Abstract
Key points
Respiratory distress is a common presenting feature among newborn infants.
Prompt investigation to ascertain the underlying diagnosis and appropriate subsequent management is important to improve outcomes.
Many of the underlying causes of respiratory distress in a newborn are unique to this age group.
A chest radiograph is crucial to assist in diagnosis of an underlying cause.
Educational aims
To inform readers of the common respiratory problems encountered in neonatology and the evidence-based management of these conditions.
To enable readers to develop a framework for diagnosis of an infant with respiratory distress.
The first hours and days of life are of crucial importance for the newborn infant as the infant adapts to the extra-uterine environment. The newborn infant is vulnerable to a range of respiratory diseases, many unique to this period of early life as the developing fluid-filled fetal lungs adapt to the extrauterine environment. The clinical signs of respiratory distress are important to recognise and further investigate, to identify the underlying cause. The epidemiology, diagnostic features and management of common neonatal respiratory conditions are covered in this review article aimed at all healthcare professionals who come into contact with newborn infants.
Introduction
The ability of the newborn infant to adapt to the extra-uterine environment is critical to survival. All systems of the body undergo important physiological changes at the time of delivery [1]. Arguably none is more critical to survival than the adaption of the lungs [2]. In utero, the fetus receives a constant supply of oxygen and nutrients via the placenta and umbilical vessels, with carbon dioxide excretion also managed via the maternal circulation. The lungs are filled with fluid secreted by the respiratory epithelium [3] which is important for promoting lung growth. Some congenital malformations of the lungs or airways may not affect the fetus or its development in utero, even anomalies incompatible with extra-uterine life. Hydrops fetalis is a recognised complication of larger lesions, including those that affect the arterial circulation. During the first gasp immediately after birth, the neonate fills the airways down to alveolar level with air to commence extra-uterine gas exchange [2]; simultaneously, decreasing pulmonary vascular pressure to allow increased blood flow to the lungs [4]; additionally, reabsorption of the fetal lung fluid occurs [5]. A preterm neonate born at <37 weeks’ gestation has the additional complication of achieving these changes with relatively immature lungs. Extremely preterm (≤28 weeks’ gestation) and late preterm neonates (≤32 weeks’ gestation) need to survive without adequate alveolar development, which generally commences after 32 weeks’ gestational age [6].
Neonatal respiratory conditions can arise for several reasons: delayed adaptation or maladaptation to extra-uterine life, existing conditions such as surgical or congenital anomalies or from acquired conditions such as pulmonary infections occurring either pre- or post-delivery. An Italian study showed that 2.2% of all births were complicated by a respiratory disorder [7] with an Indian study estimating 6.7% [8]. Respiratory conditions are the most common reason for admission to a neonatal unit in both term and preterm infants [9]. One study reported that 33.3% of all neonatal admissions at >28 weeks’ gestation, excluding infants with syndromes and those with congenital or surgical conditions, had respiratory conditions as their primary reason for admission [10]. A further study estimated that 20.5% of all neonatal admissions showed signs of respiratory distress [11]. Evidence exists of rising rates of neonatal admissions due to respiratory conditions, possibly due to the effect of increased rates of caesarean section delivery [12, 13].
This review distinguishes between those neonatal respiratory conditions witnessed primarily in preterm infants, those more common in term infants and congenital/surgical anomalies, which can occur in infants born at any gestation. Table 1 summaries the most common conditions in each category.
Table 1.
Common causes of neonatal respiratory distress
| Preterm pathology | Term pathology | Congenital anomalies/surgical conditions | Non-respiratory causes of respiratory distress |
| Respiratory distress syndrome Pneumothorax Pneumonia Pulmonary haemorrhage Aspiration Pleural effusion (chylothorax) Chronic lung disease |
Transient tachypnoea of the newborn Respiratory distress syndrome Meconium aspiration Primary or secondary persistent pulmonary hypertension of the newborn Pneumonia Pneumothorax Aspiration Pleural effusion (chylothorax) Pulmonary haemorrhage Surfactant protein deficiency syndromes Alveolar capillary dysplasia |
Congenital pulmonary airway malformation Congenital diaphragmatic hernia Tracheo-oesphageal fistula Choanal atresia Pulmonary sequestration Congenital lobar emphysema |
Heart failure (due to congenital heart disease) Neuromuscular disorders Hypoxic ischaemic encephalopathy Metabolic acidosis (due to inborn error of metabolism) |
Clinical identification and initial management of respiratory conditions
Thorough clinical assessment of the newborn infant is the most important aspect of accurately diagnosing the underlying respiratory condition. An infant with breathing difficulties displays classic clinical signs of respiratory distress regardless of the underlying cause. These consist of tachypnoea (respiratory rate >60 breaths⋅min−1), tachycardia (heart rate >160 beats⋅min−1), nasal flaring, grunting, chest wall recessions (suprasternal, intercostal and subcostal), cyanosis and apnoea. First line investigations in the assessment of a neonate with respiratory distress should include pulse oximetry, chest radiograph and blood tests (full blood count, C-reactive protein, blood culture and arterial blood gas) [14]. A chest radiograph is particularly useful for distinguishing the underlying cause. It is important to recognise that respiratory distress can be caused by non-respiratory pathology such as metabolic acidosis, neuromuscular disorders, cardiac causes or hypoxic-ischaemic encephalopathy. The scope of this review does not extend to cover non-respiratory causes of respiratory distress. Some of the important clinical considerations that should be made when assessing a newborn infant with respiratory distress to assist in diagnosing the underlying cause are shown in the box to the left [14].
Emergency treatment in cases of neonatal respiratory distress is to reverse any hypoxia with supplemental oxygen and to prevent or reverse any respiratory acidosis by ensuring adequate ventilation of the lungs. This may require noninvasive respiratory support, such as continuous positive airway pressure (CPAP) or high flow therapy [15]; or tracheal intubation and mechanical ventilation in the most severely affected cases. Feeding is generally delayed until an underlying diagnosis has been made. Further management depends on the underlying diagnosis. Antibiotics are often routinely prescribed for all infants with respiratory distress due to the difficulty in excluding respiratory infections.
Common conditions seen primarily in preterm infants
Respiratory distress syndrome
Epidemiology and risk factors
Respiratory distress syndrome (RDS) is seen primarily in preterm infants due to a deficiency of surfactant in the lungs. Often also called hyaline membrane disease, which more accurately is a histological diagnosis. Classically, RDS is observed in preterm infants, however, 6.4% [16] to 7.8% [17] of cases with RDS are diagnosed in infants born at ≥37 weeks’ gestation, many being delivered by caesarean section. Among preterm infants, the incidence varies with gestation with increasing incidence with decreasing gestations. Infants of mothers with diabetes are also at increased risk of developing RDS.
Surfactant is produced by type 2 pneumocytes from the 24th week of gestation and levels increase with increased gestational age [18]. The alveolar pool size of surfactant phospholipids in a healthy full-term infant has been estimated to be 100 mg⋅kg−1, about ten-times greater than the amount noted in lungs of infants who develop RDS [19]. The action of surfactant is not limited to reducing surface tension of alveolar lining fluid, but RDS is primarily a consequence of the failure to reduce surface tension within alveoli [20]. Reduced surfactant results in increased respiratory effort required to expand the lung with each breath and increased likelihood of alveolar collapse at the end of expiration.
Clinical aspects
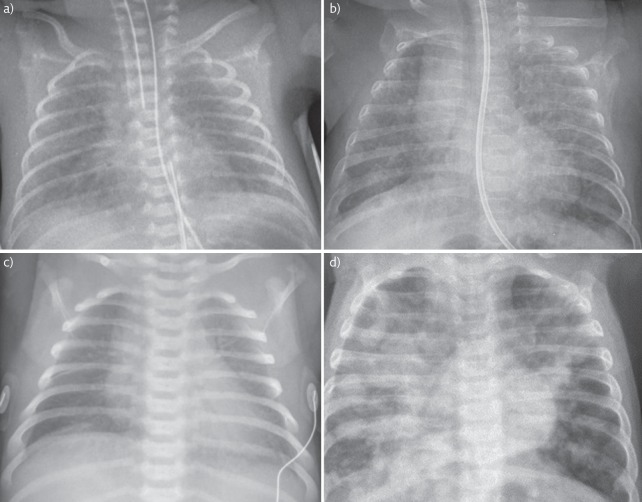
Signs of respiratory distress are usually present soon after birth. The chest radiograph demonstrates poorly inflated lungs with a “ground glass” appearance of reticular nodular shadowing throughout the lung fields and air bronchograms as demonstrated in figure 1a. The respiratory distress worsens over the first 2–3 days of life, stabilises for a further 2–3 days before clinically improving often with a diuretic phase.
Figure 1.
Chest radiograph images. a) Intubated 23+6 weeks preterm infant with RDS. Note bilateral ground glass shadowing and air bronchograms. The ET tube is low in this image and needs withdrawing. Parental consent obtained for publication. b) Ex 24-week preterm infant with CLD. Note areas of cystic changes and linear shadowing throughout both lungs. Parental consent obtained for publication. c) Term infant with TTN. Note wet silhouette around the heart and fluid in the horizontal fissure. Image: © Auckland District Health Board. d) Term infant with MAS. Widespread asymmetrical patchy shadowing throughout both lungs with hyperinflation. Reproduced from [21] with permission from the publisher.
Antenatal maternal administration of corticosteroids and exogenous surfactant therapy have revolutionised the management of RDS. Antenatal corticosteroids result in maturation of the fetal lung, by promoting maturation of the antioxidant system and of surfactant production; prepare the fetal lung for breathing and preventing or reducing the severity of RDS respectively [22]. Mothers are routinely given antenatal corticosteroids in cases of threatened preterm birth [23]. Exogenous surfactant is routinely administered prophylactically to preterm infants requiring tracheal intubation at birth to prevent RDS. New techniques of delivering surfactant with only minimal intubation time, or even without the need for an endotracheal tube are increasingly considered in the management of preterm infants at risk of developing RDS [24]. Established RDS can be treated with further doses of surfactant, but optimal timing of rescue doses of surfactant remains unclear [25]. For those infants less severely affected maintaining positive end expiratory pressure with continuous positive airway pressure (CPAP), and using supplemental oxygen where necessary is recommended [25]. “High-flow” nasal oxygen therapy as an alternative to CPAP is increasingly used in many units but requires careful evaluation [15, 26].
Prognosis
Recovery from RDS is dependent on its severity, which, in turn, is affected by gestation and birth weight. Historically 50% mortality from RDS was seen in infants <1000 g birth weight compared to 0% in those >4000 g [11]. While RDS is rarely an isolated pathology affecting extreme preterm infants, respiratory insufficiency, due to immaturity of the lungs, limits viability in extremely preterm infants.
Chronic lung disease
Epidemiology and risk factors
Chronic lung disease (CLD), also often called broncho-pulmonary dysplasia (BPD) is the most common long-term respiratory consequence of prematurity [27]. CLD is defined as supplemental oxygen dependency for at least 28 days from birth, and at 36 weeks corrected gestational age. Often the length of supplemental oxygen dependency is used to grade severity. Injuries to the developing preterm lung result in impaired alveolar and vascular development. CLD is known to be a multifactorial condition with a diverse range of contributing risk factors. Extremely preterm born infants are at the highest risk. Infants born at 23 weeks’ gestational age have an incidence of CLD of 73%, while for those born at 28 weeks’ gestation the incidence of CLD is 23% [28]. Small-for-gestational-age infants are also at a greater risk [29]. The presence of chorioamnionitis, mechanical ventilation [30], postnatal sepsis [30], oxygen toxicity [31] and fluid overload often due to the presence of a patent ductus arteriosus [32] are all risk factors for CLD. The common pathway for each of these mechanisms is thought to be the generation of an inflammatory response within the lungs of preterm infants [33]. Many studies show excessive inflammatory activity within the lungs of preterm infants who progress to develop CLD [34–36]. The preterm immune system itself may be prone to poorly regulated or excessive inflammatory activity contributing to the tissue damage [37, 38].
Despite attempts to modify all variable risk factors, the rate of CLD has failed to improve, possibly due the increased survival of more infants born extremely preterm [39]. The introduction of antenatal maternal corticosteroids administration and use of exogenous surfactant, along with lung protective ventilation strategies has, however, seen a in change in the pathology from “old CLD” to “new CLD” [40]. Old CLD was characterised by marked fibrosis; varying hyperinflation and atelectasis; and decreased alveolarisation [41]. Histologically, new CLD infants has less fibrosis, less heterogeneity of lung disease but larger, fewer alveoli than its older variant [42].
Clinical aspects
CLD usually evolves in preterm infants from their RDS. Treatment of CLD consists of supportive management and treating co-morbidities to optimise lung function. Oxygen therapy targeted to optimise oxygen saturation without causing hyperoxic damage is the mainstay of treatment. Postnatal corticosteroids are effective at decreasing inflammation within the lung, but the risk of neurodevelopmental side-effects limits its use. Current practice is to restrict use of corticosteroids to aid extubation in those infants who remain chronically mechanical ventilation dependent. Other therapies, such as diuretics and inhaled corticosteroids, have limited evidence base [43, 44] but are often used in clinical practice. Preventing preterm infants from developing CLD by modifying risk factors and optimising clinical care is the ultimate aim. Avoidance of tracheal intubation and newer methods to administer surfactant non-invasively show promise but need careful evaluation before they are routinely used.
The chest radiograph of an infant with CLD may display areas of cystic changes, linear interstitial opacities and hyper-expansion of the lungs (figure 1b) [45]. However, chest radiograph findings often do not correlate with the clinical severity in CLD [46].
Prognosis
Short-term consequences of CLD often involve the need for home oxygen therapy and a high risk of readmission to hospital [27]. CLD infants are often diagnosed with “asthma” and suffer recurrent wheeze; however, the wheeze is likely to have a different underlying cause from that of asthma in the general population. At 11 years of age, CLD infants have a higher risk of wheeze, use of inhalers and reduced lung function compared with their peers [47]. However, the impact of CLD is thought to be life-long, with survivors having reduced lung function persisting into adulthood [48].
In summary, RDS and CLD are common in preterm-born infants; although these infants may also develop other respiratory conditions especially from an infective cause. The routine use of antenatal maternal corticosteroids, exogenous surfactant and more gentle ventilation methods have improved the outcomes, there remains room for further improvements.
Common conditions primarily seen in term infants
Transient tachypnoea of the newborn
Epidemiology and risk factors
Transient tachypnoea of the newborn (TTN) is the most commonly diagnosed respiratory condition in term newborn infants [8]. When first described in 1966, it was first suggested that the self-resolving respiratory distress witnessed most often following caesarean section delivery was due to a delay in reabsorbing lung fluid [49]. This theory continues to be supported today. Delivery by caesarean section is the largest risk factor for developing TTN, particularly elective caesarean sections when mechanisms of labour have not commenced [50]. Labour is thought to induce the release of maternal catecholamines resulting in upregulation of surfactant production and trans-epithelial sodium transport causing fluid reabsorption in the infant lung [50].
The risk of TTN falls between 37 and 42 weeks’ gestation. The concept of early term birth, between 37–38 weeks’ gestation, being associated with higher risk of respiratory conditions has recently been described [51]. An increase in both early term births and caesarean sections over the past 20 years may explain the rise in respiratory admissions to neonatal units [13, 17].
It has been suggested that TTN and RDS form part of the same spectrum of disease process. Evidence suggests that full-term infants with TTN may have surfactant deficiency [52] and that antenatal maternal administration of corticosteroids may prevent TTN [53, 54], adding weight to this claim. However, the different clinical course and distinct chest radiograph appearances are evidence for different disease processes. Different findings using lung ultrasound can also be detected for the two conditions [55, 56].
Other well-established risk factors for TTN include maternal diabetes, maternal asthma, male sex, low birth weight and macrosomia [17].
Clinical aspects
An infant with TTN often but not always has mild respiratory distress from birth. A chest radiograph classically demonstrates a “wet” silhouette around the heart and fluid in the horizontal fissure. See figure 1c. The natural history of TTN is for self-resolution, so most cases are conservatively managed, with investigations to exclude more serious underlying causes, and supportive treatment using nasal cannula oxygen or non-invasive respiratory support as needed.
Prognosis
TTN generally has good prognosis. Most classifications of TTN require clinical improvement and an end to oxygen supplementation within 2–3 days to make the diagnosis. Indeed, an alternative diagnosis should be sought in cases requiring prolonged respiratory support or oxygen supplementation [57]. Two randomised trials have sought to reduce the duration of symptoms using diuretics, but no benefit has been described [58].
Persistent pulmonary hypertension of the newborn
Epidemiology and risk factors
Persistent pulmonary hypertension of the newborn (PPHN) is characterised by the failure of the pulmonary vasculature to adapt to the ex-utero environment following birth. PPHN can be primary or secondary to an associated lung condition. The incidence of PPHN is approximately one per 1000 births [59]. In utero, pulmonary vascular resistance (PVR) restricts blood flow through the lungs allowing blood to be shunted through the patent ductus arteriosus (PDA) and foramen ovale to the systemic circulation. Following birth, the combination of oxygen and respiratory movements facilitate a drop in the PVR [60]. Failure of this transition results in persisting high PVR resulting in right to left shunting at the level of the PDA and foramen ovale, leading to pulmonary hypo-perfusion, hypoxia and acidosis [61].
PPHN occurs due to, mal-development, under-development or maladaptation [61]. Mal-development and under-development are commonly associated with congenital defects which affect either the lung parenchyma or pulmonary blood vessels or both, as associated with congenital diaphragmatic hernia [62]. Infants with maladaptation have normal anatomy but fail to adapt to extra-uterine life. Most maladaptation is as a consequence of lung parenchymal disease, infection or perinatal asphyxia [61]. Maladaptation associated with primary PPHN has also been linked with chromosomal or genetic disorders, including trisomy 21 [63]; and maternal medication use during pregnancy, specifically selective serotonin uptake inhibitors; although its importance in the pathogenesis is debated [64].
Clinical aspects
PPHN is difficult to differentiate from cyanotic congenital heart disease as presentation is often very similar. Definitive diagnosis of PPHN is made using echocardiography to exclude cyanotic heart disease and estimate pulmonary arterial pressure. However, clinical findings can also assist with diagnosis. Right-to-left shunting can be evidenced by assessing pre- and post- ductal oxygen saturations, where pre-ductal saturations will be significantly higher than post-ductal. An oxygen requirement disproportionate to radiographic findings may suggest PPHN, unless PPHN is secondary to other respiratory disease [60].
Effective management of PPHN requires rapid assessment and active management to reduce PVR and address the effect of supra-systemic pulmonary pressures in those infants who may need multi-organ support. Strategies to optimise ventilation, reduce acidosis and eradicate hypoxia all contribute to the reversal of PPHN along with concurrent treatment of any underlying pathology. Continuous vital sign monitoring including pre- and post- ductal oxygen saturations and invasive blood pressure monitoring is required. Regular repeated clinical assessment with blood gas analysis and oxygenation index calculation assists in evaluating disease severity and response to treatment. Infants with PPHN are fragile and intolerant of stimulation [65]. Minimal handling, sedation, analgesia and induced paralysis are important to help avoid catastrophic changes in PVR and oxygenation.
Administering the potent vasodilator, oxygen, is key to reducing PVR. Target oxygen saturations are higher than conventional neonatal targets at a minimum level of 94% for pre-ductal readings. Invasive ventilation assists in optimising alveolar recruitment, reducing ventilation/perfusion mismatch and further reducing PVR. Inhaled nitric oxide, an endothelial derivative causing selective pulmonary vasodilation, has been demonstrated to reduce the need for extracorporeal membrane oxidation (ECMO) [66].
The effect of suprasystemic pulmonary pressures is mitigated by reducing PVR. Vasopressors improve cardiac output and increase systemic blood pressure above that in the pulmonary artery. Noradrenaline has been shown to have beneficial effects in infants with PPHN [67]. Milrinone is increasingly used due to its additional phosphodiesterase (PDE) 3 inhibitor effects.
Various additional treatments for PPHN have been tried but are not used routinely. Surfactant and magnesium sulphate may be used in selected cases. Inhibiting the breakdown of GMP with a PDE5 inhibitor such as sildenafil, or cyclic AMP with a PDE3 inhibitor such as milrinone can contribute towards reducing PVR. Prostacyclin and tolazoline are less favoured due to their side effects.
Failure of conventional treatment results in the need for ECMO. Offered at specialist centres this form of “lung by-pass” has been successfully utilised in infants with reversible disease [68].
Prognosis
The prognosis for infants with PPHN is variable. The underlying cause has a significant impact upon survival rates. At 2 years of age, survivors are noted to have severe neurodevelopment disability rates of 12% and mild disability rates of 30% [69].
Meconium aspiration syndrome
Epidemiology and risk factors
The healthy fetus does not normally pass meconium in utero. Fetal distress, usually during labour, can cause the fetus to pass meconium into the amniotic fluid before delivery. A physiological response to worsening fetal distress is for the fetus to attempt gasping respiratory effort. During such gasps the fetus may inhale meconium stained liquor into the lungs. The inhaled meconium adversely affects the lung in several ways:
Mechanical obstruction of the airways leading to ventilation/perfusion mismatch
Chemical pneumonitis
Infection
The resulting inflammatory reaction causes swelling which may block small airways; cause surfactant dysfunction; impair gaseous exchange and result in PPHN. Risk factors for meconium aspiration syndrome (MAS) are any factor increasing the risk or indicating the presence of fetal distress; post-term gestational age, reduced Apgar score, oligohydramnios and male sex [14]. Ethnicity may also affect risk of meconium staining of amniotic fluid [70].
One study demonstrated 0.43 per 1000 live births suffered from MAS requiring intubation [70]. There is evidence that the rates of meconium aspiration syndrome have fallen over the past decades, possibly due to improved antenatal care.
Clinical aspects
Most infants who have passed meconium in utero are asymptomatic, but a period of observation in hospital is routine. MAS is suspected in an infant with respiratory distress where meconium staining of the liquor has been noted. Respiratory distress will usually be present at or soon after birth. Infants may also suffer from the effects of in utero compromise and may display concurrent signs of hypoxic ischaemic encephalopathy including convulsions. The chest radiograph may show patchy changes, as seen in figure 1d.
Management of infants with MAS is largely supportive therapy while the lung inflammation resolves. The level of respiratory support will depend on severity but high frequency oscillatory ventilation or even ECMO may be required in severe cases. PPHN may develop, and should be managed as detailed above. Antibiotic therapy should be given routinely due to increased risk of infection.
Endogenous surfactant is thought to be inactivated by inhaled meconium and there is some evidence of benefit for exogenous surfactant therapy for MAS infants [71]. Lung lavage using diluted surfactant to wash out meconium from the lungs, has limited evidence of beneficial effect, with more studies need before it can be routinely recommended [72].
Outcomes
6.6% mortality is reported for infants requiring ventilation for MAS with 2.5% directly attributed to the respiratory system [70]. When all live births are studied mortality rates range between 0.96–2.00 per 100 000 live births [70, 73]. Evidence of an improving trend in mortality exists, in line with the falling incidence of MAS [73].
Pneumothorax
Epidemiology and risk factors
A pneumothorax is a leak of air from the lungs into the pleural cavity. Pneumothorax is the most common of the air-leak disorders in neonates and can occur at any gestation. Most studies report a higher risk in preterm infants [74] but a bimodal distribution with a higher risk in both the most preterms and those post-term has also been reported [75]. A recent American series showed that 0.56% of all births were complicated by pneumothorax, with low birth weight infants (<2500 g) at a higher risk [76].
Preterm infants are more likely to have underlying respiratory disease (RDS) and to receive positive pressure ventilation, both of which are associated with increased risk of developing a pneumothorax [75]. Unsurprisingly, the risk of an air leak is increased in term infants needing resuscitation and/or positive pressure ventilation, meconium aspiration and large birth weight [75].
Diagnosis and management
A spectrum of severity exists from an asymptomatic small pneumothorax that may be noted incidentally on chest radiograph, to a large tension pneumothorax causing critical respiratory failure. Diagnosis is made by chest radiograph, but using a fibre-optic light to transiluminate the chest can be useful in critical situations. Management depends on severity. A small pneumothorax will resolve spontaneously without intervention; however, a tension pneumothorax requires urgent decompression by needle thoracocentesis, prior to insertion of a chest drain. Administration of 100% oxygen to term infants to aid reabsorption of a pneumothorax is not effective [77].
Outcomes
In early preterm neonates, pneumothorax is associated with an increased risk of mortality and intraventricular haemorrhage, with the subsequent risk of developing chronic lung disease being controversial [75, 78]. One study found no increase in mortality from a pneumothorax in term infants [75].
Congenital pneumonia
Epidemiology and risk factors
Congenital pneumonia is responsible for 4.5 neonatal deaths per 100 000 birth per year in the UK [79]. Pneumonia, like neonatal sepsis, is described as being either early or late onset. Early onset, or congenital, pneumonia is associated with trans-placental infection and presents within 48 h of age [80]. Viruses, bacteria and fungi are all associated with congenital pneumonia, the most common organism responsible being group B streptococcus [81]. Chorioamnionitis is a major contributory factor for sepsis, with infected uterine fluid being inhaled by the fetus, potentially resulting in pneumonia [80]. Current guidelines support the administration of intrapartum antibiotics for chorioamnionitis, whilst prolonged rupture of membranes greater than 18 h is deemed a risk factor, and in isolation would not warrant antibiotics [82].
Congenital pneumonia has similar risk factors to neonatal sepsis. The UK National Institute for Health and Clinical Excellence guideline on early onset neonatal sepsis [83] identify risk factors to guide management:
Invasive group B streptococcal infection in a previous baby
Maternal group B streptococcal colonisation, bacteriuria or infection in the current pregnancy
Prelabour rupture of membranes
Preterm birth following spontaneous labour (before 37 weeks’ gestation)
Suspected or confirmed rupture of membranes for more than 18 h in a preterm birth
Intrapartum fever higher than 38°C or confirmed or suspected chorioamnionitis
Parenteral antibiotic treatment given to the woman for confirmed or suspected invasive bacterial infection (such as septicaemia) at any time during labour, or in the 24-h periods before and after the birth
Suspected or confirmed infection in another baby in the case of a multiple pregnancy
Newborn infants are also susceptible to late onset pneumonia. This is classified as onset >48 h of age. This occurs most commonly in infants admitted to a neonatal unit and is often associated with mechanical ventilation. The spectrum of likely causative organisms differs to early onset infection as late onset infection is considered to be hospital acquired, and therefore the choice of antibiotics used for treatment will differ.
Clinical aspects
Presentation of neonates who have congenital pneumonia is similar to those with sepsis. Signs of respiratory distress may be accompanied by temperature instability, but clinical signs of pneumonia are very difficult to elicit on examination in a neonate. Chest radiograph can show patchy consolidation with air bronchograms and a lobar distribution of consolidation, but may be initially normal. Markers of inflammation such as C-reactive protein and white blood cell count are unreliable in diagnosing infection in the neonatal population, and normal values should not be reassuring in an unwell infant.
Antibiotic treatment is the mainstay of treatment. Microbiological cultures obtained from the mother or infant can be useful in guiding treatment, although often no causative organism is identified. Supportive interventions such as oxygen and mechanical ventilation may be required.
The consequences of any neonatal infection can be overwhelming. Organ failure may ensue and intensive care support may be required. Neonates are prone to sepsis and can deteriorate rapidly. Early identification and treatment with antibiotics is vital in reducing mortality and morbidity. This is reflected in the guidance that antibiotics must be commenced within 1 h of decision to treat [83].
Prognosis
The outcomes of neonates with pneumonia vary greatly and are dependent upon the organism and its virulence. However, early identification and treatment of neonates at risk of infection or with symptoms of infection reduces both morbidity and mortality. A worse prognosis is seen in infants with low birth weights and for those with intrauterine compared to those with later onset disease [80].
Surgical and congenital conditions
Congenital anomalies within the airways and lungs may require surgical correction. The most common problems are:
Congenital diaphragmatic hernia (CDH)
Congenital pulmonary airway malformation (CPAM)
Tracheo-oesophageal fistula (TOF)
Detailed reviews of these conditions are available elsewhere [84–86] including two recent European Respiratory Society Task Force reviews on CDH and CPAM [87, 88].
Congenital diaphragmatic hernia
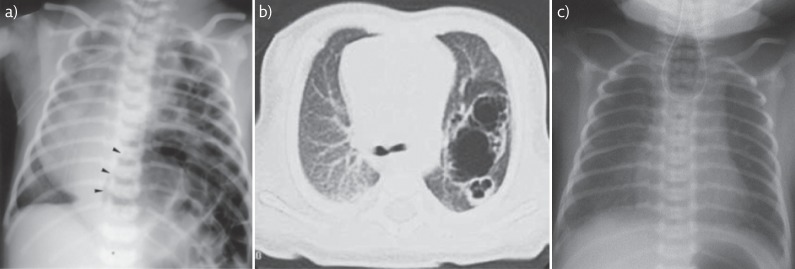
CDH was recently the focus of a recent European Respiratory Society task force review [87]. One in 2500 live births is affected by CDH [89]. A developmental failure of the diaphragm during its embryological formation allows herniation of abdominal organs into the chest affecting lung growth and alveolar development. Many genetic syndromes and chromosomal abnormalities are associated with CDH [84], but the underlying pathogenesis and pathophysiology are poorly understood. Antenatal diagnosis is made in 59% of cases [90] permitting delivery in a surgical centre. A chest radiograph of a left-sided CDH is shown in figure 2a.
Figure 2.
Radiology images of surgical conditions/congenital anomalies. a) Chest radiograph of infant with large left sided CDH. Note presence of bowel and stomach (arrowheads) within the chest. Mediastinal shift to the right. Reproduced from [91] with permission from the publisher. b) CT image of left sided CCAM demonstrating large cystic areas and c) chest radiograph demonstrating coiled nasogastric tube in the upper oesophageal pouch indicating oesophageal atresia. Note gas in stomach indicating presence of fistula between distal oesophagus and trachea. b) and c) reproduced from [21] with permission from the publisher.
Surgical correction of the diaphragmatic defect is required, usually undertaken after a period of respiratory stabilisation allowing pulmonary pressures to fall. ECMO has been used pre- and peri-operatively in cases of CDH when optimising ventilation is insufficient to overcome the respiratory failure. However this approach remains controversial [87].
Some reports suggest survival rates have shown an improving trend over recent years with in excess of 80% undergoing surgery surviving to discharge [92]; however when all cases are considered, the mortality rate remains between 42–68% [84].
Congenital pulmonary airway malformation
CPAM, also often still called congenital cystic adenomatous malformation of the lung (CCAM), affects one in 10 000 to one in 35 000 births [85]. Most cases are diagnosed by antenatal ultrasound scan. Large lesions are associated with polyhydramnios due to compression of the fetal oesophagus, impairing swallowing of amniotic fluid. Histologically, an overlap exists between CPAM and lung sequestration, a condition when a portion of the lung is not connected to the bronchial tree. A mixed picture is described of sequestration containing areas of CPAM [93]. A spectrum of postnatal clinical presentation exists from asymptomatic, to the infant in respiratory failure due to the mass effect of a large lesion or secondary pulmonary hypoplasia. Initial investigations should include a chest radiograph after delivery, and a CT scan prior to surgery. An example of a CT scan of a large left sided CPAM is given in figure 2b.
Guidelines suggest that even asymptomatic infants should have lesions excised within the first 6 months of life to avoid the risk of malignant transformation but excision of asymptomatic lesions remains controversial [94, 95] Other risks associated with a CPAM are pneumothorax, pneumonia and haemoptysis. Surgical excision can be undertaken via open thoracotomy or by a less invasive thoracoscopic approach. The prognosis after excision of a CPAM is generally good with the rare complications of air leak, bronchopulmonary fistula and sepsis. Elective surgery of asymptomatic cases has a lower rate of complications compared with surgery after symptom development [96].
Tracheo-oesophageal fistula
TOF and oesophageal atresia (OA) normally occur as part of the same congenital anomaly in about 1 in 2500 births [97]. The fistula can connect the trachea to the proximal portion, distal portion or both portions of the oesophagus. In the rare H-type fistula TOF is present without oesophageal atresia. Polyhydramnios and a small stomach on antenatal ultrasound scan should raise suspicions, but the diagnosis is usually confirmed postnatally with a chest radiograph confirming a coiled nasogastric tube in the upper pouch of the oesophagus (see figure 2c). Clinically, an infant will present with drooling of saliva and choking if feeding is attempted. The H-type fistula usually presents later in the neonatal or infancy period with evidence of recurrent aspiration. TOF and oesophageal atresia can be associated with genetic syndromes, most commonly the VACTERL (vertebral/anorectal/cardiac/tracheo-oesophageal/renal/limb anomalies) association, but most cases are sporadic [86].
Respiratory issues are related to aspiration of secretions either due to overflow from the oesophageal pouch or via the TOF. In cases of oesophageal atresia this can be minimised by placement of a dual lumen Replogal tube in the upper pouch to allow flushing and suctioning of secretions. Definitive management is by surgical correction.
The common respiratory long-term complication of TOF is tracheomalacia, resulting in a “TOF cough,” usually managed conservatively. Long-term gastrointestinal problems such as oesophageal strictures and gastro-oesophageal reflux are common.
Rare respiratory disorders in the newborn
The most common respiratory disorders affecting the neonate have been summarised. A number of less common conditions are shown in table 2.
Table 2.
Rare respiratory conditions affecting the newborn
| Diagnosis | Diagnostic features | Notes |
| Pulmonary complications of disease | ||
| Pulmonary haemorrhage | Clinical observation of blood from endotracheal tube | Usually occurs in ventilated very low birth weight infants, or complicating meconium aspiration syndrome. Risk of pulmonary haemosiderosis following severe or recurrent episodes. |
| Pleural effusion (chylothorax) | Characteristic chest radiograph appearance | Usually consequence of hydrops fetalis or chromosomal anomaly. Also iatrogenic, following surgical procedure or leak of parenteral nutrition from central venous catheter. Mortality from underlying cause. |
| Primary lung disease | ||
| Surfactant protein deficiency syndromes | Persistent and severe RDS presentation in a term infant | Mutations in surfactant proteins or lamellar body associated transport protein result in lethal respiratory distress due to deficiency in surfactant activity. |
| Alveolar capillary dysplasia | Features of severe PPHN, resistant to treatment | Diagnosis made at post mortem, with evidence of developmental failure of alveoli and reduced capillary density. Approaching 100% mortality. |
| Pulmonary Hypoplasia | Respiratory failure from birth. Small volume lungs on chest radiograph | Primary pulmonary hypoplasia rare, more commonly secondary to severe oligohydramnios, exomphalos or space occupying lesion in the chest (CDH or CPAM) Diagnosis confirmed at post mortem. |
| Congenital malformations | ||
| Pulmonary sequestration | Large lesions detected antenatally. May present with hydrops fetalis. Older child presents with chronic cough or recurrent pneumonia. |
Portion of lung not connected to bronchial tree. Can be associated with CDH or cardiac anomaly. Surgical excision recommended for symptomatic lesions, intervention for asymptomatic lesions is controversial. |
| Lobar emphysema | Large bullous cystic area on chest radiograph | Hyperinflation of one or more lobes compressing surrounding structures. Lobectomy required for symptomatic cases. |
| Choanal atresia | Inability to pass nasogastric tube. Apnoea and cyanosis when not crying (bilateral choanal atresia) | 50% of cases part of CHARGE syndrome. Bilateral atresia cases require urgent surgery to create airway. |
Conclusion
The immediate period after birth is crucial to the adaption of the infant to extra-uterine life. The newborn infant is vulnerable to a range of respiratory disorders, all presenting with signs of respiratory distress. Thorough clinical assessment and appropriate investigation is required for all infants presenting with signs of respiratory distress to ensure accurate diagnosis and correct treatment. It is important that any healthcare professional who comes into contact with newborn infants is aware of the signs of respiratory distress. Prompt recognition of the more serious underlying conditions is important to improve outcomes. The most common causes of respiratory distress in preterm and term infants have been summarised along with respiratory conditions requiring surgery.
FAQs when assessing an infant with respiratory distress
Modified from [14] with permission from the publisher.
Is it a cardiac or respiratory problem?
Consider the need for chest radiograph and echocardiogram.
Is anything else causing the respiratory distress?
Consider metabolic, renal or neurological causes.
What is the gestational age of the baby?
Preterm neonates (<37 weeks) are more likely to have respiratory distress syndrome.
Post-term neonates (>42 weeks) are more likely to have meconium aspiration syndrome.
Late preterm and term neonates are more likely to have transient tachypnoea of the newborn.
Is it severe or mild respiratory distress?
Severe distress is more likely with respiratory distress syndrome, meconium aspiration syndrome or persistent pulmonary hypertension of the neonate.
Mild distress is more likely with transient tachypnoea of the newborn.
Are there any known congenital anomalies?
Review antenatal scan reports for congenital diaphragmatic hernia, congenital cystic adenomatoid malformation, etc.
What was the delivery method?
Pre-labour section is more likely to be transient tachypnea of the newborn.
Evidence of meconium stained amniotic fluid is more likely to be meconium aspiration syndrome.
Is there poor improvement with increasing oxygen flow?
Consider persistent pulmonary hypertension of the neonate or congenital cyanotic heart disease in the case of persistent hypoxia and cyanosis despite 100% oxygen.
Are there risk factors for sepsis?
Premature rupture of membranes, group B streptococcus on high vaginal swab, maternal pyrexia or raised inflammatory markers in maternal blood would suggest pneumonia.
Footnotes
Conflict of interest None declared.
References
- 1.Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 2012; 39: 769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha SK, Donn SM. Fetal-to-neonatal maladaptation. Semin Fetal Neonatal Med 2006; 11: 166–173. [DOI] [PubMed] [Google Scholar]
- 3.Helve O, Pitkänen O, Janér C, et al. Pulmonary fluid balance in the human newborn infant. Neonatology 2009; 95: 347–352. [DOI] [PubMed] [Google Scholar]
- 4.Heymann MA. Control of the pulmonary circulation in the fetus and during the transitional period to air breathing. Eur J Obstet Gynecol Reprod Biol 1999; 84: 127–132. [DOI] [PubMed] [Google Scholar]
- 5.Swanson JR, Sinkin RA. Transition from fetus to newborn. Pediatr Clin North Am 2015; 62: 329–343. [DOI] [PubMed] [Google Scholar]
- 6.Langston C, Kida K, Reed M, et al. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis 1984; 129: 607–613. [PubMed] [Google Scholar]
- 7.Rubaltelli FF, Dani C, Reali MF, et al. Acute neonatal respiratory distress in Italy: a one-year prospective study. Italian Group of Neonatal Pneumology. Acta Paediatr 1998; 87: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian J Pediatr 1996; 63: 93–98. [DOI] [PubMed] [Google Scholar]
- 9.Pramanik AK, Rangaswamy N, Gates T. Neonatal respiratory distress: a practical approach to its diagnosis and management. Pediatr Clin North Am 2015; 62: 453–469. [DOI] [PubMed] [Google Scholar]
- 10.Parkash A, Haider N, Khoso ZA, et al. Frequency, causes and outcome of neonates with respiratory distress admitted to Neonatal Intensive Care Unit, National Institute of Child Health, Karachi. J Pak Med Assoc 2015; 65: 771–775. [PubMed] [Google Scholar]
- 11.Qian L, Liu C, Guo Y, et al. Current status of neonatal acute respiratory disorders: a one-year prospective survey from a Chinese neonatal network. Chin Med J (Engl) 2010; 123: 2769–2775. [PubMed] [Google Scholar]
- 12.Ersch J, Roth-Kleiner M, Baeckert P, et al. Increasing incidence of respiratory distress in neonates. Acta Paediatr 2007; 96: 1577–1581. [DOI] [PubMed] [Google Scholar]
- 13.Kotecha SJ, Gallacher DJ, Kotecha S. The respiratory consequences of early-term birth and delivery by caesarean sections. Paediatr Respir Rev 2015; 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev 2013; 14: 29–36. [DOI] [PubMed] [Google Scholar]
- 15.Kotecha SJ, Adappa R, Gupta N, et al. Safety and efficacy of high-flow nasal cannula therapy in preterm infants: a meta-analysis. Pediatrics 2015; 136: 542–553. [DOI] [PubMed] [Google Scholar]
- 16.Ghafoor T, Mahmud S, Ali S, et al. Incidence of respiratory distress syndrome. J Coll Physicians Surg Pak 2003; 13: 271–273. [PubMed] [Google Scholar]
- 17.Dani C, Reali MF, Bertini G, et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Eur Respir J 1999; 14: 155–159. [DOI] [PubMed] [Google Scholar]
- 18.Joshi S, Kotecha S. Lung growth and development. Early Hum Dev 2007; 83: 789–794. [DOI] [PubMed] [Google Scholar]
- 19.Hallman M, Merritt TA, Pohjavuori M, et al. Effect of surfactant substitution on lung effluent phospholipids in respiratory distress syndrome: evaluation of surfactant phospholipid turnover, pool size, and the relationship to severity of respiratory failure. Pediatr Res 1986; 20: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 20.Pickerd N, Kotecha S. Pathophysiology of respiratory distress syndrome. Paediatr Child Health (Oxford) 2009; 19: 153–157. [Google Scholar]
- 21.Morris SJ. Radiology of the chest in neonates. Paediatr Child Health (Oxford) 2003; 13: 460–468. [Google Scholar]
- 22.Wapner RJ. Antenatal corticosteroids for periviable birth. Semin Perinatol 2013; 37: 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006; 3: CD004454. [DOI] [PubMed] [Google Scholar]
- 24.Aguar M, Nuñez A, Cubells E, et al. Administration of surfactant using less invasive techniques as a part of a non-aggressive paradigm towards preterm infants. Early Hum Dev 2014; 90: Suppl 2, S57–S59. [DOI] [PubMed] [Google Scholar]
- 25.Sweet DG, Carnielli V, Greisen G, et al. European Consensus Guidelines on the Management of Neonatal Respiratory Distress Syndrome in Preterm Infants: 2013 Update. Neonatology 2013; 103: 353–368. [DOI] [PubMed] [Google Scholar]
- 26.Manley BJ, Dold SK, Davis PG, et al. High-flow nasal cannulae for respiratory support of preterm infants: a review of the evidence. Neonatology 2012; 102: 300–308. [DOI] [PubMed] [Google Scholar]
- 27.Greenough A. Long term respiratory outcomes of very premature birth (<32 weeks). Seminars in Fetal and Neonatal Medicine 2012; 73–76. [DOI] [PubMed] [Google Scholar]
- 28.Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clin Perinatol 2012; 39: 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose C, Van Marter LJ, Laughon M, et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 2009; 124: e450–e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002; 140: 171–176. [DOI] [PubMed] [Google Scholar]
- 31.Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 2010; 15: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez A, Sosenko IR, Chandar J, et al. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 1996; 128: 470–478. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty M, McGreal EP, Kotecha S. Acute lung injury in preterm newborn infants: mechanisms and management. Paediatr Respir Rev 2010; 11: 162–170; quiz 170. [DOI] [PubMed] [Google Scholar]
- 34.Kotecha S, Chan B, Azam N, et al. Increase in interleukin-8 and soluble intercellular adhesion molecule-1 in bronchoalveolar lavage fluid from premature infants who develop chronic lung disease. Arch Dis Child Fetal Neonatal Ed 1995; 72: F90–F96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneibel KR, Fitzpatrick AM, Ping X-D, et al. Inflammatory mediator patterns in tracheal aspirate and their association with bronchopulmonary dysplasia in very low birth weight neonates. J Perinatol 2013; 33: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotecha S, Wilson L, Wangoo A, et al. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res 1996; 40: 250–256. [DOI] [PubMed] [Google Scholar]
- 37.Kotecha S, Mildner RJ, Prince LR, et al. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax 2003; 58: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty M, McGreal EP, Williams A, et al. Role of serine proteases in the regulation of interleukin-877 during the development of bronchopulmonary dysplasia in preterm ventilated infants. PLoS One 2014; 9: e114524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res Part A - Clin Mol Teratol 2014; 100: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jobe AH. The New BPD. NeoReviews 2006; e531–e545. [Google Scholar]
- 41.Northway WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276: 357–368. [DOI] [PubMed] [Google Scholar]
- 42.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999; 46: 641–643. [DOI] [PubMed] [Google Scholar]
- 43.Shah VS, Ohlsson A, Halliday HL, et al. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Syst Rev 2012; 5: CD001969. [DOI] [PubMed] [Google Scholar]
- 44.Stewart A, Brion LP, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev 2011; 9: CD001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griscom NT, Wheeler WB, Sweezey NB, et al. Bronchopulmonary dysplasia: radiographic appearance in middle childhood. Radiology 1989; 171: 811–814. [DOI] [PubMed] [Google Scholar]
- 46.Rossi UG, Owens CM. The radiology of chronic lung disease in children. Arch Dis Child 2005; 90: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 2010; 182: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Northway WH, Moss RB, Carlisle KB, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med 1990; 323: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 49.Avery ME, Gatewood OB, Brumley G. Transient tachypnea of newborn. Possible delayed resorption of fluid at birth. Am J Dis Child 1966; 111: 380–385. [DOI] [PubMed] [Google Scholar]
- 50.Tutdibi E, Gries K, Bücheler M, et al. Impact of labor on outcomes in transient tachypnea of the newborn: population-based study. Pediatrics 2010; 125: e577–e583. [DOI] [PubMed] [Google Scholar]
- 51.American College of Obstetricians and Gynecologists. American Congress of Obstetricians and Gynecologists committee opinion no. 579: Definition of Term Pregnancy Obs Gynecol 2013; 122: 1139–1140. [DOI] [PubMed] [Google Scholar]
- 52.Estorgato GR, Fiori HH, da Silva Ribeiro MA, et al. Surfactant deficiency in full-term newborns with transient tachypnea delivered by elective C-section. Pediatr Pulmonol 2015. [DOI] [PubMed] [Google Scholar]
- 53.Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ 2005; 331: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dileep A, Khan NB, Sheikh SS. Comparing neonatal respiratory morbidity in neonates delivered at term by elective Caesarean section with and without dexamethasone: retrospective cohort study. J Pak Med Assoc 2015; 65: 607–611. [PubMed] [Google Scholar]
- 55.Liu J, Wang Y, Fu W, et al. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine (Baltimore) 2014; 93: e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vergine M, Copetti R, Brusa G, et al. Lung ultrasound accuracy in respiratory distress syndrome and transient tachypnea of the newborn. Neonatology 2014; 106: 87–93. [DOI] [PubMed] [Google Scholar]
- 57.Morioka I, Yamana K, Kurokawa D, et al. How long is transient tachypnea of the newborn dependent on oxygen supplementation? Pediatr Int 2015; 57: 1054–1055. [DOI] [PubMed] [Google Scholar]
- 58.Kassab M, Khriesat WM, Anabrees J. Diuretics for transient tachypnoea of the newborn. Cochrane Database Syst Rev 2015; 11: CD003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawford A, Tulloh RMR. Cardiovascular adaptation to extra uterine life. Paediatr Child Health (Oxford) 2015; 25: 1–6. [Google Scholar]
- 60.Greenough A, Khetriwal B. Pulmonary hypertension in the newborn. Paediatr Respir Rev 2005; 6: 111–116. [DOI] [PubMed] [Google Scholar]
- 61.Stayer SA, Liu Y. Pulmonary hypertension of the newborn. Best Pract Res Clin Anaesthesiol 2010; 24: 375–386. [DOI] [PubMed] [Google Scholar]
- 62.Steinhorn RH. Diagnosis and treatment of pulmonary hypertension in infancy. Early Hum Dev 2013; 89: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cua CL, Blankenship A, North AL, et al. Increased incidence of idiopathic persistent pulmonary hypertension in Down syndrome neonates. Pediatr Cardiol 2007; 28: 250–254. [DOI] [PubMed] [Google Scholar]
- 64.Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA 2015; 313: 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Storme L, Aubry E, Rakza T, et al. Pathophysiology of persistent pulmonary hypertension of the newborn: impact of the perinatal environment. Arch Cardiovasc Dis 2013; 106: 169–177. [DOI] [PubMed] [Google Scholar]
- 66.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2006; 4: CD000399. [DOI] [PubMed] [Google Scholar]
- 67.Tourneux P, Rakza T, Bouissou A, et al. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J Pediatr 2008; 153: 345–349. [DOI] [PubMed] [Google Scholar]
- 68.Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev 2008; 3: CD001340. [DOI] [PubMed] [Google Scholar]
- 69.Bendapudi P, Barr S. Diagnosis and management of pulmonary hypertension of the newborn. Paediatr Child Health (Oxford) 2014; 24: 12–16. [Google Scholar]
- 70.Dargaville PA, Copnell B. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics 2006; 117: 1712–1721. [DOI] [PubMed] [Google Scholar]
- 71.El Shahed AI, Dargaville PA, Ohlsson A, et al. Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst Rev 2014; 12: CD002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn S, Choi HJ, Soll R, et al. Lung lavage for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev 2013; 4: CD003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sriram S, Wall SN, Khoshnood B, et al. Racial disparity in meconium-stained amniotic fluid and meconium aspiration syndrome in the United States, 1989-2000. Obstet Gynecol 2003; 102: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 74.Ramesh Bhat Y, Ramdas V. Predisposing factors, incidence and mortality of pneumothorax in neonates. Minerva Pediatr 2013; 65: 383–388. [PubMed] [Google Scholar]
- 75.Duong HH, Mirea L, Shah PS, et al. Pneumothorax in neonates: trends, predictors and outcomes. J Neonatal Perinatal Med 2014; 7: 29–38. [DOI] [PubMed] [Google Scholar]
- 76.Aly H, Massaro A, Acun C, et al. Pneumothorax in the newborn: clinical presentation, risk factors and outcomes. J Matern Neonatal Med 2014; 27: 402–406. [DOI] [PubMed] [Google Scholar]
- 77.Clark SD, Saker F, Schneeberger MT, et al. Administration of 100% oxygen does not hasten resolution of symptomatic spontaneous pneumothorax in neonates. J Perinatol 2014; 34: 528–531. [DOI] [PubMed] [Google Scholar]
- 78.Bhatia R, Davis PG, Doyle LW, et al. Identification of pneumothorax in very preterm infants. J Pediatr 2011; 159: 115–120. [DOI] [PubMed] [Google Scholar]
- 79.Tambe P, Sammons HM, Choonara I. Why do young children die in the UK? A comparison with Sweden. Arch Dis Child 2015; 100: 928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nissen MD. Congenital and neonatal pneumonia. Paediatr Respir Rev 2007; 8: 195–203. [DOI] [PubMed] [Google Scholar]
- 81.Heath PT, Balfour G, Weisner AM, et al. Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet 2004; 363: 292–294. [DOI] [PubMed] [Google Scholar]
- 82.Wojcieszek AM, Stock OM, Flenady V. Antibiotics for prelabour rupture of membranes at or near term. Cochrane Database Syst Rev 2014; 10: CD001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Institute for Health and Clinical Excellence. Neonatal infection: antibiotics for prevention and treatment | NICE guideline. NICE; 2012. www.nice.org.uk/guidance/cg149 Date last updated: July 2014. Date last accessed: February 1, 2016. [PubMed]
- 84.Leeuwen L, Fitzgerald DA. Congenital diaphragmatic hernia. J Paediatr Child Health 2014; 50: 667–673. [DOI] [PubMed] [Google Scholar]
- 85.Lakhoo K. Management of congenital cystic adenomatous malformations of the lung. Arch Dis Child Fetal Neonatal Ed 2009; 94: F73–F76. [DOI] [PubMed] [Google Scholar]
- 86.Holland AJA, Fitzgerald DA. Oesophageal atresia and tracheo-oesophageal fistula: current management strategies and complications. Paediatr Respir Rev 2010; 11: 100–106. [DOI] [PubMed] [Google Scholar]
- 87.Kotecha S, Barbato A, Bush A, et al. Congenital diaphragmatic hernia. Eur Respir J 2012; 39: 820–829. [DOI] [PubMed] [Google Scholar]
- 88.Kotecha S, Barbato A, Bush A, et al. Antenatal and postnatal management of congenital cystic adenomatoid malformation. Paediatr Respir Rev 2012; 13: 162–170. [DOI] [PubMed] [Google Scholar]
- 89.Langham MR, Kays DW, Ledbetter DJ, et al. Congenital diaphragmatic hernia. Epidemiology and outcome. Clin Perinatol 1996; 23: 671–688. [PubMed] [Google Scholar]
- 90.Garne E, Haeusler M, Barisic I, et al. Congenital diaphragmatic hernia: evaluation of prenatal diagnosis in 20 European regions. Ultrasound Obstet Gynecol 2002; 19: 329–333. [DOI] [PubMed] [Google Scholar]
- 91.Arthur R. The neonatal chest X-ray. Paediatr Respir Rev 2001; 2: 311–323. [DOI] [PubMed] [Google Scholar]
- 92.Jawaid WB, Qasem E, Jones MO, et al. Outcomes following prosthetic patch repair in newborns with congenital diaphragmatic hernia. Br J Surg 2013; 100: 1833–1837. [DOI] [PubMed] [Google Scholar]
- 93.Desai S, Dusmet M, Ladas G, et al. Secondary vascular changes in pulmonary sequestrations. Histopathology 2010; 57: 121–127. [DOI] [PubMed] [Google Scholar]
- 94.Delacourt C, Hadchouel A, Khen Dunlop N. Shall all congenital cystic lung malformations be removed? The case in favour. Paediatr Respir Rev 2013; 14: 169–170. [DOI] [PubMed] [Google Scholar]
- 95.Kotecha S. Should asymptomatic congenital cystic adenomatous malformations be removed? The case against. Paediatr Respir Rev 2013; 14: 171–172. [DOI] [PubMed] [Google Scholar]
- 96.Kapralik J, Wayne C, Chan E, et al. Surgical versus conservative management of congenital pulmonary airway malformation in children: A systematic review and meta-analysis. J Pediatr Surg 2015. [DOI] [PubMed] [Google Scholar]
- 97.Smith N. Oesophageal atresia and tracheo-oesophageal fistula. Early Hum Dev 2014; 90: 947–950. [DOI] [PubMed] [Google Scholar]