Abstract
Different probiotic strains of Lactobacillus and Bifidobacterium genera possess significant and widely acknowledged health-promoting and immunomodulatory properties. They also provide an affordable means for prevention and treatment of various infectious, allergic and inflammatory conditions as demonstrated in numerous human and animal studies. Despite the ample evidence of protective effects of these probiotics against rotavirus (RV) infection and disease, the precise immune mechanisms of this protection remain largely undefined, because of limited mechanistic research possible in humans and investigated in the majority of animal models. Additionally, while most human clinical probiotic trials are well-standardized using the same strains, uniform dosages, regimens of the probiotic treatments and similar host age, animal studies often lack standardization, have variable experimental designs, and non-uniform and sometime limited selection of experimental variables or observational parameters. This review presents selected data on different probiotic strains of lactobacilli and bifidobacteria and summarizes the knowledge of their immunomodulatory properties and the associated protection against RV disease in diverse host species including neonates.
Keywords: probiotics, lactobacilli, bifidobacteria, rotavirus, diarrhea, immunomodulation
Introduction
Microbial colonization begins immediately after birth with facultative anaerobes, such as lactobacilli, enterococci and enterobacteria, being the first colonizers. Colonization by anaerobic microorganisms follows, including Bifidobacterium, Bacteroides and Clostridium, resulting in a gradual decrease of the ratio of facultative anaerobes to strict anaerobes over time (Arboleya et al., 2012). Bifidobacteria, along with lactobacilli, are an important part of normal intestinal microbiota of various mammalian species and are also the best characterized and widely commercialized probiotics. Both lactobacilli and bifidobacteria are non-spore-forming, gram-positive, lactic acid producing bacteria (LAPB). Lactobacilli have limited biosynthetic abilities and ferment refined sugars, generating lactic acid as the major end product (Wells, 2011), whereas Bifidobacteria are important producers of short chain fatty acids (SCFA) (Tojo et al., 2014). Despite some common properties, lactobacilli and bifidobacteria belong to two taxonomically distinct groups: the genus Lactobacillus in the phylum Firmicutes and the genus Bifidobacterium in the phylum Actinobacteria, respectively. In adults, Firmicutes and Bacteroidetes phyla usually dominate the intestinal microbiota, whereas Actinobacteria, Proteobacteria and Verrucomicrobia are considerably less abundant. However, in naturally delivered, breast-fed infants, bifidobacteria (Actinobacteria) appear between days 2 and 5 after birth and reach a maximum of up to 99% of all bacteria within one week becoming the predominant bacterial component of the infant fecal microbiota (Kurokawa et al., 2007; Mitsuoka and Kaneuchi, 1977; Turroni et al., 2012; Yatsunenko et al., 2012). Some studies report that Bifidobacterium infantis and Bifidobacterium breve were the most common species found in healthy infants (He et al., 2001).
Although not the most dominant in adulthood, Lactobacilli and Bifidobacteria remain stable elements of the normal intestinal microbiota, maintaining their important functions throughout life, and their dysbiosis is associated with a plethora of pathological conditions (Gerritsen et al., 2011). Numerous studies with different strains of Lactobacillus and Bifidobacterium have been performed in vitro and in vivo, in humans and animal models to investigate their immunomodulatory properties and probiotic potential to treat various infectious, allergic and inflammatory conditions (Grimm et al., 2014; Picard et al., 2005; Tojo et al., 2014; Wells, 2011) (Figure 1). While not always conclusive, most of them emphasized the beneficial effects of these probiotic bacteria, that appear to be pathogen/condition, bacteria and sometimes host species-specific. In most clinical trials, lactobacilli and bifidobacteria probiotics were demonstrated to be safe with the rare exception of probiotic-associated infections in immunosuppressed patients (Saarela et al., 2002). Historically, the most usual application of probiotics is to treat gastrointestinal disorders, including infectious diarrhea (de Vrese and Marteau, 2007).
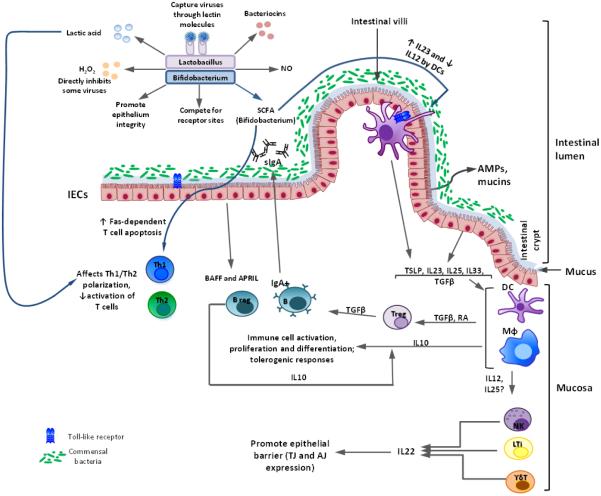
Figure 1. Interactions between probiotics of Lactobacillus and Bifidobacterium genera and the immune system modelled in vitro and in vivo (in mice and Gn pigs).
In the intestinal lumen, Lactobacillus/Bifidobacterium strains (Lacto/Bifido) inhibit some viruses directly by producing lactic acid, H2O2, bacteriocins, and other inhibitory agents; (2) Lacto/Bifido also preserve the integrity of the epithelium and compete with pathogens for Intestinal epithelial cell (IEC) receptors; (3) Lacto capture viruses by lectin-mediated binding to viral glycoproteins to prevent infection; (4) Lacto-derived nitric oxide (NO) has microbicidal and tumoricidal activities; (5) short chain fatty acids (SCFA) produced by Bifido block dendritic cell (DC) development; induce Fas-mediated T cell apoptosis; decrease IL-12 expression, but increase IL-23 production by DCs.
Intestinal epithelial cells (IECs) secrete mucins and antimicrobial peptides (AMPs) in response to the commensal microbiota/probiotics, regulating microbial replication and interaction with intestinal mucosa. IECs produce BAFF and APRIL factors, stimulating activated B (plasma) cells to produce secretory IgA (sIgA) that further limits microbial interaction with the epithelium. Under homeostatic conditions, commensal microbiota/probiotics stimulate the secretion of cytokines [including thymus stimulating lymphoprotein (TSLP), IL-33, IL-23, IL-25, and TGFβ] by IECs that promote development of antigen presenting cells [macrophages (Mφ) and DCs]. Antigen presenting cells (APCs) induce regulatory T (Treg) cell generation through TGFβ- and retinoic acid (RA)-dependent mechanisms. APC and Treg derived TGFβ and IL-10, maintain the anti-inflammatory nature of the intestine by inhibiting/reducing effector responses. Intestinal innate lymphoid cells (ILCs), including natural killer (NK) cells, lymphoid tissue inducer (LTi) cells, and γδ T cells, produce IL-22 that regulates expression of tight and adherent junction (TJ and AJ) proteins by IECs, regulating intestinal barrier function.
Acute diarrhea due to viral or bacterial infections is still a frequent cause of death, especially in hospitalized children in developing countries. Group A Rotavirus (RVA) is the leading cause of acute viral gastroenteritis in children, accounting for ~440,000 deaths annually, mostly in developing countries (Parashar et al., 2006). Current licensed human RVA vaccines have low efficacy in impoverished countries (Armah et al., 2010; Widdowson et al., 2009; Zaman et al., 2010). Similarly, RVAs are a common cause of diarrhea in young animals including nursing and weaned piglets. RVAs are responsible for 7-20% and 3-50% mortality in nursing and weaned piglets, respectively, resulting in economic losses to the pork industry (Yuan, 2006). Commercially available porcine RVA vaccines have low efficacy due to low immunogenicity, the presence of maternal antibodies in piglets, and genotypic variability of porcine RVs (Hoblet et al., 1986; Saif and Fernandez, 1996). This emphasizes the need for additional affordable host-targeted interventional strategies to alleviate the RV disease burden in children and young animals.
The initial evidence for protective effects of LAPB against RV diarrhea came from human clinical studies that in most cases do not allow evaluation of the precise biological mechanisms involved (Grandy et al., 2010). The therapeutic capacity of certain probiotic bacteria against RVA gastroenteritis has been suggested to be due to their ability to enhance and maintain mucosal integrity (Schiffrin and Blum, 2002), production of antimicrobial substances (including lactic acid, nitric oxide, H2O2, bacteriocins) (Ganzle et al., 2000) or stimulation of antimicrobial peptide and mucin production by intestinal epithelial cells (IECs), and stimulation of the local adaptive (increased production of BAFF and APRIL factors by IECs leading to an increase in secretory IgA Abs) and innate immune responses (Ganzle et al., 2000; Kaila et al., 1995) (Figure 1). Numerous cytokines produced by IECs (including IL25, IL33, TGFβ) and innate immune cells [including natural killer (IL22), antigen-presenting (APC) (IL12, IL25, IL10 TGFβ), innate lymphoid and γδ T cells (IL22)] are modulated by lactobacilli and bifidobacteria resulting in improved intestinal barrier function, reduced effector and increased regulatory immune responses (Figure 1). This review will compare the effects of lactobacilli and bifidobacteria probiotic supplementation/colonization on RVA disease and immune responses to RVAs in human clinical studies and those observed in animal experiments. We will also summarize common and distinct mechanisms observed in various studies to determine whether more guided and targeted use of these probiotics may improve the outcome in a variety of host species including livestock.
Lactobacilli and bifidobacteria probiotics and rotavirus diarrhea in human clinical studies
Probiotic efficacy in treatment of acute RV diarrhea was best exemplified for Lactobacillus rhamnosus GG (LGG), L. reuteri and some bifidobacteria in multiple randomized, double-blind, placebo-controlled trials (Table 1). In most of these trials, probiotic supplementation was combined with or preceded by oral rehydration therapy initiated within 24-48 hours after acute RV diarrhea confirmation or hospital admission (due to acute diarrhea). The statistically significant (in most cases) reduction in the duration of diarrhea was consistent among patients aged 1 month – 5 years. There were only a few trials that involved children from developing countries (China, India and Peru) with signs of clinical dehydration and generally low RV (vs other enteric pathogens) infection prevalence that did not show positive effects or only showed marginal effects of the probiotic supplementation (Mao et al., 2008; Misra et al., 2009; Salazar-Lindo et al., 2004). The authors suggested that lactose malabsorption, other underlying health conditions (unaccounted for) and dehydration might have contributed to the lack of therapeutic effects. Also, compromised nutritional status of most of the children reported in these studies could have contributed to the observed results, because malnourishment itself may aggravate RV (Uhnoo et al., 1990; Zijlstra et al., 1999) and other enteric infections via direct or indirect mechanisms (by modulating intestinal microbiota). For instance, it was recently demonstrated that severe acute malnourishment was associated with commensal microbiota immaturity (relative microbiota maturity index was lower than it should be at a certain age) that was only partially ameliorated by widely used nutritional interventions [such as ready-to-use therapeutic food (RUTF; Plumpy’Nut)] (Subramanian et al., 2014). The microbiota immaturity, often implicated in various health disorders as well unresponsiveness to vaccines, was also evident in less severe forms of malnutrition (Subramanian et al., 2015; Subramanian et al., 2014). Interestingly, another study from Peru demonstrated a prophylactic effect of 15-month long LGG supplementation against diarrhea (due bacterial or RV infections) in non-breast-fed undernourished children, especially in the toddler age group (18-29 months) (Oberhelman et al., 1999). Thus, these trials provide sufficient evidence to recommend use of at least one probiotic strain, lactobacilli or bifidobacteria, in milk, water or rehydration solution, to treat acute RV diarrhea in children under 5 years of age (Reid et al., 2003). However, additional treatments or specifically designed probiotic/symbiotic therapies may be required when supplementing undernourished children (Oberhelman et al., 1999; Salazar-Lindo et al., 2004). Finally, some studies suggested that probiotics reduce RVA diarrhea and shedding in a dose dependent manner in children (Mao et al., 2008; Shornikova et al., 1997b). After these initial observations, several potential mechanisms of probiotic-associated reduction in RV diarrhea have been discussed (Figure 1), but most of them are based on in vitro studies using different pathogens/probiotic strains and so far none have been definitively proven. The first is receptor site blockage, in which probiotic bacteria bind to receptors, thereby preventing adhesion and invasion of the virus (Bernet et al., 1994). The second suggested mechanism refers to secretory IgA and cytokine response modulation that may lead to the observed clinical effect (Christensen et al., 2002; Kaila et al., 1992). However, the fact that diarrhea appears to cease within the first 3 days after the probiotic treatment initiation emphasizes that the observed therapeutic effect is unlikely to be mediated via enhancement of adaptive immune responses. Another mechanism might involve modulation of mucin (MUC2 and MUC3 gene mRNA) expression, ultimately affecting motility defences and removal of noxious substances (Mack et al., 1999; Xu and Verstraete, 2001) and thereby alleviating diarrhea. A final theory is that some lactobacilli species (such as Lactobacillus rhamnosus GR-1 and L. fermentum RC-14) produce unidentified substances that inactivate the viral particles (Cadieux et al., 2002).
Table 1.
Human pediatric clinical trials (randomized, double-blind, placebo-controlled) demonstrating lactobacilli and bifidobacteria effects on RV diarrhea and disease.
| Probiotic bacteria species/dose | Patient age | Number of patients |
Treatment regimen | Treatment outcome | Study location |
Reference |
|---|---|---|---|---|---|---|
| Intervention studies | ||||||
|
Lactobacillus casei sp rhamnosus strain GG (1010- 1011CFU/dose) |
4-45 months (≥80% RV) |
n=71 | Orally twice a day during acute diarrhea |
Treatment significantly decreased duration of diarrhea |
Finland | (Isolauri et al., 1991) |
|
Bifidobacterium bifidum and Streptococcus
thermophilus with formula (108-35 × 108 CFU/g of formula powder) |
5-24 months | n=55 | Orally, every feeding during diarrhea |
Treatment decreased incidence of diarrhea and RV shedding |
USA | (Saavedra et al., 1994) |
| L. rhamnosus GG (1010 CFU/ml) | 5-28 months | n=42 | Twice daily for 5 days | Treatment diarrhea duration was shortened |
Finland | (Isolauri et al., 1994) |
| L. rhamnosus GG (1010-1011CFU/dose) | 13 months (mean) | n=40 | Twice daily for 2 days | Treatment decreased incidence of diarrhea and voimiting |
Pakistan | (Raza et al., 1995) |
|
L. rhamnosus GG (Lactophilus), or S.
thermophilus and L. delbruckii subsp. bulgaricus (Yalacta) |
6-35 months | n=49 | Orally twice a day for 5 days, after RV diarrhea was confirmed |
LGG improved Ab and ASC responses to RV and significantly decreased mean duration of diarrhea compared to other treatments |
Finland | (Majamaa et al., 1995) |
| L. rhamnosus GG (1010-1011CFU/dose) | 8 months (mean) | n=39 | Twice daily for 2 days | Treatment decreased duration of diarrhea |
Thailand | (Pant et al., 1996) |
| L. rhamnosus GG (3 × 109 CFU/dose) | 3-36 months (61% RV) |
n=100 | Orally twice a day during acute RV diarrhea |
Treatment decreased duration of diarrhea |
Italy | (Guarino et al., 1997) |
| L. rhamnosus GG (5 × 109 CFU/ml) | 1-36 months (27% RV) |
n=123 | Twice daily for 5 days with ORS | Treatment significantly shortened the duration of RV diarrhea and decreased frequency of stools (but not diarrhoea with confirmed bacterial etiology) |
Russia | (Shornikova et al., 1997c) |
| L. reuteri (107 or 1010/1011 CFU/dose) | 6-36 months (89% RV) |
n=97 | Orally for up to 5 days |
Treatment decreased duration of diarrhea in a dose-dependent manner |
Finland | (Shornikova et al., 1997b) |
| L. reuteri DSM 17938 (1010-1011 CFU/ml) | 6-36 months (75% RV) |
n=40 | Daily with formula for up 5 days |
Treatment decreased duration of acute diarrhea |
Finland | (Shornikova et al., 1997a) |
| L. rhamnosus GG (5 × 109 CFU/ml) | 6-36 months (92% RV) |
n=123 | Twice daily with formulafor 5 days |
Treatment decreased duration of acute diarrhea, improved weight gain, corrected acidosis |
Finland | (Rautanen et al., 1998) |
| L. acidophilus LB (1010 CFU/dose) | 3-24 months (50% RV) |
n=73 | Five doses every 12 hrs with ORS |
Treatment decreased duration of diarrhea |
Thailand | (Simakachorn et al., 2000) |
|
L. rhamnosus GG (at least 1010 CFU/250 ml), given ad libitum |
1-36 months | n=287 | Orally until diarrhea stopped |
Treatment significantly decreased mean duration of diarrhea |
Italy | (Guandalini et al., 2000) |
| L. rhamnosus GG at 6 × 109 CFU | 1-36 months | n=81 | Orally twice daily for the duration of their hospital stay |
Treatment significantly reduced the risk of rotavirus gastroenteritis (1 of 45 [2.2%] vs. 6 of 36 [16.7%] in placebo group, respectively |
Poland | (Szajewska et al., 2001) |
|
L. rhamnosus 19070-2 and L. reuteri DSM 12246 (each species 1010CFU/dose) |
6-36 months | n=71 | Orally twice a day for 5 days during acute diarrhea (≥80% RV) |
Treatment ameliorated acute and reduced the period of rotavirus excretion |
Denmark | (Rosenfeldt et al., 2002) |
| L. rhamnosus GG (109CFU/ml) | 3-36 months (with signs of mild- moderate dehydration; 24.4% RV in LGG group and 39.3% RV in placebo group) |
n=179 (all males) |
Daily with formula for diarrhea duration |
No significant differences in duration of diarrhea, rate of treatment failure, and proportion of unresolved diarrhea |
Peru | (Salazar-Lindo et al., 2004) |
|
B. lactis Bb12 and S. thermophilus TH4 (108 - 109
CFU/dose) |
3-36 months (87% RV) |
n=212 | Orally during acute RV diarrhea until 24 hrs after diarrhe subsided (~3 days?) |
Treatment only slightly decreased RV shedding in a dose dependent manner |
China | (Mao et al., 2008) |
| L. rhamnosus GG (109CFU/dose) | <36 months (moderately malnourished, 25.6% RV) |
n=229 | For 10 days | No difference in duration of diarrhea or number of stools on days 3, 6 and 10 |
India | (Misra et al., 2009) |
| L. rhamnosus GG (109CFU/dose) | 4 months - 2 years (11% RV) |
n=64 | Three times a day for 3 days |
No differences in duration or severity of diarrhea. However, the number of diarrheic stool was significantly lower on day 2 in the treatment group |
Australia, Aboroginal children |
(Ritchie et al., 2010) |
|
L. acidophilus, L. rhamnosus, B. longum and Saccharomyces boulardii (each species 9×106- 7×107CFU/dose) |
1-23 months | n=64 | Orally for 5 days with ORS, after RV diarrhea was confirmed |
Treatment decreased duration of diarrhea and incidence of voimiting |
Bolivia | (Grandy et al., 2010) |
| B. lactis (30mg/day) | 5 months - 5 years | n=75 (38 females, 37 males) |
Orally for 5 days, after RV diarrhea was confirmed |
Treatment decreased duration of diarrhea |
Turkey | (Erdogan et al., 2012) |
| L. reuteri DSM 17938 (4 × 108 CFU/ml) | 6-36 months (with clinical signs of dehydration; 62% RV) |
n=74 | Daily with formula for 7 days |
Treatment reduced the frequency, duration and recrudescence rate of acute watery diarrhea |
Italy | (Francavilla et al., 2012) |
| L. rhamnosus GG (109CFU/capsule) | 6 months - 5 years (21% RV) | n=200 | One capsule/day for 7 days |
Treatment reduced the duration of acute watery diarrhea | India | (Aggarwal et al., 2014) |
| L. rhamnosus GG (1010CFU/capsule) | 6 months - 5 years (66% RV) |
n=124 | One capsule/day in milk for 4 weeks |
Treatment reduced repeated diarrheal episodes and significantly increased RV- specific IgG levels |
India | (Sindhu et al., 2014) |
| Prophylactic studies | ||||||
| L. rhamnosus GG (3.7 × 1010 CFU/ml) | 6-24 months (undernourished) |
n=204 | Daily for 6 days/wk for 15 months |
Prophylactic effect of LGG supplementation on diarrhea incidence in non-breast-fed children |
Peru | (Oberhelman et al., 1999) |
There were limited efforts to evaluate the effects of RVA infection/diarrhea on probiotic lactobacilli and intestinal bifido- and enterobacteria in infants. These studies demonstrated that asymptomatic RVA infection did not affect colonization patterns of bifido- and enterobacteria in the gut of Indian neonates in the first month of life (Balamurugan et al., 2010), while RV diarrhea only negligibly altered the adherence properties of the evaluated probiotics (L. rhamnosus GG, L. casei Shirota, L. paracasei F19, L. acidophilus LA5, and B. lactis Bb12) or human intestinal mucus expression (Juntunen et al., 2001). The latter study also indicated that appropriate combinations of probiotics may increase their overall adhesion (possibly leading to improved immune responses), which may provide additional benefits in the treatment and prevention of RV diarrhea.
Overall, for all the reported pediatric clinical trials the exact protection mechanisms by probiotics remain unclear. Due to inability to conduct mechanistic studies in human subjects, especially in neonates, more research using animal models is critical to understand how different lactobacilli and bifidobacteria probiotic strains and various regimens of their administration modulate RV diarrhea in neonatal animals and children with variable nutritional and health status.
Lactobacilli and bifidobacteria interactions with the immune system and rotavirus in animal models
Animal models for biomedical (including probiotic) research allow for greater control of the environment, manipulations of multiple experimental variables and provide for careful monitoring of large numbers of testing parameters.
Studies in conventional or commensal microbiota transplanted animal models
So far, a few studies in conventional mouse models confirmed antagonistic effects of different lactobacilli and bifidobacteria strains against RVA diarrhea and attempted to define mechanisms of their action (Table 2). In one study, mouse pups of dams orally immunized against RVA and fed Bifidobacterium breve YIT4064 had higher levels of protection against subsequent RVA challenge than the pups born to dams immunized with RVA alone (Yasui et al., 1995). This correlated with higher levels of RV-specific Abs in the milk, feces and intestinal contents of the probiotic fed RV immunized dams. Further, in suckling rats infected with SA11 RVA and supplemented with milk fermented by L. casei DN-114 001 (known to increase small intestinal brush-border enzyme activity) (Thoreux et al., 1998), the cellular vacuolization in the small intestine was reduced, coinciding with decreased RVA load in all intestinal sections, and decreased diarrhea severity. This confirms that L. casei DN-114 001 reduced RV infection and the associated intestinal damage (Guerin-Danan et al., 2001). Another study demonstrated that B. bifidum and B. infantis supplementation mitigated rhesus RV (RRV) diarrhea and increased fecal and serum levels of RRV-specific IgA Abs in mice (Qiao et al., 2002). In agreement with previous findings in human neonates, superior results were demonstrated for L. rhamnosus GG (compared to 5 other species of lactobacilli) in reducing diarrhea severity and duration in BALB/c pups (Pant et al., 2007). Overall, these findings support major observations from human clinical trials emphasizing that RV diarrhea reduction is associated with increased local and systemic RV-specific IgA responses with effects varying for different probiotic strains. However, in these earlier (1995-2007) studies, mostly confirmatory in nature, the mechanism of the IgA increase and other related immunological modulations remained undefined. More studies on identifying novel probiotic strains (such as B. longum subsp. infantis CECT 7210) and evaluating their effects on RV diarrhea in animal preclinical experiments are underway (Munoz et al., 2011).
Table 2.
Probiotic lactobacilli and bifidobacteria effects on RVA immune responses and disease studied in animal models (rodents and pigs).
| Probiotic bacteria species/dose | Animal/Age | Treatment regimen | Treatment outcome | Reference |
|---|---|---|---|---|
| Rodent models | ||||
|
Bifidobacterium breve YIT4064
(heat-killed, 0.05% of diet) |
Neonatal suckling BALB/C mice |
Dams were treated | Treatment increased lactogenic and intestinal RV-specific Abs and passive protection of mouse pups against RV challenge |
(Yasui et al., 1995) |
|
Lactobacillus casei DN-114 001 fermented milk |
Neonatal suckling rats | Supplemented daily starting from 2 days of life |
Treatment reduced RVA infection and the associated intestinal pathology |
(Guerin-Danan et al., 2001) |
|
B. bifidum (0.75 × 108 CFU/mL) and B. infantis (0.75 × 108 CFU/mL) |
Neonatal suckling BALB/C mice |
Orally, pups received 10 μl daily, days of life 1-21, 20 μl once a week (weeks 3-5) and 40 μl once a week (weeks 6-7) ± prebiotic compounds |
Treatment reduced RVA diarrhea and increased serum and intestinal IgA Abs |
(Qiao et al., 2002) |
|
L. rhamnosus GG (ATCC 53103), L.
paracasei NCC 2461 (ST11), L. johnsonii NCC 533 (La-1), L. rhamnosus NCC 596 and Streptococcus thermophilus NCC 2496 (108/dose) |
Neonatal suckling BALB/C mice |
Orally, once daily, days of age 1-3, with or without anti-RVA immunoglobulins |
L. rhamnosus GG treatment reduced RVA diarrhea severity and duration |
(Pant et al., 2007) |
|
B. longum subsp. infantis CECT 7210 (1 × 109 CFU/dose) |
8 week-old BALB/C mice | Orally, once | Treatment reduced RVA infection | (Munoz et al., 2011) |
|
L. reuteri DSM 17938 and ATCC PTA 6475 |
Neonatal suckling CD-1 mice, overweight, underweight and normal weight |
Orally daily from days 5 to 14 of life | Treatment reduced RVA diarrhea, increased epithelial migration and increased diversity of intestinal microbiome (correlated with diarrhea reduction for both strains); also increased RVA IgA Abs, decreased pro-inflammatory cytokines, increased epithelial cell proliferation (strain-specific, did not correlate with diarrhea reduction or did not have equal effects in mice of different nutritional status) |
(Preidis et al., 2012) |
| Pig models | ||||
|
L. rhamnosus GG (10-fold incremental LGG dose increase every day from 103 to 109 CFU/dose) |
Neonatal Gn pigs colonized with human infant intestinal microbiota |
Orally, daily, 3 - 16 days of age | Treatment prevented RVA-induced shift of relative microbiota abundance from Firmicutes to Proteobacteria |
(Zhang et al., 2014) |
|
L. rhamnosus GG and B. lactis Bb12 (105 CFU/dose) |
Neonatal Gn pigs | Orally once at 3-5 days of age (colonization 28 days prior to RVA challenge) |
Treatment decreased severity of RVA infection and disease; decreased systemic, but promoted intestinal innate immune responses and immune trafficking, differentially affected TLR responses (decreased pro-inflammatory, increased B cell promoting); promoted adaptive immune (including B, effector and regulatory T cell) responses |
(Chattha et al., 2013a; Chattha et al., 2013b; Kandasamy et al., 2014; Vlasova et al., 2013) |
|
L. reuteri ATCC 23272 and L.
acidophilus NCFM (1:1 mixture, with 10-fold incremental dose increase every other day from 103 to 106 CFU/dose) |
Neonatal Gn pigs | Orally dosed at 3, 5, 7, 9, 11 days of age (first dose 2 days prior to RVA challenge, others subsequent) |
Treatment differentially affected APC frequencies in RVA infected and non-infected piglets, increased TLR expression by blood cDCs; promoted T cell responses and decreased pro-inflammatory cytokine production; did not reduce RVA diarrhea severity |
(Azevedo et al., 2012; Wen et al., 2009; Wen et al., 2011; Zhang et al., 2008a; Zhang et al., 2008c) |
|
L. acidophilus NCFM (10-fold incremental dose increase every other day from 103 to 106 CFU/dose) |
Neonatal Gn pigs | Orally dosed at 3, 5, 7, 9, 11 days of age (first dose 2 days prior to RVA vaccine 1st dose, others subsequent) |
Treatment enhanced immunogenicity of RVA vaccine | (Zhang et al., 2008b) |
|
L. rhamnosus GG (10-fold incremental dose increase every other day from 103 to 1012 CFU/dose) |
Neonatal Gn pigs | Orally dosed daily (3-19 days of age), (colonization 9 days prior to RVA challenge) |
Treatment decreased severity of RVA infection and disease; decreased intestinal epithelial damage and other effects of HRV infection |
(Liu et al., 2013; Wu et al., 2013) |
| B. lactis HN019 (109 CFU/dose) | 3 week old conventional pigs |
Orally dosed, daily until the end of experiment |
Treatment decreased severity of RVA infection and disease; promoted blood lymphocyte phagocytic and T cell proliferative responses and intestinal B cell (IgM, IgA and IgG) responses |
(Shu et al., 2001) |
In a recent study, 2 genotypically and phenotypically distinct strains of L. reuteri, DSM 17938 and ATCC PTA 6475, safe and effective in treating infantile colic (Savino et al., 2010; Savino et al., 2007), reduced RVA diarrhea duration in neonatal mouse pups and enhanced diversity of the intestinal microbiome (Preidis et al., 2012). Some observed probiotic effects were strain-specific and some were influenced by the mouse nutritional status. The antidiarrheal effects of DSM 17938, but not of ATCC PTA 6475, correlated with the rate of intestinal epithelial cell proliferation. Also, both probiotic strains increased epithelial cell migration, decreased levels of proinflammatory cytokines and increased RV-specific Abs in all but undernourished mice (Preidis et al., 2012). This study also suggested that the IgA Ab increase was not essential for probiotic disease moderation, because strain 6475 ameliorated diarrhea in underweight mice without enhancing IgA Ab production. Enhancement of IgA responses by probiotics may be facilitated by simultaneous activation of multiple signaling pathways by RV (Blutt et al., 2004) and probiotic bacteria (Iyer et al., 2008). Beneficial bacteria stimulate enterocytes, dendritic cells, or macrophages expressing innate immune receptors to produce B-cell stimulatory factors (BAFF, APRIL or TGF-β1) (He et al., 2007; Massacand et al., 2008). They may also increase the activity of the polymeric Ig receptor resulting in more efficient transport of IgA Abs from the lamina propria into the intestinal lumen (Hooper and Gordon, 2001; Nakamura et al., 2012; Norderhaug et al., 1999). Similarly, cytokine suppression did not appear to lead to diarrhea attenuation by L. reuteri 17938 in underweight mice (Preidis et al., 2012). However, epithelial cell turnover rate - one of the host defence mechanisms of expelling pathogens from the epithelium (Boshuizen et al., 2003; Cliffe et al., 2005; Mulvey et al., 2000) - appears to be generally modulated by probiotics (if evaluated) and may ultimately lead to improved protection against RV.
Experiments using humanized piglets (i.e. piglets transplanted with intestinal microbiota from human infants) revealed that RV infection shifted bacterial abundance from Firmicutes to Proteobacteria phylum, whereas LGG supplementation prevented the human RVA infection-induced changes in the microbial community (Zhang et al., 2014). These findings suggest that probiotic bacteria influence the outcome of RV infections via protecting the stability of the intestinal microbiota and the associated host metabolic profiles (Zhao et al., 2013).
Studies in the gnotobiotic (Gn) pig animal model
In humans and conventional animals, the complex microbiome, diverse diets and various underlying conditions complicate understanding of the interactions among commensals, pathogens and the immune system. Therefore, Gn animals provide the additional benefit of modelling interactions exclusively between the target organisms (single or multiple probiotic bacteria and enteric pathogens) and the host immune system without confounding factors including commensal microbiota, maternal Abs, other pathogens.
Consistent with previous observations in various species, our recent study of neonatal Gn piglets, dual colonization with Lactobacillus rhamnosus GG (LGG) and Bifidobacterium lactis Bb12 (Bb12) resulted in less severe diarrhea and reduced virus shedding titers compared to uncolonized piglets and differentially modulated mucosal and systemic innate and adaptive immunity during human RVA infection of Gn pigs (Vlasova et al., 2013) (Table 2). These probiotics exerted inhibitory effects on dendritic cell (DC) populations at the systemic level as evident by lower frequencies of activated splenic DCs (conventional and plasmacytoid) in probiotic colonized versus non-colonized vaccinated piglets post-RVA challenge. However, probiotic colonization increased frequencies of activated DCs in ileum and blood suggestive of enhanced maturation of the intestinal (mucosal) immune compartment and immune cell trafficking. We also observed a synergistic interaction between the attenuated human RVA (AttHRV) vaccine and LGG and Bb12 colonization as evident by increased frequencies of ileal TLR9+ mononuclear cells (MNCs) in intestinal tissues of probiotic colonized, RVA vaccinated piglets compared to uncolonized AttHRV vaccinated piglets pre-challenge. Further, the increased TLR9+ MNC frequencies pre-challenge coincided with a higher protective effect against virus shedding and diarrhea observed post-virulent human RVA challenge. In contrast, the LGG and Bb12 colonized, vaccinated piglets had decreased frequencies of ileal TLR2+ and TLR4+ MNCs compared to uncolonized vaccinated piglets. An earlier study of adult human subjects reported increased TLR2 and TLR4 expression in submucosal immune cells of inflamed intestinal mucosa compared to healthy mucosa (Hausmann et al., 2002) (Table 2). Thus, regulating the expression of specific TLRs by these probiotics in the small intestine might play a role in intestinal immune homeostasis and also prevent excessive inflammatory responses during viral infection.
Dual colonization of LGG and Bb12 probiotics had significant effects on human RVA vaccine induced B and T cell responses. B cell responses, including activation of intestinal B cells and RV specific IgA Ab titers were enhanced in vaccinated, probiotic colonized piglets compared to uncolonized, vaccinated piglets post-virulent human RVA challenge (Kandasamy et al., 2014). The latter effect coincided with increased TLR9 expression (see above), and according to the previous reports, TLR9 and BAFF up-regulation may be associated with increased IgA levels (Li et al., 2014), and may represent synergistic sensing of probiotics and RV by innate immune cells leading to increased IgA levels. Further, T cell responses, specifically ileal T regulatory cells, and systemic IFNγ producing T cell responses, were increased in probiotic colonized and vaccinated compared to uncolonized vaccinated piglets (Chattha et al., 2013b). Importantly, the probiotic induced immunomodulatory effects on adaptive immune responses coincided with decreased diarrhea severity and reduced fecal virus shedding.
Investigators have reported that immunomodulatory effects vary with strain (Medina et al., 2007) and composition of the probiotic bacteria (Gackowska et al., 2006). Thus, we also assessed the impact of two other lactic acid producing probiotic bacteria (LAB), Lactobacillus acidophilus (LA) and Lactobacillus reuteri (LR), on intestinal and systemic innate immune responses (Wen et al., 2009; Zhang et al., 2008a; Zhang et al., 2008b; Zhang et al., 2008c) (Table 2). Compared to uninfected negative control piglets, human RVA infection alone significantly increased monocytes/macrophages, but not the cDC population in ileum. However, LA+LR colonized human RVA infected piglets had lower frequencies of monocytes/macrophages compared to human RVA only infected piglets in ileum. Additionally, probiotic colonized piglets had lower frequency of activated macrophages post-human RVA infection. The APC populations in spleens were significantly reduced in LA+LR colonized, compared to uncolonzied piglets, post-virulent human RVA infection. Similarly, colonization of piglets with LA+LR significantly reduced TNF-α cytokine secreting cells in the ileum and spleens post-human RVA challenge (Azevedo et al., 2012) (Table 2). Thus, reduction in total, as well as activated intestinal monocyte/macrophage populations, and decreased inflammatory cytokine production in LAB colonized piglets during human RVA infection indicates that these probiotics have a protective effect on inflammatory damage during human RVA infection.
Colonization of Gn piglets with LAPB alone resulted in significant modulation of innate immunity (Table 2). LA+LR dual colonization significantly increased both monocytes/macrophages and cDC populations in ileum in comparison to uncolonized Gn piglets (Zhang et al., 2008c). Further, in the absence of human RVA infection, probiotic colonization alone increased frequencies of TLR2 and TLR9 positive cDC in blood (Wen et al., 2009). These results indicate that LA and LR alone had significant stimulatory effects on the innate immune system.
Similar to LGG and Bb12 effects on B cell responses, LA probiotic significantly enhanced the immunogenicity of AttHRV vaccine responses as indicated by higher numbers of ileal RVA specific IgA and IgG antibody secreting cells (ASCs) and increased intestinal IFNγ producing T cells compared to uncolonized piglets post RVA inoculation (Zhang et al., 2008b) (Table 2). Apart from individual effects of LA on adaptive vaccine-specific immunity, dual-colonization of LA and LR significantly modulated the types of γδ T cell (critical for early responses to infections at epithelial surfaces) responses during RVA infection of Gn piglets without vaccination (Wen et al., 2011). There were lower numbers of inflammatory type CD2+CD8− γδ T cells and higher regulatory type CD2+CD8+ γδ T cells in LA+LR probiotic colonized piglets in comparison to uncolonized piglets post-virulent human RVA infection. Additionally, higher systemic IFNγ and IL4 cytokine responses in LA+LR colonized compared to uncolonized RVA infected piglets suggest that LAPB modulated both Th1 and Th2 immunity, respectively (Wen et al., 2009). Thus, the probiotics tested had measurable beneficial effects on AttHRV vaccine protective efficacy and immunogenicity and they moderated the severity of RVA diarrhea, but only when given at least 21 days prior to human RVA challenge (Chattha et al., 2013b). However, whether these observed beneficial effects could be reproduced by these probiotics in the presence of complex microbiota remains to be determined.
Intestinal epithelial cells are the target cells for RV infection and their anatomic location facilitates interactions with probiotics and intestinal commensal bacteria. In a recent study, LGG colonization modulated human RVA effects on the levels of tight junction and adherent junction proteins (Liu et al., 2013) and down-regulated autophagy in Gn pig ileal epithelium after human RVA infection (Wu et al., 2013) (Table 2). Thus, it appears that probiotics can alleviate the RV induced pathological changes in intestinal epithelial cells and reduce diarrhea associated with the loss of mature enterocytes and subsequent malabsorption. Overall, the Gn pig represents a unique model allowing studies of the biological mechanisms of lactobacilli and bifidobacteria effects on RV and all compartments of the immune system with a high relevance to both swine and human health due to the significant immunological, digestive and anatomical similarities between the two species.
There is evidence from human pediatric trials that probiotic supplementation in the neonatal period may be affected by the breastfeeding status (Oberhelman et al., 1999). However, there are few studies on the impact of selected probiotics on responses to oral vaccines in neonates in the context of colostrum/milk (col/milk) feeding. We have recently examined how LGG+Bb12 colonization with or without col/milk (to mimic breastfed versus formula-fed infants) affects development of B cell responses to an oral AttHRV vaccine in the relevant Gn pig model (Chattha et al., 2013a).
In agreement with previous findings that breast-milk promotes growth of Bifidobacteria and Lactobacilli, supplementation of col/milk (naturally containing TGF-β and other growth factors) increased fecal probiotic shedding. This increased in probiotic shedding suggested that milk containing regulatory cytokines (such as TGFβ) and other soluble factors such as glycans can promote establishment and extend colonization by probiotics (LGG+Bb12) (Ahrne et al., 2005; Rinne et al., 2005; Yoshioka et al., 1983) (Table 2). Breast milk is a major source of TGFβ for neonates when intrinsic production is limited (Nguyen et al., 2007; Penttila, 2010) promoting intestinal immune responses, including class-switch to IgA, induction of regulatory T lymphocytes, attenuation of pro-inflammatory responses and reducing immune mediated and allergic conditions (Kalliomaki et al., 1999).
Lower counts of probiotics detected in cecum/colon of col/milk fed pigs, irrespective of vaccination, suggested a differential impact of col/milk on fecal bacterial shedding vs intestinal distribution or mucosal adherence. Maternal to bacterial components Abs in sow col/milk including peptidoglycan may prevent mucosal adhesion of probiotics resulting in lower mucosa-associated bacterial counts as observed in suckling Gn mice previously (Kramer and Cebra, 1995) (Table 2).
Combined probiotic colonization and col/milk supplementation in vaccinated pigs enhanced serum RVA-specific IgA Ab titers and intestinal IgA RVA ASC levels, which were not observed in vaccinated pigs that did not receive col/milk, suggesting complex interactions between probiotics and col/milk components (Chattha et al., 2013a). Col/milk containing human RVA Abs transiently suppressed serum IgA Ab responses after two vaccine doses irrespective of probiotic colonization, but this effect was ameliorated after three doses of the vaccine (Chattha et al., 2013a). Thus, colonization with LGG+Bb12 in breast fed vaccinated infants (with pre-existing maternal human RVA Abs) may overcome the suppressive effects of maternal Abs, at least for IgA Ab responses. Similar to our study, Isolauri et al. (1995) showed enhanced RV IgA Ab responses in LGG fed infants of unknown breastfeeding status after oral immunization with live oral RV vaccine (Isolauri et al., 1995). Thus, our results using the Gn pig model suggested that feeding LGG+Bb12 to breastfed infants may be advantageous by enhancing human IgA RVA Abs and thus preventing adverse clinical effects of human RVA gastroenteritis.
Lactobacilli and bifidobacteria prevention of RV diarrhea in livestock and the associated immune mechanisms
Increased prevalence of bacterial strains resistant to antibiotics in humans has stimulated public and federal interest in eliminating the use of antibiotics in sub-therapeutic doses for growth promotion (antibiotic-growth promoters; AGP) in livestock. An alternative approach to improve health and productivity in livestock is the use of probiotics, prebiotic substrates that serve as nutrients to certain bacteria, or their combinations (synbiotics). A variety of microbial species (bacteria of Bacillus, Escherichia, Lactobacillus, Bifidobacterium, Enterococcus, Lactococcus, Streptococcus, and Pediococcus genera, yeast and undefined mixed cultures) have been used as probiotics generally resulting in reduced mortality, enhanced immune responses, improved growth rates, feed intake and feed efficiency in poultry and livestock of different ages [reviewed in Cho et al. (2011) and Patterson et al. (2003)] (Cho, 2011; Patterson and Burkholder, 2003). While Lactobacillus and Bifidobacterium species have been used most extensively in humans; historically, various species of Bacillus, Enterococcus, and Saccharomyces yeast have been the most commonly used in livestock (Simon, 2001). Only during the past few decades, has there been an increase in research on supplementing Lactobacillus to livestock (Gusils et al., 1999; Jin et al., 2000; Pascual et al., 1999; Tellez et al., 2001) (Table 3). Further, while in some studies LAPB improved growth performance and post-weaning diarrhea (PWD) control in weanling pigs (Lessard, 1987; Shu et al., 2001), these effects were not observed in others (Walsh, 2007) (Table 3). As reviewed in Heo et al. (2013), this inconsistency in results of probiotic effects on PWD and performance in pigs may be attributed to differences in dosage and type of probiotic, management practices, diet, and age (Heo et al., 2013). One study evaluated the effects of bifidobacteria and LAPB (in place of AGPs) in newborn calves and piglets and demonstrated that these probiotics reduced mortality, improved weight gain, fecal condition and feed efficiency in both species (Abe et al., 1995). However, the effects of lactobacilli (including various strains of L. reuteri, as well as L. gasseri, L. acidophilus and L. fermentum) supplementation on infectious diarrhea occurrence, growth performance and feed conversion in neonatal and weanling piglets varied with age, feeding status (sow milk versus milk replacer) and lactobacilli strain (Chang et al., 2001; Chen et al., 2014; Huang, 2004; Liu et al., 2014; Wang et al., 2009a; Wang et al., 2013; Wang, 2011; Wang et al., 2012; Yu, 2008) (Table 3). Potential mechanisms of lactobacilli beneficial effects proposed in these studies included alleviation of oxidative stress (Wang et al., 2013; Wang et al., 2009b), protective modulation of gut microbiota (Chang et al., 2001; Huang, 2004; Liu et al., 2014) and associated metabolic profiles (Liu et al., 2014), enhancement of T-cell differentiation, ileal cytokine production (Wang et al., 2009a) and serum IgG Ab levels (Yu, 2008). Additionally, reduction in the levels of IL-1β mRNA expression in the ileum of neonatal piglets due to L. reuteri supplementation was reported (Hou, 2015; Liu et al., 2014).
Table 3.
Probiotic lactobacilli and bifidobacteria (or their derivatives) in livestock.
| Probiotic bacteria species/dose | Animal/Age | Probiotic effects | Reference |
|---|---|---|---|
|
L. acidophilus or a mixture of 12 Lactobacillus strains (2 strains of L. acidophilus, 3 strains of L. fermentum, 1 strain of L. crispatus, and 6 strains of L. brevis) |
Arbor Acres broiler chicks/1-day-old | Significantly increased the levels of amylase in the small intestine, but significantly reduced the intestinal and fecal beta-glucuronidase and fecal beta-glucosidase. |
(Jin et al., 2000) |
| Lactobacillus salivarius strain, CTC2197 | Leghorn chickens/1-day-old | Prevented Salmonella enteritidis C-114 colonization in chickens | (Pascual et al., 1999) |
|
L. acidophilus and S. faecium, given with Salmonella Enteritidis, Salmonella Typhimurium, and Salmonella Heidelberg-Specific antibodies |
Broiler chicks/3-day-old | Reduced Salmonella enteritidis intestinal colonization | (Tellez et al., 2001) |
| Lactobacillus fermentation product | Cross-bred piglets/4-5-week-old | Stimulated growth, increased feed intake and slightly increased serum concentration of IgG. | (Lessard, 1987) |
| B. lactis HN019 | Cross-bred piglets/4-5-week-old | Reduced the severity of RV and E. coli associated weanling diarrhea, improved feed conversion index and immune responses |
(Shu et al., 2001) |
| Direct fed microbials | Cross-bred piglets/4-5-week-old | No effect on growth performance and gut health | (Walsh, 2007) |
| Bifidobacterium pseudolongum or Lactobacillus acidophilus | Newborn calves and piglets | Improved body weight gain, feed conversion, reduced mortality, and decreased frequency of diarrhea | (Abe et al., 1995) |
| L. reuteri BSA131 | Landrace piglets/1-month-old | Enhanced weight gain and feed conversion; modulated intestinal microbiota (increased lactobacilli and decreased enterobacteria fecal counts) |
(Chang et al., 2001) |
| Reuteran from L. reuteri TMW1.656 and levan from L. reuteri
LTH5794 |
Crossbred gilts/4-week-old | Decreased levels of adherent ETEC K88 resulting in less outflow liquid in intestinal loops | (Chen et al., 2014) |
| Lactobacillus gasseri, L. reuteri, L. acidophilus and L. fermentum | Crossbred pigs (Duroc×Landrace×Yorkshire)/4- week-old |
Significantly improved average daily feed intake, feed conversion, average daily weight gain and improved microbial balance |
(Huang, 2004) |
| Lactobacillus fermentum I5007 | Piglets/4-day-old | Affected microbial composition (decreased numbers of Clostridium spp.), promoted intestinal development (increased villous height), and modulated immune function (reduced IL-1β in ileum of non-challenged piglets) |
(Liu et al., 2014) |
| Lactobacillus fermentum I5007 | Barrows/28-day-old | Increased CD4+ T cell frequencies, TNF-α and IFN-γ levels in ileum of E. coli K88ac challenged pigs; increased the anti- oxidative responses (increased catalase, superoxide dismutase and glutathione peroxidase levels, inhibited superoxide anion production in liver and muscle; decreased levels of malondialdehyde) |
(Wang et al., 2009a; Wang et al., 2013) |
| Bacillus subtilis M-1 and Lactobacillus reuteri X-1 | Piglets/21-day-old | Increased feed intake. average daily weight gain, but decreased immune function (serum IgG and IgM, TNF-α, IL-6 and NO levels) |
(Wang, 2011) |
| L. fermentum I5007 | Piglets/28-day-old | Alleviated weaning stress syndrome by enhancing the levels of proteins involved in energy metabolism, lipid metabolism, cell structure and mobility, protein synthesis, and immune response, thereby facilitating cellular proliferation and depressing apoptosis. |
(Wang et al., 2012) |
| Lactobacillus fermentum | Large White×Landrace barrows/28-day-old | Improved average weight gain, feed conversion and anti-OVA serum IgG levels | (Yu, 2008) |
Very few mechanistic studies addressing interactions among LAPB, immunity and RV were conducted in livestock species, and primarily in pigs. In 3-week old piglets, the administration of B. lactis HN019 led to lower concentrations of fecal RVA and reduced severity of weanling diarrhea (Shu et al., 2001) (Table 3). Indicative of immune enhancement, higher blood leukocyte phagocytic and T-lymphocyte proliferative responses, and higher intestinal RV-specific Ab (IgM, IgG and IgA) titers were detected in B. lactis HN019 fed piglets. Interestingly, another study using suckling piglets demonstrated reduced RVA shedding due to Enterococcus faecium NCIMB 10415 supplementation that was not associated with increased RV-specific Ab titers (Kreuzer et al., 2012). However, the probiotic supplementation resulted in significant differences in effector and regulatory T cell responses. These data suggest, once again that reduction in RV diarrhea/infection may be achieved via different mechanisms by different probiotic bacteria, while the increase of RVA-specific Ab levels (often found due to probiotic supplementation) is not essential for the disease attenuation.
Future research should be focused on more detailed characterization of probiotic properties of various lactobacilli and bifidobacteria, optimal regimens and doses of their supplementation, as well as immune mechanisms of the probiotic mediated protection against RV disease.
Parallels between mucosal transcriptome responses and immunomodulatory effects of lactobacilli
Molecular mechanisms of probiotic action on neonatal intestinal mucosal immunity remain largely undefined. The remarkable study by van Baarlen et al. (2011) elucidated the mucosal transcriptome responses of healthy adults to three lactobacilli strains (van Baarlen et al., 2011). Expectedly, different expression profiles were observed in response to consumption of L. acidophilus Lafti L10, L. casei CRL-431 and L. rhamnosus GG. Further, the in vivo expression profiles of distal duodenum were similar statistically to expression profiles from high-throughput pharmaceutical experiments in vitro (van Baarlen et al., 2011). The authors demonstrated that L. acidophilus Lafti L10 regulated IL-23 signaling, consistent with a role in immune tolerance, and likely to promote Th1 immune responses (important for protection against RV) as reflected by the up-regulation of expression of Th1-specific IFN-induced chemokines (such as CXCL10 and CXCL11) and IFN-responsive genes. Contrastingly, transcriptome responses to consumption of L. casei CRL-431 suggested a possible shift in the Th1/Th2 balance to a Th2 type and/or Th17 type and up-regulation of surface marker expression on antigen presenting cells. The latter mirrored an earlier study in a mouse model using L. casei CRL-431 (Galdeano and Perdigon, 2006). Finally, consumption of L. rhamnosus GG in this study was associated with induction of the cytokine-encoding genes CCL24, CCL2, and CXCL3 that are especially effective in stimulating Th1 responses (Yang et al., 1997). The observed up-regulation of several IFN-induced genes and STAT4 further emphasizes that consumption of L. rhamnosus may have promoted expression of genes that stimulate Th1 effector-cell development (Korman et al., 2008; Steinman, 2007). In two previous microarray studies (using a mouse cell line and profiling intestinal responses of humans suffering from esophagitis) the common major modulated pathways for L. rhamnosus GG were related to the regulation of the immune response, apoptosis, and cell growth and differentiation (Di Caro et al., 2005; Tao et al., 2006), suggesting that different host species exhibit at least a few similar responses to the same bacterial strain.
Recently, using a transcriptomic approach, we assessed mucosal tissue responses to LGG or LA monocolonization of neonatal Gn piglets (Kumar et al., 2014). Results suggest that transcriptomic responses vary with the strain of probiotic, duration of probiotic colonization, and region of the intestinal tract. Immediately after probiotic colonization (day 1), both LA and LGG induced higher transcriptional responses in ileum, whereas at later stages (7 days), LGG, but not LA, induced profound changes in expression of transcripts in duodenum. In agreement with previous results by van Baarlen et al. (2011), both of these probiotics seem to polarize mucosal immunity towards Th1 type (van Baarlen et al., 2011) as indicated by higher expression of chemokine (C-C motif) ligand 9 [CCL9; macrophage inflammatory protein-1 gamma (MIP-1γ)] in LA and LGG piglets and higher granzyme (serine proteases involved in apoptosis) expression in LA piglets.
Compared to LA, LGG significantly modulated genes associated with the following pathways: inflammatory response, immune cell trafficking and hematological system development in the duodenum. Pathways associated with immune modulation and carbohydrate metabolism were highly altered by LGG, whereas LA predominantly induced changes in energy and lipid metabolism-related trancriptomic responses. Further LA, but not LGG, induced prominent changes in transcription of vitamin A related genes in duodenum. Thus, LA and LGG differentially modulated major pathways in intestinal tissues. Further, both LGG and LA colonization resulted in higher expression of glucagon-like peptide 2 receptor (GLP2R) which regulates villus height and crypt depth in the small intestine (Jeppesen et al., 2001). LGG colonization also increased expression of claudin-8, a tight junction protein that regulates paracellular permeability (Ulluwishewa et al., 2011). Collectively, our intestinal tissue transcriptomic study revealed that lactobacilli have prominent impacts on the host immune and metabolic functions and that different strains have significantly varying biological effects as reported before in various in vivo and in vitro studies. These studies may illuminate the precise molecular mechanisms of probiotic action on mucosal immunity and emphasize that some mechanisms of probiotic actions may be conserved across different mammalian host species of different ages. This provides an avenue to develop optimal strategies to tailor preventive and therapeutic probiotic therapy for RV infections.
Concluding remarks
Lactobacilli and bifidobacteria provide significant health benefits to various host species, improving feed conversion and growth performance, modulating immune responses and intestinal crypt dynamics, and ultimately protecting the host from pathogens including RV. Human clinical randomized placebo controlled trials provide the strongest evidence of lactobacilli/bifidobacteria mediated protection against RV diarrhea and infection in pediatric patients, but lack mechanistic explanations for the observed protection. Animal studies confirm findings in humans and contribute substantial knowledge on the mechanisms of the probiotic mediated immune enhancement and increased protection against RV. These include modulation of effector and regulatory T cell responses resulting in Th1 or Th2 polarization of the immune response, increased activation of APCs, modulation of innate immune signaling via interactions with multiple TLRs, decrease in the levels of pro-inflammatory cytokines (Figure 1) and differential increase in enterocyte proliferation and/or migration resulting in more efficient flushing of RV from the intestinal epithelium or more rapid replacement of the epithelial layer after the necrotic RV infection. Interestingly, in different animal models as well as in human studies, the increase in systemic and mucosal IgA Ab levels was commonly observed due to lactobacilli and bifidobacteria supplementation/colonization but was not essential for reduction of RV diarrhea and infection. Recently increased interest in the use of these probiotic bacteria in livestock confirms previous results observed in humans and animal models and demonstrates that these bacteria can be universally beneficial in a variety of mammalian and avian species and provide an alternative to AGPs. Studies in animals and human subjects confirm that the probiotic action may vary with different dosages, regimens, bacterial strains, host age, health condition, and nutritional status. The presence of maternal lactogenic immune factors (in breast-fed infants or suckling animals) is of particular importance and may play a dualistic role in promoting immune maturation or interfering with probiotic actions and persistence in the gut. Finally, transcriptome research in various host species may provide additional knowledge regarding diverse effects of the probiotics and aid in designing of optimal preventative and interventional health promoting strategies.
Acknowledgemnets
We gratefully acknowledge the clinical and technical assistance of Dr. Juliette Hanson, Rich McCormick, Lindsey Good, Celina G. Vega, Marina Bok, Joshua Amimo and Kyle T. Scheuer. We also thank Dr. Gloria Solano-Aguilar (USDA) for the provided LGG-specific primers/probe. This work was supported by the National Center for Complementary and Alternative Medicine, National Institutes of Health (Grant R21 AT004716 to L.J.S.); National Institute of Allergy and Infectious Diseases, National Institutes of Health (Grant R01 A1099451 to L.J.S.); and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Abe F, Ishibashi N, Shimamura S. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. Journal of dairy science. 1995;78:2838–2846. doi: 10.3168/jds.S0022-0302(95)76914-4. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Upadhyay A, Shah D, Teotia N, Agarwal A, Jaiswal V. Lactobacillus GG for treatment of acute childhood diarrhoea: an open labelled, randomized controlled trial. The Indian journal of medical research. 2014;139:379–385. [PMC free article] [PubMed] [Google Scholar]
- Ahrne S, Lonnermark E, Wold AE, Aberg N, Hesselmar B, Saalman R, Strannegard IL, Molin G, Adlerberth I. Lactobacilli in the intestinal microbiota of Swedish infants. Microbes and infection / Institut Pasteur. 2005;7:1256–1262. doi: 10.1016/j.micinf.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Arboleya S, Solis G, Fernandez N, de los Reyes-Gavilan CG, Gueimonde M. Facultative to strict anaerobes ratio in the preterm infant microbiota: a target for intervention? Gut microbes. 2012;3:583–588. doi: 10.4161/gmic.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Coia ML, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schodel F, Ciarlet M, Neuzil KM. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- Azevedo MS, Zhang W, Wen K, Gonzalez AM, Saif LJ, Yousef AE, Yuan L. Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Beneficial microbes. 2012;3:33–42. doi: 10.3920/BM2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan R, Magne F, Balakrishnan D, Suau A, Ramani S, Kang G, Ramakrishna BS. Faecal bifidobacteria in Indian neonates & the effect of asymptomatic rotavirus infection during the first month of life. The Indian journal of medical research. 2010;132:721–727. [PMC free article] [PubMed] [Google Scholar]
- Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Crawford SE, Warfield KL, Lewis DE, Estes MK, Conner ME. The VP7 outer capsid protein of rotavirus induces polyclonal B-cell activation. Journal of virology. 2004;78:6974–6981. doi: 10.1128/JVI.78.13.6974-6981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Koopmans MP, Buller HA, Dekker J, Einerhand AW. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. Journal of virology. 2003;77:13005–13016. doi: 10.1128/JVI.77.24.13005-13016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux P, Burton J, Gardiner G, Braunstein I, Bruce AW, Kang CY, Reid G. Lactobacillus strains and vaginal ecology. Jama. 2002;287:1940–1941. doi: 10.1001/jama.287.15.1940. [DOI] [PubMed] [Google Scholar]
- Chang YH, Kim JK, Kim HJ, Kim WY, Kim YB, Park YH. Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie van Leeuwenhoek. 2001;80:193–199. doi: 10.1023/a:1012213728917. [DOI] [PubMed] [Google Scholar]
- Chattha KS, Vlasova AN, Kandasamy S, Esseili MA, Siegismund C, Rajashekara G, Saif LJ. Probiotics and colostrum/milk differentially affect neonatal humoral immune responses to oral rotavirus vaccine. Vaccine. 2013a;31:1916–1923. doi: 10.1016/j.vaccine.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattha KS, Vlasova AN, Kandasamy S, Rajashekara G, Saif LJ. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. Journal of immunology. 2013b;191:2446–2456. doi: 10.4049/jimmunol.1300678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Woodward A, Zijlstra RT, Ganzle MG. Exopolysaccharides synthesized by Lactobacillus reuteri protect against enterotoxigenic Escherichia coli in piglets. Applied and environmental microbiology. 2014;80:5752–5760. doi: 10.1128/AEM.01782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Zhao PY, Kim IH. Probiotics as a Dietary Additive for Pigs: A Review. Journal of Animal and Veterinary Advances. 2011;10:2127–2134. [Google Scholar]
- Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. Journal of immunology. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- de Vrese M, Marteau PR. Probiotics and prebiotics: effects on diarrhea. The Journal of nutrition. 2007;137:803S–811S. doi: 10.1093/jn/137.3.803S. [DOI] [PubMed] [Google Scholar]
- Di Caro S, Tao H, Grillo A, Elia C, Gasbarrini G, Sepulveda AR, Gasbarrini A. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2005;37:320–329. doi: 10.1016/j.dld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Erdogan O, Tanyeri B, Torun E, Gonullu E, Arslan H, Erenberk U, Oktem F. The comparition of the efficacy of two different probiotics in rotavirus gastroenteritis in children. Journal of tropical medicine. 2012;2012:787240. doi: 10.1155/2012/787240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, Fontana C, La Rosa MM, Cavallo L, Francavilla A. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea--a double-blind study. Alimentary pharmacology & therapeutics. 2012;36:363–369. doi: 10.1111/j.1365-2036.2012.05180.x. [DOI] [PubMed] [Google Scholar]
- Gackowska L, Michalkiewicz J, Krotkiewski M, Helmin-Basa A, Kubiszewska I, Dzierzanowska D. Combined effect of different lactic acid bacteria strains on the mode of cytokines pattern expression in human peripheral blood mononuclear cells. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 57 Suppl. 2006;9:13–21. [PubMed] [Google Scholar]
- Galdeano CM, Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clinical and vaccine immunology : CVI. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzle MG, Holtzel A, Walter J, Jung G, Hammes WP. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Applied and environmental microbiology. 2000;66:4325–4333. doi: 10.1128/aem.66.10.4325-4333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes & nutrition. 2011;6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy G, Medina M, Soria R, Teran CG, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC infectious diseases. 2010;10:253. doi: 10.1186/1471-2334-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm V, Westermann C, Riedel CU. Bifidobacteria-host interactions--an update on colonisation factors. BioMed research international. 2014;2014:960826. doi: 10.1155/2014/960826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. Journal of pediatric gastroenterology and nutrition. 2000;30:54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. Journal of pediatric gastroenterology and nutrition. 1997;25:516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Guerin-Danan C, Meslin JC, Chambard A, Charpilienne A, Relano P, Bouley C, Cohen J, Andrieux C. Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. The Journal of nutrition. 2001;131:111–117. doi: 10.1093/jn/131.1.111. [DOI] [PubMed] [Google Scholar]
- Gusils C, Gonzalez SN, Oliver G. Some probiotic properties of chicken lactobacilli. Canadian journal of microbiology. 1999;45:981–987. doi: 10.1139/w99-102. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, Scholmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- He F, Ouwehand AC, Isolauri E, Hashimoto H, Benno Y, Salminen S. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS immunology and medical microbiology. 2001;30:43–47. doi: 10.1111/j.1574-695X.2001.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. Journal of animal physiology and animal nutrition. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Hoblet KH, Saif LJ, Kohler EM, Theil KW, Bech-Nielsen S, Stitzlein GA. Efficacy of an orally administered modified-live porcine-origin rotavirus vaccine against postweaning diarrhea in pigs. American journal of veterinary research. 1986;47:1697–1703. [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Hou C, Zeng X, Yang F, Liu H, Qiao S. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. Journal of Animal Science and Biotechnology. 2015;6:14–22. doi: 10.1186/s40104-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Qiao SY, Li DF, Piao XS, Ren JP. Effects of Lactobacillus on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian-Australasian Journal of Animal Sciences. 2004;17:401–409. [Google Scholar]
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D × RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–312. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- Isolauri E, Kaila M, Mykkanen H, Ling WH, Salminen S. Oral bacteriotherapy for viral gastroenteritis. Digestive diseases and sciences. 1994;39:2595–2600. doi: 10.1007/BF02087695. [DOI] [PubMed] [Google Scholar]
- Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cellular microbiology. 2008;10:1442–1452. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- Jin LZ, Ho YW, Abdullah N, Jalaludin S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poultry science. 2000;79:886–891. doi: 10.1093/ps/79.6.886. [DOI] [PubMed] [Google Scholar]
- Juntunen M, Kirjavainen PV, Ouwehand AC, Salminen SJ, Isolauri E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clinical and diagnostic laboratory immunology. 2001;8:293–296. doi: 10.1128/CDLI.8.2.293-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari T. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Archives of disease in childhood. 1995;72:51–53. doi: 10.1136/adc.72.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatric research. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. Transforming growth factor-beta in breast milk: a potential regulator of atopic disease at an early age. The Journal of allergy and clinical immunology. 1999;104:1251–1257. doi: 10.1016/s0091-6749(99)70021-7. [DOI] [PubMed] [Google Scholar]
- Kandasamy S, Chattha KS, Vlasova AN, Rajashekara G, Saif LJ. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut microbes. 2014;5:639–651. doi: 10.4161/19490976.2014.969972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: genetics, mechanisms, and implications for autoimmunity. Current allergy and asthma reports. 2008;8:398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DR, Cebra JJ. Role of maternal antibody in the induction of virus specific and bystander IgA responses in Peyer's patches of suckling mice. International immunology. 1995;7:911–918. doi: 10.1093/intimm/7.6.911. [DOI] [PubMed] [Google Scholar]
- Kreuzer S, Machnowska P, Assmus J, Sieber M, Pieper R, Schmidt MF, Brockmann GA, Scharek-Tedin L, Johne R. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Veterinary research. 2012;43:58. doi: 10.1186/1297-9716-43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Vlasova AN, Liu Z, Chattha KS, Kandasamy S, Esseili M, Zhang X, Rajashekara G, Saif LJ. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut microbes. 2014;5:152–164. doi: 10.4161/gmic.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, Takami H, Morita H, Sharma VK, Srivastava TP, Taylor TD, Noguchi H, Mori H, Ogura Y, Ehrlich DS, Itoh K, Takagi T, Sakaki Y, Hayashi T, Hattori M. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA research : an international journal for rapid publication of reports on genes and genomes. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard M, Brisson GJ. Effect of a Lactobacillus fermentation product on growth, immune response and fecal enzyme activity in weaned pigs. Canadian Journal of Animal Science. 1987;67:509–516. [Google Scholar]
- Li W, Peng X, Liu Y, Liu H, Liu F, He L, Liu Y, Zhang F, Guo C, Chen G, Zhang L, Dong Z, Peng Y. TLR9 and BAFF: their expression in patients with IgA nephropathy. Molecular medicine reports. 2014;10:1469–1474. doi: 10.3892/mmr.2014.2359. [DOI] [PubMed] [Google Scholar]
- Liu F, Li G, Wen K, Wu S, Zhang Y, Bui T, Yang X, Kocher J, Sun J, Jortner B, Yuan L. Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. Journal of pediatric gastroenterology and nutrition. 2013;57:750–758. doi: 10.1097/MPG.0b013e3182a356e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang J, Zhang S, Yang F, Thacker PA, Zhang G, Qiao S, Ma X. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. Journal of agricultural and food chemistry. 2014;62:860–866. doi: 10.1021/jf403288r. [DOI] [PubMed] [Google Scholar]
- Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. The American journal of physiology. 1999;276:G941–950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. Journal of pediatric gastroenterology and nutrition. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- Mao M, Yu T, Xiong Y, Wang Z, Liu H, Gotteland M, Brunser O. Effect of a lactose-free milk formula supplemented with bifidobacteria and streptococci on the recovery from acute diarrhoea. Asia Pacific journal of clinical nutrition. 2008;17:30–34. [PubMed] [Google Scholar]
- Massacand JC, Kaiser P, Ernst B, Tardivel A, Burki K, Schneider P, Harris NL. Intestinal bacteria condition dendritic cells to promote IgA production. PloS one. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clinical and experimental immunology. 2007;150:531–538. doi: 10.1111/j.1365-2249.2007.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Sabui TK, Pal NK. A randomized controlled trial to evaluate the efficacy of lactobacillus GG in infantile diarrhea. The Journal of pediatrics. 2009;155:129–132. doi: 10.1016/j.jpeds.2009.01.060. [DOI] [PubMed] [Google Scholar]
- Mitsuoka T, Kaneuchi C. Ecology of the bifidobacteria. The American journal of clinical nutrition. 1977;30:1799–1810. doi: 10.1093/ajcn/30.11.1799. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz JA, Chenoll E, Casinos B, Bataller E, Ramon D, Genoves S, Montava R, Ribes JM, Buesa J, Fabrega J, Rivero M. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Applied and environmental microbiology. 2011;77:8775–8783. doi: 10.1128/AEM.05548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Terahara M, Iwamoto T, Yamada K, Asano M, Kakuta S, Iwakura Y, Totsuka M. Upregulation of Polymeric Immunoglobulin Receptor Expression by the Heat-Inactivated Potential Probiotic Bifidobacterium bifidum OLB6378 in a Mouse Intestinal Explant Model. Scandinavian journal of immunology. 2012;75:176–183. doi: 10.1111/j.1365-3083.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Saif LJ. Transfer of maternal cytokines to suckling piglets: in vivo and in vitro models with implications for immunomodulation of neonatal immunity. Veterinary immunology and immunopathology. 2007;117:236–248. doi: 10.1016/j.vetimm.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norderhaug IN, Johansen FE, Schjerven H, Brandtzaeg P. Regulation of the formation and external transport of secretory immunoglobulins. Critical reviews in immunology. 1999;19:481–508. [PubMed] [Google Scholar]
- Oberhelman RA, Gilman RH, Sheen P, Taylor DN, Black RE, Cabrera L, Lescano AG, Meza R, Madico G. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. The Journal of pediatrics. 1999;134:15–20. doi: 10.1016/s0022-3476(99)70366-5. [DOI] [PubMed] [Google Scholar]
- Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, Hart CA. Lactobacillus GG and acute diarrhoea in young children in the tropics. Journal of tropical pediatrics. 1996;42:162–165. doi: 10.1093/tropej/42.3.162. [DOI] [PubMed] [Google Scholar]
- Pant N, Marcotte H, Brussow H, Svensson L, Hammarstrom L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC microbiology. 2007;7:86. doi: 10.1186/1471-2180-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerging infectious diseases. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Hugas M, Badiola JI, Monfort JM, Garriga M. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Applied and environmental microbiology. 1999;65:4981–4986. doi: 10.1128/aem.65.11.4981-4986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poultry science. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Penttila IA. Milk-derived transforming growth factor-beta and the infant immune response. The Journal of pediatrics. 2010;156:S21–25. doi: 10.1016/j.jpeds.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents -- physiological effects and clinical benefits. Alimentary pharmacology & therapeutics. 2005;22:495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Barrish JP, Finegold MJ, Petrosino JF, Guerrant RL, Conner ME, Versalovic J. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. Journal of pediatric gastroenterology and nutrition. 2012;55:299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Duffy LC, Griffiths E, Dryja D, Leavens A, Rossman J, Rich G, Riepenhoff-Talty M, Locniskar M. Immune responses in rhesus rotavirus-challenged BALB/c mice treated with bifidobacteria and prebiotic supplements. Pediatric research. 2002;51:750–755. doi: 10.1203/00006450-200206000-00015. [DOI] [PubMed] [Google Scholar]
- Rautanen T, Isolauri E, Salo E, Vesikari T. Management of acute diarrhoea with low osmolarity oral rehydration solutions and Lactobacillus strain GG. Archives of disease in childhood. 1998;79:157–160. doi: 10.1136/adc.79.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. The Pediatric infectious disease journal. 1995;14:107–111. doi: 10.1097/00006454-199502000-00005. [DOI] [PubMed] [Google Scholar]
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clinical microbiology reviews. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne MM, Gueimonde M, Kalliomaki M, Hoppu U, Salminen SJ, Isolauri E. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS immunology and medical microbiology. 2005;43:59–65. doi: 10.1016/j.femsim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ritchie BK, Brewster DR, Tran CD, Davidson GP, McNeil Y, Butler RN. Efficacy of Lactobacillus GG in aboriginal children with acute diarrhoeal disease: a randomised clinical trial. Journal of pediatric gastroenterology and nutrition. 2010;50:619–624. doi: 10.1097/MPG.0b013e3181bbf53d. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M, Weyrehter H, Valerius NH, Paerregaard A. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. The Pediatric infectious disease journal. 2002;21:417–419. doi: 10.1097/00006454-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Saarela M, Lahteenmaki L, Crittenden R, Salminen S, Mattila-Sandholm T. Gut bacteria and health foods--the European perspective. International journal of food microbiology. 2002;78:99–117. doi: 10.1016/s0168-1605(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- Saif LJ, Fernandez FM. Group A rotavirus veterinary vaccines. The Journal of infectious diseases. 1996;174(Suppl 1):S98–106. doi: 10.1093/infdis/174.Supplement_1.S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Lindo E, Miranda-Langschwager P, Campos-Sanchez M, Chea-Woo E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: a randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048] BMC pediatrics. 2004;4:18. doi: 10.1186/1471-2431-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, Roos S, Matteuzzi D. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526–533. doi: 10.1542/peds.2010-0433. [DOI] [PubMed] [Google Scholar]
- Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics. 2007;119:e124–130. doi: 10.1542/peds.2006-1222. [DOI] [PubMed] [Google Scholar]
- Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. European journal of clinical nutrition. 2002;56(Suppl 3):S60–64. doi: 10.1038/sj.ejcn.1601489. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. Journal of pediatric gastroenterology and nutrition. 1997a;24:399–404. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Casas IA, Mykkanen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. The Pediatric infectious disease journal. 1997b;16:1103–1107. doi: 10.1097/00006454-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta paediatrica. 1997c;86:460–465. doi: 10.1111/j.1651-2227.1997.tb08913.x. [DOI] [PubMed] [Google Scholar]
- Shu Q, Qu F, Gill HS. Probiotic treatment using Bifidobacterium lactis HN019 reduces weanling diarrhea associated with rotavirus and Escherichia coli infection in a piglet model. Journal of pediatric gastroenterology and nutrition. 2001;33:171–177. doi: 10.1097/00005176-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. Journal of pediatric gastroenterology and nutrition. 2000;30:68–72. doi: 10.1097/00005176-200001000-00020. [DOI] [PubMed] [Google Scholar]
- Simon O, Jadamus A, Vahjen W. Probiotic feed additives—effectiveness and expected modes of action. Journal of Animal and Feed Sciences. 2001;10:51–67. [Google Scholar]
- Sindhu KN, Sowmyanarayanan TV, Paul A, Babji S, Ajjampur SS, Priyadarshini S, Sarkar R, Balasubramanian KA, Wanke CA, Ward HD, Kang G. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:1107–1115. doi: 10.1093/cid/ciu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature medicine. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Blanton LV, Frese SA, Charbonneau M, Mills DA, Gordon JI. Cultivating healthy growth and nutrition through the gut microbiota. Cell. 2015;161:36–48. doi: 10.1016/j.cell.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA, Jr., Ahmed T, Gordon JI. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. The Journal of pediatrics. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. American journal of physiology. Cell physiology. 2006;290:C1018–1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- Tellez G, Petrone VM, Escorcia M, Morishita TY, Cobb CW, Villasenor L, Promsopone B. Evaluation of avian-specific probiotic and Salmonella enteritidis-, Salmonella typhimurium-, and Salmonella heidelberg-specific antibodies on cecal colonization and organ invasion of Salmonella enteritidis in broilers. Journal of food protection. 2001;64:287–291. doi: 10.4315/0362-028x-64.3.287. [DOI] [PubMed] [Google Scholar]
- Thoreux K, Balas D, Bouley C, Senegas-Balas F. Diet supplemented with yoghurt or milk fermented by Lactobacillus casei DN-114 001 stimulates growth and brush-border enzyme activities in mouse small intestine. Digestion. 1998;59:349–359. doi: 10.1159/000007514. [DOI] [PubMed] [Google Scholar]
- Tojo R, Suarez A, Clemente MG, de los Reyes-Gavilan CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World journal of gastroenterology : WJG. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O'Toole PW, van Sinderen D, Marchesi JR, Ventura M. Diversity of bifidobacteria within the infant gut microbiota. PloS one. 2012;7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhnoo IS, Freihorst J, Riepenhoff-Talty M, Fisher JE, Ogra PL. Effect of rotavirus infection and malnutrition on uptake of a dietary antigen in the intestine. Pediatric research. 1990;27:153–160. doi: 10.1203/00006450-199002000-00014. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. The Journal of nutrition. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- van Baarlen P, Troost F, van der Meer C, Hooiveld G, Boekschoten M, Brummer RJ, Kleerebezem M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl. 2011;1:4562–4569. doi: 10.1073/pnas.1000079107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova AN, Chattha KS, Kandasamy S, Liu Z, Esseili M, Shao L, Rajashekara G, Saif LJ. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PloS one. 2013;8:e76962. doi: 10.1371/journal.pone.0076962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Saddoris KL, Sholly DM, Hinson RB, Sutton AL, Applegate TJ, Richert BT, Radcliffe JS. The effects of direct fed microbials delivered through the feed and/or in a bolus at weaning on growth performance and gut health. Livestock Science. 2007;108:254–257. [Google Scholar]
- Wang A, Yu H, Gao X, Li X, Qiao S. Influence of Lactobacillus fermentum I5007 on the intestinal and systemic immune responses of healthy and E. coli challenged piglets. Antonie van Leeuwenhoek. 2009a;96:89–98. doi: 10.1007/s10482-009-9339-2. [DOI] [PubMed] [Google Scholar]
- Wang AN, Cai CJ, Zeng XF, Zhang FR, Zhang GL, Thacker PA, Wang JJ, Qiao SY. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. Journal of applied microbiology. 2013;114:1582–1591. doi: 10.1111/jam.12188. [DOI] [PubMed] [Google Scholar]
- Wang AN, Yi XW, Yu HF, Dong B, Qiao SY. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. Journal of applied microbiology. 2009b;107:1140–1148. doi: 10.1111/j.1365-2672.2009.04294.x. [DOI] [PubMed] [Google Scholar]
- Wang SP, Yang LY, Tang XS, Cai LC, Liu G, Kong XF, Blachier F, Yin YL. Dietary supplementation with high-dose Bacillus subtilis or Lactobacillus reuteri modulates cellular and humoral immunities and improves performance in weaned piglets. Journal of Food, Agriculture and Environment. 2011;9:181–187. [Google Scholar]
- Wang X, Yang F, Liu C, Zhou H, Wu G, Qiao S, Li D, Wang J. Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weanling piglets. The Journal of nutrition. 2012;142:7–13. doi: 10.3945/jn.111.147074. [DOI] [PubMed] [Google Scholar]
- Wells JM. Immunomodulatory mechanisms of lactobacilli. Microbial cell factories. 2011;10(Suppl 1):S17. doi: 10.1186/1475-2859-10-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen K, Azevedo MS, Gonzalez A, Zhang W, Saif LJ, Li G, Yousef A, Yuan L. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Veterinary immunology and immunopathology. 2009;127:304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen K, Li G, Zhang W, Azevedo MS, Saif LJ, Liu F, Bui T, Yousef A, Yuan L. Development of gammadelta T cell subset responses in gnotobiotic pigs infected with human rotaviruses and colonized with probiotic lactobacilli. Veterinary immunology and immunopathology. 2011;141:267–275. doi: 10.1016/j.vetimm.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. The Journal of infectious diseases. 2009;200(Suppl 1):S1–8. doi: 10.1086/605061. [DOI] [PubMed] [Google Scholar]
- Wu S, Yuan L, Zhang Y, Liu F, Li G, Wen K, Kocher J, Yang X, Sun J. Probiotic Lactobacillus rhamnosus GG mono-association suppresses human rotavirus-induced autophagy in the gnotobiotic piglet intestine. Gut pathogens. 2013;5:22. doi: 10.1186/1757-4749-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Verstraete W. Evaluation of nitric oxide production by lactobacilli. Applied microbiology and biotechnology. 2001;56:504–507. doi: 10.1007/s002530100616. [DOI] [PubMed] [Google Scholar]
- Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- Yasui H, Kiyoshima J, Ushijima H. Passive protection against rotavirus-induced diarrhea of mouse pups born to and nursed by dams fed Bifidobacterium breve YIT4064. The Journal of infectious diseases. 1995;172:403–409. doi: 10.1093/infdis/172.2.403. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–321. [PubMed] [Google Scholar]
- Yu H, Wang A, Li X, Qiao S. Effect of viable Lactobacillus fermentum on the growth performance, nutrient digestibility and immunity of weaned pigs. Journal of Animal and Feed Sciences. 2008;17 [Google Scholar]
- Yuan L, Stevenson G, Saif LJ. Rotavirus and reovirus. In: Straw B, Zimmerman JJ, D'Allaire S, Taylor DJ, editors. Diseases of Swine. Blackwell Publishing; Ames, Iowa: 2006. pp. 435–454. [Google Scholar]
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schodel F, Steele AD, Neuzil KM, Ciarlet M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang H, Shepherd M, Wen K, Li G, Yang X, Kocher J, Giri-Rachman E, Dickerman A, Settlage R, Yuan L. Probiotics and virulent human rotavirus modulate the transplanted human gut microbiota in gnotobiotic pigs. Gut pathogens. 2014;6:39. doi: 10.1186/s13099-014-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Veterinary immunology and immunopathology. 2008a;122:175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008b;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Veterinary immunology and immunopathology. 2008c;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wu J, Li JV, Zhou NY, Tang H, Wang Y. Gut microbiota composition modifies fecal metabolic profiles in mice. Journal of proteome research. 2013;12:2987–2999. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- Zijlstra RT, McCracken BA, Odle J, Donovan SM, Gelberg HB, Petschow BW, Zuckermann FA, Gaskins HR. Malnutrition modifies pig small intestinal inflammatory responses to rotavirus. The Journal of nutrition. 1999;129:838–843. doi: 10.1093/jn/129.4.838. [DOI] [PubMed] [Google Scholar]