The first breath after birth is the most difficult in life. What happens before, during and after it?
Short abstract
The first breath after birth is the most difficult in life. What happens before, during and after it? http://ow.ly/YBOU6
The first breath after birth is the most difficult in life. What happens before, during and after it?
The breath of the human fetus
Although the placenta provides respiratory gas exchange to the fetus, active fetal breathing movements (FBMs) are present from 10 weeks of gestation. FBM incidence increases with gestational age but drops immediately before the onset of labour. FBMs not only represent the ability of the fetus to use and train the respiratory muscles in utero to be ready after birth, but they also contribute to the development of the lungs and neural circuits of respiratory control. FBMs become more frequent with increasing maternal glycaemia or in hypercapnia, whereas they are reduced or even abolished by nicotine, hypoxia, alcohol and drug (opiates) exposure in utero. Severe oxygen deprivation stimulates deep, gasping-type efforts, inducing the fetus to aspire meconium and/or other amniotic fluid [1].
Birth transition: preparing for the first breath
The uterine contractions during labour and the “vaginal squeeze” at delivery make the fetus change position, therefore compressing its highly compliant thorax. These mechanical forces represent an aid to clearance of liquid from the lungs. In utero, in fact, the lungs are not used to breathe, being completely full of the liquid secreted by the pulmonary epithelium. In order to stimulate the development of fetal lungs, the pre-natal volume of liquid inside them is higher than the resting capacity measured at birth, thanks to adduction of the glottis, which promotes fluid accumulation. The first breath, therefore, can occur only after a transition from liquid to air-filled lungs. The clearing lung process starts in parallel to labour and consists of two mechanisms:
1)uterine contraction-induced alteration of chest wall configuration of the fetus with consequent increase of transpulmonary pressure gradient that drives the fluid outside the lungs; and
2)release of fetal adrenaline to activate the sodium channels on the apical pulmonary surface that stimulate the epithelial cells to stop secreting lung fluid and to start reabsorbing it.
Compared to infants exposed to labour, infants born by caesarean section are more likely to retain more liquid in their lungs and this may limit the amount of air entering into their airways at the first breath [1, 2].
The first breath
At birth, the lungs are still full of liquid until the first breath is taken. It seems that the first inspiratory effort plays a critical role by generating an active pressure gradient to shift the fluid into the interstitial tissue, where it will be gradually removed by the pulmonary and lymphatic circulations. Near term, surfactant is secreted by alveolar cells into the lung fluid. The surfactant is a surface active agent that lowers the air/liquid interfacial tension therefore reducing the “opening pressure” (20–55 cmH2O) required to aerate the lungs and preventing alveolar collapse to facilitate their expansion. In this way, subatmospheric intrathoracic pressure swings of ∼30 cmH2O allow the generation of ∼40 mL of tidal volume. At the end of the first inspiration, produced by the contraction of the diaphragm, which develops an oesophageal pressure swing up to −70 cmH2O [3], the newborn closes the glottis in order to avoid gas loss and to maintain ∼35 cmH2O of positive intrathoracic pressure that facilitates air distribution inside the lungs further promoting liquid clearance. The first breath can take up to 30 s after birth [2].
Onset of continuous breathing: expiratory braking
The onset of continuous respiration after birth includes different factors such as asphyxia, afferent vagal input, occlusion of the umbilical circulation and rise of oxygenation secondary to the first breath. The breathing pattern of the first breaths after delivery is characterised by rapid, deep inspiration followed by prolonged expiration. During expiration, occasional breaths present alternations between low- or zero-flow periods and short or multiple expiratory flow peaks resulting in high positive airway pressure, secondary to respiratory muscle contractions that pressurise the air inside the lungs. This pattern is known as expiratory braking [1, 4].
Dynamic maintenance of end-expiratory lung volume
After the first breath, it is crucially important to maintain the lungs full of air to avoid their collapse. This is achieved by two braking mechanisms controlled by vagal reflexes present at birth [4]:
1) post-inspiratory activity of the diaphragm that contracts to counteract the passive recoil of the lung in order to slow its deflation; and
2) glottis adduction during expiration to increase the resistance to the expiratory airflow in order to retard it [5].
While end-expiratory lung volume (EELV) is normally passively determined as the balance between the outward and inward elastic recoil of chest wall and lung, respectively, infants dynamically maintain EELV by employing both braking mechanisms [2, 4, 6, 7]. Because of the highly compliant chest wall, in infancy, EELV is only 10–15% of total lung capacity, being very close to residual volume and, therefore, predisposing to airway closure development. For this reason, the braking mechanisms are very important to keep EELV above the resting volume until the chest wall stiffens with growth [5, 8].
Mechanical properties and dynamics of breathing
The maintenance of EELV depends, among others, on the compliance of both the chest wall (CCW) and the lung (CL). In infancy, CCW is three times higher than CL but they become similar after the age of 1 year. The developmental change in chest wall stiffness has important consequences for respiratory system function. In fact, it contributes to stabilising the thorax, by reducing the energy wasted to distort the compliant ribcage, therefore improving ventilatory efficiency. Not only does it allow the chest wall to withstand the inward recoil of the lung without relying on the braking mechanisms, but it also becomes the adequate scaffolding structure for optimal respiratory muscle function. It is also considered the main responsible for the transition from dynamic to passive maintenance of EELV [6, 9].
Immediately after birth, the compliance (CRS) and the resistance (RRS) of the respiratory system are, respectively, low and high, due to the residual fluid present in the lung interstitium. The price to pay to expand the lungs dynamically and actively is, therefore, a greater work of breathing that is almost entirely due to the elastic component [9].
Afterward (i.e. in the following hours and few days, depending on factors like mode of delivery and duration of labour), CL and CRS gradually rise while CCW decreases and RRS decreases because of the progressive clearing of the pulmonary fluid and lung expansion.
Chest wall geometry and respiratory muscles
At birth, the ribcage is composed primarily of cartilaginous tissue. Because ribs are horizontal and extend at right angles from the vertebral column, the cross-sectional shape of the ribcage is more circular. The ratio between the anteroposterior and lateral diameters of the thorax (thoracic index) is very high at birth and substantially decreases in the first 2 years of life [10].
The diaphragm is flattened, with consequent absence of appositional area. The contraction of the diaphragm acts mainly in the posterior part and makes the compliant ribcage displace with a paradoxical inward motion during inspiration [3, 11].
A breath-by-breath fluctuation of the relative contribution to tidal volume of the intercostal muscles and diaphragm is considered a rapid adaptive process for optimal ventilation [12]. Growth induces a progressive increase in the bulk of respiratory muscles as well as changes in their fibre composition. Hypocapnia decreases upper airway muscle and diaphragm activation. Since the carbon dioxide threshold of upper airway muscles is higher than that of the diaphragm, during carbon dioxide transitions, their dilating action could be delayed with respect to the negative pressure swings of diaphragmatic contraction so favouring obstructive apnoeas [13].
At birth, maximal inspiratory and expiratory pressures during crying are ∼90 and ∼60 cmH2O, respectively, and significantly increase during the first 6 weeks after birth reaching values of ∼120 cmH2O. Despite such high pressure demand, a consequence of elevated metabolic and ventilatory rates combined with highly compliant ribcage and small radius of curvature, the inspiratory force reserve is reduced in infants compared to adults [3, 14].
Breathing pattern and sighs
While tidal volume remains invariant (∼6 mL·kg−1) from birth to adulthood, respiratory rate progressively decreases with growth. The newborn’s ventilatory demands are generally higher and met by increasing breathing frequency (up to 40 breaths·min−1 [15, 16]), rather than tidal volume, being the most energy-efficient strategy [1]. Because of their relatively large head size, anatomic dead space in infants is greater than adults (>3 mL·kg−1) and this is important to consider when measuring tidal volume [17].
Inspiratory and expiratory time are shorter and prolonged, respectively, as consequence of expiratory braking, which is most commonly achieved by crying [5, 18–20].
Healthy, full-term newborns spend a lot of time in rapid eye movement sleep characterised by depression of phasic and tonic activity of all respiratory muscles with the exception of the diaphragm. The resulting paradoxical motion of the ribcage is associated with EELV reduction, decrease of transcutaneous oxygen partial pressure and increase in the work of breathing of the diaphragm [3, 10].
The breathing pattern of infants is also characterised by large sighs, i.e. random, spontaneous, deep inspirations, the role of which is to restore lung volume and to reset the neurorespiratory control system. In contrast to adults, in infants, sighs are followed by periods of hypoventilation or apnoea, the role and consequences of which are still unclear [21].
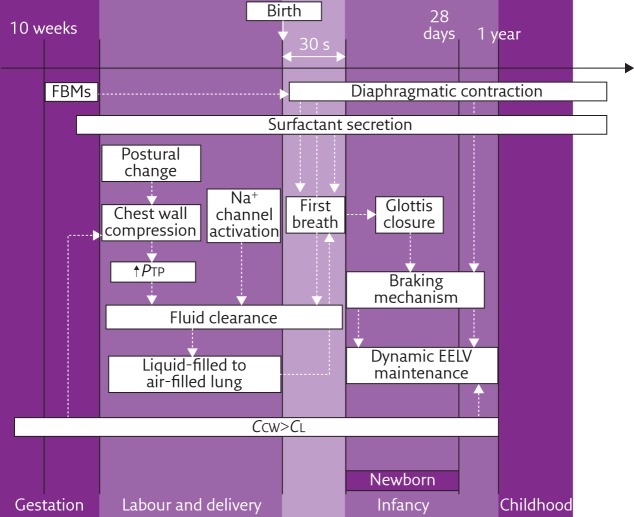
The processes described in this article are summarised in figure 1.
Figure 1.
The principal physiological adaptations of the respiratory system in utero, during labour and delivery to prepare the first breath and then during infancy. FBM: fetal breathing movement; PTP: trans-pulmonary pressure; EELV: end-expiratory lung volume; CCW: chest wall compliance; CL: lung compliance.
Footnotes
Conflict of interest: None declared
References
- 1.Koos BJ, Rajaee A. Fetal breathing movements and changes at birth. Adv Exp Med Biol 2014; 814: 89–101. [DOI] [PubMed] [Google Scholar]
- 2.te Pas AB, Davis PG, Hooper SB, et al. From liquid to air: breathing after birth. J Pediatr 2008; 152: 607–611. [DOI] [PubMed] [Google Scholar]
- 3.Gaultier C. Respiratory muscle function in infants. Eur Respir J 1995; 8: 150–153. [DOI] [PubMed] [Google Scholar]
- 4.Kosch PC, Hutchinson AA, Wozniak JA, et al. Posterior cricoarytenoid and diaphragm activities during tidal breathing in neonates. J Appl Physiol 1988; 64: 1968–1978. [DOI] [PubMed] [Google Scholar]
- 5.Kosch PC, Davenport PW, Wozniak JA, et al. Reflex control of expiratory duration in newborn infants. J Appl Physiol 1985; 58: 575–581. [DOI] [PubMed] [Google Scholar]
- 6.Papastamelos C, Panitch HB, England SE, et al. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol 1995; 78: 179–184. [DOI] [PubMed] [Google Scholar]
- 7.Zamel D, Revow M, England SJ. Expiratory airflow patterns and gas exchange in the newborn infant: results of model simulations. Respir Physiol 1989; 75: 19–27. [DOI] [PubMed] [Google Scholar]
- 8.Mortola JP. Dynamics of breathing in newborn mammals. Physiol Rev 1987; 67: 187–243. [DOI] [PubMed] [Google Scholar]
- 9.Mortola JP, Fisher JT, Smith B, et al. Dynamics of breathing in infants. J Appl Physiol 1982; 52: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 10.Gaultier C, Praud JP, Canet E, et al. Paradoxical inward rib cage motion during rapid eye movement sleep in infants and young children. J Dev Physiol 1987; 9: 391–397. [PubMed] [Google Scholar]
- 11.Devlieger H, Daniels H, Marchal G, et al. The diaphragm of the newborn infant: anatomical and ultrasonographic studies. J Dev Physiol 1991; 16: 321–329. [PubMed] [Google Scholar]
- 12.Hutten GJ, van Eykern LA, Latzin P, et al. Relative impact of respiratory muscle activity on tidal flow and end expiratory volume in healthy neonates. Pediatr Pulmonol 2008; 43: 882–891. [DOI] [PubMed] [Google Scholar]
- 13.Carlo WA, DiFiore JM. Respiratory muscle responses to changes in chemoreceptor drive in infants. J Appl Physiol 1990; 68: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 14.Kassim Z, Moxham J, Davenport M, et al. Respiratory muscle strength in healthy infants and those with surgically correctable anomalies. Pediatr Pulmonol 2015; 50: 71–78. [DOI] [PubMed] [Google Scholar]
- 15.Rusconi F, Castagneto M, Gagliardi L, et al. Reference values for respiratory rate in the first 3 years of life. Pediatrics 1994; 94: 350–355. [PubMed] [Google Scholar]
- 16.Berman S, Simoes EA, Lanata C. Respiratory rate and pneumonia in infancy. Arch Dis Child 1991; 66: 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numa AH, Newth CJ. Anatomic dead space in infants and children. J Appl Physiol 1996; 80: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hathlol K, Idiong N, Hussain A, et al. A study of breathing pattern and ventilation in newborn infants and adult subjects. Acta Paediatr 2000; 89: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 19.te Pas AB, Wong C, Kamlin CO, et al. Breathing patterns in preterm and term infants immediately after birth. Pediatr Res 2009; 65: 352–356. [DOI] [PubMed] [Google Scholar]
- 20.Latzin P, Roth S, Thamrin C, et al. Lung volume, breathing pattern and ventilation inhomogeneity in preterm and term infants. PLoS One 2009; 4: e4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi M, Khalil M, Kwiatkowski K, et al. Morphology of sighs and their role in the control of breathing in preterm infants, term infants and adults. Neonatology 2009; 96: 43–49. [DOI] [PubMed] [Google Scholar]