Abstract
Key points
Sleep disordered breathing (SDB) is common and the severity increases as pregnancy progresses.
Frequent snoring, older age and high pre-pregnancy body mass index (>25 kg⋅m−2) could be reliable indicators for SDB in early pregnancy.
SDB screening tools, including questionnaires, used in the nonpregnant population have poor predictive ability in pregnancy.
Accumulating evidence suggests that SDB during pregnancy may be associated with increased risk of adverse pregnancy outcomes, including gestational diabetes and pre-eclampsia. However, the results should be interpreted cautiously because several studies failed to adjust for potential maternal confounders and have other study limitations.
There are no pregnancy-specific practice guidelines for SDB treatment. Many clinicians and practices follow recommendations for the treatment in the general population. Women with pre-existing SDB might need to be reassessed, particularly after the sixth month of pregnancy, because symptoms can worsen with nasal congestion and weight gain.
Educational aims
To highlight the prevalence and severity of sleep disordered breathing (SDB) in the pregnant population.
To inform readers about risk factors for SDB in pregnancy.
To explore the impact of SDB on adverse maternal and fetal outcomes, and biological pathways for associated adverse maternal and fetal outcomes.
To introduce current management options for SDB in pregnancy, including medical and behavioural approaches.
Sleep disordered breathing (SDB) is very common during pregnancy, and is most likely explained by hormonal, physiological and physical changes. Maternal obesity, one of the major risk factors for SDB, together with physiological changes in pregnancy may predispose women to develop SDB. SDB has been associated with poor maternal and fetal outcomes. Thus, early identification, diagnosis and treatment of SDB are important in pregnancy. This article reviews the pregnancy-related changes affecting the severity of SDB, the epidemiology and the risk factors of SDB in pregnancy, the association of SDB with adverse pregnancy outcomes, and screening and management options specific for this population.
Short abstract
Sleep disordered breathing should be sought and treated during #pregnancy http://ow.ly/U2UO3
Introduction
Sleep disordered breathing (SDB) is highly prevalent during pregnancy as consequences of pregnancy-related changes and increasing rates of maternal obesity [1–3]. SDB represents a spectrum of abnormal respiratory events that include habitual snoring, increased upper airway resistance and obstructive sleep apnoea (OSA) [2]. Although snoring is the most common manifestation of SDB, it may not be seen in all patients with these disorders. OSA is the most severe version of the SDB and refers to repetitive episodes of complete or partial obstruction of the airway during sleep that lead to intermittent hypoxaemia and poor sleep quality [2]. There is growing evidence that SDB is associated with adverse pregnancy outcomes, especially pregnancy-induced hypertension and gestational diabetes [4–7]. Taken together, the data suggests that SDB during pregnancy is an important condition to identify and manage in order to improve maternal and fetal wellbeing. This article reviews SDB in pregnancy, pregnancy-related changes affecting the severity of SDB, the epidemiology and the risk factors of SDB in pregnancy, the association of SDB with adverse pregnancy outcomes, and screening and management options specific for this population.
Epidemiology of SDB in pregnancy
Although the prevalence of OSA is low among reproductive-age women compared to men or older women, snoring and other SDB symptoms are more common among pregnant women compared to their nonpregnant counterparts [3, 8, 9]. The prevalence of habitual snoring among pregnant women has been estimated to be between 11.9% and 49% in the third trimester in cross-sectional studies [10–12]. Longitudinal studies have shown that habitual snoring (three or more nights per week) increases from 7–11% [13–15] in the first trimester to 16–25% in the third trimester [13–16]. Pien et al. [17] reported that the frequency of SDB symptoms including snoring, breathing pauses, gasping and choking increased significantly from first trimester to the month of delivery. These women also experienced a significant increase in subjective daytime sleepiness [17]. A study using an Internet-based survey found that 19% of 2427 women reported symptoms of SDB across all months of pregnancy [18].
The prevalence of OSA in pregnant women, diagnosed by polysomnography (PSG), is not known because large population-based epidemiological studies using objective sleep measures are lacking. A small prospective cohort study of 105 pregnant women who underwent first- and third-trimester PSG noted that 10.5% women in the first and 26.7% of women in the third trimester had OSA (apnoea–hypopnoea index (AHI) ≥5 events per h sleep) [3]. However, the majority of women were overweight or obese in this study. The authors corrected this restriction using data on the body mass index (BMI) distribution in their local clinical population and estimated that overall OSA prevalence is 8.4% (95% CI 5.6–11.9%) in the first trimester and 19.7% (95% CI 15.6–24.4%) in the third trimester in their general obstetric population [3]. A recent prospective study of high-risk women also demonstrated that objectively assessed SDB in early pregnancy is common and new-onset SDB occurs in 20% of these women [19].
Risk factors of SDB and pregnancy
Pregnancy as a risk factor for SDB
The reason for the increased pregnancy-onset SDB has not been well established. The physiological hormonal, mechanical and cardiovascular changes of pregnancy may place women at risk of developing SDB or exacerbate existing sleep disorders [2]. A clinician who examines a pregnant woman for potential SDB needs to be familiar with these pregnancy changes that may predispose to SDB.
Anatomical narrowing and increased resistance within the respiratory system may occur because increased levels of oestrogen and progesterone induce capillary engorgement, hypersecretion and mucosal oedema of the upper airway [20, 21]. These reduce dimensions of the nasopharynx, oropharynx and larynx, with an increased Mallampati score as pregnancy progresses [10–12].
Pregnancy rhinitis, which causes nasal congestion, occurs in up to 42% of women by the third trimester of pregnancy, without known allergic reaction [21]. Increased nasal congestion may also cause increased nasopharyngeal resistance and produce more intrapharyngeal pressure during sleep [2]. Thus, pregnant women with narrowed airways are more likely to snore and have obstructed breathing during sleep compared to nonpregnant women and the prevalence of habitual snoring increases from the first to the third trimester [11, 13, 17]. Pregnant women with a larger neck circumference and higher baseline BMI report more symptoms of SDB than other women [3, 10, 11].
Maternal blood volume also peaks at 40–50% over baseline by the third trimester. The combination of increased blood volume, interstitial fluid and recumbent position during sleep causes nasal congestion and displaces fluid, which could adversely affect upper airway patency [20]. Evidence regarding nocturnal displacement of fluid is conflicting. While some studies have indicated that nocturnal fluid shifting from the legs into the neck increases susceptibility or severity of pharyngeal obstruction [22], others have reported that such rostral fluid shifts do not increase the frequency of obstructed breathing events [6].
A compensatory increase in the anterior–posterior diameter of the chest and elevated diaphragm caused by the enlarging uterus result in tracheal shortening and progressive reductions in functional residual capacity by 20–25%, expiratory reserve volume by 15–20% and residual volume by 22% [20, 23]. These alterations can lead to the closure of small airways during normal tidal breathing [10, 11]. In late pregnancy, airway closure results in ventilation–perfusion mismatch and reduced gas exchange [24], especially in the supine position, due to gravity, intra-abdominal pressure and loss of muscle tone during sleep [10, 20].
Oxygen consumption and minute ventilation progressively increase during pregnancy by 20% and 30–50%, respectively [20]. The increased ventilatory drive may induce obstructive respiratory events by increasing diaphragmatic effort that creates negative inspiratory pressure on the hyperaemic upper airway [25]. Furthermore, higher ventilatory drive, along with resultant respiratory alkalosis, may cause instability in respiratory control pathways and potentially increase the likelihood of central apnoea episodes at sleep onset and during sleep [20, 26]. However, findings from one recent study suggest that pregnancy does not increase risk of central apnoea [27].
Finally, frequent awakenings due to pregnancy-related discomfort may cause respiratory instability and periodic breathing at sleep onset [26]. The resulting sleep deprivation can also increase arousal threshold, impair upper airway muscle activity, and increase upper airway collapsibility.
Known risk factors of SDB and pregnancy
Obesity and ageing are known risk factors for SDB in general population. These features were also found to be risk factors for SDB in a pregnant population [3, 6, 28]. Obese women prior to pregnancy are more likely to suffer from SDB than lean women [1, 6, 29]. However, Pien et al. [3] found that gestational weight gain was not associated with third trimester SDB. A study on twin pregnancies also reported that weight gain was not significantly different between women with new-onset SDB and women without SDB [30]. These findings suggest that increased fat deposition within a specific body part could be more important than total weight gain in the development of new onset SDB during pregnancy. For example, fat deposition in the soft tissue regions of a woman’s neck can contribute to development of SDB [10, 11].
In addition to obesity and ageing, any condition causing upper airway narrowing including abnormal upper airway structures, such as tonsillar and adenoid hypertrophy and macroglossia, will predispose women to have SDB. Subtle abnormalities may not necessarily cause SDB before pregnancy, but these abnormalities in combination with pregnancy-related changes may trigger SDB [28]. Chronic hypertension is a known risk factors for SDB in the nonpregnant population and are also associated with SDB in early pregnancy [9, 30]. Furthermore, studies in the pregnant population have reported that smoking may facilitate airway obstruction on a restrictive pattern of airway disorder, and contribute to loud snoring and breathing problems during sleep [23].
Adverse pregnancy outcomes associated with SDB
Emerging evidence suggests that women with SDB are at increased risk of developing adverse maternal and fetal outcomes such as gestational diabetes mellitus (GDM), pre-eclampsia and intrauterine growth retardation [3, 4, 7, 10]. However, there are significant discrepancies in research designs, settings (laboratory or home), assessment methods (laboratory or portable PSG), sample sizes, gestational periods when data collected or comparison groups (e.g. comparing pregnant women at various gestational ages with nonpregnant controls in different menstrual cycles) among the studies. These studies also failed to adjust for potential maternal confounders and have other study limitations. Thus, the results should be interpreted cautiously.
Potential pathophysiologic mechanisms for adverse pregnancy outcomes
The underlying mechanistic pathways linking sleep disturbances and pregnancy adverse outcomes are likely to be multifactorial. Pregnancy adaptations including maternal inflammatory response, mild insulin resistance, hyperlipidaemia and decreased cardiorespiratory reserve make women vulnerable to pregnancy complications [2, 31]. SDB is a major contributor to adverse pregnancy outcomes. Even small changes in sleep parameters and subtle obstructive respiratory events could exacerbate the pregnancy adaptations and potentially increase the risk of adverse pregnancy outcomes. Recent evidence supporting this concept indicated that even treating very mild SDB (e.g. snoring or flow limitation) with nasal continuous positive airway pressure (CPAP) improves haemodynamic parameters and fetal wellbeing [32–36].
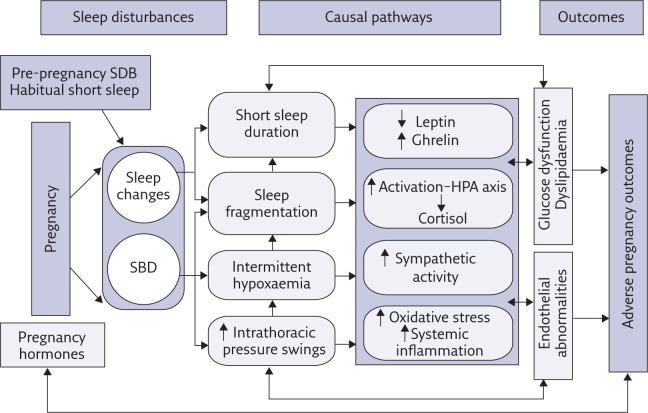
Figure 1 provides guidance for our proposed model explaining potential pathways linking sleep disturbances and pregnancy complications. These pathways are mostly hypothetical and remain to be investigated. Repeated episodes of partial or complete pharyngeal collapse during sleep result in intermittent hypoxia, reoxygenation, intrathoracic pressure swings and sleep fragmentation secondary to repetitive arousals, decreasing total sleep time in general and slow-wave sleep (SWS) in particular [37]. These physiological consequences of SDB may increase the risk of adverse outcomes during pregnancy through an intermediary mechanism that includes oxidative stress, systemic inflammation and sympathetic nervous system overactivity, which lead to endothelial dysfunction and, possibly, metabolic dysfunction (impaired glucose and lipid metabolism) [38].
Figure 1.
Potential causal pathways linking sleep disturbances during pregnancy with adverse pregnancy outcomes. HPA: hypothalamic–pituitary–adrenal. Reproduced and modified from [31] with permission from the publisher.
Oxidative stress in particular plays a pivotal role in the development of adverse pregnancy outcomes, inducing pro-inflammatory cytokines that trigger further oxidative stress and sympathetic activity [38–40], and endothelial dysfunction due to hypoxia–reoxygenation and respiratory efforts [2]. Increased oxidative stress is also a physiological trigger in the mechanism of insulin resistance, glucose intolerance and dyslipidaemia, and thus, potentially, in the onset of GDM and pre-eclampsia. In animal models, gestational exposure to hypoxia was associated with increased pancreatic β-cell proliferation, cell death, impaired fetal growth and hyperlipidaemia [40, 41].
Sleep fragmentation, intermittent hypoxia and intrathoracic pressure changes linked to SDB also activate the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, which in turn leads to increased release of the glucocorticoid cortisol [42–46]. Disproportionate sympathetic activation persists into the daytime, leading to increased peripheral vascular reactivity and catecholamine production, blunted baroreflex sensitivity, hindered pancreatic insulin secretion and excited hepatic glucose release [40]. All of these might contribute to the development of endothelial dysfunction, impaired glucose metabolism, elevated systemic arterial blood pressure and decreased maternal cardiac output, which compromises uteroplacental blood flow, as is observed in pre-eclampsia [33, 40, 47]. Prolonged secretion of the cortisol increases susceptibility to insulin resistance, hyperglycaemia and elevation of blood pressure, and restrains further development of the inflammatory process [44, 45].
Besides SDB-related events, extremes of sleep duration, poor sleep quality and daytime sleepiness in general have also been found to be strongly linked with systemic inflammation, including elevated interleukin-6, tumour necrosis factor-α, C-reactive protein levels and leukocyte counts [48, 49]. Increased expression of inflammatory cytokines may contribute to abnormal glucose metabolism, decreasing insulin sensitivity, endothelial dysfunction and subsequent pregnancy complications [2, 31]. Increased inflammation, especially in early pregnancy, is also likely to disrupt the normal remodelling of the maternal uteroplacental spiral arteries and may lead to abnormal placentation, which is associated with endothelial dysfunction, pre-eclampsia and preterm birth [2]. Abnormal placentation may result in fetal growth retardation, mitochondrial dysfunction and metabolic dysfunction in offspring after delivery [50, 51].
Experimental studies of healthy subjects showed that disruption of sleep (especially SWS) by itself (for a few nights), independent of sleep duration, can adversely affect insulin and glucose metabolism and sympathovagal balance, and increase cortisol circulation [52]. This evidence, along with other data, suggests that SWS is important for fetal wellbeing and glucose and cardiovascular regulation in pregnancy because it increases the secretion of growth hormone and uteroplacental blood flow to the fetus [33], and decreases peripheral sympathetic nervous system activity, HPA activation, cortisol secretion and brain glucose utilization [45, 52, 53].
Sleep loss and intermittent hypoxia have been reported to induce changes in lipid metabolism, leptin sensitivity and ghrelin, which regulate appetite, satiety and energy metabolism [53, 54]. Recent data suggest that insulin resistance, dyslipidaemia, and dysregulation of leptin and ghrelin may contribute to the pathophysiology of GDM and pre-eclampsia [7, 31, 55]. However, it is unclear if these occur independently of obesity. Obesity is one of the foremost causes for SDB and all adverse pregnancy outcomes. Short sleep, daytime sleepiness and insulin resistance associated with SDB may place women at risk for obesity due to changes in lipid, leptin and ghrelin levels, which increase appetite and caloric intake without equal energy expenditure [54].
Adverse maternal outcomes associated with SDB
Gestational diabetes mellitus
GDM, glucose intolerance with onset or recognition during pregnancy, affects 7% of pregnancies [56]. The prevalence rises with increasing rates of maternal obesity [1]. GDM is associated with numerous adverse maternal, fetal and neonatal outcomes, including pre-eclampsia, caesarean section, preterm labour, macrosomia, and death [57].
Emerging data indicate that there is a link between SDB and GDM. Two systematic reviews and meta-analyses of observational studies reported that SDB was associated with a two- to three-fold increased odds of GDM (pooled adjusted OR 1.86 (95% CI 1.29–2.37) [4] and OR 3.06 (95% CI 1.89–4.96) [5]). The existence of maternal SDB was identified mostly through the use of questionnaires. Findings of studies with small sample sizes objectively assessing the impact of SDB on the risk of GDM were contradictory [31]. A cohort study using longitudinal, objective assessment of SDB demonstrated a dose–response relationship between SDB and composite adverse pregnancy outcomes including GDM, pregnancy-related hypertension and preterm birth. The rates of GDM among women with no SDB, mild SDB and moderate/severe SDB were 25%, 43% and 63%, respectively [57]. Chen et al. [58] also reported that the risk of GDM increased almost two-fold (OR 1.6, 95% CI 1.07–2.8) among 791 pregnant women with PSG-diagnosed OSA in a retrospective cohort study. These two studies, however, did not fully control the effect of obesity [57, 58]. In another cohort study of 175 obese pregnant women, SDB was not associated with increased prevalence of GDM [29].
Hypertensive disorders of pregnancy
All hypertensive disorders (chronic hypertension, gestational hypertension, pre-eclampsia–eclampsia and pre-eclampsia superimposed on chronic hypertension) affect about 5–10% of pregnancies [56]. They are associated with maternal and neonatal morbidity and mortality. Of particular concern is pre-eclampsia, a pregnancy-specific syndrome of reduced organ perfusion secondary to vasospasm and endothelial activation [56]. Pre-eclampsia shares similar risk factors with SDB, which makes it difficult to explore possible association between these diseases.
A systematic review including retrospective and small cohort studies reported a two-fold increase in pre-eclampsia among women with SDB (adjusted OR 2.34, 95% CI 1.60–3.09) [4]. A recent case–control study, objectively assessing SDB, also reported that hypertensive disorders (chronic hypertension, gestational hypertension and pre-eclampsia) and frequent snoring are associated with OSA in pregnancy [9]. However, data from prospective observational cohorts are conflicting. O’Brien et al. [14] found that new-onset snoring during pregnancy, but not chronic snoring, was independently associated with gestational hypertension (OR 2.4, 95% CI 1.5–3.8) and pre-eclampsia (OR 1.6, 95% CI 1.1–2.4) after adjusting for known risk factors in the largest longitudinal study of 1700 pregnant women. The largest cohort study of 791 women with PSG-diagnosed OSA reported that women with existing OSA diagnosed prior to pregnancy had an increased risk of pre-eclampsia (adjusted OR 1.6, 95% CI 2.16–11.26), compared to women without the SDB diagnosis [58]. The effect of obesity was not fully controlled but the obesity rate is reported to be only 1.6% in the Taiwanese population. Conversely, two prospective studies employing diagnostic PSG and a portable diagnostic device did not confirm the link between pre-eclampsia and SDB [3, 7].
Severe maternal morbidity
A study of 55 781 965 women using data from a large database of delivery-related hospital discharges in the USA reported that OSA was associated with an increased risk of severe morbidity: cardiomyopathy (OR 9.0, 95% CI 7.5–10.9), pulmonary embolism (OR 4.5, 95% CI 2.3–8.9), eclampsia (OR 5.4, 95% CI 3.3–8.9) and hospital death (OR 5.3, 95% CI 2.4–11.5) [1].
Adverse fetal and infant outcomes associated with SDB
Despite increasing evidence to link SDB with adverse maternal outcomes, the findings of studies investigating the clinical fetal and infant outcomes of SDB during pregnancy are conflicting. While some studies suggest an association of SDB with fetal growth, Apgar score and prematurity, other studies did not find such an effect on the fetus [6, 51, 59]. These conflicting data may be attributed to studies having small sample sizes, lacking objective measures, including women with complicated pregnancies and failing to control for important confounders such as obesity, diabetes and hypertension.
Case reports and small studies have identified recordable fetal heart decelerations but findings of a larger prospective study of 20 women with PSG-diagnosed OSA did not support this outcome. None of the apnoea episodes was accompanied by any fetal tracing abnormality [59].
Several studies reported no differences in birthweight between women with and without SDB, but Pamidi et al. [4] found a link between SDB and low-birthweight infants among pooled studies (unadjusted OR 1.39, 95% CI 1.14–1.65). A cohort of women with PSG-diagnosed OSA also reported that OSA-exposed infants weigh less than unexposed infants [60]. However, infants’ weights were found to be higher among women with SDB symptoms in a larger prospective study [61]. Women with SDB may give birth to large infants or large for gestational age infants because the prevalence of obesity and/or diabetes is high among these women [60, 61].
Miscarriage and preterm delivery
In a retrospective study of clinical chart review, overweight/obese women with confirmed OSA (moderate–severe) were more likely to have had a miscarriage than women without SDB [62]. This study did not have a control group, and the timing of miscarriage and the age of onset of each patient’s SDB were not known. Another retrospective study reported a higher risk of preterm birth among pregnant women with confirmed OSA than normal weight controls (29.8 versus 12.3%; OR 2.6, 95% CI 1.02–6.6) after adjusting for comorbid conditions, but these pregnancies were complicated with pre-eclampsia [60].
Clinical manifestations and diagnosis
Clinical manifestations
A comprehensive sleep history in a patient suspected of SDB should include an evaluation of manifestations of SDB. Night-time features of SDB include snoring, gasping, choking, breathing pauses, shortness of breath (dyspnoea) and restlessness. Daytime symptoms of SDB consist of excessive daytime sleepiness, morning headaches, daytime fatigue and possibly cognitive impairment [63]. Even though snoring and daytime sleepiness are very common among pregnant women [8, 17], women in pregnancy, and women generally, are less likely to report snoring, snorting, breathing pauses or daytime sleepiness as a symptom of SDB. They describe their sleepiness with words like “unrefreshed,” “fatigue” or “tired” [8]. It is likely that some symptoms of SDB, including daytime sleepiness, are nonspecific or are similar to conditions caused by pregnancy adaptations such as fetal movement, urination urge, back pain or general discomfort [6, 8, 56]. Thus, they may consider these symptoms as a normal part of pregnancy. Additionally, pregnant women may not be aware of their snoring or other nocturnal symptoms because these symptoms may occur for the first time in their life. Partner-reported snoring and breathing pauses may be more reliable in pregnancy [8].
Studies using objective sleep measures indicated that pregnant women are more likely to have mild or moderate disease [3, 35]. The prevalence and severity of SDB increase as pregnancy progress [3, 17].
Screening for SDB in the pregnant population
Given the evidence of the high prevalence of SDB in pregnancy and its association with adverse pregnancy outcomes, early identification, diagnosis and treatment of SDB, especially OSA, is important in pregnancy. However, SDB is probably underdiagnosed during pregnancy. Obstetric care providers screen poorly for sleep disorders [64] probably due to reasons mentioned above and do not routinely refer or treat women with SDB. Furthermore, current SDB screening tools including questionnaires used in the nonpregnant population have poor predictive ability in pregnancy [8, 65, 66]. Thus, generic screening tools are not recommended in the pregnant population.
Snoring is strongly associated with the overnight PSG-diagnosed OSA in general population [14, 63]. As mentioned earlier, cohort studies of pregnant women indicated that older age and high pre-pregnancy BMI are risk factors for SDB [3, 14]. These studies suggest that habitual snoring, older age and high pre-pregnancy BMI could be reliable indicators for pre-existing SDB in early pregnancy. Women with these predisposing factors require careful surveillance as pregnancy progress. In fact, a pregnancy-specific tool including frequent snoring (yes or no), chronic hypertension (yes or no), maternal age and baseline BMI predicted SDB well among high-risk women in early pregnancy [66]. Thus, examining clinical and pregnancy history regarding risk factors of SDB (habitual snoring, witnessed apnoeas, chronic hypertension and pre-pregnancy obesity) may help to identify pre-existing SDB or pregnancy-onset SDB. Women with pre-existing SDB might also need to be reassessed, particularly after the sixth month of pregnancy, because symptoms can reoccur or worsen with nasal congestion and weight gain [28].
Objective sleep measures
There are no specific guidelines for diagnostic evaluation of SDB in pregnancy because of limited data. Current standard procedures developed for the general population are applied to pregnant women to evaluate SDB. Healthcare providers should have a high index of suspicion for sleep disorders, and evaluate women for sleep-related history and physical examination [63]. Pregnant women with suspected SDB should be referred to a sleep centre to obtain evidence about abnormal respiratory events by undergoing an overnight PSG. Home portable sleep monitors can be a convenient and cost-effective alternative to laboratory PSG, but data are insufficient on their validity and reliability in the pregnant population [6]. Home sleep monitors may be used for the diagnosis of OSA in women who are not able to spend a night at a sleep centre. As in the general population, laboratory PSG is appropriate for the diagnostic evaluation of women suspected of having comorbid sleep disorders. Additionally, results of home monitors should be viewed cautiously due to their negative predictive value [67].
Management and treatment
There is no pregnancy-specific practice guideline for SDB treatment in women with or without complications. Despite having no clinical practice guidelines or well-designed randomised controlled trials to direct clinical management decisions, many clinicians and practices follow recommendations for treatment in the general population [63]. These treatment modalities include medical devices (CPAP and oral mandibular repositioning devices (MADs)), lifestyle modifications, medications and upper airway surgery [63].
Medical devices
CPAP is a safe and well-tolerated therapy with good compliance among pregnant women. The target is to eliminate abnormal respiratory events (AHI <5 events per h) and prevent recurrent oxyhaemoglobin desaturations to <90%. Small studies have reported that CPAP improves maternal and fetal outcomes for women with OSA, pre-eclampsia or risk factors such as chronic hypertension [28, 35, 36]. These results are promising, but well-controlled, large-scale studies need to examine the effect of CPAP treatment on all pregnancy outcomes. Autotitrating CPAP is better suited for pregnant women’s condition to adjust therapeutic CPAP pressure when the severity changes during pregnancy and improve compliance. Regular follow-up is also necessary in pregnancy after initiation of CPAP therapy [6, 28]. Women with pre-existing OSA and established CPAP treatment should continue treatment during pregnancy. CPAP may need to be readjusted around 24 weeks of pregnancy because of pregnancy-induced nasal congestion and increased BMI [28]. Untreated women with pre-existing OSA should immediately receive CPAP treatment.
Oral MADs are most likely to be effective for women with mild–moderate OSA. However, Reid et al. [68] reported that autotitrating CPAP was superior in treating SDB than a MAD plus a nasal strip in pregnant women. Additionally, a MAD is custom made and fitted by a specialised dentist. Its production sessions may take a long time for a pregnancy period. Commercial devices are also available with less cost. However, this treatment has side-effects, including soreness, nausea and permanent musculoskeletal changes that are associated with pregnancy. It is important to have a MAD fitted by a dentist specialised in sleep medicine.
Lifestyle modifications
Although weight loss often decreases the severity of SDB and has potential to prevent development of SDB in the general population [63], it is less of an option for the pregnant population. However, achieving normal weight before pregnancy and control weight gain during pregnancy may decrease the risk of new-onset SDB and associated complications. Women with position-dependent OSA, without significant oxyhaemoglobin desaturations or hypertensive complications, may possibly benefit from sleeping in the lateral recumbent or head-elevated position. Other lifestyle modifications that can be helpful in SDB include avoidance of respiratory-destabilising drugs, an irregular sleep–wake schedule, smoking, nasal congestion at night, or heavy or spicy meals close to bedtime [63].
Drug treatment
There is no medication that prevents or treats SDB. Modafinil has been used to treat the sleepiness associated with SDB, but not snoring or breathing pauses [63]. It has been recommended to discontinue it during pregnancy and lactation due to limited or lack of safety data on these periods [6]. When discontinued, individuals may need to be warned to take appropriate safety measures, especially when they are driving.
Surgery
Although one study used tracheostomy [2], which resolved SDB, no kind of surgery is preferred as a treatment option for SDB [6] because it increases the risks of adverse outcomes.
Follow-up
The decision to manage SDB post partum must be individualised according to the severity of symptoms, weight loss and comorbidities. The severity of SDB subsides after delivery due to weight loss and decreased nasopharyngeal oedema [2, 11, 31, 69]. The severity of SDB and the management plan need to be re-assessed by a sleep specialist for all women who had pre-existing SDB, were diagnosed with new-onset SDB or were suspected for SDB during pregnancy. A post partum PSG might be necessary for these women after their weight stabilisation (approximately 3 months post partum) [6, 11, 28, 69]. Weight loss/management strategies will be beneficial for obese women with SDB to relieve their symptoms as well as improving their comorbid conditions. All women with gestational SDB should be monitored closely for symptom recurrence in following pregnancies.
Selected reading
Chen YH, Kang JH, Lin CC, et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol 2012; 206: 136.e1–136.e5.
This is the largest retrospective cohort study of pregnant women with PSG-confirmed SDB showing that the risk of GDM increased almost two-fold (OR 1.6, 95% CI 1.07–2.8) among women known to have antenatal SDB after controlling a number of maternal characteristics such as age, education level and marital status.
Facco FL, Ouyang DW, Zee PC, et al. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med 2012; 8: 389–394.
Facco et al. developed a risk prediction model in this high-risk pregnancy cohort combining snoring, BMI, a history of chronic hypertension and age ((15 if frequent snoring)+(15 if chronic hypertension)+age+BMI). The Berlin score and Epworth Sleepiness Scale did not accurately predict sleep apnoea in this cohort, with areas under the receiver operating characteristic curves (AUCs) of 0.54 (p=0.6) and 0.57 (p=0.3), respectively. However, the risk prediction model performed significantly better (AUC 0.86, p>0.001).
Pamidi S, Pinto LM, Marc I, et al. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol 2014; 210: 52.e51–52.e14.
Pamidi et al. found 31 of the 4386 studies that met the criteria for their meta-analysis. Of these, 21 studies observational in design, reported dichotomous results; nine of these adjusted for potential confounders. Together, they showed that maternal SDB was significantly associated with gestational hypertension/pre-eclampsia (pooled adjusted odds ratio (aOR) 2.34, 95% CI 1.60–3.09; five studies) and GDM (pooled aOR 1.86, 95% CI 1.30–2.42; five studies).
Louis JM, Mogos MF, Salemi JL, et al. Obstructive sleep apnea and severe maternal–infant morbidity/mortality in the United States, 1998–2009. Sleep 2014; 37: 843–849.
Louis et al. used a large database of delivery-related hospital discharges of 55 781 965 in the USA in this study. They found that OSA was associated with an increased risk of severe morbidity: cardiomyopathy (OR 9.0, 95% CI 7.5–10.9), pulmonary embolism (OR 4.5, 95% CI 2.3–8.9), eclampsia (OR 5.4, 95% CI 3.3–8.9) and hospital death (OR 5.3, 95% CI 2.4–11.5).
Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010; 16: 574–582.
This article reviews current data on pathophysiological mechanisms by which SDB during pregnancy may cause harm, and explores biological pathways for associated adverse maternal and fetal outcomes.
Footnotes
Conflict of interest None declared.
References
- 1.Louis JM, Mogos MF, Salemi JL, et al. Obstructive sleep apnea and severe maternal–infant morbidity/mortality in the United States, 1998–2009. Sleep 2014; 37: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med 2010; 16: 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pien GW, Pack AI, Jackson N, et al. Risk factors for sleep-disordered breathing in pregnancy. Thorax 2014; 69: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pamidi S, Pinto LM, Marc I, et al. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol 2014; 210: 52.e51–52.e14. [DOI] [PubMed] [Google Scholar]
- 5.Luque-Fernandez MA, Bain PA, Gelaye B, et al. Sleep-disordered breathing and gestational diabetes mellitus: a meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care 2013; 36: 3353–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis J, Pien GW. Obstructive sleep apnea in pregnancy. www.uptodate.com/contents/obstructive-sleep-apnea-in-pregnancy Date last updated: June 29, 2015.
- 7.Facco FL, Lappen J, Lim C, et al. Preeclampsia and sleep-disordered breathing: A case-control study. Pregnancy Hypertens 2013; 3: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izci B, Martin SE, Dundas KC, et al. Sleep complaints: snoring and daytime sleepiness in pregnant and pre-eclamptic women. Sleep Med 2005; 6: 163–169. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien LM, Bullough AS, Chames MC, et al. Hypertension, snoring, and obstructive sleep apnoea during pregnancy: a cohort study. BJOG 2014; 121: 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med 2003; 167: 137–140. [DOI] [PubMed] [Google Scholar]
- 11.Izci B, Vennelle M, Liston WA, et al. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J 2006; 27: 321–327. [DOI] [PubMed] [Google Scholar]
- 12.Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth 1995; 74: 638–642. [DOI] [PubMed] [Google Scholar]
- 13.Facco FL, Kramer J, Ho KH, et al. Sleep disturbances in pregnancy. Obstet Gynecol 2010; 115: 77–83. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol 2012; 207: 487 e481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarberg M, Svanborg E, Wirehn AB, et al. Snoring during pregnancy and its relation to sleepiness and pregnancy outcome – a prospective study. BMC Pregnancy Childbirth 2014; 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourjeily G, Raker CA, Chalhoub M, et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J 2010; 36: 849–855. [DOI] [PubMed] [Google Scholar]
- 17.Pien GW, Fife D, Pack AI, et al. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep 2005; 28: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 18.Mindell JA, Cook RA, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med 2015; 16: 483–488. [DOI] [PubMed] [Google Scholar]
- 19.Facco FL, Ouyang DW, Zee PC, et al. Sleep disordered breathing in a high-risk cohort prevalence and severity across pregnancy. Am J Perinatol 2014; 31: 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med 2011; 32: 1–13. [DOI] [PubMed] [Google Scholar]
- 21.Dzieciolowska-Baran E, Teul-Swiniarska I, Gawlikowska-Sroka A, et al. Rihinitis as a cause of respiratory disorders during pregnancy. Adv Exp Med Biol 2013; 755: 213–220. [DOI] [PubMed] [Google Scholar]
- 22.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med 2009; 179: 241–246. [DOI] [PubMed] [Google Scholar]
- 23.Hirnle L, Lysenko L, Gerber H, et al. Respiratory function in pregnant women. Adv Exp Med Biol 2013; 788: 153–160. [DOI] [PubMed] [Google Scholar]
- 24.Bourne T, Ogilvy AJ, Vickers R, et al. Nocturnal hypoxaemia in late pregnancy. Br J Anaesth 1995; 75: 678–682. [DOI] [PubMed] [Google Scholar]
- 25.Remmers JE, Degroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978; 44: 931–938. [DOI] [PubMed] [Google Scholar]
- 26.Thomson S, Morrell MJ, Cordingley JJ, et al. Ventilation is unstable during drowsiness before sleep onset. J Appl Physiol 2005; 99: 2036–2044. [DOI] [PubMed] [Google Scholar]
- 27.Mazer J, Fung J, Sharkey K, et al. Pregnant women are unlikely to develop central sleep apnea. Chest 2013; 144: 991A. [Google Scholar]
- 28.Guilleminault C, Kreutzer M, Chang JL. Pregnancy, sleep disordered breathing and treatment with nasal continuous positive airway pressure. Sleep Med 2004; 5: 43–51. [DOI] [PubMed] [Google Scholar]
- 29.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol 2012; 120: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Facco FL, Ouyang DW, Zee PC, et al. Sleep Disordered Breathing in a High-Risk Cohort Prevalence and Severity across Pregnancy. Am J Perinatol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izci-Balserak B, Pien GW. The relationship and potential mechanistic pathways between sleep disturbances and maternal hyperglycemia. Curr Diab Rep 2014; 14: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blyton DM, Skilton MR, Edwards N, et al. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep 2013; 36: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep 2004; 27: 79–84. [DOI] [PubMed] [Google Scholar]
- 34.Edwards N, Blyton CM, Kesby GJ, et al. Pre-eclampsia is associated with marked alterations in sleep architecture. Sleep 2000; 23: 619–625. [PubMed] [Google Scholar]
- 35.Guilleminault C, Palombini L, Poyares D, et al. Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: Preliminary findings. Sleep Med 2007. [DOI] [PubMed] [Google Scholar]
- 36.Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med 2007. [DOI] [PubMed] [Google Scholar]
- 37.Polotsky VY, Rubin AE, Balbir A, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 2006; 7: 7–16. [DOI] [PubMed] [Google Scholar]
- 38.Arnardottir ES, Mackiewicz M, Gislason T, et al. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 2009; 32: 447–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci 2012; 4: 1391–1403. [DOI] [PubMed] [Google Scholar]
- 40.Levy P, Ryan S, Oldenburg O, et al. Sleep apnoea and the heart. Eur Respir Rev 2013; 22: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gozal D, Reeves SR, Row BW, et al. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med 2003; 167: 1540–1547. [DOI] [PubMed] [Google Scholar]
- 42.Qiu C, Enquobahrie D, Frederick IO, et al. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health 2010; 10: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamisier R, Pepin JL, Remy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 2011; 37: 119–128. [DOI] [PubMed] [Google Scholar]
- 44.Szelenyi J, Vizi ES. The catecholamine cytokine balance: interaction between the brain and the immune system. Ann NY Acad Sci 2007; 1113: 311–324. [DOI] [PubMed] [Google Scholar]
- 45.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008; 177: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards N, Blyton DM, Kirjavainen T, et al. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med 2000; 162: 252–257. [DOI] [PubMed] [Google Scholar]
- 48.Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? J Reprod Immunol 2007; 73: 158–165. [DOI] [PubMed] [Google Scholar]
- 49.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med 2012; 13: 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalyfa A, Carreras A, Hakim F, et al. Sleep disruption during late gestation of pregnancy induces metabolic dysfunction in offspring. Am J Respir Crit Care Med 2013; 187.23668455 [Google Scholar]
- 51.Sahin FK, Koken G, Cosar E, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet 2008; 100: 141–146. [DOI] [PubMed] [Google Scholar]
- 52.Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008; 105: 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiegel K, Tasali E, Penev P, et al. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–850. [DOI] [PubMed] [Google Scholar]
- 54.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol 2012; 24: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reutrakul S, Zaidi N, Wroblewski K, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab 2013; 98: 4195–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunningham FG, Leveno KL, Bloom SL, et al. Williams Obstetrics. New York, McGraw-Hill Medical, 2014. [Google Scholar]
- 57.Facco FL, Ouyang DW, Zee PC, et al. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol 2014; 210: 559.e1–559.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen YH, Kang JH, Lin CC, et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol 2012; 206: 136.e1–136.e5. [DOI] [PubMed] [Google Scholar]
- 59.Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol 2010; 202: 552.e551–552.e557. [DOI] [PubMed] [Google Scholar]
- 60.Louis JM, Auckley D, Sokol RJ, et al. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol 2010; 202: 261 e261–265. [DOI] [PubMed] [Google Scholar]
- 61.Higgins N, Leong E, Park CS, et al. The Berlin Questionnaire for assessment of sleep disordered breathing risk in parturients and non-pregnant women. Int J Obstet Anesth 2011; 20: 22–25. [DOI] [PubMed] [Google Scholar]
- 62.Lee EK, Gutcher ST, Douglass AB. Is sleep-disordered breathing associated with miscarriages? An emerging hypothesis. Med Hypotheses 2014; 82: 481–485. [DOI] [PubMed] [Google Scholar]
- 63.Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5: 263–276. [PMC free article] [PubMed] [Google Scholar]
- 64.Bourjeily G, Raker C, Paglia MJ, et al. Patient and provider perceptions of sleep disordered breathing assessment during prenatal care: a survey-based observational study. Ther Adv Respir Dis 2012; 6: 211–219. [DOI] [PubMed] [Google Scholar]
- 65.Bourjeily G, El Sabbagh R, Sawan P, et al. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath 2013; 17: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 66.Facco FL, Ouyang DW, Zee PC, et al. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med 2012; 8: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3: 737–747. [PMC free article] [PubMed] [Google Scholar]
- 68.Reid J, Glew RA, Skomro R, et al. Sleep disordered breathing and gestational hypertension: postpartum follow-up study. Sleep 2013; 36: 717–721B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edwards N, Blyton DM, Hennessy A, et al. Severity of sleep-disordered breathing improves following parturition. Sleep 2005; 28: 737–741. [DOI] [PubMed] [Google Scholar]