects were walking on a treadmill as opposed to over ground require further attention.
Conclusion
Locomotor function in iSCI patients is clinically well-addressed by measures of speed and distance as they represent levels of walking capacity required for daily activities but should be complemented by refined assessments of movement quality and spinal conductivity. The modulation of specific gait parameters is distinctly dependent on spinal cord integrity, and gait alterations after iSCI are specific and differ from brain disorders (e.g. Parkinson’s disease, stroke). The distinction and understanding of the dynamic changes of neural control after injury as well as during recovery and rehabilitation interventions may contribute to improved outcome evaluation and advanced treatments for iSCI.
Acknowledgments
Funding
This study was supported by the European Commission’s Seventh Framework Program [CP-IP 258654, NEUWalk]; the Wolf Foundation, Switzerland; the Clinical Research Priority Program CRPP Neurorehab UZH; Wings for Life WFL-US-008/12 and by NIH/NINDS [R01 NS067092].
We would like to thank B. Huber for assistance with patient recruitment and data acquisition, W. Popp and L. Tanadini for their help with statistical analyses. We would also like to thank the participants of this study.
Footnotes
Conflict of interest disclosures: None.
References
- 1.Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 2.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. New Engl J Med. 2012;366:489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 3.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil. 2001;82:818–824. doi: 10.1053/apmr.2001.23752. [DOI] [PubMed] [Google Scholar]
- 4.Lucareli PR, Lima MO, Lima FP, de Almeida JG, Brech GC, D’Andrea Greve JM. Gait analysis following treadmill training with body weight support versus conventional physical therapy: a prospective randomized controlled single blind study. Spinal Cord. 2011;49:1001–1007. doi: 10.1038/sc.2011.37. [DOI] [PubMed] [Google Scholar]
- 5.Petersen JA, Spiess M, Curt A, Dietz V, Schubert M. Spinal cord injury: one-year evolution of motor-evoked potentials and recovery of leg motor function in 255 patients. Neurorehabil Neural Repair. 2012;26:939–948. doi: 10.1177/1545968312438437. [DOI] [PubMed] [Google Scholar]
- 6.Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci. 2013;33:2365–2375. doi: 10.1523/JNEUROSCI.3968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenzweig ES, Courtine G, Jindrich DL, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hedel HJ, Wirz M, Curt A. Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord. 2006;44:352–356. doi: 10.1038/sj.sc.3101853. [DOI] [PubMed] [Google Scholar]
- 10.Ditunno JF, Jr, Barbeau H, Dobkin BH, et al. Validity of the walking scale for spinal cord injury and other domains of function in a multicenter clinical trial. Neurorehabil Neural Repair. 2007;21:539–550. doi: 10.1177/1545968307301880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of disability in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma. 2011;28:1413–1430. doi: 10.1089/neu.2009.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field-Fote EC, Fluet GG, Schafer SD, et al. The Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI) J Rehabil Med. 2001;33:177–181. doi: 10.1080/165019701750300645. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz DJ, Datta S, Harkema SJ. Longitudinal patterns of functional recovery in patients with incomplete spinal cord injury receiving activity-based rehabilitation. Arch Phys Med Rehabil. 2012;93:1541–1552. doi: 10.1016/j.apmr.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepin A, Norman KE, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 1. Adaptation to changes in speed. Spinal Cord. 2003;41:257–270. doi: 10.1038/sj.sc.3101452. [DOI] [PubMed] [Google Scholar]
- 16.Ivanenko YP, Poppele RE, Lacquaniti F. Distributed neural networks for controlling human locomotion: lessons from normal and SCI subjects. Brain Res Bull. 2009;78:13–21. doi: 10.1016/j.brainresbull.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Nooijen CF, Ter Hoeve N, Field-Fote EC. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J Neuroeng Rehabil. 2009;6:36. doi: 10.1186/1743-0003-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil-Agudo A, Perez-Nombela S, Forner-Cordero A, Perez-Rizo E, Crespo-Ruiz B, del Ama-Espinosa A. Gait kinematic analysis in patients with a mild form of central cord syndrome. J Neuroeng Rehabil. 2011;8:7. doi: 10.1186/1743-0003-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82:707–715. [PubMed] [Google Scholar]
- 20.Awai L, Curt A. Intralimb coordination as a sensitive indicator of motor-control impairment after spinal cord injury. Front Hum Neurosci. 2014;8:148. doi: 10.3389/fnhum.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curt A, Dietz V. Traumatic cervical spinal cord injury: relation between somatosensory evoked potentials, neurological deficit, and hand function. Arch Phys Med Rehabil. 1996;77:48–53. doi: 10.1016/s0003-9993(96)90219-1. [DOI] [PubMed] [Google Scholar]
- 22.Pearson K. On lines and planes of closest fit to systems of points in space. Philosophical Magazine. 1901;2:559–572. [Google Scholar]
- 23.Cattell RB. Scree test for the number of factors. Multivar Behav Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 24.Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Modular control of limb movements during human locomotion. J Neurosci. 2007;27:11149–11161. doi: 10.1523/JNEUROSCI.2644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guadagnoli E, Velicer WF. Relation of sample size to the stability of component patterns. Psychol Bull. 1988;103:265–275. doi: 10.1037/0033-2909.103.2.265. [DOI] [PubMed] [Google Scholar]
- 26.Guadagnoli E, Velicer W. A Comparison of Pattern Matching Indices. Multivar Behav Res. 1991;26:323–343. doi: 10.1207/s15327906mbr2602_7. [DOI] [PubMed] [Google Scholar]
- 27.Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 28.Jacobs SR, Yeaney NK, Herbison GJ, Ditunno JF., Jr Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch Phys Med Rehabil. 1995;76:635–641. doi: 10.1016/s0003-9993(95)80632-6. [DOI] [PubMed] [Google Scholar]
- 29.Zorner B, Blanckenhorn WU, Dietz V, Curt A. Clinical algorithm for improved prediction of ambulation and patient stratification after incomplete spinal cord injury. J Neurotrauma. 2010;27:241–252. doi: 10.1089/neu.2009.0901. [DOI] [PubMed] [Google Scholar]
- 30.van Middendorp JJ, Hosman AJ, Donders AR, et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet. 2011;377:1004–1010. doi: 10.1016/S0140-6736(10)62276-3. [DOI] [PubMed] [Google Scholar]
- 31.Curt A, Dietz V. Ambulatory capacity in spinal cord injury: significance of somatosensory evoked potentials and ASIA protocol in predicting outcome. Arch Phys Med Rehabil. 1997;78:39–43. doi: 10.1016/s0003-9993(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 32.Curt A, Keck ME, Dietz V. Functional outcome following spinal cord injury: significance of motor-evoked potentials and ASIA scores. Arch Phys Med Rehabil. 1998;79:81–86. doi: 10.1016/s0003-9993(98)90213-1. [DOI] [PubMed] [Google Scholar]
- 33.Tyrell CM, Roos MA, Rudolph KS, Reisman DS. Influence of systematic increases in treadmill walking speed on gait kinematics after stroke. Phys Ther. 2011;91:392–403. doi: 10.2522/ptj.20090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Exp Brain Res. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- 35.Wuehr M, Schniepp R, Pradhan C, et al. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp Brain Res. 2013;224:287–294. doi: 10.1007/s00221-012-3310-6. [DOI] [PubMed] [Google Scholar]
- 36.Morris M, Iansek R, Matyas T, Summers J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord. 1998;13:61–69. doi: 10.1002/mds.870130115. [DOI] [PubMed] [Google Scholar]
- 37.von Schroeder HP, Coutts RD, Lyden PD, Billings E, Jr, Nickel VL. Gait parameters following stroke: a practical assessment. J Rehabil Res Dev. 1995;32:25–31. [PubMed] [Google Scholar]
- 38.Nakamura R, Handa T, Watanabe S, Morohashi I. Walking cycle after stroke. Tohoku J Exp Med. 1988;154:241–244. doi: 10.1620/tjem.154.241. [DOI] [PubMed] [Google Scholar]
- 39.Nadeau S, Betschart M, Bethoux F. Gait analysis for poststroke rehabilitation: the relevance of biomechanical analysis and the impact of gait speed. Phys Med Rehabil Clin N Am. 2013;24:265–276. doi: 10.1016/j.pmr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Wirth B, van Hedel H, Curt A. Foot control in incomplete SCI: distinction between paresis and dexterity. Neurol Res. 2008;30:52–60. doi: 10.1179/174313208X297030. [DOI] [PubMed] [Google Scholar]
- 41.van Hedel HJ, Wirth B, Curt A. Ankle motor skill is intact in spinal cord injury, unlike stroke: implications for rehabilitation. Neurology. 2010;74:1271–1278. doi: 10.1212/WNL.0b013e3181d9ed7c. [DOI] [PubMed] [Google Scholar]
- 42.Bachmann LC, Matis A, Lindau NT, Felder P, Gullo M, Schwab ME. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med. 2013;5:208ra146. doi: 10.1126/scitranslmed.3005972. [DOI] [PubMed] [Google Scholar]
- 43.Dietz V. Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol. 2010;6:167–174. doi: 10.1038/nrneurol.2009.227. [DOI] [PubMed] [Google Scholar]
- 44.Beauparlant J, van den Brand R, Barraud Q, et al. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain. 2013;136:3347–3361. doi: 10.1093/brain/awt204. [DOI] [PubMed] [Google Scholar]
- 45.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 46.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 47.Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet. 1994;344:1260–1263. doi: 10.1016/s0140-6736(94)90751-x. [DOI] [PubMed] [Google Scholar]
- 48.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- 49.Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- 50.van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]