Abstract
Purpose
Osteogenesis imperfecta (OI) predisposes to recurrent fractures. The moderate-to-severe forms of OI present with antenatal fractures and the mode of delivery that would be safest for the fetus is not known.
Methods
We conducted systematic analyses on the largest cohort of individuals (n=540) with OI enrolled to-date in the OI Linked Clinical Research Centers. Self-reported at-birth fracture rates were compared in individuals with OI types I, III, and IV. Multivariate analyses utilizing backward-elimination logistic regression model building were performed to assess the effect of multiple covariates including method of delivery on fracture-related outcomes.
Results
When accounting for other covariates, at-birth fracture rates did not differ based on whether delivery was by vaginal route or by cesarean section (CS). Increased birth weight conferred higher risk for fractures irrespective of the delivery method. In utero fracture, maternal history of OI, and breech presentation were strong predictors for choosing CS for delivery.
Conclusion
Our study, the largest to analyze the effect of various factors on at-birth fracture rates in OI shows that delivery by CS is not associated with decreased fracture rate. With the limitation that the fracture data were self-reported in this cohort, these results suggest that CS should be performed only for other maternal or fetal indications, but not for the sole purpose of fracture prevention in OI.
Keywords: Osteogenesis imperfecta, at-birth fracture, cesarean section, in utero fracture, natural history study
Introduction
Osteogenesis imperfecta (OI), a genetically heterogeneous connective tissue disorder, is the most common skeletal dysplasia predisposing to significantly increased bone fragility and fractures.1 Whereas the classification of OI has undergone a significant change with the discovery of new genes implicated in OI, the vast majority of patients (85–90%) can be categorized into types I, II, III, and IV that were originally proposed by Sillence.2–4 Type I is the mildest form, type II the most severe and perinatally lethal form, while types III and IV are moderate-to-severe forms of the disorder.
The widespread utilization of ultrasound during pregnancy and the availability of genetic testing have increased the antenatal diagnosis of OI.5–7 Most fetuses with moderate-to-severe OI and some with the mild forms sustain in utero fractures and a valid clinical concern would be to minimize trauma during delivery. Cesarean section (CS) has been suggested as a more favorable delivery method by some based on the notion that this method is “more controlled” and “less traumatic” than vaginal delivery and thus could potentially decrease mortality and morbidity.8–10 However, there is no evidence to show superiority of CS over vaginal delivery. The only study to address at-birth fracture rates based on mode of delivery found that CS neither decreased fracture rates in the nonlethal forms nor prolonged the survival in those with lethal forms of OI; however, limitations in sample size precluded a robust analysis of covariates related to fracture outcomes.11
Providing evidence-based answers to clinically relevant questions in OI has been made difficult by the rarity of the condition. To advance clinical research and improve the care of patients with OI, the Linked Clinical Research Centers (LCRC), a network of five clinical centers with significant experience in treating patients with OI was established in 2009.3 Using the data from 540 individuals with OI enrolled in the “Longitudinal Study of Osteogenesis Imperfecta” conducted by the LCRC, we systematically analyzed the effects of various factors including mode of delivery on the at-birth fracture rates in OI. This study is the largest to date to address whether CS has an effect on at-birth fracture rates and whether an antenatal diagnosis of OI influences the choice of the delivery method. In addition, we attempt to define novel correlations for fractures associated with birth trauma.
MATERIALS AND METHODS
Study Population
The establishment of the LCRC and the subjects enrolled in the “Longitudinal Study of Osteogenesis Imperfecta” have been previously described.3 The LCRC comprised five clinical sites across North America: Baylor College of Medicine (Houston, TX); Kennedy Krieger Institute (Baltimore, MD) in collaboration with Nemours/Alfred I. DuPont Hospital for Children (Wilmington, DE); Oregon Health & Science University (Portland, OR); Shriners Hospital for Children (Chicago, IL); and Shriners Hospital for Children (Montreal, Canada). The Data Collection and Analysis Center at the University of South Florida College of Medicine (Tampa, FL) served as the central facility for data collection and analyses. The Collagen Diagnostic Laboratory at the University of Washington (Seattle, WA) served as the center for molecular and biochemical analysis. The Institutional Review Boards of participating sites approved the research protocol. Informed consent was obtained from subjects or their legal guardians.
Five hundred and forty individuals with OI were enrolled in the longitudinal study based on the protocol that has been previously published.3 Of these individuals, 239 had OI type I, 147 had OI type IV, and 101 had OI type III (n=487) (Table 1) and were included in further analyses. The numbers of individuals with other subtypes of OI were very small (OI type V n=16; OI type VI n=12; OI type VII n=6; unclassified OI n=19) and were hence excluded from this study. Individuals with OI type II were also excluded due to the severity of the phenotype. The classification was predominantly based on clinical features, however, genotypic information was used to reclassify patients whenever available. Ten subjects originally classified as OI types III or IV were reclassified into types V, VII, or the ‘unclassified’ categories based on genotype.
Table 1.
Characteristics of patients with non-lethal, type I collagen-related OI enrolled in the LCRC. Percentages in parentheses depict the proportion within each OI subtype.
| OI type I | OI type III | OI type IV | |
|---|---|---|---|
| Total Number | 239 | 101 | 147 |
| Male (%) | 113 (47.3) | 43 (42.6) | 64 (43.5) |
| Female (%) | 126 (52.7) | 58 (57.4) | 83 (56.5) |
| Family History Present (%) | 148 (61.9) | 7 (6.93) | 42 (28.6) |
| In Utero Fractures Present (%) | 14 (5.85) | 65 (64.4) | 52 (35.4) |
| Prenatal Diagnosis (%) | 78 (32.6) | 38 (37.6) | 45 (30.6) |
| Presentation at birth* | |||
| Vertex | 189 | 50 | 101 |
| Breech | 18 | 33 | 23 |
Information about presentation at birth was unavailable in 73 individuals.
The data collected for the present study included gender, age at enrollment, antenatal diagnosis (yes or no), presentation at birth (breech or vertex), family history (yes or no), family members with OI (mother, father, or sibling), in utero fractures (yes or no), number of fractures at birth, specific locations of at-birth fractures (long bone, rib, clavicle, and/or skull), gestational age at delivery, birth weight, birth length, and mode of delivery. Mode of delivery was categorized as vaginal (spontaneous or induced) or CS. A total of 454 subjects had data on all the covariates and were included in the primary analyses. The data were collected in identical fashion at every site using on-line case report forms in accordance to the instructions outlined in the Manual of Operations. The Data Collection and Analysis Center monitored the data delinquency and sent queries to centers to further improve the quality of the data.
Statistical Analysis
Differences in the demographic characteristics were assessed using chi-square test. The Mann-Whitney U test was used to determine the differences between median birth weight and length. The proportions of individuals with at-birth fracture based on OI subtype and delivery method (vaginal vs. CS) were analyzed using chi-square test (n=454); the Fisher-Exact Test was applied when the numbers were less than five in any cell. In order to identify whether mode of delivery had an effect on fracture rates at birth, we further examined the data using logistic regression. We applied a backward-elimination strategy where all variables were included in a full model and at each iteration an independent variable that did not improve the model at p=0.05 level by utilizing the likelihood-ratio chi-squared test was removed. Backward elimination was stopped when all included variables led to model improvement. In the model, birth fracture was included as a binary dependent variable. Independent variables that were included include delivery method (CS or vaginal delivery), OI subtype (type I, III or IV), presence of in utero fractures (yes or no), gender (male or female), family history of OI (yes or no), family members with OI (mother, father, or sibling), antenatal diagnosis of OI (yes or no), presentation (breech or vertex), gestational age (in weeks), birth weight, and birth length. Interaction terms were not included in the full model given concerns for over-fitting given the large number of potential interactions. For variables that remained in the model after backward-elimination, interaction terms were evaluated. The interaction between that variable with independent effect and each other variable, stated above, was added and evaluated for model improvement by the likelihood-ratio chi-squared test with a threshold of p=0.05 for inclusion. We also examined the interaction between antenatal diagnosis and delivery method.
To analyze whether certain characteristics influenced the decision to choose a particular delivery method, we utilized logistic regression with a backward-elimination strategy to identify novel correlations where mode of delivery was the binary dependent variable. Covariates considered included in utero fractures, maternal history of OI and presentation at birth. Interaction terms were also evaluated to discern whether each variable independently correlated with the outcome measure.
RESULTS
Study Population
The analysis of demographic characteristics showed that the proportions of individuals with each subtype in the study were generally representative of the prevalence observed in the broader population (Table 1). 3,12 Patients with non-lethal dominant forms of type I collagen-related OI, i.e. types I, III, and IV, accounted for 90% of all subjects. Family history of OI was present in 62% of those with OI type I while only 29% with OI type IV and 7% with OI type III had a parent with OI (p<0.001). As expected, in utero fracture rates were highest in those with OI type III and lowest in those with OI type I and differed significantly between the three subtypes (p<0.001). The frequency of antenatal diagnosis in the various subtypes ranged from 31–38% and there were no intergroup differences; this is most likely due to the fact that majority of subjects with type I OI also had a family history of the condition, thus increasing the rate of detection of this milder form which is otherwise difficult to diagnose antenatally.
The proportion of preterm deliveries with fetuses with the three subtypes of OI was not different (0.13, 0.16, 0.12 for OI types I, III, and IV, respectively) and the majority of deliveries occurred at full term gestation (37–42 weeks). The prevalence of breech presentation was highest in OI type III, followed by types IV and I (39.2%, 18.6%, and 8.6%, respectively, p<0.001). The likelihood of having a breech presentation at term in OI type III was 6.93 (95% Confidence Interval (CI) 3.61–13.3; p <0.001), and 2.39 (CI 1.23–4.64; p =0.002) as compared to types I and IV, respectively.
The median birth weights were significantly different between the OI subtypes delivered at term gestation (37–42 weeks) with those with OI type I having the highest birth weight (type I vs. III, p<0.001; type I vs. IV, p<0.001; type III vs. IV, p=0.004) (Figure 1). However such differences were not observed in those who were delivered preterm (prior to 37 weeks). The median birth lengths were highest in those with OI type I and lowest in OI type III with statistically significant differences between the various subtypes (type I vs. III; type I vs. IV; type III vs. IV, p<0.001) (Figure 1). In those delivered preterm, significant differences were observed only between those with OI types I and III (p=0.005).
Figure 1. Birth weight and length of subjects with OI.
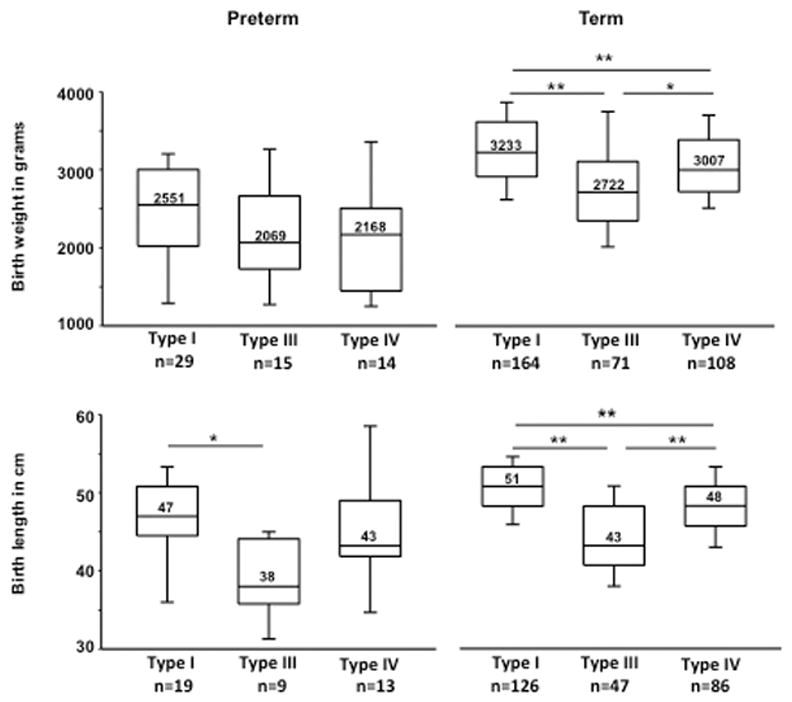
Box plots of the birth weight and length in subjects with OI depicting the median, 25th and 75th centiles. The error bars represent the 5th and 95th centiles. (Pre-term – gestational age < 37 weeks, term gestation - 37–42 weeks; *p<0.01; **p<0.001, Mann-Whitney U Test).
Frequency of at-birth fractures by OI subtype
As expected, individuals with type III OI had the highest rate of at-birth fractures (92.6%) followed by those with type IV (50.7%) and type I (17.2%) (Table 2). The odds ratio for the presence of at-birth fracture in OI type III was 60.5 as compared to OI type I (CI 25.9–140.9; p <0.001) and 12.2 as compared to OI type IV (CI 5.27 – 28.3.4; p<0.001). Subjects with OI type IV were 5 times more likely to have at-birth fractures compared to those with OI type I (OR 4.95, CI 3.06–8.04; p<0.001). These results are consistent with the well-known clinical severity of the subtypes.
Table 2.
At-birth fracture rates based on OI subtype and mode of delivery.
| Number of Patients With
| |||||
|---|---|---|---|---|---|
| OI Type | Delivery Type | Fractures | No Fractures | Proportion with fractures | p value* |
| I | Vag. Del. | 25 | 130 | 0.161 | 0.561 |
| CS | 13 | 53 | 0.197 | ||
| Total | 38 | 183 | 0.172 | ||
| III | Vag. Del. | 39 | 4 | 0.907 | 0.698 |
| CS | 49 | 3 | 0.942 | ||
| Total | 88 | 7 | 0.926 | ||
| IV | Vag. Del. | 42 | 35 | 0.545 | 0.313 |
| CS | 28 | 33 | 0.459 | ||
| Total | 70 | 68 | 0.507 | ||
| All Types | Vag. Del. | 106 | 169 | 0.385 | 0.014 |
| CS | 90 | 89 | 0.503 | ||
| Total | 196 | 258 | 0.432 | ||
denotes alpha value for comparison between vaginal delivery and CS within each OI subtype.
At-birth fracture rates based on delivery method
To determine whether CS was a safer mode of delivery for those with OI, we assessed the at-birth fracture rates within each subtype and found that they did not differ based on whether the delivery was by vaginal route or by CS (Table 2). The proportion of individuals with at-birth fractures with vaginal delivery and CS were 0.16 and 0.19 for OI type I (p=0.561), 0.91 and 0.94 for OI type III (p=0.698), 0.55 and 0.46 for OI type IV (p=0.313), respectively. However, when the data were pooled together, the fracture rate was higher in those who were delivered by CS as compared to vaginal delivery (0.5 vs. 0.39, p=0.014) (Table 2). The odds ratio (OR) for fractures with CS was significantly higher than with vaginal delivery (1.6, 95% CI 1.10–2.36; p=0.014). This could either imply that CS is indeed associated with a higher risk for fracture or be secondary to an ascertainment bias wherein CS was chosen as the method of delivery in those who were diagnosed with OI antenatally. If the latter were to be the case, such increased risk would only be observed in those individuals diagnosed with OI antenatally and not in those who were diagnosed after birth. In individuals diagnosed antenatally with OI, analysis of pooled data from all subjects showed that the risk for at birth fracture was elevated with CS (OR 2.27, CI 1.20–4.30; p=0.011); however, in those diagnosed after birth no such increase was evident (OR 1.20, CI 0.72–2.01; p=0.484) (Table 3). When examining just cases with the presence of in utero fracture there was an increased delivery by CS (OR 4.53, CI 2.31–8.90; p<0.001). This suggests that CS was likely not the driver behind increased at-birth fracture rates but rather the delivery method chosen in those who had in utero fractures, and thus at-birth fracture. Further analyses with logistic modelling showed that the mode of delivery had no effect on the fracture rates at birth when accounting for the type of OI and the presence of in utero fractures.
Table 3.
Fracture rates in antenatally and postnatally diagnosed OI. The increased fracture rate with CS observed only in those with antenatal but not postnatal diagnosis of OI suggest that this is due to ascertainment bias and CS is not the driver behind the increased fractures.
| Number of Patients With:
| |||||
|---|---|---|---|---|---|
| Delivery Type | Fractures | No Fractures | Proportion with Fracture | p value* | |
| Antenatally diagnosed OI | Vag Del. | 31 | 46 | 0.403 | 0.011 |
| CS | 49 | 32 | 0.605 | ||
| Postnatally diagnosed OI | Vag Del. | 69 | 116 | 0.373 | 0.475 |
| CS | 38 | 53 | 0.418 | ||
denotes alpha value for comparison between vaginal delivery and cesarean section within each subgroup.
The presence or absence of fracture at birth as a categorical variable does not address the question of whether any fractures were sustained during the process of delivery. The limitations in the timing of the ultrasound examination and antenatal quantification of the fractures make this a difficult question to address. However, within the limitations of the dataset, we attempted to address this by analyzing the subgroup of subjects who were antenatally diagnosed with OI but were not known to have any antenatal fractures; presence of birth fractures in these individuals was presumed to be due to occur during delivery. The fracture rate analysis in this subgroup also showed no difference within each subtype or in the pooled data based on whether delivery was by vaginal route or CS (p=1.00 for type I; p=1.00 for type III; p=0.631 for type IV, p= 0.768 for pooled data) (Table 4). We further attempted to evaluate the effect of delivery method for fetuses with breech presentation separately from vertex presentation and did not identify significant differences for at-birth fracture rate (breech presentation p=0.334; vertex presentation p=0.496).
Table 4.
Subset of patients with fractures presumably sustained during delivery.
| Number of Patients With:
| |||||
|---|---|---|---|---|---|
| OI Type | Delivery Type | Presumed Fracture at birth | Total (antenatally diagnosed but no prenatal fractures) | Proportion of Patients with Fractures | p value* |
| I | Vag. Del. | 6 | 42 | 0.143 | 1.00 |
| C-section | 2 | 21 | 0.095 | ||
| III | Vag. Del. | 1 | 2 | 0.500 | 1.00 |
| C-section | 1 | 2 | 0.500 | ||
| IV | Vag. Del. | 4 | 12 | 0.333 | 0.631 |
| C-section | 1 | 7 | 0.143 | ||
| All Types | Vag. Del. | 11 | 56 | 0.196 | 0.768 |
| C-section | 4 | 30 | 0.133 | ||
denotes alpha value for comparison between vaginal delivery and CS within each OI subtype.
A unique scenario is where the mother affected with OI is pregnant with a fetus also affected with OI. In the LCRC data set, 45 such women (36 with OI type I and 9 with OI type IV) were identified. All women with type IV OI delivered by CS. Among the women with OI type I, 18 delivered by CS while 18 delivered by vaginal route. The at-birth fracture rate in the newborns was not significantly different even in this subcategory of subjects between vaginal delivery and CS (0.27 vs. 0.25). These results taken collectively show that delivery by CS is not associated with a decrease in fracture rates at birth.
Factors Influencing the Choice of Delivery Method
The decision to choose a delivery method is dependent on patient and physician preferences in addition to multiple variables specific for OI. We hence looked for factors that correlated with choosing CS as a delivery method. Antenatal diagnosis of OI correlated with an increased chance being delivered by CS (OR 2.139, CI 1.433–3.190; p<0.001). To further clarify potential correlates, we used backward-elimination logistic regression model building to examine correlations between mode of delivery and three factors that clinicians often consider while deciding the delivery method, namely, presentation at birth, maternal history of OI, and presence of in utero fractures. There was an improvement in the fit of the model when mode of delivery was correlated with all three of these variables (p<0.001). Interactions between these variables did not improve the model suggesting that presentation at birth, maternal history of OI, and presence of in utero fractures independently and individually influence the decision to perform a CS.
Factors affecting at-birth fracture rate
The severity of the type of OI was the predominant determinant of the fracture rates at birth. Using a backward-elimination strategy, we assessed the correlation between at-birth fractures and many covariates and found that in utero fracture, OI subtype and birth weight independently correlated (p<0.001) with fracture. Higher birth weight conferred a higher risk for fracture. Overall, though there was a trend towards higher fracture risk with vaginal delivery as compared to CS with increasing birth weight, these results were statistically insignificant (p=0.092). We examined interaction terms and included an interaction of antenatal diagnosis with delivery type and this did not improve the model (p=0.145).
Discussion
Antenatal suspicion for a skeletal dysplasia such as OI raises many clinically relevant issues including confirmation of diagnosis, assessing the severity of the condition and possible lethality, evaluating the risks posed to the mother, and planning for a delivery that minimizes the risk for fracture. While there have been studies that have addressed the diagnosis and assessment of severity in the antenatal period, there have been no systematic studies that provide guidance on further management of pregnancy or delivery.13–18 Using systematically collected data from a large cohort of subjects with non-lethal type I collagen-related OI, we tried to address issues often encountered in clinical practice including, the implications of OI on fetal presentation, and growth parameters, the effect of mode of delivery on at-birth fracture rates, characteristics that confer a higher risk for fractures, and factors influencing the choice of delivery method.
The presentation of the fetus during delivery correlates with the severity of OI with nearly 40% with OI type III having breech presentation. Overall, irrespective of the type of OI, the incidence of breech presentation was significantly higher than in the general population. While nearly a third of fetuses could be in the breech presentation during second trimester, most of these rotate so that the overall incidence of breech in the general population is less than 5 percent. 19–21 There is increased incidence of breech presentation in fetuses with chromosomal aneuploidy, disorders affecting neuromuscular function such as Prader-Willi syndrome, Zellweger syndrome, spinal muscular atrophy, and developmental abnormalities such as meningomyelocele.20 The reasons for increased breech presentation in OI may be due to the increased head-to-body ratio, long bone fractures, and other bony deformities that impede fetal rotation. Our results are consistent with observations made by Cubert and colleagues in a smaller cohort of subjects with OI.11 The incidence of preterm delivery in OI was not significantly elevated as compared to data from the general population and the majority were delivered at term gestation.22 The birth weight and length were smallest in OI type III followed by OI types IV and I. These data suggest growth restriction correlates with type of OI. Similar differences were not discernable in those delivered prior to 37 weeks likely due to the fact that the varying gestational ages at delivery contributed to a large dispersion around the measure of central tendency.
The in utero and at-birth fracture rates were highest in OI type III and those with this severe form of OI were ~60 times more likely to have an at-birth fracture than those with type I OI. The subtype of OI was the single most important factor that determined the fracture rates. Some have advocated that CS be preferentially performed with antenatal diagnosis of OI as this method is quicker and is supposed to provide more control over the delivery.8–10 However, such recommendations do not account for the fact that delivery by CS involves significant traction forces on the fetal limbs through a relatively small incision in the uterine wall as compared to the relatively uniform pressure exerted by the muscular birth canal during vaginal delivery. To date, there has been no evidence to show that delivery by CS leads to decrease in fracture rates at birth or increases survival in the perinatal period. Our results clearly show that delivery by CS is not associated with a decrease in the at-birth fracture rates when accounting for the type of OI. About 90% of subjects with OI type III, ~50% with OI type IV, and ~17% with OI type I have fractures at birth irrespective of the mode of delivery. Interestingly, when data were analyzed collectively without categorizing subjects by OI subtype, CS seemed to be associated with an increase in the risk for fracture. However, this was from an ascertainment bias due to CS being the ‘chosen’ method of delivery in individuals who were antenatally diagnosed with OI. The large size of the cohort allowed us to further investigate potential correlates that had an outcome on fracture rates. We found that only birth weight independently correlated with fracture and those with higher birth weight were at an elevated risk. Fetal macrosomia, in general, has been associated with increased trauma during delivery and macrosomic fetuses (weight > 4 kg) have higher rates of clavicular and shoulder fractures.23–25 There was a trend towards higher fracture risk with increasing birth weight with vaginal delivery though these results were statistically insignificant. Given the risks for shoulder dystocia with higher birth weights, CS could be potentially considered in OI in this specific scenario.
The question of which method results in fewer fractures during the process of delivery is a difficult one to answer. The practicality and limitations of sensitivity and expertise in quantifying antenatal fractures preclude an accurate differentiation between antenatal fractures and those that occur during the intrapartum period. We presumed that the at-birth fractures in the subset of subjects who were antenatally diagnosed with OI but were not known to have any antenatal fractures occurred during delivery and analyzed the fracture rate based on delivery method. This analysis also showed that fracture rates were not different between CS and vaginal delivery.
A situation that is infrequently encountered is that of a woman with OI who is pregnant with a fetus affected with OI. Women with types III and IV OI can have pelvic and long bone deformities and often CS would be required for delivery of the fetus due to cephalo-pelvic disproportion or concerns of maternal fractures during delivery 9,10,26,27. In fact there have been anecdotal reports wherein CS had to be performed to deliver even a non-viable fetus.28 In our cohort, all 9 women with OI type IV delivered by CS. However in OI type I wherein equal numbers of individuals were delivered by CS and vaginal routes, the fracture rate did not differ based on mode of delivery. Data regarding maternal fractures during delivery was not available.
The choice of delivery method was dependent on many factors including antenatal diagnosis, presence of in utero fractures, breech presentation, and maternal history of moderate form of OI. These results are discordant from the results of Cubert et al. who noted that fetal malpresentation was the main reason behind CS in their cohort.11 The discrepancy in the findings is explicable by the smaller sample size and the unavailability of pregnancy-related data in their study.
The results from our study have to be interpreted within the context of the limitations of the data. All of the data points of interest including antenatal diagnosis, mode of delivery, and the presence or absence of fractures at birth were self-reported. These data could not be confirmed by the review of medical records. Any such data set is bound to have some element of recall bias, especially in subjects who are older. The at-birth fracture rate is a categorical data and cannot answer whether there was a difference in the number of fractures between the two delivery methods. In addition, clinically relevant questions, such was whether a trial of labor in those that were delivered by CS was associated with increased fracture risk or whether the location of fracture was significantly different based on delivery type cannot be answered from the current data. We were also unable to account for complications that arise at the time of delivery, which may be associated with fracture risk, such as protracted labor or use of instrumentation in a vaginal delivery. In spite of these limitations, our analyses on the largest cohort of subjects with OI have much strength as well. The systematic collection of specific data points as defined by preset conditions and monitoring by a central committee assures uniformity and high quality data. The large sample size allows for statistical analysis with sufficient power to answer clinically relevant questions and can inform clinical decisions. The lessons learnt from this experience are being used to prospectively collect data in the natural history study conducted by the Brittle Bone Disease Consortium, a Rare Disease Clinical Research Network of the National Institutes of Health. These efforts ultimately aim to develop guidelines for management of pregnancy in OI and underscore the utility of collaborative research networks in improving the clinical care of patients with rare disorders.
In summary, our study, the largest to assess the factors that determine fracture rate at birth in OI show that delivery by CS is not associated with decreased risk for at-birth fractures. Thus, CS should be performed only for other maternal or fetal indications, but not for sole purpose of fracture prevention in OI.
Acknowledgments
This work was supported by The Brittle Bone Disease Consortium (1U54AR068069-0), a part of the National Center for Advancing Translational Sciences (NCATS) Rare Disease Clinical Research Network (RDCRN), and is funded through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Brittle Bone Disease Consortium is also supported by the Osteogenesis Imperfecta Foundation. The project was also supported by Baylor College of Medicine IDDRC Grant Number 1 U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. SB was the recipient of the Texas Department of Health Services Summer Scholarship program for 2014. MJ was funded by NIH training grant (T32GM07526). SCN is a recipient of the DDCF Clinical Scientist Development Award and this work was supported by the Doris Duke Charitable Foundation, Grant #2013095.
We thank the Members of the Brittle Bone Disease Consortium, David Eyre, Gerald Harris, Eric Orwoll, Cathleen Raggio, Laura Tosi, and Anne Tsai. We would also like to recognize the contributions of clinical research teams at the respective sites: Mary Mullins, Alyssa Tran, Susan Carter (Baylor College of Medicine and Texas Children’s Hospital); Vonda Vensel, Jill Christie, and Abigail Hata (Oregon Health & Science University); Michaela Durigova (Shriners Hospital Montreal); Lauren Davey (A.I. duPont Hospital for Children).
References
- 1.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–85. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 2.Marini JC, Blissett AR. New genes in bone development: what’s new in osteogenesis imperfecta. J Clin Endocrinol Metab. 2013;98:3095–103. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel RM, et al. A cross-sectional multicenter study of osteogenesis imperfecta in North America - results from the linked clinical research centers. Clin Genet. 2014 doi: 10.1111/cge.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–16. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aerts M, Van Holsbeke C, de Ravel T, Devlieger R. Prenatal diagnosis of type II osteogenesis imperfecta, describing a new mutation in the COL1A1 gene. Prenat Diagn. 2006;26:394. doi: 10.1002/pd.1428. [DOI] [PubMed] [Google Scholar]
- 6.Byers PH, Krakow D, Nunes ME, Pepin M. Genetic evaluation of suspected osteogenesis imperfecta (OI) Genet Med. 2006;8:383–8. doi: 10.1097/01.gim.0000223557.54670.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner RD, Adsit J, Basel D. COL1A1/2-Related Osteogenesis Imperfecta. In: Pagon RA, et al., editors. GeneReviews(R) University of Washington, Seattle University of Washington, Seattle; Seattle (WA): 1993. All rights reserved. [Google Scholar]
- 8.Marini JC. Osteogenesis imperfecta: comprehensive management. Adv Pediatr. 1988;35:391–426. [PubMed] [Google Scholar]
- 9.Roberts JM, Solomons CC. Management of pregnancy in osteogenesis imperfecta: new perspectives. Obstet Gynecol. 1975;45:168–70. [PubMed] [Google Scholar]
- 10.Sharma A, George L, Erskin K. Osteogenesis imperfecta in pregnancy: two case reports and review of literature. Obstet Gynecol Surv. 2001;56:563–6. doi: 10.1097/00006254-200109000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Cubert R, Cheng EY, Mack S, Pepin MG, Byers PH. Osteogenesis imperfecta: mode of delivery and neonatal outcome. Obstet Gynecol. 2001;97:66–9. doi: 10.1016/s0029-7844(00)01100-5. [DOI] [PubMed] [Google Scholar]
- 12.Lindahl K, et al. Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krakow D, Lachman RS, Rimoin DL. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genet Med. 2009;11:127–33. doi: 10.1097/GIM.0b013e3181971ccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkova E, et al. Fetal skeletal dysplasias in a tertiary care center: radiology, pathology, and molecular analysis of 112 cases. Clin Genet. 2014 doi: 10.1111/cge.12434. [DOI] [PubMed] [Google Scholar]
- 15.Krakow D, et al. Evaluation of prenatal-onset osteochondrodysplasias by ultrasonography: a retrospective and prospective analysis. Am J Med Genet A. 2008;146A:1917–24. doi: 10.1002/ajmg.a.32269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepin M, Atkinson M, Starman BJ, Byers PH. Strategies and outcomes of prenatal diagnosis for osteogenesis imperfecta: a review of biochemical and molecular studies completed in 129 pregnancies. Prenat Diagn. 1997;17:559–70. [PubMed] [Google Scholar]
- 17.Schramm T, et al. Prenatal sonographic diagnosis of skeletal dysplasias. Ultrasound Obstet Gynecol. 2009;34:160–70. doi: 10.1002/uog.6359. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, et al. Diagnosis of Fetal Osteogenesis imperfecta by Multidisciplinary Assessment: A Retrospective Study of 10 Cases. Fetal Pediatr Pathol. 2015;34:57–64. doi: 10.3109/15513815.2014.962198. [DOI] [PubMed] [Google Scholar]
- 19.Berendes HW, Weiss W, Deutschberger J, Jackson E. Factors Associated with Breech Delivery. Am J Public Health Nations Health. 1965;55:708–19. doi: 10.2105/ajph.55.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun FH, Jones KL, Smith DW. Breech presentation as an indicator of fetal abnormality. J Pediatr. 1975;86:419–21. doi: 10.1016/s0022-3476(75)80977-2. [DOI] [PubMed] [Google Scholar]
- 21.Vartan CK. The behaviour of the foetus in utero with special reference to the incidence of breech presentation at term. J Obstet Gynaecol Br Emp. 1945;52:417–34. doi: 10.1111/j.1471-0528.1945.tb07745.x. [DOI] [PubMed] [Google Scholar]
- 22.Beck S, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd ME, Usher RH, McLean FH. Fetal macrosomia: prediction, risks, proposed management. Obstet Gynecol. 1983;61:715–22. [PubMed] [Google Scholar]
- 24.Lam MH, Wong GY, Lao TT. Reappraisal of neonatal clavicular fracture: relationship between infant size and neonatal morbidity. Obstet Gynecol. 2002;100:115–9. doi: 10.1016/s0029-7844(02)02055-0. [DOI] [PubMed] [Google Scholar]
- 25.Wollschlaeger K, Nieder J, Koppe I, Hartlein K. A study of fetal macrosomia. Arch Gynecol Obstet. 1999;263:51–5. doi: 10.1007/s004040050262. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy U, Vausse S, Donnai P. Management of pregnancy complicated by maternal osteogenesis imperfecta. Report of a case with uterine rupture. J Obstet Gynaecol. 2002;22:316. doi: 10.1080/01443610220130689. [DOI] [PubMed] [Google Scholar]
- 27.Vogel TM, Ratner EF, Thomas RC, Jr, Chitkara U. Pregnancy complicated by severe osteogenesis imperfecta: a report of two cases. Anesth Analg. 2002;94:1315–7. doi: 10.1097/00000539-200205000-00049. table of contents. [DOI] [PubMed] [Google Scholar]
- 28.Feng ZY, et al. A type IV osteogenesis imperfecta family and pregnancy: a case report and literature review. Chin Med J (Engl) 2012;125:1358–60. [PubMed] [Google Scholar]


