Abstract
OBJECTIVE
To evaluate the efficacy of surgery for patients with isolated breast cancer liver metastases (BCLM).
BACKGROUND
Single-arm retrospective studies have demonstrated promising results associated with surgery for isolated BCLM, but this treatment remains controversial and its role is not well-defined.
METHODS
A review of 2150 patients with BCLM who underwent treatment in a single institution was performed, and 167 (8%) patients with isolated BCLM were identified. A case-control study was performed to compare outcomes in patients with isolated BCLM who underwent surgery and/or ablation to patients who underwent conventional medical therapy.
RESULTS
A total of 167 patients were included (surgery/ablation: 69; medical: 98) with a median follow-up for survivors of 73 months. Patients in the surgical cohort more frequently had ER positive tumors and received adjuvant chemotherapy and radiotherapy for their primary breast tumor. Hepatic tumor burden was less and the interval from breast cancer diagnosis to BCLM was significantly longer (53 vs. 30 months) in the surgical cohort. Patients undergoing surgical treatment had a median recurrence-free interval of 28.5 months (95%CI: 19-38) with 10 patients (15%) recurrence free after 5 years. There was no significant difference in overall survival(OS) between the surgical and medical cohorts (median OS: 50 vs. 45 months; 5-year OS: 38 vs. 39%).
CONCLUSION
Hepatic resection and/or ablation was not associated with a survival advantage. However, significant recurrence-free intervals can be accomplished with surgical treatment. Surgical intervention might be considered in highly selected patients with the goal of providing time off of systemic chemotherapy.
Introduction
Breast cancer is the most frequent potentially life-threatening cancer among women.1,2 In the United States, it is estimated that the incidence of invasive breast cancer is over 230,000 and more than 40,000 patients will die from it in 2014.3 It has been estimated that in about 5-10% of patients with metastatic breast cancer, liver metastases are the only sign of disseminated disease.4-6
The standard of care for patients with metastatic breast cancer is palliative therapy consisting of the sequential application of hormonal agents and/or cytotoxic chemotherapy agents (with or without anti-HER2 agents).1,7 It is well established that tumor resistance to palliative therapy is inevitable and the results of systemic therapy for metastatic breast cancer have been disappointing with a 5-year relative survival of approximately 24%, depending on the molecular subtypes (i.e., hormone sensitive, HER2 status, or triple negative).8-12
Despite the limitations of systemic therapies, many physicians have been reluctant to refer patients with BCLM for surgical evaluation since there is no convincing evidence of a survival advantage. Although promising results reporting 5-year survival of up to 61% after hepatic resection or ablation have been published in recent case series these studies are limited by their retrospective nature, small cohorts and lack of controls.4,13-18
The aim of this study was to analyze long-term results of patients with isolated BCLM treated with liver resection and/or ablative techniques as compared to a control cohort of medically treated patients. We also sought to define and identify factors associated with survival in order to potentially optimize selection of patients for surgical therapy.
Methods
A retrospective review of consecutive patients with isolated BCLM from January 1991 to January 2014 treated at Memorial Sloan Kettering Cancer Center (MSKCC) was performed from the prospective MSKCC Breast Medicine service and Hepatopancreatobiliary surgery service databases. Patients were identified from these databases using a designated query in combination with ICD-9 code for liver metastases (197.7). Patient demographics, clinicopathologic variables and survival data were obtained from these databases, verified and supplemented with additional chart review when necessary. All pathological specimens were reviewed and confirmed by MSKCC pathologists. Staging is based on the American Joint Committee on Cancer 7th edition.1 The hormonal [estrogen receptor (ER) progesterone receptor (PR)] and HER2 status were based on the primary breast cancer. Cumulative size of BCLM was defined as the largest diameter of each liver metastases added together. Synchronous presentation of BCLM was defined as a time interval between the diagnosis of the primary breast cancer and development of BCLM of 6 months or less. Patients with isolated BCLM treated with liver resection and/or ablation (surgical cohort) and those receiving medical therapy alone (medical cohort) were analyzed and compared. Approval for the study was obtained from the MSKCC institutional review board.
Preoperative Assessment and Surgery
Prior to treatment, the diagnosis of BCLM was confirmed by percutaneous liver biopsy and/or radiological criteria combined with clinical history. Surgical cases were discussed on a case-by- case basis at a multidisciplinary meeting where consensus concerning resectability and appropriateness of liver resection were determined. All liver resections were performed using a standard technique as reported previously.19 Major resections were defined as those consisting of 3 or more liver segments.20 Some patients underwent a combination of surgery and intraoperative ablation at the same setting. Percutaneous ablation techniques, such as radiofrequency and microwave ablation, were performed as previously reported.21,22
Postoperative morbidity and mortality were defined as complications or deaths within 90 days of surgery. For the patients who underwent resection, morbidity events were recorded prospectively into a departmental database and graded in severity with a score of 1 to 5, consistent with the “Common Terminology Criteria for Adverse Events Version 4.0”.23 Morbidity events for patients who underwent ablation only were retrospectively reviewed from charts and electronic medical records.
Statistical Analysis
Continuous variables were compared using the Student's t-test or Mann-Whitney test, as appropriate by the type of distribution. Categorical variables were compared using χ2 or the Fisher exact test depending on the number of observations. A p-value ≤0.05 was considered significant. Overall survival (OS) and recurrence-free interval (RFI) were calculated from the date of diagnosis of BCLM to the time of death and recurrence (only for surgical cohort), respectively. Chemotherapy-free interval was calculated from the date of BCLM resection or ablation to the time that the first chemotherapy was administered. Patients who did not experience the event of interest by the end of the study were censored at the time of last follow- up. Survival outcomes were estimated using the Kaplan-Meier method and were compared using a stratified log-rank test. A Cox regression model was used to adjust for confounders. Parameters with p<0.1 on univariate analysis and clinically relevant were included in the regression model.
A propensity score analysis was utilized to control for selection bias, which resulted in uneven distribution of covariates among the surgical and medical cohorts.24 The matching algorithm was based on logistic regression and included the following covariates: ER status, adjuvant chemotherapy after breast surgery, adjuvant radiotherapy after breast surgery, trastuzumab (Herceptin, Roche) treatment, number of BCLM, time from breast cancer diagnosis to BCLM, and type of breast surgery. The impact of surgery/ablation on OS was assessed with Cox regression model adjusting for the propensity score as a linear variable. In addition, 3 subcohorts were generated with high (0.71-1), intermediate (0.4-0.7), and low (0-0.39) propensity scores. Differences in survival were further analyzed between the matched sub-cohorts to assess the impact of surgery/ablation on OS independent of confounders. All statistical analyses were performed using statistical software (Statistical Package for the Social Sciences, Inc., Chicago, IL, SPSS software version 21).
Results
Clinicopathologic characteristics of the study population
There were 2150 consecutive patients with BCLM who received treatment at MSKCC between January 1991 and January 2014, and the prevalence of isolated BCLM was 8% (n=167). These 167 patients constituted the study cohort; 41% (n=69) underwent a partial hepatectomy and/or ablation (surgical cohort), and 59% (n=98) received medical treatment (medical cohort). The percentage of patients who underwent partial hepatectomy and/or ablation declined from 54% (25 patients) in the earlier half of the study period (1991-2002) to 36% (44 patients) in the latter half (2003-2014). For the entire study population (n=167) the median follow-up for survivors from primary breast cancer diagnosis and from BCLM was 73 months [Interquartile range (IQR):46-135] and 26 months (IQR: 12-48), respectively. Patient, primary and BCLM tumor characteristics of the entire study population are summarized in Table 1. Sixty-nine patients underwent liver resection and/or ablation (resection only, n=48; percutaneous ablation only, n= 18; combination, n=3). Their median follow-up for survivors from primary breast cancer diagnosis and from BCLM was 89 months (IQR: 55-190) and 31 months (IQR: 18-63), respectively. Of the patients who underwent resection ± ablation (n=51), major liver resections were performed in 24 (47%). A margin negative liver resection was achieved in 84% of the patients (n=43) and was not associated with overall survival.
Table 1.
Clinicopathologic characteristics and Survival in patients with isolated Breast cancer liver metastases
| Variable | All patients (n=167) | BCLM surgery (n=69) | BCLM medical (n=98) | P* |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis of primary breast tumor, years | 47 (39-57) | 47 (39-56) | 47( 40-59) | 0.2 |
| Age at diagnosis of BCLM, years | 52 (43-61 ) | 51 (43-59) | 52 (42-62) | 0.7 |
| Follow-up period1 (primary breast to last follow-up), months | 73 (46-135) | 89 (55-190) | 62 (42-100) | 0.09 |
| Follow-up period1 (From BCLM to last follow-up), months | 26 (12-48) | 31 (18-63) | 24 (10-43) | 0.1 |
| Time interval from breast cancer diagnosis to BCLM diagnosis, months | 42(16-82) | 53(27-94) | 30(8-46) | 0.001 |
| Breast Cancer | ||||
| Primary tumor, T | 0.5 | |||
| 1 | 53(43%) | 29(45%) | 24(41%) | |
| 2 | 59(48%) | 29(45%) | 30(52%) | |
| 3 | 8(7%) | 4(6%) | 4(7%) | |
| 4 | 2(2%) | 2(3%) | 0 | |
| Nodal status -negative | 66(46%) | 29(43%) | 37(49%) | 0.5 |
| American Joint Committee on Cancer (AJCC) staging (7th edition) | 0.07 | |||
| 1 | 24(19%) | 7(21%) | 17(19%) | |
| 2 | 38(31%) | 16(47%) | 22(25%) | |
| 3 | 28(23%) | 6(18%) | 22(25%) | |
| 4 | 33(27%) | 5(15%) | 28(31%) | |
| Tumor grade | 0.1 | |||
| 1 | 0 | 0 | 0 | |
| 2 | 32(24%) | 15(31%) | 17(20%) | |
| 3 | 104(76%) | 34(69%) | 70(80%) | |
| Histologic subtype | 0.06 | |||
| -Intraductal carcinoma | 143(93%) | 65(98%) | 84(89%) | |
| -Intralobular carcinoma | 4(2%) | 1(2%) | 3(3%) | |
| -Others e.g. mixed | 7(5%) | 0 | 7(7%) | |
| Estrogen Receptor(ER) status positive | 97(66%) | 42(78%) | 55(59%) | 0.02 |
| Progesterone Receptor(PR) status | 78(53%) | 32(59%) | 46(50%) | 0.3 |
| Human epidermal growth factor receptor 2 status positive§ | 47(38%) | 19(45%) | 28(34%) | 0.2 |
| Adjuvant chemotherapy after breast surgery# | 91(71%) | 52(81%) | 39(60%) | 0.008 |
| Adjuvant hormonal therapy after breast surgery# | 74(46%) | 31(51%) | 43(61%) | 0.4 |
| Adjuvant radiotherapy after breast surgery# | 81(50%) | 44(68%) | 37(53%) | <0.001 |
| Trastuzumab (Herceptin) treatment§ | 41(25%) | 11(16%) | 30(31%) | 0.03 |
| Type of breast surgery | <0.001 | |||
| -None | 27(16%) | 0 | 27(28%) | |
| -Breast Conservation Surgery | 65(39%) | 35(51%) | 30(31%) | |
| -Mastectomy | 73(45%) | 33(49%) | 40(41%) | |
| Breast cancer liver metastases | ||||
| Synchronous presentation | 35(21%) | 7(10%) | 28(29%) | 0.004 |
| Number of liver metastases | 2(1-4) | 1 (1-2) | 3 (1-6) | <0.001 |
| Number of liver metastases >5 | 39(24%) | 7(10%) | 32(34%) | <0.001 |
| Unilobar distribution | 95(59%) | 46(69%) | 49(52%) | 0.03 |
| Solitary | 73(44%) | 44(64%) | 29(30%) | <0.001 |
| Largest size, cm | 2.6 (1.7- 4.4) | 3(2-5) | 2.5(1.4- 4.1) | 0.04 |
| Largest tumor Size>5cm | 36(22%) | 18(26%) | 18(19%) | 0.3 |
| Cumulative size, cm | 4 (2.1-7) | 3.8(2-5.5) | 4.5(2.2-8) | 0.1 |
| Cumulative size>5cm | 69(43%) | 42(63%) | 51(54%) | 0.3 |
| Survival | ||||
| Overall survival from BCLM diagnosis , months median; 95%CI | 43(26-60) | 50(32-67) | 45(21-68) | 0.5 |
| 5 -year Overall survival from BCLM diagnosis, months %; 95%CI | 37%(27-47) | 38%(25-52) | 39%(24-53) | 0.98 |
Continuous variables are reported as median(IQR) and continuous variables as number (%); BCLM: Breast cancer liver metastasis
Only for patients who underwent breast surgery
p value is for analysis between the medical and surgical cohorts only, not for the entire study population
Follow-up calculated for survivors
The discrepancy in HER2 status and Herceptin treatment can be explained by patient refusing treatment, Herceptin not in clinical use yet or in some patients, HER2 was positive only in the liver metastasis but not in primary breast cancer.
Comparison of surgical and medical cohorts
The median time interval from breast cancer diagnosis to BCLM was significantly longer in the surgical cohort (53 vs. 30 months, p=0.001). Patients in the surgical cohort more commonly had ER positive tumors (78 vs. 59%, p=0.02) and more frequently received adjuvant chemotherapy after breast surgery (81 vs. 60%, p=0.008) and adjuvant radiotherapy after breast surgery (68 vs. 53%, p<0.001). There was no significant difference between the surgical and medical cohort in HER2 positivity (45 vs. 35% respectively, p=0.2) but fewer surgical patients received trastuzumab (16 vs. 31%, p=0.03) during the course of their treatment. All of the patients in the surgical cohort had some form of breast surgery for their primary tumor while 28% in the medical cohort did not have any breast surgery (p<0.001). With regard to the characteristics of BCLM in the 2 cohorts, there was a lower incidence of synchronous disease (10 vs. 29%, p=0.004); fewer liver metastases (1 vs. 3, p<0.001), fewer patients with more than 5 liver metastases (10 vs. 34%, p<0.001), more unilobar distribution (69 vs. 52%, p=0.03) and solitary liver disease (64 vs. 30%, p<0.001) in the surgical cohort. These results are summarized in Table 1.
Survival outcome
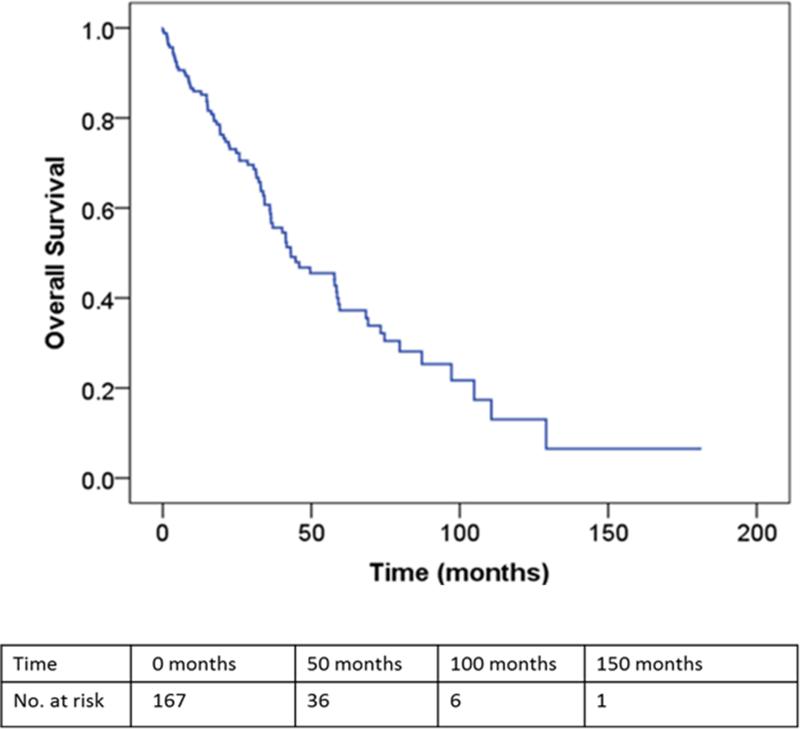
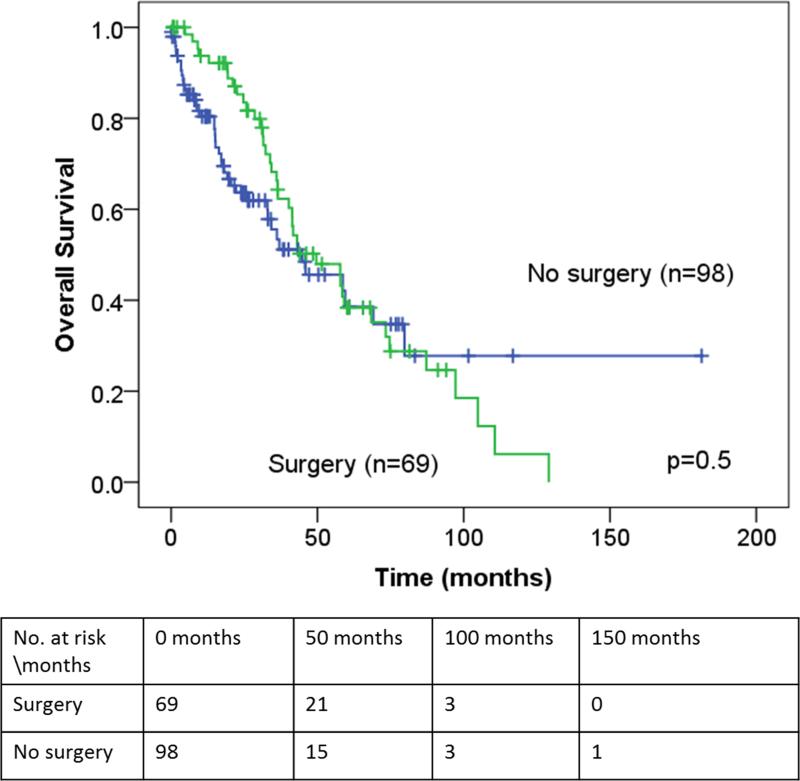
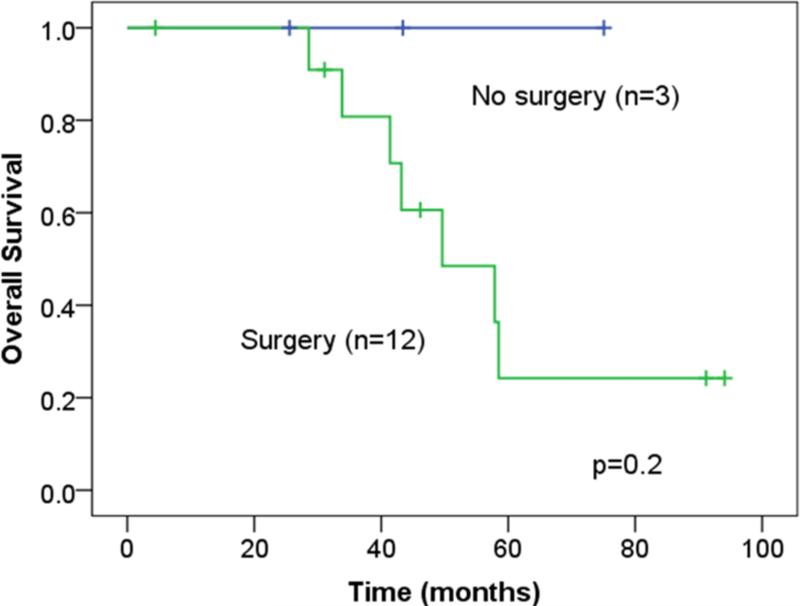
The median and predicted 5-year OS, as measured from the time of BCLM treatment, for the entire study population was 43 months (95%CI: 26-60) and 37% (95% CI: 27-47), respectively (Figure 1). In the surgical cohort, the median RFI after resection was 28.5 months (95% CI: 19-38) and the median chemotherapy-free interval was 25 months (95%CI 17-34). Disease recurrence was observed in 42 patients (63%): liver recurrence in 16 patients and extrahepatic recurrence in 26 patients. There were 10 patients (15%) recurrence free for more than 5 years (one percutaneous ablation only). Of these 10 patients, only 3 patients have recurred at a median follow-up of 6.6 years (range: 5.4-9.8). There were no significant differences in the median and predicted 5-year OS between the surgical and medical cohorts (median OS: 50 vs. 45 months, p=0.5; 5-year OS: 38 vs. 39%, p=0.98, respectively)(Table 1 and Figure 2). In multivariate analysis, absence of lymph node metastases in the primary tumor, presence of trastuzumab therapy and solitary BCLM were independently associated with an improved OS (Table 3).
Figure 1.
Overall survival curve for all patients with isolated breast cancer liver metastasis (n=167) as measured from the time of initial BCLM treatment.
Figure 2.
Overall survival for the surgical and medical cohort as measured from the time of initial BCLM treatment.
Table 3.
Predictors of Overall Survival in all patients with isolated Breast cancer liver metastases (n=167)
| Variable | Category | No. of patients | Median survival (95%CI) | p | Multivariate analysis OR (95%CI); p-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at BCLM, years | < 50 ≥ 50 |
68 99 |
46(36-56) 41(20-62) |
0.5 | NA |
| Breast Cancer | |||||
| Primary tumor, T | T1 T2 T3 T4 |
53(43%) 59(48%) 8(7%) 2(2%) |
58(38-78) 43(31-55) 31(12-51) 25(24-NR) |
0.2 | NA |
| Nodal | Negative Positive |
66(46%) 78(54%) |
46(28-64) 40(31-49) |
0.02 | 2 (1.1-3.3); 0.01 |
| Tumor grade/ differentiation | Grade 1 Grade 2 Grade 3 |
0 32(24%) 104(76%) |
0 58(38-77) 45(27-62) |
0.7 | NA |
| American Joint Committee on Cancer (AJCC) staging (7th edition) | 1 2 3 4 |
24(19%) 38(31%) 28(23%) 33(27%) |
34(30-38 50(28-71) 26(10-41) NR |
0.07 | NA |
| Estrogen Receptor(ER) status | Negative Positive |
50(34%) 97(66%) |
58(32-83) 50(33-66) |
0.8 | NA |
| Progesterone Receptor(PR) status | Negative Positive |
69(47%) 78(53%) |
43(16-71) 58(31-86) |
0.1 | NA |
| Human epidermal growth factor receptor 2 (HER2) status | Negative Positive |
78(62%) 47(38%) |
40(32-49) 60(47-72) |
0.04 | NA |
| Adjuvant chemotherapy after breast surgery | Without With |
38(29%) 91(71%) |
33(16-50) 43(28-59) |
0.2 | NA |
| Adjuvant hormonal therapy after breast surgery | Without With |
59(44%) 74(56%) |
40(31-49) 46(26-66) |
0.7 | NA |
| Adjuvant radiotherapy after breast surgery | Without With |
55(40%) 81(60%) |
46(25-67) 42(27-56) |
0.6 | NA |
| Trastuzumab (Herceptin) treatment | No Yes |
126(75%) 41(25%) |
40(33-47) 69(52-86) |
0.04 | 0.5 (0.3-0.9); 0.02 |
| Liver metastases | |||||
| Number | Solitary Multiple |
73(44%) 92(56%) |
50(22-77) 41(31-52) |
0.02 | 1.2 (1.06-1.4); 0.006 |
| Site of BCLM | Unilobar Bilobar |
95(59%) 67(41%) |
58(39-77) 34(22-46) |
0.01 | 1.04 (0.6-2); 0.9 |
| Largest size | <5 cm ≥ 5cm |
128(78%) 36(22%) |
45(30-60) 36(5-69) |
0.8 | NA |
| Cumulative size | <5 cm ≥ 5cm |
93(57%) 69(43%) |
50(32-67) 36(25-47) |
0.07 | 1.3 (0.7-2.2); 0.4 |
| Presentation of BCLM | Synchronous Metachronous |
35(21%) 132(79%) |
NR 42(30-53) |
0.1 | NA |
| Time interval of breast cancer diagnosis to BCLM diagnosis | <5 years ≥ 5 years |
100(70%) 42(30%) |
42(34-49) 58(30-87) |
0.1 | NA |
Continuous variables are reported as median (IQR) and continuous variables as number; BCLM: Breast cancer liver metastasis; CI: Confidence interval; OR: Odds ratio; NR - not reached.
Within the resection group, no differences in OS or RFI were observed between the patients who were treated with ablation alone and the patients who underwent liver resection (Figure S1, S2). Seven patients who underwent BCLM resection had more than 5 lesions. The median age of these patients at the diagnosis of the primary breast cancer and BCLM was 38 (range: 26-55) and 42 years (range: 38-57), respectively. The median interval between breast surgery and BCLM surgery was 47 months (range: 18-164). From BCLM resection, the median OS was 34 months (95%CI: 29-39) and the median RFI was 19 months (95%CI: 6-31) with 4 patients experiencing recurrence at last follow-up.
Propensity score match analysis
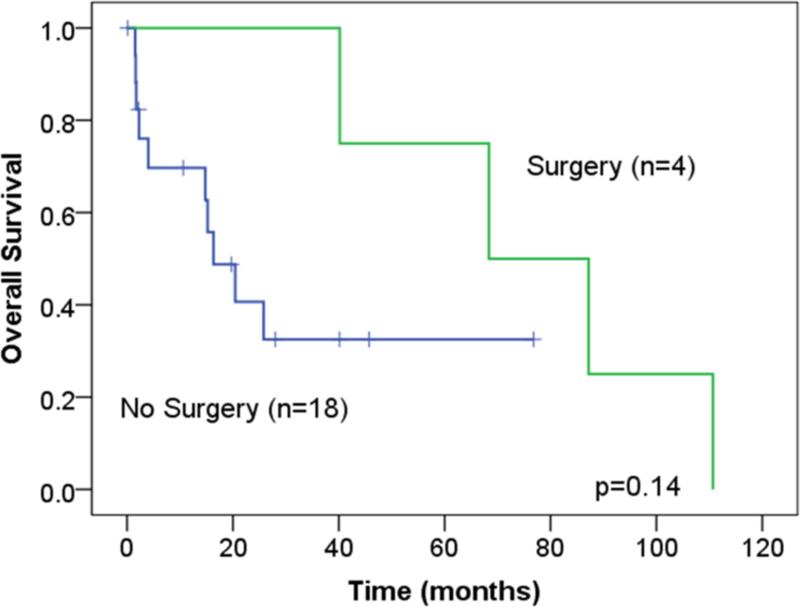
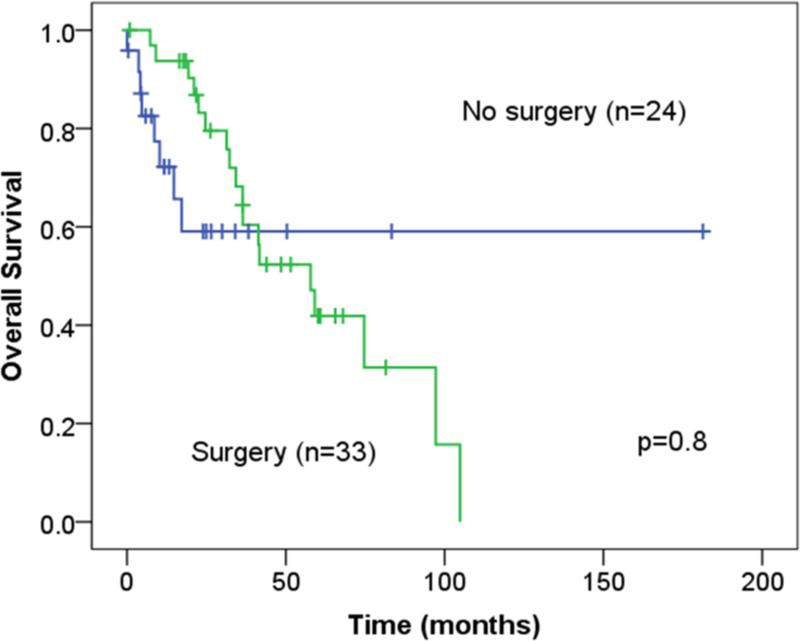
Forty-five patients in the medical cohort were matched with another 49 patients in the surgical cohort. In a Cox regression model, which accounted for the propensity score as a linear variable and the surgical intervention, no survival differences were found [(OR: 0.3, CI: 0.06-1.2, p=0.09; OR: 0.8, CI:0.4-1.5, p=0.5;respectively]. Subcohort analysis of patients with low, intermediate, and high propensity scores that were stratified by surgical intervention did not demonstrate survival advantage (Figure 3).
Figure 3.
Overall survival of the different propensity score subgroups stratified by surgical intervention.
Discussion
The first report of a liver resection for BCLM was from MSKCC and the patient survived for 20 months.25 Currently, it is not clear if surgery should have a role in the treatment of isolated BCLM as there are no level 1 or convincing natural history data to support it.1,7,15,26,27
There are several theoretical reasons to consider metastasectomy for BCLM. The presence of oligometastases could represent a state of limited metastatic spread, when potential cure may be achieved by locally directed therapy.15,28,29 Limited and resectable BCLM may be either an intermediate state in the evolution of metastatic disease, the consequence of indolent natural history due to either tumor or host factors, or the result of an excellent response to systemic treatments.28 This phenomenon may also be explained in part by a genetic basis for metastatic tropism.30,31 Furthermore, unique metastatic clones have successfully negotiated the metastatic cascade and may not respond as well to systemic treatment as the primary cancer.32 Resecting chemotherapy-resistant clones and necrotic tumor poorly accessible to drugs, may improve the efficacy of systemic therapy.29,33 Studies have also demonstrated that the hormonal/HER2 status of BCLM does not always correspond to the primary breast cancer.34-36 Conversion of hormonal/HER2 status in BCLM occurs mainly from positive to negative, and can occur up to 13% of the time for ER, 41% for PR and 10% for HER2. It must be highlighted that these hormonal/HER2 status changes may be attributed to and are also inherently confounded by a significant rate of discordant laboratory testing of ER, PR and HER2 status.34,35,37 As much as a third of BCLM are triple negative (ER-/PR-/HER2-). This phenomenon provides another rationale for surgical treatment of BCLM as there is no effective systemic therapy for triple negative breast cancer.29,34
Hepatic resection is now an established part of the treatment of colorectal and neuroendocrine liver metastasis because of an associated prolonged survival and curative potential.38,39 This fact coupled with the improved safety of liver surgery in high volume centers has resulted in an increasing number of hepatic resections and/or ablations for non-colorectal non-neuroendocrine (NCNN) liver metastases and specifically BCLM.39-47 The median survival for this heterogeneous group of NCNN liver metastases patients has been reported to range from 27 to 49 months.16-18,39-47 In a large multi-institutional study by Adam et al., 460 patients with isolated BCLM who underwent resection experienced a median survival of 45 months and a 5- and 10-year OS of 41% and 22%, respectively.42 These results were comparable to those obtained with resection of colorectal and neuroendocrine liver metastases.38,39 In a recent systematic review of liver resection for isolated BCLM consisting of 19 studies and 553 patients, the median overall survival was 40 months (range: 15–74) and the median 5-year survival rate was 40%(range: 21–80%).48 As a result, some have advocated that liver resection for selected patients with isolated BCLM should follow a similar management guideline for colorectal liver metastases.15,49-53 However, these published series and promising results are significantly limited by their retrospective nature, selection bias and the lack of a comparable cohort of patients treated with best medical therapy. Further, there are no prospective randomized data to answer if resection of isolated BCLM is beneficial.7,15,27 It is noteworthy that in contrast to colorectal and neuroendocrine liver metastasis, breast cancer metastases are not confined to the portal venous drainage system and thus, in theory, should rarely be confined to the liver without other systemic metastases. Interestingly, these previously published results are comparable to the results of our surgical cohort. Our study, however, is the first to perform a case-control comparison of patients with isolated BCLM treated with liver resection and/or ablation to conventional medical therapy.
The survival figures demonstrated by previous single-arm surgical case series may be attributed to better medical therapy rather than surgery alone. In a recent meta-analysis of 1361 patients with metastatic breast cancer, survival stratified according to four different 4-year time periods significantly improved over time54. The median and 3-year survival rate improved from 15 months and 18% to 31 months and 42%, respectively. This improvement in survival was attributed to the use of newer and more effective systemic therapies and not a more liberal use of surgical therapy. Although these figures were not as favorable as those reported in surgical series, this study included all patients with metastatic breast cancer as compared to the highly selected patients chosen for surgery. It is, however, difficult to differentiate the impact of surgical treatment from that of the improved systemic therapies for BCLM.
The patients who underwent hepatectomy for BCLM in our series were highly selected for favorable biologic characteristics from a much larger cohort of patients. Most presented as metachronous disease, had one or two liver metastases, unilobar in distribution, and a longer interval between breast cancer detection to BCLM diagnosis as compared to the medical cohort. Even with cautious and stringent selection criteria the surgical cohort had nearly identical survival characteristics to those of the medical cohort. Based on the propensity score match, surgical intervention was not associated with improved survival (p=0.5). Even for the patients in the subcohort most likely to receive surgery, survival was not associated with surgical intervention (p=0.2). These data suggest that the previous seemingly promising results of hepatic resection for isolated BCLM cannot be attributed to surgery alone and may be more likely due to the favorable tumor biology and improving systemic therapies. Notably, at our institution, the percentage of patients treated with resection/ablation has decreased over time, reflecting the evolving medical therapies and lack of associated survival benefit.
Despite the lack of improved survival, an important potential benefit of surgical treatment for oligometastatic BCLM could be the potential to provide patients with a significant period of time free of detectable disease during which they might avoid palliative systemic therapy. This is important because it can provide time off cytotoxic chemotherapy in particular. In our surgical cohort, over half the patients were free of recurrent disease and off chemotherapy for 2 years. Moreover, there were 10 patients (15%) free of disease for more than 5 years. Of these 10 patients, only 3 patients have recurred at a median follow-up of 6.6 years. This “treatment-free holiday” can potentially have significant improvements in quality of life and cost of therapy. It is important to also note in this context that surgery and ablation are now safe therapies associated with short lived and manageable morbidity.
As a single institution retrospective cohort analysis, this study has inherent limitations such as selection bias and limited sample size. These patients were treated over a very long period of over 2 decades during which many current treatments were not yet available. This is one of several unavoidable confounders for our survival results. Moreover, combining resection and ablation therapies can be interpreted as a potential weakness; however, their outcomes were not different. Ideally, the question of how to manage isolated BCLM would be answered in a randomized prospective trial. However, given the low incidence, relatively indolent course, as well as the strongly held preferences of groups that manage these patients, such a trial is unlikely to be performed. However, this is the only case-control study, which matches patients with isolated BCLM based on clinicopathologic factors and attempts to elucidate the additive effect of liver metastasectomy over systemic therapy alone.
Conclusion
Surgical therapy for BCLM is safe and in a subset of carefully selected cases may provide a substantial period of time free of recurrent disease during which systemic chemotherapy might be avoided. This does not appear to compromise overall survival. At the same time, there are no associated overall survival benefits in these selected patients when compared to patients receiving standard medical care. Surgical intervention should only be considered in highly selected patients with the goal of providing time off systemic therapy and this may be most appropriate for patients requiring cytotoxic chemotherapy.
Supplementary Material
Table 2.
Perioperative variables for patients undergoing surgerv and/or ablation
| Variable | BCLM surgery n (%) |
|---|---|
| No. of patients – Total | 69 |
| - Resection only | 48 (70) |
| - Percutaneous ablation only | 18 (26) |
| - Combination (resection and operative ablation) | 3 (4) |
|
Ablation technique (n=21) | |
| - Radiofrequency Ablation | 18 (86) |
| - Microwave Ablation | 3(14) |
|
Surgery (n=51) | |
| - Major resection | 24(47) |
| - Minor resection | 27 (53) |
| Operative time, mins, median | 181 (160-226) |
| Estimated blood loss, mls, median | 300 (150-550) |
| R0 resection | 43 (84) |
| Length of Hospital stay, days, median | 6 (5-8) |
| 90- day mortality | 0 |
| 90- day morbidity | 12 (23) |
| ≥ Grade 3 complications | 5 (10) |
| 90- day readmissions | 2 (3) |
| Morbidity- Patients undergoing resection (n=51)* | |
| Grade 1 and 2 | 7 (14) |
| Grade 3 | 5 (10) |
| Grade 4 and 5 | 0 |
| Morbidity- Patients undergoing percutaneous ablation only (n=18)* | |
| Grade 1 and 2 | 3 (17) |
| Grade 3 | 1 (6) |
| Grade 4 and 5 | 0 |
Continuous variables are reported as median (IQR) and continuous variables as number(%).
Grade 1 are mild and asymptomatic observations requiring only oral medication or bedside medical care; Grade 2 are moderate or minimal complications that requires intravenous medical therapy with resolution, antibiotics or total parenteral nutrition; Grade 3 are severe or medically significant but not immediately life-threatening complications, requiring radiologic, endoscopic, or operative intervention; Grade 4 are life-threatening consequences resulting in chronic deficit or disability. Grade 5 pertains to death related to adverse events.
Acknowledgments
Funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
No disclosures.
References
- 1.Gradishar WJ, Anderson BO, Blair SL, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12:542–90. doi: 10.6004/jnccn.2014.0058. [DOI] [PubMed] [Google Scholar]
- 2.Ruiterkamp J, Voogd AC, Bosscha K, et al. Impact of breast surgery on survival in patients with distant metastases at initial presentation: a systematic review of the literature. Breast Cancer Res Treat. 2010;120:9–16. doi: 10.1007/s10549-009-0670-0. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Elias D, Maisonnette F, Druet-Cabanac M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003;185:158–64. doi: 10.1016/s0002-9610(02)01204-7. [DOI] [PubMed] [Google Scholar]
- 5.Martinez SR, Young SE, Giuliano AE, et al. The utility of estrogen receptor, progesterone receptor, and Her-2/neu status to predict survival in patients undergoing hepatic resection for breast cancer metastases. Am J Surg. 2006;191:281–3. doi: 10.1016/j.amjsurg.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67–78. doi: 10.1023/a:1006285726561. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242–52. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012–9. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagemeister FB, Buzdar AU, Luna MA, et al. Causes of death in breast cancer: a clinicopathologic study. Cancer. 1980;46:162–7. doi: 10.1002/1097-0142(19800701)46:1<162::aid-cncr2820460127>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Atalay G, Biganzoli L, Renard F, et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39:2439–49. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]
- 11.Schneebaum S, Walker MJ, Young D, et al. The regional treatment of liver metastases from breast cancer. J Surg Oncol. 1994;55:26–31. doi: 10.1002/jso.2930550108. discussion 32. [DOI] [PubMed] [Google Scholar]
- 12.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 13.Díaz R, Santaballa A, Munárriz B, et al. Hepatic resection in breast cancer metastases: should it be considered standard treatment? Breast. 2004;13:254–8. doi: 10.1016/j.breast.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Vlastos G, Smith DL, Singletary SE, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–74. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meloni MF, Andreano A, Laeseke PF, et al. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009;253:861–9. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobs TF, Hoffmann RT, Schrader A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32:38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 18.Tasci Y, Aksoy E, Taskin HE, et al. A comparison of laparoscopic radiofrequency ablation versus systemic therapy alone in the treatment of breast cancer metastasis to the liver. HPB (Oxford) 2013;15:789–93. doi: 10.1111/hpb.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito K, Ito H, Are C, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg. 2009;13:2276–83. doi: 10.1007/s11605-009-0993-5. [DOI] [PubMed] [Google Scholar]
- 20.Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257:377–82. doi: 10.1097/SLA.0b013e31825a01f6. [DOI] [PubMed] [Google Scholar]
- 21.Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189:883–9. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 22.Sofocleous CT, Sideras P, Petre EN. “How we do it” - a practical approach to hepatic metastases ablation techniques. Tech Vasc Interv Radiol. 2013;16:219–29. doi: 10.1053/j.tvir.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Grobmyer SR, Pieracci FM, Allen PJ, et al. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–64. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 24.West SG, Cham H, Thoemmes F, et al. Propensity scores as a basis for equating groups: Basic principles and application in clinical treatment outcome research. J Consult Clin Psychol. 2014 doi: 10.1037/a0036387. [DOI] [PubMed] [Google Scholar]
- 25.BRUNSCHWIG A. Hepatic lobectomy for metastatic cancer. Cancer. 1963;16:277–82. doi: 10.1002/1097-0142(196303)16:3<277::aid-cncr2820160302>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Ehrl D, Rothaug K, Hempel D, et al. Importance of liver resection in case of hepatic breast cancer metastases. Hepatogastroenterology. 2013;60:2026–33. [PubMed] [Google Scholar]
- 27.Weinrich M, Weiß C, Schuld J, et al. Liver resections of isolated liver metastasis in breast cancer: results and possible prognostic factors. HPB Surg. 2014;2014:893829. doi: 10.1155/2014/893829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–82. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 29.Senkus E, Cardoso F, Pagani O. Time for more optimism in metastatic breast cancer? Cancer Treat Rev. 2014;40:220–8. doi: 10.1016/j.ctrv.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Minn AJ, Gupta GP, Padua D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–5. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almendro V, Kim HJ, Cheng YK, et al. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res. 2014;74:1338–48. doi: 10.1158/0008-5472.CAN-13-2357-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan SC, Parker CC. Local treatment of metastatic cancer--killing the seed or disturbing the soil? Nat Rev Clin Oncol. 2011;8:504–6. doi: 10.1038/nrclinonc.2011.88. [DOI] [PubMed] [Google Scholar]
- 34.Hoefnagel LD, Moelans CB, Meijer SL, et al. Prognostic value of estrogen receptor α and progesterone receptor conversion in distant breast cancer metastases. Cancer. 2012;118:4929–35. doi: 10.1002/cncr.27518. [DOI] [PubMed] [Google Scholar]
- 35.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo JS, Jung W, Jeong J. Metastatic breast cancer shows different immunohistochemical phenotype according to metastatic site. Tumori. 2010;96:424–32. doi: 10.1177/030089161009600308. [DOI] [PubMed] [Google Scholar]
- 37.McCullough AE, Dell'orto P, Reinholz MM, et al. Central pathology laboratory review of HER2 and ER in early breast cancer: an ALTTO trial [BIG 2-06/NCCTG N063D (Alliance)] ring study. Breast Cancer Res Treat. 2014;143:485–92. doi: 10.1007/s10549-013-2827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frankel TL, D'Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol. 2014;109:2–7. doi: 10.1002/jso.23371. [DOI] [PubMed] [Google Scholar]
- 39.Lee SY, Cheow PC, Teo JY, et al. Surgical treatment of neuroendocrine liver metastases. Int J Hepatol. 2012;2012:146590. doi: 10.1155/2012/146590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ercolani G, Grazi GL, Ravaioli M, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–66. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 41.Reddy SK, Barbas AS, Marroquin CE, et al. Resection of noncolorectal nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204:372–82. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lendoire J, Moro M, Andriani O, et al. Liver resection for non-colorectal, non neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB (Oxford) 2007;9:435–9. doi: 10.1080/13651820701769701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Rourke TR, Tekkis P, Yeung S, et al. Long-term results of liver resection for non colorectal, non-neuroendocrine metastases. Ann Surg Oncol. 2008;15:207–18. doi: 10.1245/s10434-007-9649-4. [DOI] [PubMed] [Google Scholar]
- 45.Weitz J, Blumgart LH, Fong Y, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–76. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metcalfe MS, Mullin EJ, Maddern GJ. Hepatectomy for metastatic noncolorectal gastrointestinal, breast and testicular tumours. ANZ J Surg. 2006;76:246–50. doi: 10.1111/j.1445-2197.2006.03689.x. [DOI] [PubMed] [Google Scholar]
- 47.Groeschl RT, Nachmany I, Steel JL, et al. Hepatectomy for noncolorectal non neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. 2012;214:769–77. doi: 10.1016/j.jamcollsurg.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 48.Chua TC, Saxena A, Liauw W, et al. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer. 2011;47:2282–90. doi: 10.1016/j.ejca.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. discussion 318-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–8. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 51.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 52.Hwang M, Jayakrishnan TT, Green DE, et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer. 2014;50:1747–57. doi: 10.1016/j.ejca.2014.03.277. [DOI] [PubMed] [Google Scholar]
- 53.Howlader M, Heaton N, Rela M. Resection of liver metastases from breast cancer: towards a management guideline. Int J Surg. 2011;9:285–91. doi: 10.1016/j.ijsu.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Dafni U, Grimani I, Xyrafas A, et al. Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res Treat. 2010;119:621–31. doi: 10.1007/s10549-009-0630-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.