Abstract
MicroRNA-181a binds to the 3′ untranslated region of messenger RNA (mRNA) for renin, a rate-limiting enzyme of the renin-angiotensin system. Our objective was to determine whether this molecular interaction translates into a clinically meaningful effect on blood pressure and whether circulating miR-181a is a measurable proxy of blood pressure. In 200 human kidneys from the TRANScriptome of renaL humAn TissuE (TRANSLATE) study, renal miR-181a was the sole negative predictor of renin mRNA and a strong correlate of circulating miR-181a. Elevated miR-181a levels correlated positively with systolic and diastolic blood pressure in TRANSLATE, and this association was independent of circulating renin. The association between serum miR-181a and systolic blood pressure was replicated in 199 subjects from the Genetic Regulation of Arterial Pressure of Humans In the Community (GRAPHIC) study. Renal immunohistochemistry and in situ hybridization showed that colocalization of miR-181a and renin was most prominent in collecting ducts where renin is not released into the systemic circulation. Analysis of 69 human kidneys characterized by RNA sequencing revealed that miR-181a was associated with downregulation of four mitochondrial pathways and upregulation of 41 signaling cascades of adaptive immunity and inflammation. We conclude that renal miR-181a has pleiotropic effects on pathways relevant to blood pressure regulation and that circulating levels of miR-181a are both a measurable proxy of renal miR-181a expression and a novel biochemical correlate of blood pressure.
INTRODUCTION
High blood pressure (BP) is a leading risk factor for cardiovascular disease burden worldwide (1). Both systolic and diastolic BP (SBP and DBP, respectively) are complex, polygenic traits; many genetic, molecular and environmental factors act separately or together on major mechanisms of BP regulation (2). A growing body of evidence suggests that noncoding RNAs (ncRNAs) control a number of regulatory networks in the cardiovascular system (3–5).
MicroRNAs (miRNAs) are small (approximately 22 base pairs in length) ncRNAs, commonly referred to as “master regulators” of posttranscriptional regulation because of their prominent role in the control of downstream messenger RNA (mRNA) levels (6). By interacting with complementary sequences of the 3′ untranslated region (UTR) of target mRNAs, miRNAs are responsible for triggering mRNA degradation or repression of translation, leading to a decrease in mRNA and protein levels (7). Besides tissue miRNAs, circulating miRNAs have recently emerged as mediators of intercellular communication and as potential biomarkers of cardiovascular disease (8). The specific role of miRNAs in BP regulation has been poorly investigated, whereas their role in other complex disorders is better established (3–5).
Our previous studies showed that hsa-miR-181a-5p (miR-181a) binds to the 3′UTR of renin mRNA in kidney cells transfected with reporter gene constructs, and also leads to reduced gene expression (9). Both reduced renal expression of miR-181a and an increase in renin mRNA abundance were associated with hypertension in a small collection of human kidneys (9). miR-181a exhibited an inverse correlation with renin in a genetically hypertensive strain of mice (10). Taken together, these data strongly suggest that this miRNA may constitute a new posttranscriptional layer of control for the rate-limiting enzyme of the renin-angiotensin system (RAS) and, as such, may contribute to BP regulation, thus making it an attractive target for the development of new antihypertensive therapies.
Here, in a unique resource of 200 matched human kidney and serum samples from the TRANScriptome of renaL humAn TissuE (TRANSLATE) study (11–13), we examined the potential relevance of miR-181a to BP regulation in both anatomical compartments. We further used an independent population sample of similar size from the Genetic Regulation of Arterial Pressure of Humans In the Community (GRAPHIC) study (14) to replicate some of our association findings. Finally, in searching for the renal mechanisms that may link miR-181a to BP, we explored the transcriptome of 69 normal control renal samples from The Cancer Genome Atlas (TCGA) cohort characterized by next-generation RNA sequencing.
MATERIALS AND METHODS
For detailed methodology, please see the online supplementary file.
Subjects
Human kidney samples from the TRANSLATE study were collected after surgery in 200 patients who underwent elective unilateral nephrectomy because of noninvasive renal cancer in one of three nephrology-urology centers: Silesian Renal Tissue Bank (11,12), TRANSLATE P (recruitment conducted in Western Poland) and TRANSLATE Z (recruitment conducted in Southern Poland) (13). Serum from all subjects was collected before surgery at the time of recruitment.
An additional set of 199 serum samples from biologically unrelated subjects was secured from the GRAPHIC study (14).
All individuals were of white-European ancestry and gave written informed consent for participation. The studies adhered to the Declaration of Helsinki and were approved by the local ethics committees.
Renal Tissue and Serum Processing, RNA Extraction, Gene Expression Studies, Histology and Biochemistry
Renal tissue samples in the TRANSLATE study were collected immediately after nephrectomy from a healthy (unaffected by cancer) pole of the kidney and immersed in RNAlater (Life Technologies) before storage at −80°C, as described previously (11–13). RNA was extracted from both kidney tissues and serum samples using appropriate miRNeasy kits (Qiagen). The expression levels of miR-181a, along with renal renin mRNA and housekeeping control genes, were measured by quantitative real-time PCR (qPCR; Supplementary Table S1). Serum renin levels were measured by immunoradiometric assay. Formalin-fixed and paraffin-embedded kidney samples from a TRANSLATE individual were used to localize the expression of miR-181a (by in situ hybridization, Supplementary Table S2) and renin (by immunohistochemistry).
Statistical Analysis
In the TRANSLATE study, unadjusted association analysis between two quantitative phenotypes was explored first by Pearson linear correlation. Further analyses were conducted using step-wise multiple linear regression models in which clinical (age, sex, body mass index [BMI], recruitment center) and experimental (cDNA or qPCR plate) variables were independent parameters. Three types of sensitivity analysis were used after examination of association between mRNA expression/biochemical phenotypes and BP values. In the first type of sensitivity analysis, BP from patients on antihypertensive treatment were corrected for BP lowering effect of medications by adding a constant of 15 mmHg and 10 mmHg to measured SBP and DBP, respectively, in line with algorithms used previously (15). The second sensitivity analysis was based on exclusion of all subjects on antihypertensive therapy. The third sensitivity analysis included only individuals with clinic SBP ≤40 mmHg and DBP ≤90 mmHg, who were not taking antihypertensive medication.
In the GRAPHIC study, none of the subjects were on antihypertensive treatment. The analyses of association between serum levels of miR-181a and clinic BP were conducted by multiple linear regression after adjusting for age, sex and BMI. Findings at the P <0.05 level were considered statistically significant.
Pathway Analysis of Human Kidney Transcriptomes Characterized by Next-Generation RNA Sequencing
Next-generation RNA sequencing and small RNA sequencing data from kidney tissues unaffected by cancer (labeled as “normal-matched”) collected from 69 individuals diagnosed with clear cell renal carcinoma were downloaded from TCGA database. Apart from age and sex, no other demographic or clinical information (including BP and hypertension status) were available for those individuals. Analysis of association between miR-181a and mRNAs was conducted using limma (16) with each mRNA expression value as the response variable and miR-181a expression, age and sex as independent parameters included in the regression model.
To identify renal pathways associated with expression of miR-181a, we used gene set enrichment analysis (GSEA) (17) with KEGG, BioCarta and Reactome as pathway repositories. Correction for multiple testing in both individual mRNA- and pathway-based analysis was conducted by calculation of false discovery rate (FDR) q-values and the corrected threshold for statistical significance was set at q <0.01.
Hierarchical Clustering Analysis of Renal Transcriptomes Characterized by Next-Generation RNA Sequencing
To exclude the potential effect of coexistent cancer on the transcriptome of the tumor-unaffected renal tissue from where the specimen was sampled, we compared global expression profiles of three different cohorts: healthy tissue from nephrectomies due to cancer, noncancer kidneys and clear cell renal carcinoma by hierarchical clustering of multidimensional scaling dimension values. The RNA sequencing data were collected from three sources: TCGA database (69 normal-matched and 10 randomly chosen renal cancer samples), GTEx Project (8 noncancer kidneys harvested from donors identified through low postmortem interval) (18) and TRANSLATE study (in which 32 kidneys underwent next-generation RNA sequencing).
All supplementary materials are available online at www.molmed.org.
RESULTS
Demographic and Clinical Characteristics
Table 1 provides characteristics of 200 individuals from the TRANSLATE study included in the renal and serum expression analyses and 199 subjects from the GRAPHIC study used in the replication analyses. Supplementary Table S3 provides a breakdown of TRANSLATE subject demographics by recruitment center. Basic demographic characteristics of 69 TCGA individuals, whose kidneys underwent next-generation sequencing, are shown in Supplementary Table S4.
Table 1.
Clinical characteristics of individuals in the TRANSLATE and the GRAPHIC study.a
| Variable | TRANSLATE | GRAPHIC |
|---|---|---|
| N | 200 | 199 |
| Age (years) | 61.6 ± 10.6 | 52.7 ± 4.3 |
| BMI (kg/m2) | 27.7 ± 4.3 | 27.3 ± 4.5 |
| Male sex | 120 (60%) | 99 (50%) |
| SBP (mmHg) | 136.3 ± 13.4 | 135.8 ± 25.5 |
| DBP (mmHg) | 82.8 ± 8.7 | 84.8 ± 13.7 |
| Hypertension | 134 (67%) | 102 (51%) |
| Antihypertensive treatment | 114 (57%) | 0 (0%) |
Data are shown as mean ± standard deviations or numbers and percentages.
Expression of miR-181a and Renin mRNA Are Negatively Correlated in the Human Kidney
There was a negative linear correlation between miR-181a and renin in 200 human kidneys (r = −0.15, P = 0.015). In a multiple regression model adjusted for both clinical and experimental variables, renal miR-181a was the only independent parameter associated with renin mRNA in the kidney (β = −0.35, SE = 0.16, P = 0.028).
Renal Expression of miR-181a Is Associated with Circulating miR-181a Levels
miR-181a was measured in available serum from 189 TRANSLATE individuals whose kidneys were examined at the earlier stage of the project. There was a positive linear correlation between serum levels of miR-181a and its expression in human kidneys (r = 0.34, P < 0.001). This association remained significant after full adjustment for clinical and laboratory variables (β = 0.52, SE = 0.11, P < 0.001).
Circulating Concentrations of Renin Are Associated with its Renal Expression but Not with Serum Levels of miR-181a
Serum levels of renin protein were measured in 192 TRANSLATE samples. There was a linear correlation between serum renin and renal renin mRNA; lower dCt values, and thus higher expression in the kidney, was associated with higher levels of renin in serum (r = −0.20, P = 0.003). After adjustment for clinical and laboratory variables, this association retained its statistical significance (β = −0.039, SE = 0.014, P = 0.007). Serum concentrations of renin were not associated with serum levels of miR-181a in fully adjusted main or sensitivity analyses (data not shown).
Serum and Renal Levels of miR-181a Are Associated with Blood Pressure, Independent of Renin
Circulating levels of renin showed moderate or borderline associations with clinic SBP and DBP in the main adjusted and sensitivity analyses (Table 2). There was no statistically significant association between BP and renal renin mRNA (Supplementary Table S5).
Table 2.
Associations between blood pressure and miR-181a level in serum and the kidney.a
| Systolic blood pressure | Diastolic blood pressure | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Model Ab | Model Bc | Model Cd | Model De | Model A | Model B | Model C | Model D | |
| Serum log10 renin (pg/mL) | 4.2 ± 1.8 | 7.8 ± 2.3 | 10.2 ± 3.0 | 4.5 ± 2.4 | 2.0 ± 1.2 | 4.2 ± 1.5 | 5.3 ± 2.2 | NS |
| P = 0.020 | P = 0.001 | P = 0.001 | P = 0.067 | P = 0.098 | P = 0.004 | P = 0.018 | ||
| Kidney miR-181a (dCt) | −1.8 ± 0.9 | NS | −3.0 ± 1.2 | −2.7 ± 0.8 | −1.5 ± 0.6 | NS | −1.9 ± 0.9 | −1.5 ± 0.7 |
| P = 0.041 | P = 0.016 | P = 0.002 | P = 0.010 | P = 0.042 | P = 0.038 | |||
| Serumf miR-181a (dCt) | −1.7 ± 0.5 | NS | −2.4 ± 0.8 | −1.5 ± 0.6 | −1.7 ± 0.3 | −1.1 ± 0.4 | −2.5 ± 0.5 | −2.0 ± 0.4 |
| P = 0.002 | P = 0.002 | P = 0.009 | P < 0.001 | P = 0.015 | P < 0.001 | P < 0.001 | ||
| Serumg miR-181a (dCt) | −1.7 ± 0.5 | NS | −2.5 ± 0.7 | −1.9 ± 0.5 | −1.8 ± 0.3 | −1.1 ± 0.4 | −2.5 ± 0.5 | −2.0 ± 0.4 |
| P = 0.002 | P = 0.001 | P = 0.001 | P < 0.001 | P = 0.010 | P < 0.001 | P < 0.001 | ||
P, level of statistical significance; NS, not significant.
Data are expressed as β-coefficients (with standard errors and P values) from linear regression models in which clinic blood pressure (BP) was the dependent variable and log10-transformed serum levels of renin or delta cycle thresholds (dCt) (inverse measure of miR-181 expression) as one of the independent variables.
Model A: all subjects with available clinical and molecular data and measured clinic BP.
Model B: all subjects with BP transformed using the therapy-adjustment algorithm.
Model C: subjects who were not on antihypertensive medication.
Model D: subjects who were not on antihypertensive medication and had a clinic SBP ≤ 140 mmHg and DBP ≤ 90 mmHg.
Serum model without serum levels of renin.
Serum model adjusted for log10 circulating levels of renin included.
There was a strong association between BP and both renal and serum miR-181a abundance (measured as dCt) in crude and adjusted analyses of TRANSLATE; lower dCt (higher expression) was related to higher BP values in the crude, adjusted analysis and at least two of three sensitivity analyses (Table 2). The most significant association was identified between serum miR-181a levels and BP in subjects not on antihypertensive therapy; each unit decrease in dCt (doubling of expression) of miR-181a was associated with ≈2.4 mmHg higher SBP (β = −2.4, SE = 0.8, P = 0.002) and ≈2.5 mmHg higher DBP (β = −2.5, SE = 0.5, P < 0.001) (Table 2). The association between serum miR-181a and both SBP and DBP remained statistically significant after adjustment for circulating levels of renin (Table 2) and also remained statistically significant in models that included only normotensive individuals (Table 2). An independent analysis of 199 GRAPHIC study subjects who were not on antihypertensive treatment showed the same direction of association between serum levels of miR-181a and BP; each unit decrease in dCt (doubling of expression) of serum miR-181a was associated with ≈3.4 mmHg increase in SBP (β = −3.4, SE = 1.7, P = 0.043). The association between serum miR-181a and DBP showed the same trend but did not reach the level of nominal statistical significance (β = −1.3, SE = 0.9, P = 0.158).
Renal Coexpression of Renin and miR-181a Is Most Apparent in the Distal Nephron of the Human Kidney
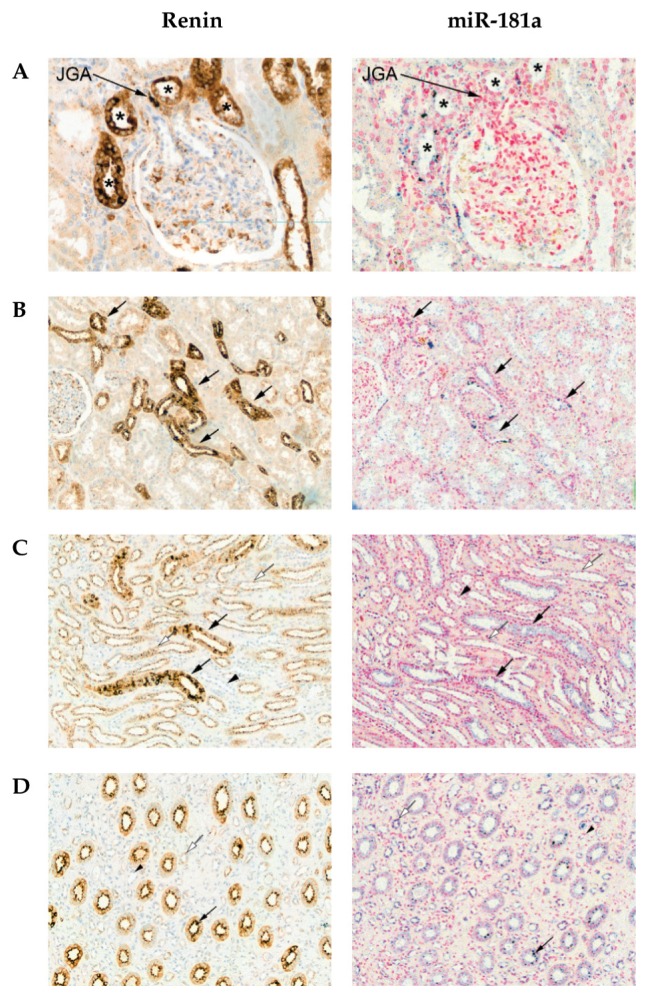
There was strong immunostaining for renin in the juxtaglomerular apparatus, as expected (Figure 1A). The expression of renin within cortical tubules was polarized, with tubules at the vascular pole of glomeruli (distal convoluted tubules) showing stronger immunostaining compared with segments most consistent with identity of proximal tubules (Figures 1A, B). Renin was expressed in the distal segments of the nephron within both the outer and inner medulla, most prominently in collecting ducts (Figures 1C, D). There was some weak diffuse staining for miR-181a surrounding the juxtaglomerular apparatus (Figure 1A). miR-181a was colocalized with renin in distal tubules of the cortex (Figure 1B) and within the distal medullary segments of the nephron (most prominently in collecting ducts; Figures 1C, D). Both renin and miR-181a showed staining in the apical (supranuclear) part of the tubular epithelial cells in a globular manner (Figures 1C, D). The colocalization of miR-181a and renin in the distal collecting duct is shown in higher magnification in Supplementary Figure S1.
Figure 1.
Localization of renin and miR-181a in the human kidney by immunohistochemistry and in situ hybridization, respectively. (A–D) Sections through histological compartments of the kidney: (A) glomerulus; JGA, juxtaglomerular apparatus; *, distal convoluted tubule; (B) cortical tubules (arrows indicate distal convoluted tubules); (C) outer medulla; black arrows, collecting ducts; black arrowhead, loop of Henle; white arrows, loop of Henle (thick ascending limb); (D) inner medulla; black arrows, collecting duct; short black arrows, peritubular capillaries; white arrows, loop of Henle; all images in row A, 200×, B, 100×, C, 200× and D, 100×.
miR-181a Is Associated with Downregulation of Mitochondrial Respiration and Upregulation of Immunity/Inflammation Pathways in 69 Human Kidneys Characterized by Next-Generation RNA Sequencing
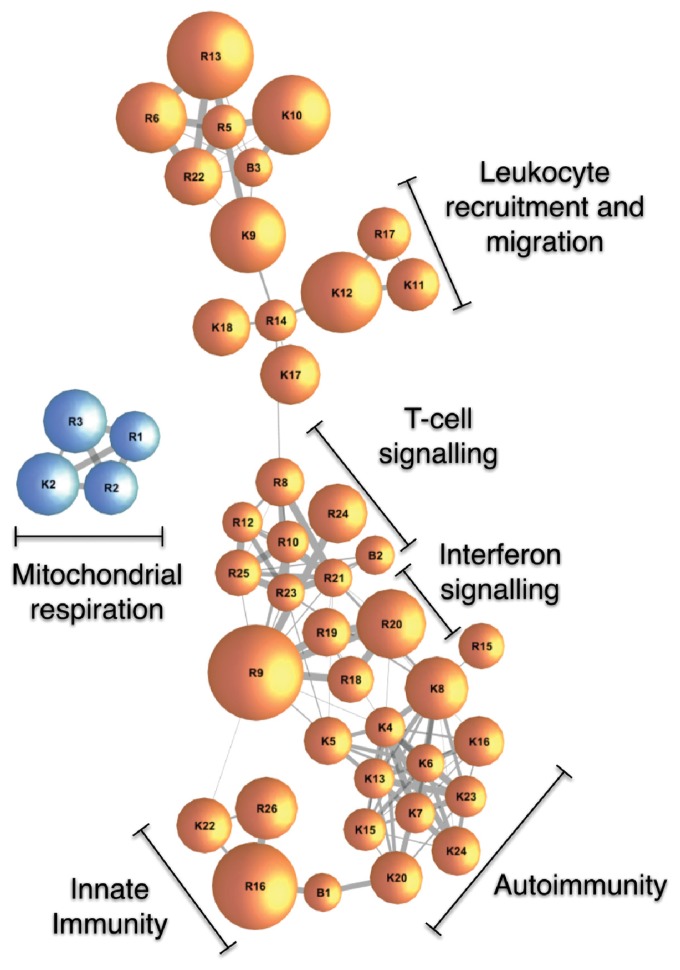
A total of 54,043 transcripts were expressed in the normal-matched kidneys from TCGA subjects. Individually, no renal transcript was associated with miR-181a after correction for multiple testing. Indeed, the most significant association was assigned a FDR q-value of approximately 0.31. Renal expression of miR-181a was, however, associated with 53 pathways from KEGG, BioCarta and Reactome repositories (all FDR q < 0.01; Supplementary Table S6). The majority of miR-181a-associated pathways clustered in two master coexpression networks. The largest network was composed of 41 upregulated pathways underpinned primarily by adaptive immunity and inflammation, while a smaller network consisted of four downregulated pathways responsible for mitochondrial respiratory function (Figure 2).
Figure 2.
Pathways associated with expression of miR-181a in the human kidney. Gene set enrichment analysis of renal transcriptomes characterized by next-generation RNA sequencing. Nodes represent pathways from Kyoto Encyclopedia of Genes and Genomes (KEGG, pathways marked with K), Reactome (pathways marked with R) and BioCarta (pathways marked with B), identified as positively (orange nodes) or negatively (blue nodes) associated with miR-181a expression. Nodes are sized according to the number of constituent genes. Connections between nodes are sized according to the number of shared genes between pathways (expressed as the overlap coefficient). Full names of the pathways are in the supplementary online material (Supplementary Table S6).
Apparently Healthy Tissue from Kidneys after Cancer Nephrectomies Clusters with Kidneys Having No Apparent Pathology–Hierarchical Clustering Analysis of Renal Transcriptomes Determined by Next-Generation RNA Sequencing
Hierarchical clustering of the first three dimensions extracted by multidimensional scaling from the available GTEx Project, TRANSLATE and TCGA expression profiles showed strong clustering of the normal (noncancer) GTEx kidneys with both TRANSLATE and normal-matched TCGA samples. The TCGA cancer samples showed a tight clustering with each other in clear separation from the other samples (Supplementary Figure S2).
DISCUSSION
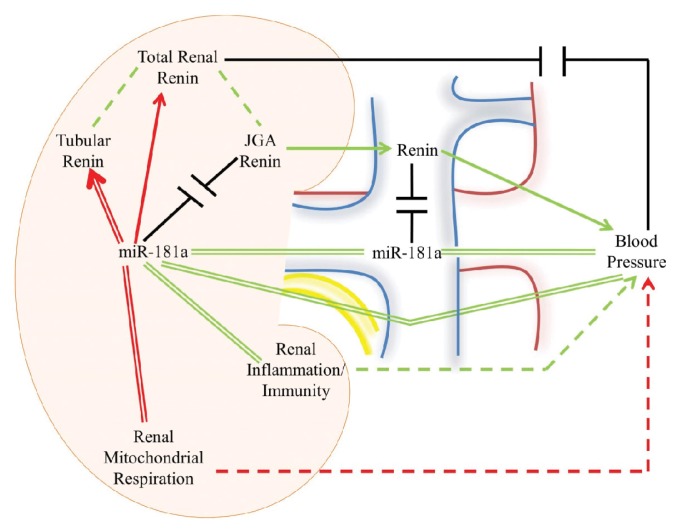
Our study has provided several important and novel insights into the interactions between miR-181a, its renal mRNA target (renin), the kidney transcriptome and BP. Firstly, we identified a new association between circulating levels of miR-181a and clinic BP in two separate populations. Secondly, we demonstrated that this association is independent of circulating renin. Thirdly, we provided a new biologically relevant context to this finding through demonstrating that renal miR-181a does not colocalize with juxtaglomerular renin (that is released into the circulation) but is expressed in the distal nephron together with tubular renin (that acts in autocrine/paracrine manner). Finally, we have identified a network of renal pathways with strong prior evidence of a role in hypertension that may account (at least to some extent) for an association between miR-181a and BP. A schematic representation of the findings provided by our study is shown in Figure 3.
Figure 3.
miR-181a, renin, and blood pressure: a schematic representation of the novel and existing associations. Green lines indicate positive associations, red lines indicate negative associations and black lines indicate a lack of association. Solid lines represent associations tested in the present study, double lines indicate novel associations and dashed lines indicate associations demonstrated in the literature. Arrowheads, where present, indicate a direction of effect and JGA is an abbreviation of juxtaglomerular apparatus. Renal miR-181a was negatively associated with total renal renin, with immunochemistry/in situ hybridization demonstrating that this association was driven through tubular, but not JGA renin. Total renal renin was not associated with blood pressure but, through JGA renin, was positively associated with circulating renin, a known correlate of blood pressure. Renal miR-181a was positively associated with circulating miR-181a levels, which in turn showed a positive association with blood pressure, independent of renin. Renal miR-181a showed also a negative association with renal expression of mitochondrial respiration pathways and positive association with pathways of renal adaptive immunity and inflammation. Downregulation of renal mitochondrial respiration and upregulation of adaptive immunity and response to inflammation in the kidney are well-known contributors to hypertension.
In keeping with some (19–21), but not all (22,23) previous studies, the direction of the association between circulating renin and BP in our analysis was positive. In subjects with hypertension (67% of TRANSLATE patients) the strong negative feedback effect of BP on renin release (and thus circulating renin) is disturbed (24). This is particularly apparent in individuals aged >50 years (21), who were most prevalent in our study. It should also be noted that measurement of serum levels of renin may be confounded by many factors, including body position, diet, exercise (25), intake of sodium (26) and use of antihypertensive medication. With the exception of antihypertensive treatment, we could not fully account for the interindividual differences in other parameters when examining the relationship between serum levels of renin and BP. Variation in these parameters may, to some extent, explain discrepancies in the association between circulating renin and BP between various studies.
An understanding of the role of miRNAs in renin control is still in its infancy. Two miRNAs, miR-330 and miR-125b-5p, are known to control renin cell lineage (27). Our initial study was the first to identify a miRNA (miR-181a) as a potential negative regulator of renin mRNA expression in the human kidney (9). The present data, together with findings from previous in vitro functional assays (9), clearly indicate that renal miR-181a is indeed related to the expression of renin in the human kidney. The direction of the association between BP and miR-181a in the present study, however, was opposite to the one we anticipated from its negative correlation with renin mRNA in kidney, but consistent with the upregulation of miR-181a in preeclampsia, a disorder complicated by hypertension (28,29). Indeed, adjustment for circulating renin had very little effect on the association between miR-181a and BP. Furthermore, our analysis colocalized miR-181a and renin primarily to the distal segments of the nephron, most prominently within the principal cells of the medullary collecting duct (Supplementary Figure S1), from where renin is not released into the circulation and where it acts in an autocrine/paracrine manner independent of the systemic RAS and of an effect on BP (30, 31).
Tubular renin and other components of the local RAS, including angiotensinogen, angiotensin converting enzyme (ACE) and angiotensin II type 1 receptor (AGTR1), play an important role in human and several forms of experimental hypertension (30,31). The apparent suppressive effect of miR-181a on renin mRNA expression should (at least theoretically) translate into reduction of RAS activity in this histological compartment and lead to BP reduction rather than elevation. Indeed, targeted knock-out of renin in this segment of the nephron reduced medullary and cortical collecting duct renin mRNA and attenuated angiotensin II-induced hypertension, while overexpression of collecting duct renin was reported to lead to hypertension in mice (32,33). Our study revealed no association between renal expression of renin and BP, but the mRNA quantification could not separate juxtaglomerular-derived from distal nephron-related renin (Figure 3). It should also be noted that response mechanisms of collecting duct renin to known biological stimuli (such as angiotensin II) differ significantly from those observed for juxtaglomerular renin. Future studies should focus on measuring miR-181a and renin together with other components of the RAS, specifically within the collecting ducts, to fully elucidate the role of miR-181a in the local RAS in the context of BP regulation.
Since any given miRNA can bind to and control expression of hundreds of mRNA targets within a wide array of signaling pathways operating in a range of regulatory systems (34,35), the net effect of a single miRNA on complex phenotypes such as BP may be difficult to predict simply from its binding to just one mRNA target. Next-generation RNA sequencing may provide unbiased high resolution insights into the coexpression networks associated (either directly or indirectly) with a target mRNA/miRNA; to this end, we used it to explore the signatures of miR-181a on the global renal transcriptome. We should stress that these data are not an outcome of predictions and theoretical modeling in silico, but directly reflect patterns of coexpression between miR-181a and its partner mRNAs in the human kidney. To the best of our knowledge this is one of the first examples of using RNA sequencing of human kidney to gain insight into transcriptional mechanisms of potential relevance to BP regulation. Indeed, our analysis of 69 human kidneys characterized by next-generation RNA sequencing revealed that renal miR-181a level was associated with decreased expression of pathways underpinned by mRNAs common to mitochondrial respiratory function and increased expression of signaling cascades of immunity (mainly adaptive) and response to inflammation. The role of miR-181a in inflammation and immunity was reported previously in several types of cells and tissues including thymus (36) and stem/progenitor cells (37), but we are the first to identify the existence of this association in the human kidney. miR-181a is a recognized contributor to control of adaptive immunity; it regulates the development of T cells (38). miR-181a was also shown to have antiinflammatory actions by lowering interleukin-1a, interleukin-1b, interleukin-6 and TNFa (39, 40). miR-181a is also a known mitochondrial miRNA (“mitomiR”), acting on the expression of genes at the intersection of mitochondrial function, cellular senescence and response to inflammation (41). Upregulation of miR-181a was postulated to promote apoptosis, mitochondrial dysfunction and oxidative stress, possibly as a result of direct suppression of the mitochondrial antiapoptotic Bcl2 gene family (42). Indeed, transfection of a miR-181a mimic was associated with a decrease in mitochondrial function and heightened oxidative stress in central nervous system cells in vitro (42). To the best of our knowledge, our study is the first to demonstrate that miR-181a might also operate as a mitomiR in the human kidney.
Both adaptive immunity (43–45) and impairment of mitochondrial electron transport (46) have been causally linked to hypertension, possibly via oxidative stress. Data from different rodent models of hypertension including spontaneously hypertensive rats and manganese superoxide dismutase deficient mice have implicated renal mitochondrial injury in the development and progression of hypertension (47–49). Similarly, the key role of T-cell signaling (a central part of miR-181a-associated pathways) in the development of hypertension is increasingly recognized (50,51). The kidney is particularly important in this context; it has been proposed to play a central role in the effects of adaptive immunity/autoimmunity on BP (52,53). Causal connections between both mitochondrial respiration and adaptive immunity/autoimmunity also have been reported (48).
There are some limitations to our findings. Firstly, we recognize that while both coexpression networks have a clear biological potential to confer prohypertensive effects, the extent to which they may actually account for the observed miR-181a-associated increase in BP is difficult to determine. Indeed, because of the inherent limitation of association analysis, we cannot assign a direction of effect or causality to the correlations detected (Figure 3). Further research will be required to establish if increased levels of miR-181a indeed act directly to elevate BP or whether it is a part of response mechanism to BP elevation.
Secondly, we acknowledge that our analyses focused only on two physiological/anatomical compartments (serum and the kidney). We recognize that miR-181a is abundant in many other tissues, including those relevant to BP regulation, and that other kidney-independent pathways and mechanisms may contribute to the association between miR-181a and BP. This will require further investigations in populations in which other cells and tissues relevant to BP regulation are available. Thirdly, because of lack of BP phenotyping in the population used in the RNA sequencing analysis of the renal transcriptome (TCGA), we could not stratify our analysis on hypertension status or explore directly the extent to which the renal pathways associated with expression of miR-181a were associated with SBP and DBP. Ideally, the identification of renal pathways associated with miR-181a would have been performed in the TRANSLATE cohort, where information on BP is available. Unfortunately, the number of TRANSLATE kidneys with available next-generation RNA sequencing data was too small to allow a meaningful analysis.
Our study has several strengths. Firstly, to the best of our knowledge, the data reported here come from the only population of this size with available human renal tissues together with serum and data on BP. Difficulty in obtaining human tissue (the TRANSLATE study took several years to recruit 200 individuals) may explain the significantly smaller numbers of subjects (usually not exceeding 30 to 50) in previous studies on tissue-serum profiling of miRNAs (54). Secondly, we provided an independent replication of the findings from our analysis of association between circulating levels of miR-181a and BP. Thirdly, our analysis is relatively immune to the potential confounding effects of certain medications, particularly heparin (55) and antiplatelet drugs (56), on circulating miR-181a levels; none of the TRANSLATE or GRAPHIC patients were receiving heparin and just six of the GRAPHIC patients were receiving antiplatelet treatment. In addition, we have also minimized any potential confounding effect of antihypertensive treatment on the association between serum miR-181a and BP by selecting individuals who were not on BP-lowering medications (GRAPHIC) and by conducting sensitivity analyses restricted to subjects not on antihypertensive therapy and those without high blood pressure (TRANSLATE). Finally, we employed a cautious strategy to mitigate any effect of coexistent cancer on the renal transcriptome. In TRANSLATE, specimens were carefully sampled from the contralateral (cancer unaffected) healthy pole of the kidney. This way of securing “control” tissue for gene expression analysis was used previously by others (57). More importantly, our MDS of renal transcriptomes characterized by next-generation RNA sequencing showed tight clustering of global expression patterns of healthy tissue sampled from kidneys obtained after nephrectomy due to cancer and subjects who did not have cancer. Thus, it is unlikely that the transcriptome programs in cells within neoplastically unaffected apparently healthy renal tissue were largely influenced by the presence of cancer in other parts of the kidney.
CONCLUSION
We show a novel association between serum miR-181a and BP, and we demonstrate that this association is independent of circulating renin. Instead, our data suggest that the key signatures of miR-181a on the renal transcriptome, including the suppression of mitochondrial respiration pathways and upregulation of adaptive immunity are likely to account for the association between miR-181a and BP elevation. The data presented here provide novel insights into intrarenal expression of renin and highlight the potential of a miR-181a-driven mechanism of renin control located primarily within the tubular epithelium of the distal nephron. Our study also illustrates the importance of moving beyond the concept of a single miRNA–single mRNA target to fully elucidate the ultimate biological mechanisms underlying the associations between miRNAs and complex phenotypes. Future experiments with antagomiRs or synthetic miRNA mimics to miR-181a should provide further insights into the causal mechanisms underlying interactions between miR-181a and its mRNA targets in the kidney and the relevance of these to BP regulation.
Supplemental Data
ACKNOWLEDGMENTS
We thank Boye Schnack Nielsen from Bioneer S/A (Denmark) for his help with the in situ hybridization and immunohistochemistry. This work was supported by grants from British Heart Foundation (PG/12/9/29376 to M Tomaszewski and J Eales), the National Health & Medical Research Council of Australia (NHMRC project grant 526662 to FJ Charchar) and the Federation University Australia Self-Sustaining Regions Research and Innovation Initiative, an Australian Government Collaborative Research Network grant (to FJ Charchar and FZ Marques). FZ Marques is supported by a NHMRC (APP1052659) and National Heart Foundation (PF12M6785) co-shared Early Career Fellowships. SPR Romaine is supported by Health Education East Midlands.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Marques FZ, et al. (2015) Signatures of miR-181a on the renal transcriptome and blood pressure. Mol. Med. 21:739–48.
REFERENCES
- 1.Rapsomaniki E, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charchar F, Zimmerli L, Tomaszewski M. The pressure of finding human hypertension genes: new tools, old dilemmas. J Hum Hypertens. 2008;22:821–8. doi: 10.1038/jhh.2008.67. [DOI] [PubMed] [Google Scholar]
- 3.Iaconetti C, Gareri C, Polimeni A, Indolfi C. Non-coding RNAs: the “dark matter” of cardiovascular pathophysiology. Int J Mol Sci. 2013;14:19987–20018. doi: 10.3390/ijms141019987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marques FZ, Booth SA, Charchar FJ. The emerging role of non-coding RNA in essential hypertension and blood pressure regulation. J Hum Hypertens. 2015;29:459–67. doi: 10.1038/jhh.2014.99. [DOI] [PubMed] [Google Scholar]
- 5.Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101:921–8. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid SM, Akgul B. Master regulators of posttranscriptional gene expression are subject to regulation. Methods Mol Biol. 2014;1107:303–10. doi: 10.1007/978-1-62703-748-8_18. [DOI] [PubMed] [Google Scholar]
- 7.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 8.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 9.Marques FZ, et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58:1093–8. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 10.Jackson KL, et al. A novel interaction between sympathetic overactivity and aberrant regulation of renin by miR-181a in BPH/2J genetically hypertensive mice. Hypertension. 2013;62:775–81. doi: 10.1161/HYPERTENSIONAHA.113.01701. [DOI] [PubMed] [Google Scholar]
- 11.Tomaszewski M, et al. Pathway analysis shows association between FGFBP1 and hypertension. J Am Soc Nephrol. 2011;22:947–55. doi: 10.1681/ASN.2010080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomaszewski M, et al. Fibroblast growth factor 1 gene and hypertension: from the quantitative trait locus to positional analysis. Circulation. 2007;116:1915–24. doi: 10.1161/CIRCULATIONAHA.107.710293. [DOI] [PubMed] [Google Scholar]
- 13.Tomaszewski M, et al. Renal mechanisms of association between fibroblast growth factor 1 and blood pressure. J Am Soc Nephrol. 2015;26:3151–60. doi: 10.1681/ASN.2014121211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomaszewski M, et al. Genetic architecture of ambulatory blood pressure in the general population: insights from cardiovascular gene-centric array. Hypertension. 2010;56:1069–76. doi: 10.1161/HYPERTENSIONAHA.110.155721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehret GB, Caulfield MJ. Genes for blood pressure: an opportunity to understand hypertension. Eur Heart J. 2013;34:951–61. doi: 10.1093/eurheartj/ehs455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth GK. limma: Linear Models for Microarray Data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer; New York: c2005. pp. 397–420. [Google Scholar]
- 17.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abd-Allah NM, Hassan FH, Esmat AY, Hammad SA. Age dependence of the levels of plasma norepinephrine, aldosterone, renin activity and urinary vanillylmandelic acid in normal and essential hypertensives. Biol Res. 2004;37:95–106. doi: 10.4067/s0716-97602004000100010. [DOI] [PubMed] [Google Scholar]
- 20.Uckaya G, et al. Plasma leptin levels strongly correlate with plasma renin activity in patients with essential hypertension. Horm Metab Res. 1999;31:435–8. doi: 10.1055/s-2007-978769. [DOI] [PubMed] [Google Scholar]
- 21.Vetter H, Glanzer K, Vetter W. Essential hypertension: relationship between renin and blood pressure. Clin Exp Hypertens. 1980;2:553–61. doi: 10.3109/10641968009037129. [DOI] [PubMed] [Google Scholar]
- 22.Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens. 1998;16:853–62. doi: 10.1097/00004872-199816060-00017. [DOI] [PubMed] [Google Scholar]
- 23.He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin-angiotensin system and blood pressure: differences between blacks and whites. Am J Hypertens. 1999;12:555–62. doi: 10.1016/s0895-7061(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 24.Lucas CP, et al. Disturbed relationship of plasma-renin to blood-pressure in hypertension. Lancet. 1974;2:1337–9. doi: 10.1016/s0140-6736(74)92211-9. [DOI] [PubMed] [Google Scholar]
- 25.Williams B, Baschiera F, Lacy PS, Botha J, Prescott MF, Brunel P. Blood pressure and plasma renin activity responses to different strategies to inhibit the renin-angiotensin-aldosterone system during exercise. J Renin Angiotensin Aldosterone Syst. 2013;14:56–66. doi: 10.1177/1470320312454766. [DOI] [PubMed] [Google Scholar]
- 26.He FJ, Markandu ND, MacGregor GA. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension. 2001;38:321–5. doi: 10.1161/01.hyp.38.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Medrano S, Monteagudo MC, Sequeira-Lopez ML, Pentz ES, Gomez RA. Two microRNAs, miR-330 and miR-125b-5p, mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol. 2012;302:F29–37. doi: 10.1152/ajprenal.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med. 2009;47:923–9. doi: 10.1515/CCLM.2009.228. [DOI] [PubMed] [Google Scholar]
- 29.Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci. 2011;18:46–56. doi: 10.1177/1933719110374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–62. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: A major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2014;307:F931–8. doi: 10.1152/ajprenal.00367.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens. 2013;26:965–72. doi: 10.1093/ajh/hpt071. [DOI] [PubMed] [Google Scholar]
- 34.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 36.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–61. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, et al. MicroRNA-181a regulates local immune balance by inhibiting proliferation and immunosuppressive properties of mesenchymal stem cells. Stem Cells. 2012;30:1756–70. doi: 10.1002/stem.1156. [DOI] [PubMed] [Google Scholar]
- 38.Kroesen BJ, et al. Immuno-miRs: critical regulators of T-cell development, function and ageing. Immunology. 2015;144:1–10. doi: 10.1111/imm.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie W, et al. miR-181a regulates inflammation responses in monocytes and macrophages. PLoS One. 2013;8:e58639. doi: 10.1371/journal.pone.0058639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie W, Li Z, Li M, Xu N, Zhang Y. miR-181a and inflammation: miRNA homeostasis response to inflammatory stimuli in vivo. Biochem Biophys Res Commun. 2013;430:647–52. doi: 10.1016/j.bbrc.2012.11.097. [DOI] [PubMed] [Google Scholar]
- 41.Rippo MR, Olivieri F, Monsurro V, Prattichizzo F, Albertini MC, Procopio AD. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56C:154–63. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213–9. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10:203–7. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lob HE, et al. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–83. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens. 2010;19:181–6. doi: 10.1097/MNH.0b013e3283360a2e. [DOI] [PubMed] [Google Scholar]
- 46.Chan SH, Wu KL, Chang AY, Tai MH, Chan JY. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension. 2009;53:217–27. doi: 10.1161/HYPERTENSIONAHA.108.116905. [DOI] [PubMed] [Google Scholar]
- 47.de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1616–25. doi: 10.1152/ajpregu.00615.2005. [DOI] [PubMed] [Google Scholar]
- 48.Eirin A, Lerman A, Lerman LO. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur Heart J. 2014;35:3258–66. doi: 10.1093/eurheartj/ehu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin K, Vaziri ND. Salt-sensitive hypertension in mitochondrial superoxide dismutase deficiency is associated with intra-renal oxidative stress and inflammation. Clin Exp Nephrol. 2014;18:445–52. doi: 10.1007/s10157-013-0851-3. [DOI] [PubMed] [Google Scholar]
- 50.Harrison DG, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–40. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014;126:267–74. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol. 2014;10:56–62. doi: 10.1038/nrneph.2013.248. [DOI] [PubMed] [Google Scholar]
- 53.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–8. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 54.Schena FP, Serino G, Sallustio F. MicroRNAs in kidney diseases: new promising biomarkers for diagnosis and monitoring. Nephrol Dial Transplant. 2014;29:755–63. doi: 10.1093/ndt/gft223. [DOI] [PubMed] [Google Scholar]
- 55.Kaudewitz D, et al. Impact of intravenous heparin on quantification of circulating microRNAs in patients with coronary artery disease. Thromb Haemost. 2013;110:609–15. doi: 10.1160/TH13-05-0368. [DOI] [PubMed] [Google Scholar]
- 56.Willeit P, et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ Res. 2013;112:595–600. doi: 10.1161/CIRCRESAHA.111.300539. [DOI] [PubMed] [Google Scholar]
- 57.Rodwell GEJ, et al. A transcriptional profile of aging in the kidney. PLoS Biol. 2004;2:e427. doi: 10.1371/journal.pbio.0020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.