Abstract
The adoptive transfer of alternatively activated macrophages (AAMs) has proven to attenuate inflammation in multiple mouse models of colitis; however, the effect of cryopreservation on AAMs, the ability of previously frozen AAMs to block dinitrobenzene sulfonic acid (DNBS) (Th1) and oxazolone (Th2) colitis and their migration postinjection remains unknown. Here we have found that while cryopreservation reduced mRNA expression of canonical markers of interleukin (IL)-4–treated macrophages [M(IL-4)], this step did not translate to reduced protein or activity, and the cells retained their capacity to drive the suppression of colitis. The anticolitic effect of M(IL-4) adoptive transfer required neither T or B cell nor peritoneal macrophages in the recipient. After injection into the peritoneal cavity, M(IL-4)s migrated to the spleen, mesenteric lymph nodes and colon of DNBS-treated mice. The chemokines CCL2, CCL4 and CX3CL1 were expressed in the colon during the course of DNBS-induced colitis. The expression of integrin β7 on transferred M(IL-4)s was required for their anticolitic effect, whereas the presence of the chemokine receptors CCR2 and CX3CR1 were dispensable in this model. Collectively, the data show that M(IL-4)s can be cryopreserved M(IL-4)s and subsequently used to suppress colitis in an integrin β7-dependent manner, and we suggest that these proof-of-concept studies may lead to new cellular therapies for human inflammatory bowel disease.
INTRODUCTION
There is increasing interest in cell-based therapies to treat diseases such as cancer (1,2), spinal cord injuries (3) and inflammatory bowel disease (IBD) (4), for which the main subtypes include Crohn’s disease (CD) and ulcerative colitis. Mesenchymal stem cells are currently being evaluated experimentally and clinically for their efficacy in IBD, particularly in fistulizing CD (5). Autologous T cells differentiated into Tregs in vitro are also thought to have an antiinflammatory effect when adoptively transferred to patients with CD, since a dose-dependent reduction in the Crohn’s disease activity index (CDAI) was observed in a small safety trial (6). Here we propose the use of interleukin (IL)-4–treated alternatively activated macrophages—M(IL-4)—as a potential therapeutic for the management of IBD.
Alternatively activated macrophages (AAMs) are considered antiinflammatory because of their ability to dampen T-cell expansion through their expression of programmed-death ligands (PD-L1, PD-L2) (7,8), decreased proinflammatory cytokine and superoxide secretion (9) and their ability to regulate fibrogenesis through arginase 1 activity in mice (10) and CCL18-mediated fibroblast stimulation in humans (11). In addition to their association with tissue restitution, this macrophage phenotype is also associated with infection with parasitic helminths (and other strong Th2 environments), and infection with a variety of helminths has been shown to ameliorate inflammatory disease in rodents, including models of colitis (12).
There is a plethora of evidence to support the use of adoptively transferred M(IL-4)s as an antiinflammatory therapy. Whereas the method of inducing alternative activation may differ—alternative activation being comprised of a spectrum of differentiation states (13)—collectively, it has been shown that treatment with M(IL-4)s or M(IL-4)-like cells is antiinflammatory in mouse models of colitis (14–19). AAMs have also been demonstrated to reduce the severity of nephropathy (20) and diabetes (21) in mice. This effect is thought to be dependent on the macrophage’s ability to express IL-10 (18) and arginase 1 (22) and the ability to retain its antiinflammatory phenotype posttransfer (23). However, despite significant proof-of-principle data that adoptive transfer of M(IL-4)s, or other regulatory macrophages, can block colitis, there are significant gaps in the knowledge of how this effect is mediated, which would stand as an impediment to the development of M(IL-4) to treat IBD. Thus, the current project, building on our earlier observations on M(IL-4) amelioration of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice (14,18), addresses the following questions: (a) are cryopreserved M(IL-4)s capable of suppressing DNBS-induced colitis; (b) would any anticolitic effect of cryopreserved M(IL-4)s apply to the oxazolone model of colitis; (c) are macrophages or T cells in the recipients required for the anticolitic effects of transferred M(IL-4)s; and (d) is recruitment to the colon or peripheral lymphoid tissue important for the anticolitic effect of M(IL-4)s?
MATERIALS AND METHODS
Animal Cell Retrieval and Experimentation
All animal experiments adhered to the Canadian Council on Animal Welfare as administered by the University of Calgary Animal Care Committee under protocol AC13-0015.
Murine Macrophages and Cryopreservation
Bone marrow cells were isolated from the femurs and tibia of mice and then treated with ammonium chloride potassium (ACK) buffer to remove erythrocytes. Cells were plated onto plastic Petri dishes and differentiated into macrophages by culture for 7 d in RPMI-1640 medium (Sigma-Aldrich) supplemented with 2% Pen/Strep, 1 × GlutaMAX™, 20% fetal bovine serum (FBS) (all Gibco) and 20 ng/mL recombinant macrophage-colony stimulating factor (M-CSF) (R&D Systems), changing medium every 2–3 d. To dissociate cells, a 1:1 mix of TrypLE Express™ (Gibco) and 1 mmol/L ethylenediaminetetraacetic acid (EDTA) in Dulbecco’s phosphate-buffered saline (DPBS) was added for 30 min at 37°C followed by gentle scraping with a rubber policeman. Cells were then reseeded and differentiated for 48 h with recombinant IL-4 ± IL-13 (both at 20 ng/mL) (Cedarlane Labs) in RPMI-1640 medium supplemented with 2% penicillin-streptomycin (10,000 U/mL) from Thermo Fisher Scientific, 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) and 10% FBS (all Gibco). In some experiments, macrophages were treated with interferon (IFN)-γ (10 ng/mL, 48 h; Cedarlane Labs). For cryopreservation, differentiated macrophages were resuspended in 10% dimethyl sulfoxide (DMSO) in FBS at 5 × 106 cells/mL and frozen for ≥7 d by placing in a Mr. Frosty™ Freezing Container (Thermo Fisher) containing isopropanol to slow the freezing rate to ~1°C per minute (overnight −80°C) and then transferring the vials to liquid nitrogen the next day. For use of frozen cells, vials were quickly thawed by removing from liquid nitrogen storage, immersed into a 37°C water bath, washed in complete medium and resuspended for use. For fresh versus frozen comparisons, cell cultures were grown and analyzed (“fresh”), and a fraction of the population was frozen and analyzed at a later time point (“frozen”).
Arginase activity and Griess assays (to measure nitrite as a surrogate for nitric oxide) were performed as previously described (18). Arginase activity is represented in units, where 1 unit represents the amount of arginine hydrolyzed within 1 min per 1 million cells. Lipopolysaccharide (LPS)-stimulated secretion of cytokines were analyzed by stimulating 1 × 106 macrophages with 1 μg LPS (Sigma-Aldrich) in 1 mL fresh medium, collecting the supernatant after 24 h and analyzing samples using DuoSet® ELISA kits (R&D Systems) as per the manufacturer’s protocol.
Human Macrophages
Experiments with human samples were conducted under a protocol approved by the University of Calgary Conjoint Health Research Ethics Board (protocol 24827).
Peripheral blood from healthy donors was collected in heparinized tubes and separated by using a Ficoll-Paque™ PREMIUM (GE Healthcare Bio-Sciences AB) density gradient, as per the manufacturer’s protocol. Buffy coat cells were incubated for 4 h in serum-free medium (RPMI-1640 medium supplemented with 2% Pen/Strep, 20 mmol/L HEPES; all Gibco), which was then replaced with complete medium (containing 10% FBS) and incubated for 7 d with 10 ng/mL recombinant human M-CSF. Macrophages were differentiated for 48 h with recombinant IL-4 ± IL-13, both at 10 ng/mL (Cedarlane Labs). Cells were dissociated and/or cryopreserved as described for murine macrophages.
Mice and Induction of Colitis
Male BALB/c mice (Charles River Laboratories) were 8–10 wks old at the time of experimentation. The following other mice were used: Itgb7null (C57BL6-Itgb7tm1Cgn/J); RAG1 KO (B6129S7-Rag1tm1Mom/J); BALB/c eGFP; C57BL/6J mice (all the Jackson Laboratory); CCR2 KO (C57BL6-Ccr2rfp/rfp) and CX3CR1 KO (C57BL6-Cx3cr1gfp/gfp) mice were gifts from the laboratory of Paul Kubes, University of Calgary. Following a previously established protocol, 1 × 106 M(IL-4)s in 250 μL PBS (106 spleen cells or PBS alone for naive controls) were injected intraperitoneally 48 h before the induction of colitis (18) (earlier studies had shown that a threshold for an anticolitic effect of AAMs was 0.75 × 106 cells [14]). Acute colitis was induced by intrarectal administration of DNBS (MP Biomedicals) (3 mg for BALB/c or 5 mg for C57BL/6) or 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone; Sigma-Aldrich) (4 mg) in 100 μL 50% ethanol (or 100 μL PBS for naive controls) by using a polystyrene catheter 3 cm proximal from the anus, while mice received 3% inhaled isoflurane (Pharmaceutical Partners of Canada). In some experiments, clodronate-loaded liposomes (Nico van Rooijen, Amsterdam, Netherlands) were rocked for 5 min at room temperature to yield a homogeneous preparation and 200 μL was intraperitoneally injected on d −3 and −5 before the administration of DNBS (14). Macrophage depletion in the peritoneal cavity was confirmed by the absence of F4/80+ cells on d 0, as measured by flow cytometry on peritoneal exudate cells; neutrophils (Gr1+) were still detectable in lavages after treatment with clodronate intraperitoneally. Animals were monitored daily for weight changes, and on d 3 postcolitis, a necropsy was performed. The colon was excised and measured and a mid-10% portion fixed in 10% neutral-buffered formalin, processed into paraffin, sectioned at 7 μm onto positively charged slides and stained with hematoxylin and eosin. Macroscopic and histological damage scores were assessed as previously described (18).
In Vivo Localization of Adoptively Transferred Macrophages
Animals were fed a modified AIN-93M diet (TD.00102, Harlan Laboratories) starting on d −6 to reduce background fluorescence. Cells (6.0–7.5 × 106 macrophages or splenocytes) were stained with VivoTrack 680 NIR (PerkinElmer) as per their instructions, just before injection on d −2. Spleen cells were isolated by mashing a naive control spleen through a 100-μm nylon strainer using the plunger from a 10-mL syringe and then treating the isolate with ACK buffer for 90 s to remove erythrocytes. DNBS-induced colitis was induced on d 0 as described above and mice assessed 3 d later.
Noninvasive whole-body imaging was performed by using In-Vivo Xtreme 4MP (Bruker, formerly Carestream). Mice were shaved 24 h before imaging. Macrophages were visualized using 670-nm excitation and 750-nm emission wavelengths. Every 24 h, animals were imaged ventrally, laterally and dorsally. The imaging protocol contained three steps: reflectance imaging (2-s exposure time), fluorescent imaging at 670/750 (6-s exposure time) and X-ray imaging (10-s exposure time). During the whole imaging procedure the mice were anesthetized with 1–3% isoflurane and kept at 37°C in the imaging chamber. Images from the In-Vivo Xtreme were acquired and analyzed by using Bruker molecular imaging software MI SE (version 7.1.3.20550). Whole-body images were quantified daily by measuring fluorescence (corrected by background) in a constant region of interest for all animals covering the ventral abdominal area.
After whole-body in vivo imaging, mice were necropsied on d 3 post-DNBS, and their organs (lungs, heart, liver, small intestine, mesenteric lymph nodes, colon) were removed, flushed and washed with PBS for further imaging. The whole colon and randomly selected sections of the liver and spleen were mounted onto a slide underneath a glass coverslip with fluorescence-mounting medium (Dako). Spinning disk confocal intravital microscopy was performed using an Olympus BX51W1 (Olympus) upright microscope equipped with a 20×/0.95 XLUM Plan Fl water immersion objective. The microscope was equipped with a confocal light path (WaveFx, Quorum) on a Yokogawa CSU-10 head (Yokogawa Electric Corporation). Laser excitation at 640 nm (Cobalt) was used, and fluorescence in the blue channel was visualized with the appropriate long pass filter (624 ± 40 nm, respectively; Semrock). Exposure time was constant at 200 ms. Sensitivity settings were maintained at the same level for all experiments. A 512 × 512–pixel back-thinned EMCCD camera (C9100-13, Hamamatsu) was used for fluorescence detection. Volocity Acquisition software (Improvision) was used to drive the confocal microscope. Images captured using the spinning disk were processed and analyzed in Volocity 4.20. Fluorescence-labeled cells in the colon were enumerated by selecting three random fields of view at 20× magnification.
Real-Time Polymerase Chain Reaction
Real-time polymerase chain reaction (PCR) of murine and human macrophages was done as previously described (18). Primers used are listed in Table 1.
Table 1.
PCR primers used to assess murine bone marrow–derived macrophages, murine colon tissue and human peripheral blood–derived macrophages.
| Species | Gene | Alias | PubMed accession number | Forward | Reverse |
|---|---|---|---|---|---|
| Mouse | Rn18s | 18S | NR_003278.3 | ATGGCCGTTCTTAGTTGGTG | CGCTGAGCCAGTCAGTGTAG |
| Arg1 | Arginase 1 | NM_007482.3 | AACACTCCCCTGACAACCAG | CCAGCAGGTAGCTGAAGGTC | |
| Nos2 | iNOS | NM_010927.3 | AGACCTCAACAGAGCCCTCA | GCAGCCTCTTGTCTTTGACC | |
| Chi3l3 | Ym1 | NM_009892.2 | TGGAGGATGGAAGTTTGGAC | AATGATTCCTGCTCCTGTGG | |
| Retnla | Relm-α | NM_020509 | AGCAATCCCATGGCGTATAA | CAGTAGCAGTCATCCCAGCA | |
| Ccl4 | CCL4 | NM_013652.2 | GCTCTGCGTGTCTGCCCTCT | TCTGTGAAGCTGCCGGGAGGT | |
| Cx3cl1 | Fractalkine | NM_009142.3 | GCCCGCCGAATTCCTGCACT | CAATGGCACGCTTGCCGCAG | |
| Ccl5 | CCL5 | NM_013653.3 | TCTCTGCAGCTGCCCTCACCA | GCGCGAGGGAGAGGTAGGCA | |
| Ccl25 | CCL25 | NM_009138.3 | GACCAGAAAGGCATTGGTGGCCC | CGGCATCCAGGCCCCAACAAAA | |
| Human | RN18S5 | 18S | NR_003286.2 | ATGGCCGTTCTTAGTTGGTG | CGCTGAGCCAGTCAGTGTAG |
| CD14 | CD14 | NM_000591.3 | GCCGCTGTGTAGGAAAGAAG | GCTGAGGTTCGGAGAAGTTG | |
| MRC1 | Mannose receptor | NM_002438.3 | GGCGGTGACCTCACAAGTAT | ACGAAGCCATTTGGTAAACG | |
| CCL18 | CCL18 | NM_002988.3 | CCCCAAGCCAGGTGTCATCCTC | GGGCCATTGCCCTGGCTCAG |
Flow Cytometry
Annexin V and propidium iodide staining were conducted by using the Annexin V Binding Buffer (BioLegend) and following the manufacturer’s instructions.
For flow cytometry staining with antibodies, cells were resuspended in flow buffer (PBS containing 0.1% sodium azide and 1% FBS) and blocked (TruStain fcX™ anti-mouse CD16/32 or human TruStain FcX Fc receptor blocking solution; BioLegend) for 15 min at 4°C. Surface staining was conducted for 30 min at 4°C in the dark. Fixation, permeabilization and intracellular staining was done at room temperature using the FOXP3 Fix/Perm buffer set (BioLegend). Data were analyzed using Attune® software (Applied Biosystems) or FlowLogic™ (Inivai). Mouse and human macrophages were previously assessed for purity and found to be >96% F4/80+ or CD68+, respectively (data not shown). Cells were derived from culture and gated on a single forward and side scatter population.
Flow cytometry antibodies and stains used in this study were as follows: FITC-conjugated Annexin V (5 μL; 640906, BioLegend), propidium iodide solution (10 μL; 421301, BioLegend), APC-conjugated anti-mouse/human arginase 1 (5 μL; IC5868A, R&D), PE-conjugated anti-mouse MRC1 (intracellular staining, 0.2 μg; 141705, BioLegend), APC-conjugated anti-mouse F4/80 (0.2 μg; 123115, BioLegend), PE/Cy7-conjugated Ly-6C (0.06 μg; 128018, BioLegend); PE-conjugated anti-mouse Gr1 (0.2 μg; 108407, BioLegend), PE-conjugated anti-human MMR (intracellular staining, 5 μL; FAB25342P, R&D) and pacific blue–conjugated anti-human CD14 (1 μg; 325615, BioLegend).
Coculture of Peritoneal Cells with Bone Marrow–Derived Macrophages
Mice were humanely sacrificed using 4% isofluorane followed by cervical dislocation. A peritoneal lavage was conducted by injecting 8 mL 4°C PBS with a 10-mL syringe and 18-G needle into naive control BALB/c mice, massaging the abdomen for a minute and then collecting fluid with the same apparatus. Peritoneal exudate was pooled from two mice and divided equally into three Petri dishes in RPMI-1640 medium supplemented with 2% Pen/Strep, 20 mmol/L HEPES and 10% FBS. Bone marrow–derived M(IL-4)s from BALB/c eGFP mice (for which cells express GFP under the human ubiquitin C promoter) were generated as described above and added to each Petri dish at 1 × 106 cells to a final volume of 10 mL THP-1 medium per dish. Cells were cocultured for 48 h, and then all cells (floating and adhered, as described above for bone marrow–derived macrophages) were collected for analysis by flow cytometry.
T-Cell Proliferation
The T-cell proliferation experiment was conducted by using methods similar to Huber et al. (8). The 24-well plates were coated with anti-CD3 (2 μg/mL; BioLegend) for 24 h at 4°C. CD4+ T cells from naive control BALB/c mice were isolated from the spleen by using a magnetic isolation kit (EasySep, StemCell Technologies), stained with CFSE (5 μm for 10 min at 37°C, CellTrace™ CFSE Cell Proliferation Kit, Thermo Fisher) and added to the plate at 4 × 105 cells per well with anti-CD28 (1 μg/mL; BioLegend). Fresh and frozen macrophages (1 × 104), derived as previously described, were added to the wells at a final 1:4 macrophage to T cell ratio. Cells were collected 96 h later, spun down, fixed with 4% paraformaldehyde (PFA) and analyzed by flow cytometry.
Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM). Data were analyzed and graphed using GraphPad Prism 5 (GraphPad Software). Unless otherwise stated, data were analyzed using a one-way analysis of variance with Tukey or Newman-Keuls post hoc test for parametric data and Dunn multiple comparison test for nonparametric data (that is, macroscopic and histological scores); p <0.05 was accepted as a level of statistically significant difference.
All supplementary materials are available online at www.molmed.org.
RESULTS
Addition of IL-13 with IL-4 Does Not Synergistically Enhance the M(IL-4) Phenotype
Previously, IL-4 and IL-13 alternatively activated murine peritoneal macrophages and bone marrow–derived macrophages were found to inhibit colitis in mice (14,18). To optimize this potential therapeutic option, the abilities of IL-4 and IL-13 alone, and in combination, to induce an alternatively activated phenotype in murine bone marrow–derived macrophages and human blood–derived macrophages were compared. Real-time PCR analyses revealed that IL-4 and IL-4 + IL-13 resulted in similar upregulation in mRNA expression of canonical markers of murine and human AAMs: IL-13 by itself was generally less effective in inducing an AAM phenotype (Supplementary Table S1). This pattern was confirmed by analysis of arginase 1 expression and activity, LPS-evoked nitric oxide production (all for mouse; Supplementary Figure S1A), CD14 and MRC1 expression and CCL18 production (all for human; Supplementary Figure S1B). These data indicated that IL-4 was sufficient to induce alternative activation in both murine and human macrophages. Because the macrophages treated with IL-4 or IL-4 + IL-13 were not substantially different (at least by the parameters assessed herein) and given the primary goal to optimize AAM therapy to treat colitis in murine model systems, all subsequent experiments focused on IL-4–treated macrophages [M(IL-4)s].
Cryopreserved Macrophages Retain an M(IL-4) Phenotype and Reduce the Severity of Colitis
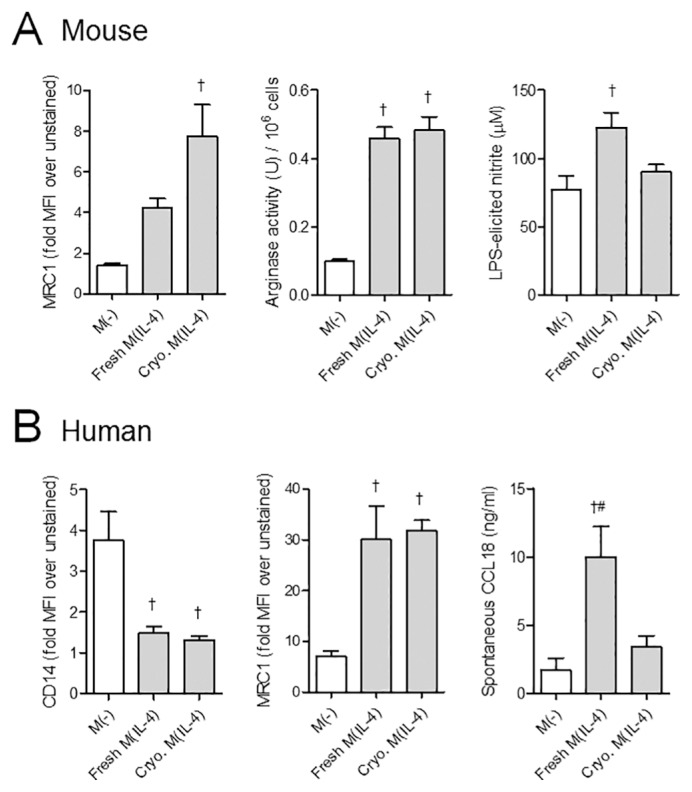
From a practical standpoint, M(IL-4) anticolitic therapy would be best served by the immediate availability of the cells from cryopreserved stocks. Freshly differentiated murine and human M(IL-4)s showed no differences in viability compared with thawed cryopreserved M(IL-4)s, as measured by propidium iodide staining and flow cytometry analysis (% dead cells before cell calculations for adoptive transfer studies: mouse fresh 17.8 ± 6.0% versus frozen 11.5 ± 3.0%, n = 4–8; human fresh 16.5 ± 3.5% versus frozen 17.6 ± 6.6%, n = 3). Comparison of fresh with cryopreserved M(IL-4)s revealed reduced transcripts for Arg1, Chi3l3 (Ym1), Retnla (Relm-α) in murine cryopreserved M(IL-4) and reduced MRC1 and CCL18 mRNA in human cryopreserved M(IL-4)s. CD14 mRNA levels were similarly reduced in the two human macrophage groups (Table 2). Despite these mRNA changes, protein differences were minimal between the groups. Thus, arginase activity and MRC1 were similarly upregulated in fresh and cryopreserved M(IL-4)s (Figure 1A), and, notably, the increase in LPS-induced nitric oxide (NO) in fresh M(IL-4)s was not observed with cryopreserved murine M(IL-4)s. For human M(IL-4)s, both fresh and frozen cells displayed reduced CD14 protein expression, and similar upregulation of MRC1 expression, although the cryopreserved M(IL-4)s had a compromised ability to spontaneously produce CCL18 (Figure 1B).
Table 2.
Comparison of murine and human fresh versus cryopreserved M(IL-4) by real-time PCR.
| Fold-change over M(−) | |||
|---|---|---|---|
|
|
|||
| Species | Gene | Fresh M(IL-4) | Cryopreserved M(IL-4) |
| Mouse | Arg1 | 183,966 ± 54,719a,b | 1,254 ± 552 |
| Nos2 | 11.06 ± 3.05a,b | 2.31 ± 0.825 | |
| Chi3l3 (Ym1) | 2,296 ± 678a,b | 177 ± 17 | |
| Retnla (Relm-α) | 1,955,323 ± 470,371a,b | 72,331 ± 20,697 | |
| Human | CD14 | 0.110 ± 0.0367a,b | 0.0231 ± 0.0135a |
| MRC1 | 10.66 ± 1.64a,b | 5.49 ± 1.41a | |
| CCL18 | 64.75 ± 27.91a,b | 4.27 ± 1.72 | |
Mouse bone marrow–derived macrophages were treated with IL-4 for 48 h and compared before and after cryopreservation for AAM marker expression (Arg1, Chi3l3, Retnla) and Nos2 as a negative control. Likewise, fresh and frozen human monocyte–derived M(IL-4)s from healthy blood donors were compared on their capacity to downregulate CD14 and upregulate MRC1 and CCL18 transcripts. M(−), undifferentiated macrophage. Mouse, n = 7; human, n = 9. p < 0.05:
compared with M(−);
compared with cryopreserved M(IL-4).
Figure 1.
Comparison of fresh and cryopreserved (Cryo.) mouse and human M(IL-4)s. (A) Mouse macrophages were assessed by their capacity to express the mannose receptor (MRC1) by flow cytometry (n = 3), arginase activity and ability to produce nitric oxide in response to LPS, as measured by nitrite levels in a Griess assay (n = 7, two experiments). (B) Human M(IL-4)s from healthy control blood donor monocytes were similarly assessed by their capacity to downregulate CD14 and upregulate MRC1 as measured by flow cytometry (n = 3) and spontaneously secrete CCL18 into the culture supernatant, as measured by ELISA (n = 5, two experiments). MFI, mean fluorescence intensity. p < 0.05: †compared with M(−); #compared with Cryo. M(IL-4). Data are represented as the mean ± SEM.
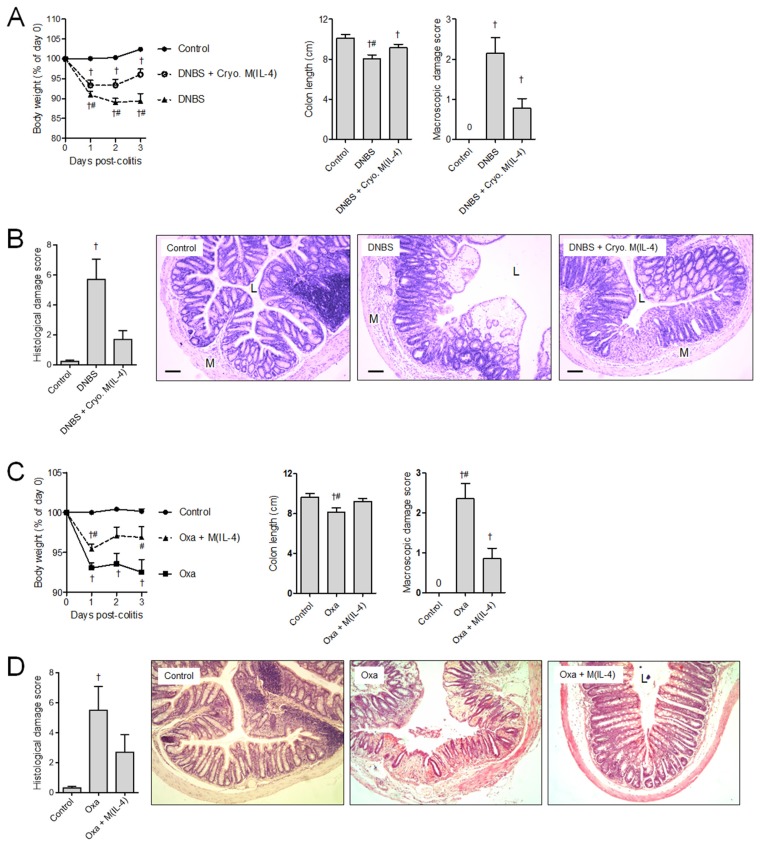
Adoptively transferred cryopreserved M(IL-4)s protected mice against DNBS colitis-associated cachexia, colon shortening, macroscopic and histological damage (Figures 2A, B). Testing the broad ability of M(IL-4)s to suppress intestinal inflammation, experiments were conducted in which mice were treated with M(IL-4)s and colitis evoked by intrarectal delivery of oxazolone, a hapten that induces colitis by activation of NKT cells and mobilization of IL-13 secretion (24). M(IL-4)s significantly limited the severity of colitis in mice induced by oxazolone (Figures 2C, D).
Figure 2.
Transfer of cryopreserved (Cryo.) M(IL-4)s protect against murine colitis. One million M(IL-4)s were transferred intraperitoneally 48 h before the induction of colitis. The extent of damage in DNBS (Th1) colitis was measured by weight loss, shortened colon lengths and extent of colon macroscopic damage (A) and histopathology (B). Experiments with DNBS were repeated twice: naive control (n = 9), DNBS (n = 10) and DNBS + Cryo. M(IL-4) (n = 10). (C, D) By using the same experimental timeline, M(IL-4)-mediated protection against oxazolone (Th2)-induced colitis was assessed by the same parameters. Experiments with oxazolone were repeated four times: naive control (n = 15), Oxa (n = 14), Oxa + M(IL-4) (n = 17). p < 0.05: †compared with naive control; #compared with DNBS + Cryo. M(IL-4). Bar represents 50 μm; M, muscularis; L, lumen. Data are represented as the mean ± SEM.
M(IL-4) Suppression of Colitis Does Not Require Macrophages or Adaptive Immunity in Recipients
Assessing the mechanism of M(IL-4) suppression of colitis, we posited a requirement for either regulatory macrophages or mobilization of regulatory T or B cells in the recipients of M(IL-4)s.
Using a validated strategy to deplete macrophages before adoptive transfer of M(IL-4)s, mice were treated with clodronate liposomes (25), and effective depletion of peritoneal macrophages was confirmed by flow cytometry (data not shown). With the protocol used (see Materials and Methods), the number of neutrophils was minimally affected. Assessment of the colitis in clodronate-treated mice was not markedly different from that observed in liposome only–treated mice, and regardless of clodronate or liposome treatment, M(IL-4)s retained their anticolitic activity, indicating that the M(IL-4)s did not need to activate/recruit endogenous macrophages to mediate or participate in the suppression of colitis (Supplementary Figure S2A). An in vitro experiment supported this finding. Peritoneal cells were isolated by peritoneal lavage and were cocultured with 106 GFP-expressing bone marrow–derived M(IL-4)s for 48 h. Protein analysis by flow cytometry showed that there was no difference in the expression of the AAM markers MRC1 or arginase 1 in cocultured peritoneal macrophages compared with non-cocultured controls, indicating that the transfer of M(IL-4)s did not enhance the expression of these proteins (data not shown).
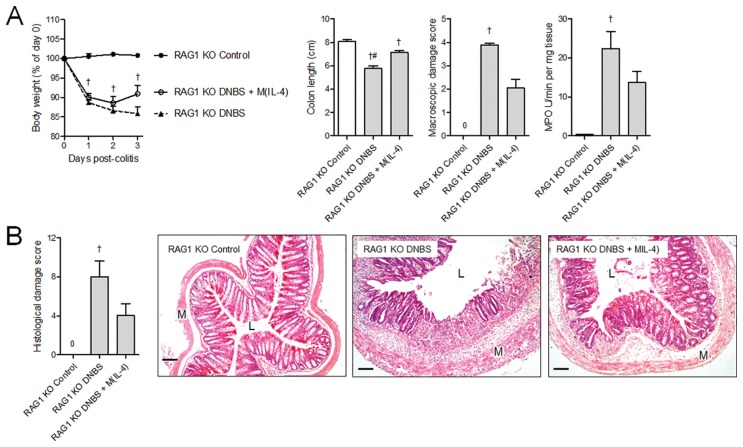
While the mechanism of DNBS-induced colitis in RAG1−/− mice may differ from that in wild-type animals, the macroscopic and histological presentation of the disease in both mice is remarkably similar. Colitis induced in RAG1−/− mice by DNBS was significantly reduced by pretreatment of the mice with M(IL-4)s, demonstrating that neither T cells nor B cells were essential to the anticolitic effect of M(IL-4)s (Figure 3). In addition, in vitro coculture with neither fresh nor cryopreserved M(IL-4)s suppressed anti-CD3 and anti-CD28 antibody-induced proliferation of splenic T cells [dividing cells: unstimulated T cells 7.7 ± 0.2%, CD3- and CD28-stimulated T cells 34.7 ± 0.9%, CD3- and CD28-stimulated T cells cocultured with fresh M(IL-4)s 37.8 ± 1.0%, CD3- and CD28-stimulated T cells cocultured with cryopreserved M(IL-4)s 44.8 ± 0.9%; n = 3–4].
Figure 3.
Intraperitoneal transfer of M(IL-4)s does not require host lymphocytes to protect against DNBS colitis. The M(IL-4) transfer model was repeated in RAG1 KO mice (lacking T and B cells) to determine whether host lymphocytes were essential for the anticolitic effect. The data show that wild-type M(IL-4) were still able to attenuate (A) colon shortening, and only mice treated with DNBS alone were significantly different from naive controls in terms of macroscopic damage, MPO activity, and (B) histological damage. Experiments were repeated twice: naive control (n = 5), DNBS (n = 10), DNBS + M(IL-4) (n = 10). p < 0.05: †compared with naive control; #compared with DNBS + M(IL-4). Bar represents 50 μm; M, muscularis; L, lumen. Data are represented as the mean ± SEM.
Transferred M(IL-4)s Localize to the Colon during DNBS Colitis
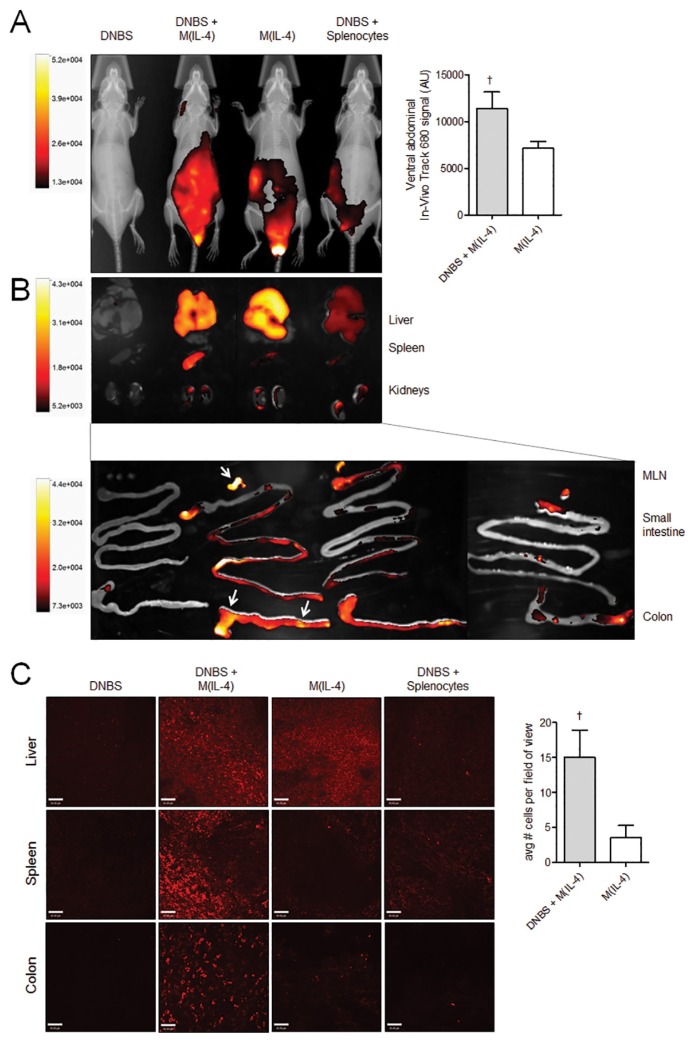
Given that macrophages were injected into the peritoneal cavity before the induction of DNBS, we sought to investigate where the cells might preferentially locate in the presence of colitis. Splenocytes were used as a non-macrophage-cell control and indicator of random cell distribution. On d 3, fluorescence intensity was strongest in the peritoneal area of the M(IL-4)-treated mice (Figure 4A), a pattern that was similar to d −2 just immediately after the injection of cells (not shown). Fluorescence quantification in the ventral abdomen shows that the signal was significantly brighter by d 3 in DNBS + M(IL-4) mice compared with M(IL-4) alone, suggesting that macrophages were being actively recruited to the colon during DNBS colitis. Dissected organs revealed that the signal was most intense in the liver; however, the mesenteric lymph nodes, colon and spleen contained increased fluorescence in mice treated with both M(IL-4) and DNBS. Negligible fluorescence was detected in the heart and lungs (data not shown). Whole mount imaging (Figure 4C) confirmed that macrophages localized to the liver of all M(IL-4)-treated mice, whereas DNBS induced localization of macrophages to the spleen and colon. Quantification of macrophages within the colon revealed that whereas M(IL-4)s were found in colons of both control and DNBS mice, there was a higher number of macrophages found in mice with colitis. Macrophages were found in patchy areas throughout the length of the colon (data not shown).
Figure 4.
Transferred M(IL-4)s home to the colon, liver, mesenteric lymph nodes and spleen during DNBS colitis. (A) Macrophages and spleen cells (as a nonspecific control cell) were labeled with fluorescent dye VivoTrack 680 NIR and localized using whole-body imaging; fluorescence on d 3 just before the necropsy is depicted overlying the X-ray images. Throughout the course of the experiment, the majority of cells remained within the peritoneal cavity in a pattern similar to d 3 (data not shown). (B) Whole organs were dissected and reexamined by using the whole-body imaging system to confirm localization to the liver, spleen, mesenteric lymph nodes and colon in M(IL-4) + DNBS–treated mice. Arrows indicate the areas of peak fluorescence. (C) Cellular localization in the liver, spleen and colon was confirmed further with spinning disk confocal microscopy on mounted organ sections. VivoTrack 680–labeled macrophages at 20× magnification were enumerated in triplicate random fields of view. Whole mounted colons from DNBS mice treated with M(IL-4)s contained significantly more macrophages compared with M(IL-4)s into naive control mice. Data are representative of two to three experiments: DNBS, n = 2; DNBS + M(IL-4), n = 3–4; M(IL-4), n = 3–4; DNBS + splenocytes, n = 2. †p < 0.05 (Mann-Whitney U test, one-tailed). Data are represented as the mean ± SEM.
Integrin β7 Expression on Transferred M(IL-4)s Is Necessary in Their Ability to Block Colitis
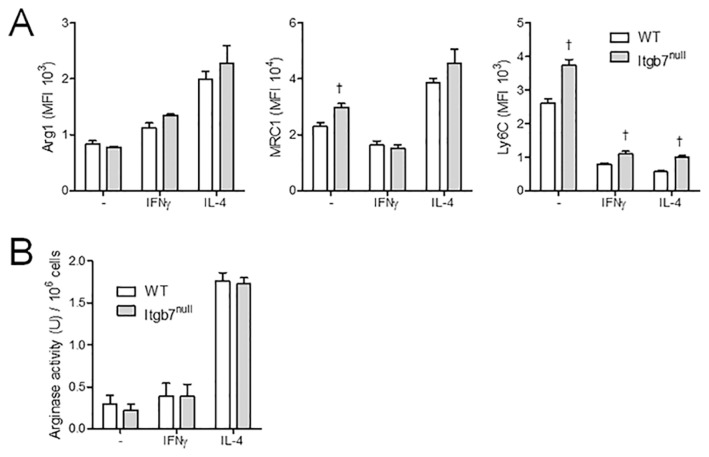
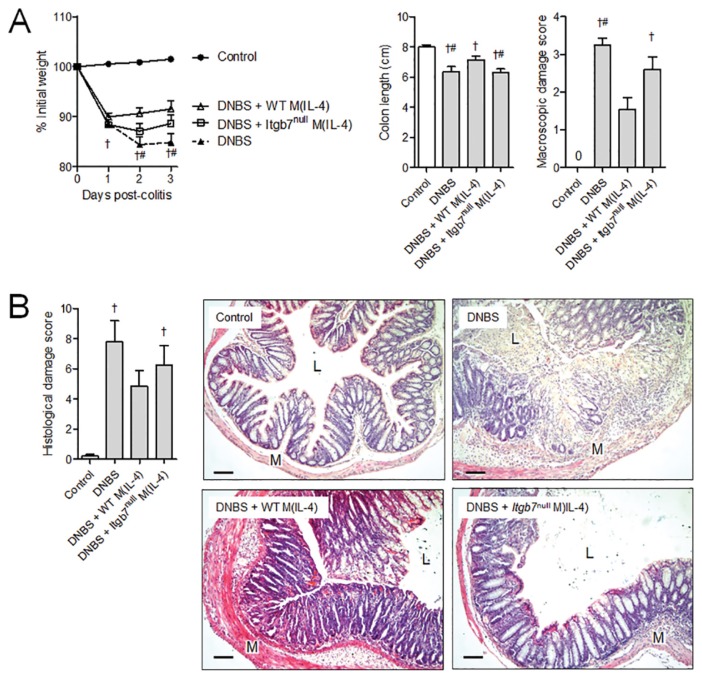
The adhesion molecule α4β7 is important in leukocyte trafficking to the gut during both homeostatic and inflammatory conditions. Given that transferred M(IL-4)s localized to the gut preferentially during colitis, we hypothesized that a lack of β7 would prevent this migration and the ability of M(IL-4)s to protect against inflammation. Initial studies revealed no difference in the ability to generate an M(IL-4) from bone marrow from Itgb7null mice, as assessed by arginase and Ym1 expression (Figure 5); however, in comparison with M(IL-4)s from WT mice, Itgb7null M(IL-4)s were incapable of suppressing DNBS-induced colitis, as assessed by any of the indices used here (Figure 6).
Figure 5.
Alternative activation of bone marrow–derived macrophages from WT and Itgb7null mice is comparable. (A) Expression of AAM markers Arg1 and MRC1 are not significantly different in Itgb7null mouse macrophages, as measured by flow cytometry, although there is a significant increase in Ly6C expression in all Itgb7null macrophages compared with WT. (B) This result was confirmed at the functional level where arginase activity was similar between WT and KO M(IL-4)s. All groups (n = 3), one experiment. p < 0.05: †compared with WT. Data are represented as the mean ± SEM.
Figure 6.
ITGB7 expression on transferred M(IL-4)s is required for protection against DNBS colitis. Protection mediated by pretransfer of M(IL-4)s intraperitoneally was measured on d 3 of DNBS colitis. (A) In these experiments, mice treated with WT but not Itgb7null M(IL-4)s were significantly protected against colitis-associated cachexia, colon shortening, macroscopic damage and (B) histopathology. Experiments were repeated twice: naive control, n = 5; DNBS, n = 7; DNBS + WT M(IL-4), n = 10; DNBS + Itgb7null M(IL-4); n = 10. p < 0.05: †compared with naive control; #compared with DNBS + WT M(IL-4). Bar represents 50 μm; M, muscularis; L, lumen. Data are represented as the mean ± SEM.
Absence of CCR2 and CX3CR1 on Transferred M(IL-4)s Does Not Hinder Their Anticolitic Activity
A time course analysis of DNBS-induced colitis revealed similar disease activity scores 1–3 d after treatment (Supplementary Figure S3A), and quantitative PCR on colonic tissue extracts showed increased expression of mRNA for the chemokines Ccl2 and Cx3cl1 (fractalkine) (Supplementary Figure S3B). Recognizing that redundancy is a feature within chemotaxis signaling, we nevertheless tested a strict requirement for either of the receptors for these chemokines (that is, CCR2 and CX3CR1, respectively) on the anticolitic effect of M(IL-4)s. Hence, WT, CCR2 KO and CX3CR1 KO M(IL-4)s were compared in their ability to attenuate DNBS colitis. As with Itgb7null M(IL-4)s, CCR2 KO and CX3CR1 KO M(IL-4)s were not significantly different compared with WT M(IL-4)s using arginase and Ym1 as the canonical markers of a M(IL-4) (data not shown). In the analysis of DNBS-induced colitis, adoptive transfer of WT, CCR2 and CX3CR1 M(IL-4)s all had similar abilities to reduce the severity of disease (Supplementary Figure S4).
DISCUSSION
The macrophage is an incredibly versatile cell (26), and definition of its role in the suppression of inflammation and tissue restitution after injury has led to the speculation that the AAM could be used in cellular immunotherapy to treat or prevent inflammatory disease (14). Indeed, we, and others, have shown that adoptive transfer of AAMs retrieved from mice or differentiated in vitro from bone marrow precursors or peritoneal macrophages can suppress colitis (14–19). In the current study, we sought to extend these provocative data by determining whether cryopreserved M(IL-4)s, a defined AAM subtype, could be used to suppress colitis and to assess roles for the recipient’s macrophages and T and B cells, and mucosal homing in the M(IL-4)-driven suppression of colitis.
With the ultimate goal of translating the proof-of-principle data from murine models to the treatment of human disease, it was important to consider two practical issues before conducting mechanistic studies: would M(IL-4)s suppress colitis in a manner equivalent to that observed with M(IL-4 + IL-13)s, and would cryopreserved M(IL-4)s retain their ability to inhibit colitis. It is generally accepted that IL-4 is the key cytokine in the alternative activation of macrophages and that IL-13 complements or enhances the differentiation of the AAM via STAT3 and STAT6 signaling (27,28). Assessment of canonical markers of mouse and human AAMs revealed almost identical responses to IL-4 and IL-4 + IL-13 (IL-13 alone was a less potent stimulus of an AAM phenotype), a finding in accordance with data showing that bone marrow–derived macrophages and human monocytes were more sensitive to stimulation by IL-4 than IL-13 (28). Macrophages can be frozen and upon thawing retain their phenotype and function as defined by in vitro assays (29). While murine and human cryopreserved M(IL-4)s had fewer mRNA transcripts for marker genes, this result did not translate into reduced protein expression/enzyme activity that was virtually identical when fresh and previously frozen cells were compared, suggesting (a) upregulation of mRNA levels in freshly differentiated M(IL-4) greatly surpasses that needed to mediate significant increases in protein expression and (b) that the fresh and frozen M(IL-4)s were likely to be functionally equivalent.
When tested in the DNBS model of colitis, cryopreserved M(IL-4)s significantly ameliorated disease and, to a degree, were no different from previous observations with M(IL-4)s freshly differentiated from peritoneal macrophages or bone marrow–derived macrophages (14,18). Murine colitis evoked by intrarectal administration of oxazolone is driven, at least in part, by epithelial cell apoptosis as a result of excessive IL-13 expression by NKT cells (24) and has been compared with human ulcerative colitis. The striking finding that adoptive transfer of cryopreserved M(IL-4)s inhibited oxazolone-induced colitis suggests an elegant natural solution to disease in an IL-4–skewed microenvironment, that of the mobilization of an IL-4–dependent antiinflammatory macrophage. In addition, DNBS- and oxazolone-induced colitis present some features of Crohn’s disease and ulcerative colitis, respectively, and the ability of systemically delivered M(IL-4)s to suppress both DNBS- and oxazolone-induced disease suggests that this strategy might be broadly applicable to patients with IBD.
The suppression of colitis by AAMs has been attributed to their ability to produce IL-10, a skewing in the balance of their response to LPS, where IL-10 synthesis is favored over that of IL-12 and the production of arginase (14–19). In addition, AAMs may promote wound healing via effects on epithelial cells or fibroblasts (11). However, it is unknown if the anticolitic effect of M(IL-4)s is critically dependent on an immune cell(s) in the recipient mice.
By using sensitizing protocols and chronic repeated regimens, T cells have been implicated in DNBS-induced colitis; however, T cells are not a prerequisite for the acute colitis that develops within 1–3 d post-DNBS, since DNBS-treated RAG1−/− mice develop macroscopic and histological signs of disease that are virtually indistinguishable from similarly treated wild-type mice (30). T cells can influence AAM function, and vice versa (8,31), so we speculated that M(IL-4) mobilization of a regulatory T cell could be important to the suppression of colitis (6). Contrary to this, RAG1−/− mice treated with DNBS and M(IL-4)s developed significantly less disease than time-matched DNBS only–treated mice, revealing that neither T nor B cells (neither effector nor regulatory cell) in the recipient mice are an absolute requirement for M(IL-4)s to suppress colitis. This does not, however, negate the possibility that in an immunocompetent animal, T or B cells could contribute, to some degree, to the M(IL-4)-driven suppression of colitis. Moreover, should these findings translate to humans, it suggests that individuals with compromised T-cell activity because of recent medications would still experience the therapeutic benefit of M(IL-4)s.
The concept of macrophage-macrophage interaction is not unprecedented (32), and it was intriguing to consider the possibility that the therapeutic benefit of adoptive transfer of M(IL-4)s could be amplified by co-opting macrophages in the recipient mice. By using clodronate-filled liposomes to deplete macrophages (minor effects on neutrophils and dendritic cells have been described with this technique [25]), it was found that peritoneal macrophages in recipient mice were not essential for the anticolitic effect of treatment with M(IL-4)s [a finding in accordance with data showing that endogenous macrophages in the peritoneal cavity were not affected by coculture with M(IL-4)s, as gauged by expression of MRC1, Ly6C and Arg1 by flow cytometry]. However, it is possible that colonic macrophages could escape depletion via clodronate, and subsequent studies need to be directed at this issue. There remains the possibility that gut macrophages were not eliminated, either in part or fully after clodronate treatment intraperitoneally, and therefore M(IL-4)s that have migrated to the gut could be locally affecting resident intestinal macrophages and/or newly recruited monocytes. Nevertheless, collectively, the data are consistent with the hypothesis that M(IL-4)s have an endogenous anticolitic capacity (for example, antiinflammatory cytokine response) or directly interact with stromal cells to protect the tissue or promote tissue resistance. It is also clear that, while we have provided data on the lack of requirement for T cells, B cells and macrophages in the recipients, the potential involvement of other immune cells, such as NK cells, or fibroblasts (via arginase activity) in the recipient cannot be dismissed and warrants further study.
With cellular immunotherapy, it is intuitive to ask, do the cells traffic to the target organ to suppress disease? Adoptive transfer of regulatory T cells inhibits colitis in mice, and this was accompanied by accumulation of the cells in secondary lymphoid organs and/or the colon (33). It has also emerged that the peritoneal cavity may be an inductive site for immune activity (34). Indeed, in conjunction with testing the effect of mesenchymal stem cell (MSC) transfer in patients with Crohn’s disease (4), it has been shown that this approach is effective in the mouse model of dextran sodium-sulfate–induced colitis and that the MSCs that remained in the peritoneal cavity exerted an anticolitic effect (34). In addition, intraperitoneal administration of splenic B cells from Hymenolepis diminuta–infected mice blocked DNBS-induced colitis, with no obvious infiltration of the transferred cells into either the spleen or the colon (30). Furthermore, in patients treated intravenously with donor macrophages after kidney transplantation, transferred cells were found to localize initially to the lung, and within 24 h, disseminated nonspecifically throughout the body (35).
In vivo imaging of transferred M(IL-4)s revealed that at the time of necropsy (that is, 3 d post-DNBS), substantial M(IL-4) fluorescence was detected in the peritoneal cavity, and little or no fluorescence was detected in the heart, lungs or kidney. In both naive control and colitic mice, M(IL-4)s migrated to the liver, and this hepatic localization may represent normal drainage from the peritoneal cavity. However, DNBS-treated mice did display a preferential accumulation of the transferred M(IL-4)s in the spleen, mesenteric lymph nodes and colon. Despite the aforementioned data on MSCs in the peritoneum, earlier studies with trinitrobenzene sulphonic acid (TNBS)-induced colitis had revealed that a portion of the transferred cells homed to the gut (36,37). Observation of the anticolitic effect of M(IL-4)s in RAG1−/− mice suggested that activity in the peritoneal cavity or the gut may be important to the inhibition of disease.
The integrin α4β7 is important for homing to the gut via interaction with the mucosal addressin, MAdCAM (38,39), and αEβ7 interaction with E-cadherin in the gut mucosa can facilitate the retention of T cells (40). Use of M(IL-4)s differentiated from the bone marrow of Itgb7 knockout (KO) mice failed to suppress DNBS-induced colitis, identifying mucosal homing of M(IL-4)s as being critical to their ability to suppress inflammation in this model of colitis. Subsequent analysis of the DNBS model revealed upregulation of mRNA for Ccl2 and Cx3cl1 in the colon, both of which could drive the recruitment of cells via CCR2 and CX3CR1, respectively. However, adoptive transfer of M(IL-4)s from CCR2-KO and CX3CR1-KO mice were as effective as M(IL-4)s from wild-type mice in reducing the severity of DNBS-induced colitis, indicating that neither receptor is essential for the anticolitic effect of M(IL-4)s: given the redundancy that resides within chemokine to chemokine receptor chemotaxis, these data do not unequivocally rule out a role for either CCR2 or CX3CR1 in M(IL-4)s from wild-type mice.
CONCLUSION
Cellular immunotherapy has gained significant momentum in recent years with a focus on regulatory T cells and MSCs to treat inflammatory disease (6,36). Macrophages have received lesser attention, although macrophage transfer in two patients after kidney transplantations was found to enhance organ engraftment and patient outcome (35). Here, we provide support for the concept of adoptive transfer of macrophages to treat colitis and specifically show that cryopreserved M(IL-4)s can inhibit oxazolone and DNBS-induced colitis (disorders with very different immune spectrums). The M(IL-4) suppression of colitis required neither macrophages nor T and B cells in the recipient, and while the anticolitic effect was independent of CCR2 or CX3CR1 on the M(IL-4)s, the ability to home to mucosal tissue was an essential prerequisite for suppression of inflammation. We speculate that additional mechanistic studies, comparisons of murine and human M(IL-4)s and analyses of putative side effects following the delivery of M(IL-4)s delivery will pave the way for M(IL-4) treatment of inflammatory bowel disease.
Supplemental Data
ACKNOWLEDGMENTS
The authors would like to thank Michael Dicay and Evelyn Lailey for their phlebotomy assistance. This work was supported by the Mouse Phenomics Resources Laboratory and Live Cell Imaging Facility, both of which were funded by the Snyder Institute at the University of Calgary. This research is funded by the following: Crohn’s and Colitis Canada operating grant (to DM McKay), Alberta Innovates Health Solutions (AIHS) Scientist Award (to DM McKay), Canada Research Chair (Tier 1) in Intestinal Immunophysiology Award (DM McKay), AIHS Fellowship (to JL Reyes), an AIHS Summer Studentship (to J Iannuzzi) and an AIHS Doctoral Studentship (to G Leung).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Leung G, et al. (2015) Cryopreserved interleukin-4–treated macrophages attenuate murine colitis in an integrin β7–dependent manner. Mol. Med. 21:924–36.
REFERENCES
- 1.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122:3824–34. doi: 10.1172/JCI64124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazouli M, Roubelakis MG, Theodoropoulos GE. Stem cells as potential targeted therapy for inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:952–5. doi: 10.1097/01.MIB.0000442922.85569.21. [DOI] [PubMed] [Google Scholar]
- 5.van Deen WK, Oikonomopoulos A, Hommes DW. Stem cell therapy in inflammatory bowel disease: which, when and how? Curr Opin Gastroenterol. 2013;29:384–90. doi: 10.1097/MOG.0b013e328361f763. [DOI] [PubMed] [Google Scholar]
- 6.Desreumaux P, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207–17.e1202. doi: 10.1053/j.gastro.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 7.Loke Pn, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–41. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–20. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 9.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song E, et al. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 12.McKay DM. The therapeutic helminth? Trends Parasitol. 2009;25:109–14. doi: 10.1016/j.pt.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:6–13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter MM, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Weisser SB, et al. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J Leukoc Biol. 2011;90:483–92. doi: 10.1189/jlb.0311124. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo A, et al. Inhibition of colitis by IL-25 associates with induction of alternatively activated macrophages. Inflamm Bowel Dis. 2012;18:449–59. doi: 10.1002/ibd.21799. [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, et al. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013;62:1131–41. doi: 10.1136/gutjnl-2012-302152. [DOI] [PubMed] [Google Scholar]
- 18.Leung G, Wang A, Fernando M, Phan VC, McKay DM. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: participation of IL-10. Am J Physiol Gastrointest Liver Physiol. 2013;304:G781–92. doi: 10.1152/ajpgi.00055.2013. [DOI] [PubMed] [Google Scholar]
- 19.Shouval Dror S, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–19. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–9. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 21.Parsa R, et al. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61:2881–92. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisser SB, et al. Arginase activity in alternatively activated macrophages protects PI3Kp110δ deficient mice from dextran sodium sulfate induced intestinal inflammation. Eur J Immunol. 2014;44:3353–67. doi: 10.1002/eji.201343981. [DOI] [PubMed] [Google Scholar]
- 23.Cao Q, et al. Failed renoprotection by alternatively activated bone marrow macrophages is due to a proliferation-dependent phenotype switch in vivo. Kidney Int. 2014;85:794–806. doi: 10.1038/ki.2013.341. [DOI] [PubMed] [Google Scholar]
- 24.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–38. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 25.van Rooijen N, Hendrikx E. Liposomes for Specific Depletion of Macrophages from Organs and Tissues. In: Weissig V, editor. Liposomes: Methods and Protocols. Humana Press; New York: 2010. pp. 189–203. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee A, et al. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junttila IS, et al. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Rα, IL-13Rα1, and γc regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marim FM, Silveira TN, Lima DS, Jr, Zamboni DS. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One. 2010;5:e15263. doi: 10.1371/journal.pone.0015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes JL, et al. Splenic B cells from Hymenolepis diminuta–infected mice ameliorate colitis independent of T cells and via cooperation with macrophages. J Immunol. 2015;194:364–78. doi: 10.4049/jimmunol.1400738. [DOI] [PubMed] [Google Scholar]
- 31.Tiemessen MM, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiwon M, et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156:456–68. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leithäuser F, et al. Foxp3-expressing CD103+ regulatory T cells accumulate in dendritic cell aggregates of the colonic mucosa in murine transfer colitis. Am J Pathol. 2006;168:1898–909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sala E, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149:163–176.e120. doi: 10.1053/j.gastro.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson JA, et al. Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072–8. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 36.Castelo-Branco MTL, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonçalves FdC, et al. Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J Gastroenterol. 2014;20:18228–39. doi: 10.3748/wjg.v20.i48.18228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luck H, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Picarella D, et al. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–106. [PubMed] [Google Scholar]
- 40.Strauch UG, et al. Integrin αE(CD103)β7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J Immunol. 2001;166:3506–14. doi: 10.4049/jimmunol.166.5.3506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.