Abstract
Key points
Asthma in the elderly can be difficult to identify due to modifications of its clinical features and functional characteristics.
Several comorbidities are associated with asthma in the elderly, and this association differs from that observed in younger patients.
In clinical practice, physicians should treat comorbidities that are correlated with asthma (i.e. rhinitis or gastro-oesophageal reflux), assess comorbidities that may influence asthma outcomes (i.e. depression or cognitive impairment) and try to prevent comorbidities related to ‘drug-associated side-effects (i.e. cataracts, arrhythmias or osteoporosis).
“Geriatric asthma” should be the preferred term because it implies the comprehensive and multidimensional approach to the disease in the older populations, whereas “asthma in the elderly” is only descriptive of the occurrence of the disease in this age range.
Educational aims
To present critical issues in performing differential diagnosis of asthma in the elderly.
To offer the instrument to implement the management of asthma in the most advanced ages.
Asthma is a chronic airway disease that affects all ages, but does this definition also include the elderly? Traditionally, asthma has been considered a disease of younger age, but epidemiological studies and clinical experience support the concept that asthma is as prevalent in older age as it is in the young. With the ever-increasing elderly population worldwide, the detection and proper management of the disease in old age may have a great impact from the public health perspective. Whether asthma in the elderly maintains the same characteristics as in young populations is an interesting matter. The diagnostic process in older individuals with suspected asthma follows the same steps, namely a detailed history supported by clinical examination and laboratory investigations; however, it should be recognised that elderly patients may partially lose reversibility of airway obstruction. The correct interpretation of spirometric curves in the elderly should take into account the physiological changes in the respiratory system. Several factors contribute to delaying the diagnosis of asthma in the elderly, including the age-related impairment in perception of breathlessness. The management of asthma in advanced age is complicated by the comorbidities and polypharmacotherapy, which advocate for a comprehensive approach with a multidimensional assessment. It should be emphasised that older age frequently represents an exclusion criterion for eligibility in clinical trials, and current asthma medications have rarely been tested in elderly asthmatics. Ageing is associated with pharmacokinetic changes of the medications. As a consequence, absorption, distribution, metabolism and excretion of antiasthmatic medications can be variably affected. Similarly, drug-to-drug interactions may reduce the effectiveness of inhaled medications and increase the risk of side-effects. For this reason, we propose the term “geriatric asthma” be preferred to the more generic “asthma in the elderly”.
Short abstract
Asthma in the elderly: is it time think differently? http://ow.ly/Y71zN
Introduction
Asthma in the elderly is largely under- or misdiagnosed and, thus, undertreated. This is mainly due to the erroneous belief that asthma is a disease of childhood. It is not rare to fall into the trap of attributing respiratory symptoms suggestive of asthma to conditions other than asthma, i.e. chronic obstructive pulmonary disease (COPD), when they occur at older ages. Old observational reports and new epidemiological studies confirm that asthma is as frequent in older as it is in younger populations. Lee and Stretton [1] in 1972 and later Burr et al. [2] in 1979 showed that features of asthma could be documented in individuals aged 70 years and over, to the same proportion of asthma as in younger ages. A MEDLINE search using “asthma” and “elderly” as MeSH (Medical Subject Headings) terms has yielded a steadily increase in articles with time, implying a better understanding of the disease and greater attention to the topic. The prevalence of asthma in the elderly is difficult to establish, and this is due on one hand to the heterogeneity of the clinical and functional presentations of the disease in the aged population (including the poor perception of symptoms and the tendency to lose the airway reversibility), and on the other hand, to the definition of “elderly”, which is usually, but not always, considered to begin at 65 years of age. In addition, little information is available on the rate of relapse after asthma remission in later life. Several studies in European and US populations have shown that asthma prevalence among people aged over 65 years ranges from 1.8% to 10.9% [3]. If anything, the burden of asthma in the elderly is more relevant than in young asthmatics, regarding costs, quality of life, hospitalisation and mortality [4, 5].
The recognition of age of onset is one of the mandatory steps when dealing with older asthmatics, because it allows us to distinguish between two entities that may even behave as different diseases, referred as “early” and “late” onset asthma. The late-onset disease appears to be more severe and less atopic. Is it possible that asthma occurring for the first time in the most advanced ages is a different disease than asthma that begins early in life and carries over to older ages? Asthma that starts in childhood may persist until the most advanced ages, or may disappear in adult ages and relapse at older ages. In these cases, the disease can be definitely regarded as early-onset asthma. The latter also includes a condition in which the clinical manifestations of asthma occur in older age but recall bias does not allow establishment of the age of onset. The opposite scenario includes asthma that makes its appearance for the first time at older ages (late-onset asthma). The question is at which age to set the threshold for the definition of “late” onset. We propose that 65 years of age should be the cut-off to identify a late-onset disease, as opposed to, for example, 45 years.
It must be stated that the knowledge of asthma in the elderly suffers from the fact that there has been very little original research in this field. This is because advanced age is invariably an exclusion criterion in almost all randomised clinical trials (RCTs), thus denying old asthmatic individuals access to experimental observations and interventional studies. Our group has recently shown that asthmatics enrolled in RCTs are not fully representative of real-life patients [6], and this is particularly true in elderly patients in whom the odds ratio to be excluded from RCTs increases to 18.6 at the age of 75–84 years compared to the youngest populations. The other factor that contributes to excluding elderly asthmatics from RCTs is the frequent occurrence of comorbidities, which are the norm in older individuals and tend to increase with ageing. The conclusion from the study by Battaglia et al. [6] is that almost half of elderly asthmatics are treated with drugs that have not been tested in such populations.
The frequent coexistence of comorbid conditions in older patients, which are higher in number and different than those detected in younger asthmatics, may complicate the management of asthma. Comorbidities (and polypharmacy) can influence the metabolism and excretion of respiratory drugs, and can negatively impact adherence to and persistence with chronic treatment. For the above reasons, the optimal control of the disease in older ages cannot be achieved without including a multidimensional approach with a multidisciplinary intervention. We propose to use the more comprehensive “geriatric asthma” instead of the generic and somewhat restrictive “asthma in the elderly”, which is only descriptive of the prevalence in a specific age group. A holistic approach is advocated when physicians deal with elderly subjects who suffer from asthma and may be the key factor to attain optimal control of the disease in this age range.
Diagnosis of asthma in advanced age
One of the crucial issues related to asthma in the most advanced ages is the proper recognition of the disease. The question is whether the traditional diagnostic algorithm can be applied in older individuals. Are the tools employed and the criteria used suitable also for older populations or should they be modified (or used differently) based on age-related changes? In approaching the differential diagnosis of asthma in the elderly, physicians may be faced with several issues, including the objective (i.e. spirometric) evaluation of airway obstruction and modifications of clinical presentation of asthma. It has been shown that <50% of patients with a new diagnosis of asthma receive objective pulmonary function testing, and this percentage decreases to 42%, 29% and 9.5% in patients aged 70–79, 80–89, and 90–99 years, respectively [7]. It is plausible to hypothesise that both overdiagnosis and underdiagnosis of asthma in the elderly would be lower if objective diagnostic testing were used widely. Obvious difficulties exist in performing spirometry in the elderly, mainly because of the not trivial prevalence of cognitive impairments; several tests of cognition have been investigated as predictors of inability to perform spirometry in old ages [8, 9]. These include the Mini Mental State Examination, inability to copy intersecting pentagons and clock drawing tests, with the first two being more appropriate in predicting inability to perform spirometry in old age [8]. All of them are easy to perform and require only a few minutes, which suggests that a rapid cognitive assessment should be part of the multidimensional management of geriatric asthma. It has been demonstrated that the vast majority of elderly subjects is capable of performing acceptable and reproducible spirometries [10, 11] and carbon monoxide diffusion capacity tests [11], if the personnel are adequately trained. Moreover, specific “age-tailored” tests, such as the measurement of the forced expiratory volume in the first 6 s (FEV6), has been shown to be useful in those patients in whom the complete expiration and, thus, the achievement of a valid forced vital capacity (FVC) can be difficult [12]. The use of FEV6 as substitute for FVC has the advantage of requiring an easier and faster manoeuvre, with less physical effort for patients.
Correct interpretation of spirometric curves in the elderly is probably the most difficult task for nontrained physicians. The physiological changes in the respiratory system due to the normal ageing process may confuse the picture. One of the most debated issues about spirometry in the elderly is the need for appropriate reference values in order to identify obstruction and restriction without misclassification [13]. As mentioned, physiological changes of the respiratory system with ageing may mimic the presence of airway obstruction. In this context, the so-called senile lung presents with features of lung hyperinflation and the healthy elderly may appear to be emphysematous patients [14]. However, in the senile lung, the destruction of the lung parenchyma (i.e. emphysema) is absent, whereas a homogeneous enlargement of the alveolar airspaces is documented. The senile lung can be easily distinguished from the emphysematous lung, by measuring the lung density by computed tomography [15]. In this context, the static elastic recoil pressure of the lung diminishes [16], causing reduction in flow rates and vital capacity. The increased compressibility of the airways with age becomes evident at low lung volumes, where the reduction in lung elastic recoil overcomes that of the airways, leading to airflow limitation and airway closure. In recent years, several research groups have published reference equations for spirometric parameters obtained from cohorts of elderly subjects [17–22]. In evaluating a spirometric test in the elderly, physicians need to use software that allows the selection of the appropriate set of reference values in order to prevent misclassifications; in particular, for borderline values, the main risk is of classifying the healthy elderly as mildly obstructed. To date, reference values covering all ages (from infancy to geriatric age in a unique dataset [17]) are available. The use of these equations, instead of separate equations for adults and the elderly, may prevent confusion in interpreting spirometry in older individuals [23].
Perhaps the most challenging task in interpreting airway obstruction in the elderly is the differentiation of asthma from COPD. The above-described structural changes of the lung lead to a condition of the airways that is no longer susceptible to the effects of treatment, accounting for the loss of reversibility and the persistence of airway obstruction. The possibility of development of fixed airflow limitation in elderly asthmatics may contribute to generating confusion, as the scholastic distinction between the two chronic respiratory diseases based on the degree of response to a rapidly acting bronchodilator agent loses its strength. However, it has been demonstrated that elderly subjects with a history of asthma have different pathology compared to subjects with a history of COPD, even in the presence of fixed airflow limitation [24]. Moreover, the presence of physical disability may lead to misdiagnosis of COPD in elderly asthmatics [25]. Even smoking history is not a clear-cut condition to distinguish asthmatics from individuals with COPD: about one out of three adult asthmatics is a current smoker [26]. What are the clinical implications of this assumption? An obstructed elderly subject with a long history of asthma should be treated as an asthmatic (not as COPD), regardless of the presence of reversibility of bronchial obstruction.
What are then the additional tools to help to recognise asthma or to exclude other respiratory conditions? Emphysema is not a feature of long-standing asthma in the elderly, and the diffusing capacity of the lung for carbon monoxide (DLCO) should be normal and therefore used to differentiate asthma from COPD [27]. Nevertheless, Gelb et al. [28] demonstrated the presence of unsuspected mild emphysema in adult nonsmoking patients with asthma and airway obstruction. However, these observations are limited to two cases that were autopsied, thus limiting the applicability in clinical practice. The Global Allergy and Asthma European Network (GA2LEN) survey [29] demonstrated that subjects aged >45 years were less likely to have at least one positive skin prick tests for each tested allergen (including grass, cat, dog, house dust mite, birch, Artemisia, olive and Alternaria) except for Parietaria and Blattella, compared with subjects aged <45 years. The GA2LEN findings are in line with previous literature that showed a decline of allergen sensitisations with age [30]. Ageing is associated with modifications of the immune system, defined as immunosenescence. This could contribute to a reduced prevalence of allergic diseases in elderly populations. In this regard, atopy has rarely been considered in the clinical assessment of the geriatric respiratory patient. Although information on allergies in the elderly are scanty, positive skin tests have been reported in a variable percentage of older adults ranging from 8% to 12%, or even higher [31]. Indeed, elderly individuals with asthma have more evidence of atopy than age-matched controls without asthma [32]. In this context, some authors have proposed the evaluation of atopy as a useful tool in the differential diagnosis between asthma and COPD in the elderly [33]. However, this matter has not been investigated thoroughly and current guidelines rightly do not include the assessment of the allergic component to distinguish asthma from COPD. However, it is commonly accepted that the airways of atopic subjects are inflamed. The question is whether the state of atopy-related airway inflammation is responsible for the development of the obstructive phenotype that persists even when the inflammation diminishes as a consequence of reduction in the atopic state. From a clinical perspective, allergic reactions in older adults can have the same or even worse manifestations than in young people. A significant association between indoor allergen sensitisation and the occurrence of asthmatic symptoms was found in the Normative Aging Study, which was conducted in older men [34]. Taken together, these observations warrant the evaluation of atopy in the elderly [30]. It must be recognised that the two disorders may coexist in the same individual, leading to the “asthma–COPD overlap syndrome”. This nosological entity becomes relevant in patients aged >70 years in whom the proportion of patients with airflow obstruction who have an overlap syndrome may be as high as 60% [35, 36].
Exhaled nitric oxide fraction (FeNO) has been proposed as a marker of airway eosinophilic inflammation; therefore, it is plausible that it could play a role in the diagnosis of asthma in elderly patients. Unfortunately, data that have specifically addressed this issue are limited and mostly influenced by the use of inhaled corticosteroids (ICS) [37]. Of note, in healthy subjects, FeNO levels appear to show a triphasic shape with an ascendant loop during adolescence, a plateau in adults and a linear increase in the elderly until age 80 years [38]. Modern technology, not yet available in clinical practice, may open new frontiers in the diagnosis of asthma, even in cases of fixed airway obstruction. A promising procedure is the analysis of exhaled air molecular profiles by electronic nose. This innovative methodology can adequately distinguish patients with fixed asthma and COPD and, intriguingly, it can do it regardless of current smoking and the use of inhaled drugs [39].
From a clinical point of view, it has been reported that clinical features of asthma do not differ between very old (>80 years) and younger patients [40]. However, the possibility exists that subjects aged 80 years and over represent a high selected group of “survivors”, which limits the extrapolation to all elderly patients. Indeed, other studies in asthmatics aged >65 years showed a more severe and uncontrolled asthma than in younger asthmatics. Table 1 describes the main pitfalls in the diagnostic approach to elderly patients with symptoms suggestive of asthma.
Table 1.
Common pitfalls in diagnosing asthma in the elderly
| Pitfall | Is it useful in diagnosing asthma? | Comments | Ref. |
| Absence of reversibility | No | Elderly asthmatics may develop fixed airflow limitation | [24] |
| Impaired DLCO | Yes | DLCO should be normal in asthmatics | [27] |
| Smoking | No | Unfortunately, asthmatic patients do not avoid smoking | [26] |
| Disability | No | Presence of disability is not confined to COPD | [25] |
| Absence of allergy | No | Elderly asthmatics often are nonatopic | [30, 31] |
| Presence of allergy | Possibly | If associated with a long disease duration | [25, 30] |
| Long disease duration | Possibly | Asthma starting in young age does persist | [24] |
Pharmacological challenges in the treatment of geriatric asthma
Asthma in the geriatric population appears to be widely undertreated [41]. The treatment of asthma in geriatric age follows international guidelines [27], although most recommendations are extrapolated from findings in younger subjects. As already discussed, older age has always represented an exclusion criterion for eligibility in clinical trials and current asthma medications have never been tested in elderly asthmatics. The care of older asthmatics should address different domains such as comorbidities and polypharmacotherapy. The latter has been demonstrated to be one of the strongest predictors and the most important risk factor for adverse drug reactions in the elderly [42]. Therefore, particular attention should be given to concomitant nonrespiratory medications, since they can interfere with respiratory drugs or with the disease [43]. For instance, β-blockers, frequently prescribed in elderly subjects who suffer from cardiovascular diseases or administered as eye drops for glaucoma, can facilitate bronchoconstriction.
Ageing is associated with pharmacokinetic changes that are primarily due to the decline in the function of the liver and the kidneys. As a consequence, absorption, distribution, metabolism and excretion of anti-asthmatic medications can be affected to a variable extent. Absorption is the pharmacokinetic parameter least affected by ageing; however, the prevalence of defects in the absorption may delay the time of onset of action of some drugs [44]. The prevalence of chronic renal failure increases with age and has been demonstrated to have negative prognostic implications [45]. In this case, the risk of adverse drug reactions is augmented by the fact that renal failure is often unrecognised due to normal levels of serum creatinine. Reduced hepatic clearance of drugs increases the potential for drug interactions. From a clinical perspective, to limit the risk of drug interaction, the dosage should be reduced for hepatically or renally cleared drugs. In addition, appropriate monitoring plans should be in place and the drug interaction should be documented in medical records. Whenever possible, clinicians should be encouraged to consider substitution of the suspected drug with another drug of similar efficacy but lower potential for interactions. Importantly, caregivers should be informed about the potential risks and should be asked to be vigilant [46]. In elderly subjects, the lack of coordination between activation of the device and inhalation of the active drug may increase the oral deposition and decrease the lung deposition, thus reducing the efficacy and increasing local and systemic side-effects.
ICS are the cornerstone of the pharmacological management of patients with persistent asthma [27] at all ages. However, particularly in the geriatric population, the long-term and high-dose use of ICS may be associated with increased risk of adverse events. Factors such as the patient’s inhalation technique and peak inspiratory flow, which are variably impaired in elderly patients, can increase the occurrence of side-effects. These include skin bruising, osteoporosis and bone fractures, cataracts, glaucoma, oral candidiasis, diabetes, and pneumonia.
The pharmacokinetic and pharmacodynamic features of the ICS are influenced by protein binding and bioactivation by first-pass metabolism in the liver. In clinical practice, clinicians should be aware that the hepatic metabolism can be the cause of drug interactions. For example, the concomitant use of itraconazole, clarithromycin or calcium-channel blockers may increase the bioavailability of ICS and the risk of toxicity [47]. In addition, the increased systemic bioavailability can be responsible for the potential suppression of the hypothalamic–pituitary–adrenal axis. When the risk of interaction of ICS with other drugs is suspected, the use of the minimum efficacy dose of ICS and a closer clinical follow-up are recommended. If prolonged co-administration of enzymatic inhibitors is required, the alternative approach is the choice of a corticosteroid that is metabolised through a process of hydrolysis (i.e. beclomethasone).
Bronchodilators are also affected by ageing-related changes of their pharmacokinetic and pharmacodynamic properties [48]. This has been demonstrated for β2-agonists, anticholinergics and theophylline [49]. β2-adrenergic agonists, either short-acting β2-agonists or long-acting β2-agonists (LABAs), bind the β-adrenoceptor, whose response is different in elderly asthmatics due to increased sympathetic system activity, reduction in adenyl cyclase responses, and reduction in β2-adrenergic receptor number and affinity with ageing [50]. β2-agonists induce a net influx of intravascular potassium into cells, with subsequent electrolyte disorder. Thus, in older asthmatics, hypokalaemia, QT prolongation, tachycardia and tremor, which are mediated by the systemic drug absorption and are dose dependent, are the most serious adverse effects. Of course, the coexistence of cardiovascular disorders increases the frequency and severity of side-effects [51]. Anticholinergic drugs might represent a valid alternative to β2-agonists; however, their use in the elderly should take into consideration the potential risk of side-effects. The anticholinergic response is different in older asthmatics due to a decrease in parasympathetic activity and reduction in receptor numbers or post-receptor coupling with age [49]. The most frequent side-effects are dry mouth and unpleasant taste, contributing in older people to reduced ability to speak, mucosal damage and respiratory infection due to the reduction of antimicrobial activity of saliva [52]. Reduction of gastrointestinal motility, urinary hesitancy and exacerbations of glaucoma can be also observed in older individuals. In addition, due to the reduced metabolism and drug elimination in older patients, chronic use of anticholinergic drugs may induce mild cognitive impairment. Finally, in three out of 1000 patients a paradoxical bronchoconstriction may occur [49]. Theophylline treatment is limited by its narrow therapeutic range, the risk of interaction with several drugs and variable pharmacokinetics among patients. Since it is administered in tablets, theophylline may overcome the common issues related to the proper use of inhalers; however, this drug carries the highest frequency of side-effects in the elderly, in whom its clearance is reduced and increased blood levels are observed. The most frequent side-effects are gastrointestinal symptoms and arrhythmias. Several studies have demonstrated that theophylline metabolism and clearance decrease in the elderly [53–55]. The clearance of theophylline is reduced by 22–35% in elderly people and it is further decreased by concomitant diseases, particularly liver and heart diseases [56]. The phosphodiesterase 4 inhibitor roflumilast is available for COPD treatment and its use in asthma can be an interesting add-on therapeutic option in severe asthma with frequent exacerbations. Its use in clinical practice is, however, limited by the risk of side-effects, which are dose-dependent, making the range of efficacy/tolerability very narrow.
Leukotriene receptor antagonists (LTRA), such as montelukast, are an interesting alternative treatment to ICS and LABA in the elderly. On one hand, they can contribute to improving patients’ adherence in those with problems in managing inhaler devices; on the other hand, they may increase the safety of asthma therapy by avoiding ICS and LABA side-effects, which are more frequent in the elderly. As an example, LTRA can be proposed as an alternative to β2-agonists when hearth failure and/or ischaemic heart disease are present, becoming the first-line drugs for elderly asthmatics with a recent history of cardiovascular events.
An exciting field in the treatment of asthma is the possibility to treat elderly patients with severe asthma with monoclonal antibodies (i.e. omalizumab). Previously published data have confirmed that omalizumab is as effective in elderly asthmatics as it is in younger patients: in different studies, anti-IgE treatment was shown to reduce exacerbations and symptoms in patients aged 50 years and over [57–59]. In addition to the anti-IgE monoclonal antibody, novel therapies are currently being explored in experimental studies for severe asthma, although to our knowledge, no specific treatment is dedicated to older asthmatic patients. Prospective trials are needed to understand the effectiveness of monoclonal antibodies in the treatment of severe asthma in the geriatric ages.
Comorbid conditions
The geriatric patient is (almost by definition) characterised by the concomitant occurrence of multiple diseases, the number of which increases with ageing. Elderly asthmatics are not an exception. The number of comorbidities in older asthmatics is higher than that encountered in younger subjects, as recently demonstrated [60], and the pattern is different from that observed in younger asthmatics. Soriano et al. [61] demonstrated that in elderly asthmatics, the spectrum of comorbidities in primary care resembles that of COPD, with angina (3.5%), cataract (3.0%) and osteoporosis (2.7%) being the most prevalent conditions. These observations have obvious clinical implications. In this perspective, the higher prevalence of comorbidities observed in elderly asthmatics does not seem to be associated with worsened asthma control. It is rather the excessive number of medications that indirectly affects asthma control in elderly asthmatics, by worsening the adherence to antiasthmatic treatment and increasing the risk of drug-to-drug interactions, with consequent impaired efficacy and safety.
What are the most common comorbid conditions experienced in elderly patients in real life? Mood changes, such as depression and anxiety, are very common in the geriatric patient, because asthma symptoms influence emotional well-being dramatically. The impact of depression on asthma in the elderly has been widely demonstrated; depression is associated with poor asthma outcomes, including a higher rate of exacerbations [62] and higher mortality rates [4]. It is logical to assume that the negative effect of depression on the natural history of asthma is through the worsening of the level of adherence to treatment, which is common in depressed individuals. Elderly patients with asthma are also frequently affected by cognitive impairments. This comorbid condition can be underdiagnosed in clinical practice and have detrimental consequences for the control of asthma. Indeed, subjects suffering from even mild cognitive impairment may experience impaired perception of asthma symptoms and poor medication adherence [63]. An increased risk of new onset atrial fibrillation was recently demonstrated in asthmatics [64]. This observation deserves attention in clinical practice based on the chronic use of bronchodilators that could worsen the cardiac disease. Table 2 provides an attempt to distinguish the comorbidities in geriatric asthma in order to improve outcome in real life.
Table 2.
Comorbidities in geriatric asthma
| Comorbidities | Advice | |
| Related to shared risk factors | Rhinitis Gastro-oesophageal reflux |
Treat independently to improve asthma outcomes |
| Related to ageing | Depression Cognitive impairment |
Assess to avoid drug interaction or treatment failure |
| Related to asthma treatment | Cataracts Osteoporosis Arrhythmias Diabetes |
Monitor during asthma treatment to reduce drug dosages |
Inhalation technique
The pharmacological management of asthma is mainly based on inhalation therapy. In the elderly, the patients’ ability to handle the inhalers may then play an important role. Inhaler technique is strongly influenced by the cognitive impairments, both those clinically evident (i.e. dementia) and those subclinical, which are more difficult to detect and more prevalent in the geriatric population. Elderly asthmatics have been demonstrated, however, to improve their inhaler technique if practical demonstration and coaching are provided [65]. This implies that older asthmatics should receive appropriate instructions and the inhaler technique should be constantly assessed. Moreover, physical impairments, including arthritis, visual problems or inability to generate an adequate inspiration flow for a specific device, should be taken into account for the proper choice of device.
Unanswered questions
In the evaluation of the functional determinants of asthma in the elderly, the short-term response to a course of oral corticosteroids or the long-term response to ICS could be a surrogate of the acute response to short-acting bronchodilators. This is an interesting area of research in the geriatric population.
A myth in the functional assessment of asthma in the elderly is the assumption that obtaining optimal spirometries of good quality in older ages is an impossible mission. No doubt, spirometry is an effort-dependent manoeuver, and the collaboration of the patient together with the interaction between the patient and the operator are mandatory. However, elderly subjects may perform good quality spirometry to the same extent as younger patients. Bellia et al. [10] demonstrated that as much as 90% acceptable and reproducible spirometries were obtained in elderly subjects with or without chronic respiratory diseases.
An interesting area of research is to explore whether multiple comorbid conditions share a common systemic pathway; for instance, the metabolic syndrome could be pathogenetically linked to asthma through the pro-inflammatory low-density lipoproteins (LDL), as suggested by Scichilone et al. [66] and by Barochia et al. [67]. Small LDL could lead to the amplification of the inflammatory cascade in asthma: larger studies specifically designed to confirm the association between asthma and dyslipidaemia in the geriatric asthma are strongly needed.
The use of complementary/alternative medicines (CAMs) is a widespread phenomenon, common at any age. In asthma, homeopathy, acupuncture, herbal medicines and yoga are the most utilised techniques [68]. Asthmatics ask for CAMs because of the common belief that CAMs are more natural and safe, and this is particularly true in elderly patients who are administered a large amount of medications and have fear of drug interactions. However, the vast majority of the clinical trials published on CAMs do not seem to meet the acceptable level of quality [69], often making the results difficult to interpret. Obviously, some of the CAM techniques are self-applied (yoga and relaxation techniques) and, therefore, blinded randomised trials are impossible.
Conclusions
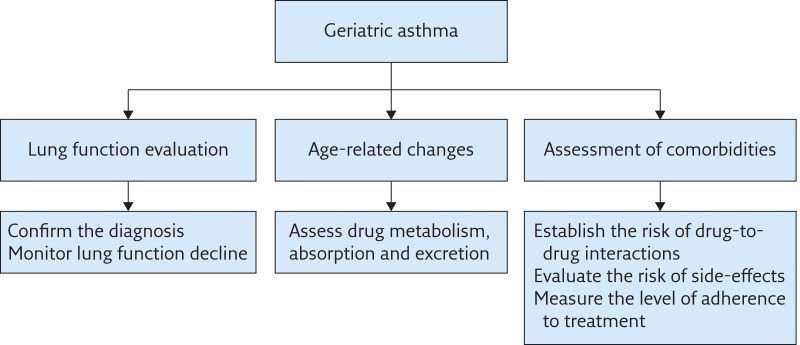
The above-described features of geriatric asthma call for a different approach to this pathological condition. Based on what has been discussed, the management of asthma in the elderly should switch from a disease-oriented scheme to a dysfunction-oriented behaviour. In addition to a comprehensive lung function assessment, which should incorporate measurements of static and dynamic lung volumes together with DLCO, a multidimensional approach is recommended to establish the complexity of the disease. A comprehensive algorithm for the management of geriatric asthma is proposed in figure 1. We suggest that the term “geriatric” asthma should be preferred to “senile” asthma, which confines the disease to the age-related structural and functional changes in the lung, or the more generic “asthma in the elderly”, which is only descriptive of the prevalence in old age groups. Geriatric asthma is no longer an enigma for general practitioners, nor is it an “orphan” disease for specialists; on the contrary, accumulating evidence confirms that the time has come to implement its dedicated (multidimensional) management in clinical practice.
Figure 1.
Algorithm for the management of geriatric asthma.
Self-evaluation questions
- Which of the following is true for a 75-year-old patient with fixed airflow limitation and a history of asthma from adolescence?
- a. They should be treated as a “COPD” patient, i.e. using inhaled bronchodilators as first-line therapy
- b. They should be treated as an “asthmatic” patient, i.e. using inhaled steroids as first-line therapy along with bronchodilators
- c. Skin-prick tests are mandatory before initiating any treatment
- d. Radiography is necessary to exclude COPD
- Which of the following algorithms should be used in diagnosing asthma in the elderly?
- a. Clinical history; spirometry; bronchodilator test; DLCO; skin prick test
- b. Skin prick test; chest radiography; spirometry; DLCO; bronchodilator test
- c. High-resolution computed tomography of the chest; spirometry; bronchodilator test
- d. Clinical history; skin-prick test; chest X-ray
- A 70-year-old man has a poor controlled asthma despite the use of controller medications. Could the physicians evaluate the patient’s eligibility to anti-IgE treatment?
- a. No, because clinical trials have not investigated the effectiveness of monoclonal antibodies in the treatment of severe asthma in the geriatric population
- b. No, due the modifications of the immune system (immunosenescence) associated with ageing
- c. Yes, published data have confirmed that omalizumab in elderly asthmatics is effective as in younger asthmatics
- d. No, its use in clinical practice is strongly limited by the risk of side-effects.
- A 72-year-old female who has never smoked is diagnosed with moderate asthma. She suffers from depression, diabetes, arterial hypertension and diffuse arthritis of the hands. What is the most appropriate pharmacological approach?
- a. She should not undergo any inhaled treatment because of difficulties in handling the device. Adrenaline should be prescribed for acute attacks.
- b. Depression will certainly affect adherence to treatment. Oral treatment (antileukotrienes, theophylline and oral corticosteroids) should be preferred on regular basis.
- c. Based on the arthritis of her hand, the most suitable inhaler device should be selected, and the patient should be properly informed and coached.
- d. A metered-dose inhaler is to be preferred to dry-powder inhaler because of ease of use.
Suggested answers
b
a
c
c
Footnotes
Conflict of interest None declared.
References
- 1.Lee HY, Stretton TB. Asthma in the elderly. Br Med J 1972; 4: 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burr ML, Charles TJ, Roy K, et al. . Asthma in the elderly: an epidemiological survey. Br Med J 1979; 1: 1041–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oraka E, Kim HJ, King ME, et al. . Asthma prevalence among US elderly by age groups: age still matters. J Asthma 2012; 49: 593–599. [DOI] [PubMed] [Google Scholar]
- 4.Bellia V, Pedone C, Catalano F, et al. . Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest 2007; 132: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 5.Plaza V, Serra-Batlles J, Ferrer M, et al. . Quality of life and economic features in elderly asthmatics. Respiration 2000; 67: 65–70. [DOI] [PubMed] [Google Scholar]
- 6.Battaglia S, Basile M, Spatafora M, et al. . Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration 2015; 89: 383–389. [DOI] [PubMed] [Google Scholar]
- 7.Gershon AS, Victor JC, Guan J, et al. . Pulmonary function testing in the diagnosis of asthma: a population study. Chest 2012; 141: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 8.Allen SC, Baxter M. A comparison of four tests of cognition as predictors of inability to perform spirometry in old age. Age Ageing 2009; 38: 537–541. [DOI] [PubMed] [Google Scholar]
- 9.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age Ageing 2006; 35: 304–306. [DOI] [PubMed] [Google Scholar]
- 10.Bellia V, Pistelli R, Catalano F, et al. . Quality control of spirometry in the elderly. The SA.R.A. study. SAlute Respiration nell’Anziano = Respiratory Health in the Elderly. Am J Respir Crit Care Med 2000; 161: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 11.Haynes JM. Pulmonary function test quality in the elderly: a comparison with younger adults. Respir Care 2014; 59: 16–21. [DOI] [PubMed] [Google Scholar]
- 12.Sorino C, Sherrill D, Guerra S, et al. . Prognostic value of FEV1/FEV6 in elderly people. Clin Physiol Funct Imaging 2011; 31: 101–107. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Guzman E, Mannino DM. Airway obstructive diseases in older adults: from detection to treatment. J Allergy Clin Immunol 2010; 126: 702–709. [DOI] [PubMed] [Google Scholar]
- 14.Verbeken EK, Cauberghs M, Mertens I, et al. . The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest 1992; 101: 800–809. [DOI] [PubMed] [Google Scholar]
- 15.Bellia M, Benfante A, Menozzii M, et al. . Validation of lung densitometry threshold at CT for the distinction between senile lung and emphysema in elderly subjects. Monaldi Arch Chest Dis 2011; 75: 162–166. [DOI] [PubMed] [Google Scholar]
- 16.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J 1999; 13: 197–205. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochat MK, Laubender RP, Kuster D, et al. . Spirometry reference equations for central European populations from school age to old age. PLoS One 2013; 8: e52619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loth DW, Ittermann T, Lahousse L, et al. . Normal spirometry values in healthy elderly: the Rotterdam Study. Eur J Epidemiol 2013; 28: 329–334. [DOI] [PubMed] [Google Scholar]
- 20.Karrasch S, Flexeder C, Behr J, et al. . Spirometric reference values for advanced age from a South German population. Respiration 2013; 85: 210–219. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rio F, Dorgham A, Pino JM, et al. . Lung volume reference values for women and men 65 to 85 years of age. Am J Respir Crit Care Med 2009; 180: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 22.Pistelli R, Bellia V, Catalano F, et al. . Spirometry reference values for women and men aged 65–85 living in southern Europe: the effect of health outcomes. Respiration 2003; 70: 484–489. [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Hall GL, Stanojevic S, et al. . Age- and height-based prediction bias in spirometry reference equations. Eur Respir J 2012; 40: 190–197. [DOI] [PubMed] [Google Scholar]
- 24.Fabbri LM, Romagnoli M, Corbetta L, et al. . Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167: 418–424. [DOI] [PubMed] [Google Scholar]
- 25.Bellia V, Battaglia S, Catalano F, et al. . Aging and disability affect misdiagnosis of COPD in elderly asthmatics: the SARA study. Chest 2003; 123: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 26.de Marco R, Cappa V, Accordini S, et al. . Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J 2012; 39: 883–892. [DOI] [PubMed] [Google Scholar]
- 27.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. www.ginasthma.org/local/uploads/files/GINA_Report_2015_Aug11.pdf Date last updated: August 11, 2015.
- 28.Gelb AF, Yamamoto A, Mauad T, et al. . Unsuspected mild emphysema in nonsmoking patients with chronic asthma with persistent airway obstruction. J Allergy Clin Immunol 2014; 133: 263–265. [DOI] [PubMed] [Google Scholar]
- 29.Newson RB, van Ree R, Forsberg B, et al. . Geographical variation in the prevalence of sensitization to common aeroallergens in adults: the GA2LEN survey. Allergy 2014; 69: 643–651. [DOI] [PubMed] [Google Scholar]
- 30.Scichilone N, Augugliaro G, Togias A, et al. . Should atopy be assessed in elderly patients with respiratory symptoms suggestive of asthma? Expert Rev Respir Med 2010; 4: 585–591. [DOI] [PubMed] [Google Scholar]
- 31.Hanania NA, King MJ, Braman SS, et al. . Asthma in the elderly: current understanding and future research needs – a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol 2011; 128: S4–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burney PG, Britton JR, Chinn S, et al. . Descriptive epidemiology of bronchial reactivity in an adult population: results from a community study. Thorax 1987; 42: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sin BA, Akkoca O, Saryal S, et al. . Differences between asthma and COPD in the elderly. J Investig Allergol Clin Immunol 2006; 16: 44–50. [PubMed] [Google Scholar]
- 34.Litonjua AA, Sparrow D, Weiss ST, et al. . Sensitization to cat allergen is associated with asthma in older men and predicts new-onset airway hyperresponsiveness. The Normative Aging Study. Am J Respir Crit Care Med 1997; 156: 23–27. [DOI] [PubMed] [Google Scholar]
- 35.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009; 64: 728–735. [DOI] [PubMed] [Google Scholar]
- 36.Soriano JB, Davis KJ, Coleman B, et al. . The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003; 124: 474–481. [DOI] [PubMed] [Google Scholar]
- 37.Columbo M, Wong B, Panettieri RA Jr, et al. . Asthma in the elderly: the role of exhaled nitric oxide measurements. Respir Med 2013; 107: 785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacinto T, Malinovschi A, Janson C, et al. . Evolution of exhaled nitric oxide levels throughout development and aging of healthy humans. J Breath Res 2015; 9: 036005. [DOI] [PubMed] [Google Scholar]
- 39.Fens N, Roldaan AC, van der Schee MP, et al. . External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin Exp Allergy 2011; 41: 1371–1378. [DOI] [PubMed] [Google Scholar]
- 40.Bozek A, Filipowski M, Fischer A, et al. . Characteristics of atopic bronchial asthma in seniors over 80 years of age. Biomed Res Int 2013; 2013: 689782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enright PL, McClelland RL, Newman AB, et al. . Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest 1999; 116: 603–613. [DOI] [PubMed] [Google Scholar]
- 42.Onder G, Petrovic M, Tangiisuran B, et al. . Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med 2010; 170: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 43.Braman SS. Growing old with asthma: what are the changes and challenges? Expert Rev Respir Med 2010; 4: 239–248. [DOI] [PubMed] [Google Scholar]
- 44.Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J 2012; 105: 437–445. [DOI] [PubMed] [Google Scholar]
- 45.McAlister FA, Ezekowitz J, Tonelli M, et al. . Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 46.Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007; 370: 185–191. [DOI] [PubMed] [Google Scholar]
- 47.Daveluy A, Raignoux C, Miremont-Salame G, et al. . Drug interactions between inhaled corticosteroids and enzymatic inhibitors. Eur J Clin Pharmacol 2009; 65: 743–745. [DOI] [PubMed] [Google Scholar]
- 48.Bellia V, Battaglia S, Matera MG, et al. . The use of bronchodilators in the treatment of airway obstruction in elderly patients. Pulm Pharmacol Ther 2006; 19: 311–319. [DOI] [PubMed] [Google Scholar]
- 49.Gupta P, O’Mahony MS. Potential adverse effects of bronchodilators in the treatment of airways obstruction in older people: recommendations for prescribing. Drugs Aging 2008; 25: 415–443. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer MA, Weinberg CR, Cook D, et al. . Differential changes of autonomic nervous system function with age in man. Am J Med 1983; 75: 249–258. [DOI] [PubMed] [Google Scholar]
- 51.Centanni S, Carlucci P, Santus P, et al. . Non-pulmonary effects induced by the addition of formoterol to budesonide therapy in patients with mild or moderate persistent asthma. Respiration 2000; 67: 60–64. [DOI] [PubMed] [Google Scholar]
- 52.Scichilone N, Ventura MT, Bonini M, et al. . Choosing wisely: practical considerations on treatment efficacy and safety of asthma in the elderly. Clin Mol Allergy 2015; 13: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antal EJ, Kramer PA, Mercik SA, et al. . Theophylline pharmacokinetics in advanced age. Br J Clin Pharmacol 1981; 12: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otero MJ, Barrueco M, Marino EL, et al. . Individualization of theophylline dosage in adults with bronchial asthma. Drug Intell Clin Pharm 1986; 20: 704–707. [DOI] [PubMed] [Google Scholar]
- 55.Shin SG, Juan D, Rammohan M. Theophylline pharmacokinetics in normal elderly subjects. Clin Pharmacol Ther 1988; 44: 522–530. [DOI] [PubMed] [Google Scholar]
- 56.Ohta K, Fukuchi Y, Grouse L, et al. . A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir Med 2004; 98: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 57.Maykut RJ, Kianifard F, Geba GP. Response of older patients with IgE-mediated asthma to omalizumab: a pooled analysis. J Asthma 2008; 45: 173–181. [DOI] [PubMed] [Google Scholar]
- 58.Korn S, Schumann C, Kropf C, et al. . Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol 2010; 105: 313–319. [DOI] [PubMed] [Google Scholar]
- 59.Verma P, Randhawa I, Klaustermeyer WB. Clinical efficacy of omalizumab in an elderly veteran population with severe asthma. Allergy Asthma Proc 2011; 32: 346–350. [DOI] [PubMed] [Google Scholar]
- 60.Wardzynska A, Kubsik B, Kowalski ML. Comorbidities in elderly patients with asthma: association with control of the disease and concomitant treatment. Geriatr Gerontol Int 2015; 15: 902–909. [DOI] [PubMed] [Google Scholar]
- 61.Soriano JB, Visick GT, Muellerova H, et al. . Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005; 128: 2099–2107. [DOI] [PubMed] [Google Scholar]
- 62.Park HW, Kim TW, Song WJ, et al. . Prediction of asthma exacerbations in elderly adults: results of a 1-year prospective study. J Am Geriatr Soc 2013; 61: 1631–1632. [DOI] [PubMed] [Google Scholar]
- 63.Cousens NE, Goeman DP, Douglass JA, et al. . The needs of older people with asthma. Aust Fam Physician 2007; 36: 729–731. [PubMed] [Google Scholar]
- 64.Chan WL, Yang KP, Chao TF, et al. . The association of asthma and atrial fibrillation – a nationwide population-based nested case-control study. Int J Cardiol 2014; 176: 464–469. [DOI] [PubMed] [Google Scholar]
- 65.Crane MA, Jenkins CR, Goeman DP, et al. . Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. Prim Care Respir Med 2014; 24: 14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scichilone N, Rizzo M, Benfante A, et al. . Serum low density lipoprotein subclasses in asthma. Respir Med 2013; 107: 1866–1872. [DOI] [PubMed] [Google Scholar]
- 67.Barochia AV, Kaler M, Cuento RA, et al. . Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am J Respir Crit Care Med 2015; 191: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziment I. Alternative therapies for asthma. Curr Opin Pulm Med 1997; 3: 61–71. [DOI] [PubMed] [Google Scholar]
- 69.Bloom BS, Retbi A, Dahan S, et al. . Evaluation of randomized controlled trials on complementary and alternative medicine. Int J Technol Assess Health Care 2000; 16: 13–21. [DOI] [PubMed] [Google Scholar]