Abstract
A novel functional assay of antiplatelet drug efficacy was designed by utilizing the phenomena of platelet margination in flowing blood and transient platelet contacts with surface-immobilized platelet agonists. Flow margination enhances transient contacts of platelets with the walls of flow chambers covered with surface-immobilized proteins. Depending on the type and the surface density of the immobilized agonists, such transient interactions could “prime” the marginated platelet subpopulation for enhanced activation and adhesion downstream. By creating an upstream surface patch with an immobilized platelet agonist, platelet flow margination was used to test how effective antiplatelet drugs are in suppressing downstream platelet activation and adhesion. The platelet adhesion downstream was measured by a so-called “capture” patch region close to the distal end of the flow chamber. Platelet adhesion downstream was found to be dose-dependent on the upstream surface coverage of the “priming” patch, with immobilized fibrinogen acting as a platelet agonist. Several antiplatelet agents (acetylsalicylic acid, eptifibatide, and tirofiban) were evaluated for their efficacy in attenuating downstream adhesion after upstream platelet priming. The activation of the platelet population was found to be dependent on both the extent of the upstream agonist stimulus and the antiplatelet drug concentration. Such a relationship provides an opportunity to measure the efficacy of specific antiplatelet agents against the type and concentration of upstream platelet agonists.
I. INTRODUCTION
Each year, millions of individuals require surgical intervention to deal with cardiovascular diseases, oftentimes requiring the assistance of a vascular device such as a stent, shunt or graft.1 A major failure mode of these devices is the formation of a thrombus, leading to an occlusion of the device or an embolic event. This hemostatic response of the body to foreign materials often necessitates that patients be placed on systemic anticoagulants, many of which result in a considerable loss in quality of life. The development of antiplatelet agents is hindered by the fact that no current in vitro platelet activation assay fully takes into account the conditions under which platelets interact with different agonists in vivo and the downstream consequences of such interactions. Our group has recently shown that there is a quantitative relationship between transient contacts of platelets with upstream immobilized agonists and downstream platelet adhesion and activation, leading to a new perspective on vascular device failures.2 This finding was utilized here to design a novel antiplatelet drug efficacy assay that mimics transient platelet encounters with exposed agonists at a blood vessel wall lesion, or with procoagulant proteins adsorbed to the surface of an implanted vascular device.
A. Platelet activation and adhesion
It is commonly accepted that upon encountering a blood vessel injury or biomaterial, platelets initiate the process of repair by recognizing exposed subendothelial proteins via membrane receptors and tethering to the surface.3,4 Platelets roll along the surface of the injured vessel or material due to short term interactions of glycoprotein Ib and von Willebrand factor, then arrest, activate, and aggregate through the interactions of glycoprotein IIb/IIIa (GPIIbIIIa) and fibrinogen or collagen.5–7 Upon adhering, platelets undergo a morphological change and release the contents of their granules, which contain additional activation factors.8 These processes lead to an amplification of the activation cascade and the formation of a fibrin clot.9 Most platelets that contact a locus of injury, however, do not immediately adhere at the site of initial contact.10 Those platelets that have made transient contacts with a procoagulant surface stimulus remain “primed” for downstream activation as they continue to circulate. We have recently demonstrated that a platelet population allowed to transiently interact with a stimulating surface patch has an increased propensity to activate and adhere downstream.2,11 This phenomenon is largely due to the margination of platelets in flowing blood which was utilized here to create a new type of antiplatelet agent assay that takes into account the upstream history of platelet–agonist interactions.
B. Antiplatelet agents
Anticoagulant or antiplatelet therapy is often used during and after the surgical introduction of a vascular device or repair of a damaged blood vessel to reduce the risk of thrombotic complications.12 A large proportion of patients continue to receive these therapies indefinitely due to the increased risk of thrombosis and embolism associated with damaged vessel walls or with blood contacting implants. Examples of antiplatelet drugs currently prescribed include thromboxane inhibitors [acetylsalicylic acid (ASA)], GPIIb/IIIa inhibitors (abciximab, eptifibatide, and tirofiban) and adenosine diphosphate (ADP) inhibitors (clopidogrel, prasugrel, and cangrelor). Despite the prevalence of antiplatelet and anticoagulation agents, the lack of relevant platelet function assays has limited the scope of antiplatelet drugs tests in vitro. There is currently no optimal system in which to test the effects of antiplatelet therapies on the dynamics of platelet activation and adhesion in circulation, since current assays for antiplatelet agents tend to approximate only the local effects of vascular injury or procoagulant device surfaces. Practically, no current in vitro platelet function assay takes into account the upstream priming that can occur in vessel injury conditions. Platelet aggregometry, for example, in which a platelet agonist is added to whole blood or plasma and aggregate formation is recorded, is considered the “gold standard” for platelet functionality assays.13 Although aggregometry is capable of providing a large amount of information on platelet functionality, it does not provide a circulatory environment that accurately mimics adhesion, activation, and aggregation onto an injured blood vessel wall or an implanted device.3,14 Other methods, including analysis of activation markers using flow cytometry or aggregation using techniques such as thromboelastography, only detect bulk platelet activation and do not directly assess surface-induced adhesion and activation.15
C. Platelet function assays
Most platelet function assays are unable to model the effects of a specific activating region and actual vessel flow conditions and specific injury site conditions are typically not presented. One exception is the IMPACT cone and plate analyzer (CPA), which was developed to more accurately represent natural haemostasis.16 In the CPA, a polystyrene plate is used to monitor platelet adhesion and aggregation in a well-defined shear environment. Plasma proteins are allowed to adsorb to the surface of a polystyrene plate from whole blood, creating a thrombogenic surface. The type and amount of plasma proteins that adsorb to the surface are not controlled, however, so no direct correlations between specific plasma proteins and platelet function can be elucidated. The platelet function analyzer (PFA-100/200®, Siemens) is another assay commonly used to assess platelet function. The PFA-100/200 is a shear activated, cartridge based platelet assay, which was originally designed to replace or automate bleeding time tests by mimicking an injured blood vessel.17 In this assay, whole blood is drawn through a membrane with a small (150 μm) aperture, which is coated with either collagen and ADP or collagen and epinephrine.18 These agonists, combined with the elevated shear at the aperture, serve to activate platelets that eventually occlude the membrane. While this assay does directly assess surface-induced activation and adhesion, it does so in the presence of high shear rates and does not take into account transient surface interactions. Recent years have also seen the rise in use of microfluidic flow cells as essential tools for studying platelet activation and adhesion.19,20 Gutierrez et al. used extracellular matrix (ECM) coated substrates to investigate the influence of elevated shear rates on platelet adhesion with respect to the GPIIb/IIIa receptor.21 Similar assays have also been used by Maloney et al. to investigate patient-specific responses to antiplatelet therapies.22
While these studies have come closer to simulating vessel wall lesion conditions by presenting physiologically relevant substrates to shear and/or ECM activated platelets, the influence of transient contacts between platelets and surface bound agonists has been overlooked. To investigate this phenomenon, we have developed a flow-based method to test the downstream activation of platelets after specific upstream stimulation of platelets by transient contacts with mimetic procoagulant agonists. The surface-immobilized procoagulant proteins act as platelet agonists in order to mimic exposed subendothelium in a blood vessel lesion or a procoagulant surface of an implanted vascular device. Varying the surface density of an upstream agonist can thus mirror a range of pathological conditions in patients with lesions on a blood vessel wall.
D. Novel flow assay
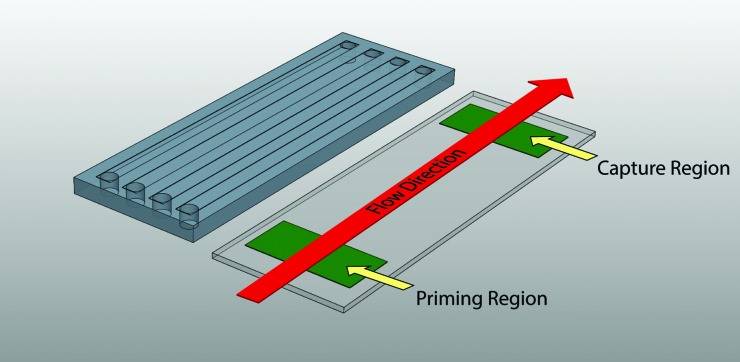
The central premise of the flow assay described here is that by controlling the presentation of an upstream agonist (a so-called “priming” region made of covalently tethered procoagulant proteins), the subsequent activation of platelets can be determined using a downstream platelet “capture” region. This assay is carried out in a miniaturized array of flow chambers through which a small volume of blood is perfused. Figure 1 shows the geometry of the upstream agonist priming and downstream capture regions in rectangular flow chambers. Due to the geometry of the flow cell, this assay takes advantage of a margination phenomenon observed in flowing blood in which platelets are concentrated near the vessel wall. Such margination of platelets toward the chamber walls is due to the existence of a red blood cell depletion zone established during flow. Spatial fractionation of platelets ensures a higher number of contacts between the marginated platelet subpopulation and upstream priming and downstream capture regions. This assay requires a small sample volume (<1 ml of whole blood) per run, and a typical perfusion lasts 5 min, thus making it an ideal candidate for a variety of clinical uses.
Fig. 1.
Schematic of a flow assay assembly. Protein agonists are deposited on a glass substrate in the priming and capture regions by microcontact printing and covalent attachment of proteins is achieved through the use of a commercial NHS ester chemistry (Nexterion-H®, Schott). A relief-molded PDMS flow channel is inverted on the stamped surface to create the final assembled device. Flow through the device brings blood past the priming region followed by the capture region before exiting the device. Platelets near the substrate surface are therefore able to sequentially interact with two surface-bound agonist regions in a controlled flow environment.
II. METHODS
A. Flow cell design and creation
Flow chambers contained an upstream platelet priming area (10 mm in length) and a downstream capture region (also 10 mm in length). Flow channel dimensions were 3 mm wide, 0.1 mm deep, and 70 mm long. Such dimensions were selected to match physiologically relevant venous (∼100 s−1) and arterial (500–1000 s−1) shear rates depending on the volumetric flow rate used in perfusion.23–26 Each device consisted of a parallel array of four flow channels and was designed to fit on a standard 25 × 75 mm microscope slide (Fig. 1). The flow chambers were manufactured through relief molding of polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning) on patterned polymeric tape. Patterns for the channels were cut out of tape on a xurographic plotter (CE5000-60, Graphtec). These patterns were then transferred to the bottom of a mold and PDMS was cast into the mold at a ratio of 15:1 polymer to crosslinker by weight. The flow chambers were allowed to cure, then released from the mold and cut to final dimensions (25 × 75 mm). Fluid vias were bored from the top of the PDMS into the flow channels to provide access for the inlet and outlet of blood.
To create platelet priming and capture regions, patches of fibrinogen were deposited at specific upstream and downstream locations in each flow cell using a microcontact printing (μCP) process described in detail elsewhere.11,27 Briefly, soft lithographic stamps were created out of PDMS, corresponding to random patterns of micrometer-sized islands of varying surface density coverage. The surface of these stamps was coated with a fibrinogen solution (1 mg/ml in phosphate-buffered saline (PBS), Sigma-Aldrich) and allowed to dry, then brought into contact with the substrate. Covalent linkages between proteins and the glass-based bottom of the flow assay were achieved by using commercially available Nexterion-H® (Schott) slides coated with a poly(ethylene oxide)-based polymer containing reactive N-hydroxysuccinimide (NHS) esters.28,29 Through the use of a constant concentration of protein solution and a uniform surface chemistry, the density of fibrinogen transferred to the surface remained constant in spite of varying total surface coverage densities provided by stamp patterns. After μCP of fibrinogen, the slides were rinsed in distilled deionized water prior to assembly to eliminate any nonbound protein.
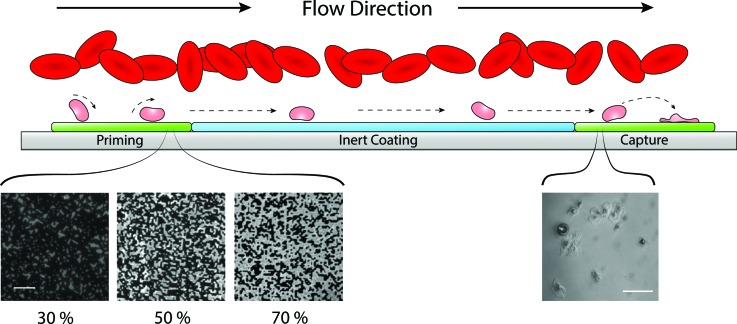
The downstream analysis of platelet activation was carried out via a surface capture assay.2 A printed protein capture region consisting of a 10 mm long patch of 100% surface density coverage of fibrinogen was covalently immobilized to the surface of the flow cell 45 mm downstream of the priming region. This region served to capture activated platelets from the marginated platelet subpopulation. Figure 2 shows a schematic of a flow assay during platelet perfusion, highlighting the micrometer-sized islands of covalently attached agonist in the priming region at three different surface densities. Devices were assembled by inverting the PDMS fluidic channels onto the stamped Nexterion-H glass substrate. Capillary forces held the PDMS in place strongly enough to prevent leakage for the duration of the experiments. Prior to all experiments, a solution of human serum albumin (HSA, 1 mg/ml in PBS, Sigma-Aldrich) was perfused through each flow cell and was allowed to incubate at room temperature for 1 h. The introduction of HSA served to both coat the PDMS walls of the chamber and react with the remaining NHS ester groups between stamped priming and capture regions. The presence of covalently attached HSA intermediate to the priming and capture regions serves to reduce the adsorption of proteins from serum as well as provides a platelet-inert substrate over which perfusion is conducted.30
Fig. 2.
Schematic of flow assay function. The presence of red blood cells in flow creates a margination effect which drives platelets toward the chamber walls. An increased number of platelets near the substrate surface relative to the bulk flow increases chances of platelet–surface interactions. Platelets near the substrate transiently roll along the surface and become primed through interactions with agonists. A variable density of printed agonists in the priming region provides a range of probabilities that a platelet contacting the surface in the priming region will interact with a printed agonist. Shown here are fluorescent images of microcontact printed fibrinogen at three different surface coverage densities, 30%, 50%, and 70% (scale bar represents 10 μm). Primed platelets continue to flow downstream along a platelet-inert surface. Primed platelets encountering the downstream agonist patch have an increased propensity to form stable adhesions compared to nonprimed populations. The number of adhered platelets in the capture region is used as an indication of overall priming in the platelet population. The inset image is a representative view of platelets adhered to a fibrinogen capture region (scale bar represents 20 μm).
B. Flow cell operation
Whole human blood was drawn from healthy donors into buffered 3.2% (0.105 M) sodium citrate and was treated with Phe-Pro-Arg-chloromethylketone (PPACK, 80 μM, Haematologic Technologies) within 5 min of draw to prevent thrombin-induced coagulation. Any additional antiplatelet agent was added at this time as experimental conditions dictated. All samples were used within 10 min of draw from donors. Blood was kept in a water bath at 37 °C for the duration of the experiment, and polyethylene microfluidic tubing connected the flow cell to the vial of blood. All microfluidic tubing was perfused with HSA prior to experiments in order to preadsorb albumin onto the surface and thereby reduce the adsorption of serum proteins. Additionally, the microfluidic tubing was kept as short as possible to minimize any possible priming effect it might have on the platelet population. Flow was achieved by drawing blood through the device using a syringe-pump (Kent Scientific). A flow rate of 3.6 ml/h produced a shear rate of 200 s−1, which was used in all experiments. Flow was sustained for 5 min, after which the device was rinsed with prewarmed Tyrode's buffer to remove any nonattached cells. Attached cells were fixed in 4% paraformaldehyde, imaged using a differential interference contrast microscope (Diaphot 300, Nikon), and quantified. Adhered platelets were counted in ten randomly selected fields (300 × 400 μm) within the downstream capture region.
III. RESULTS
A. Upstream agonist titration
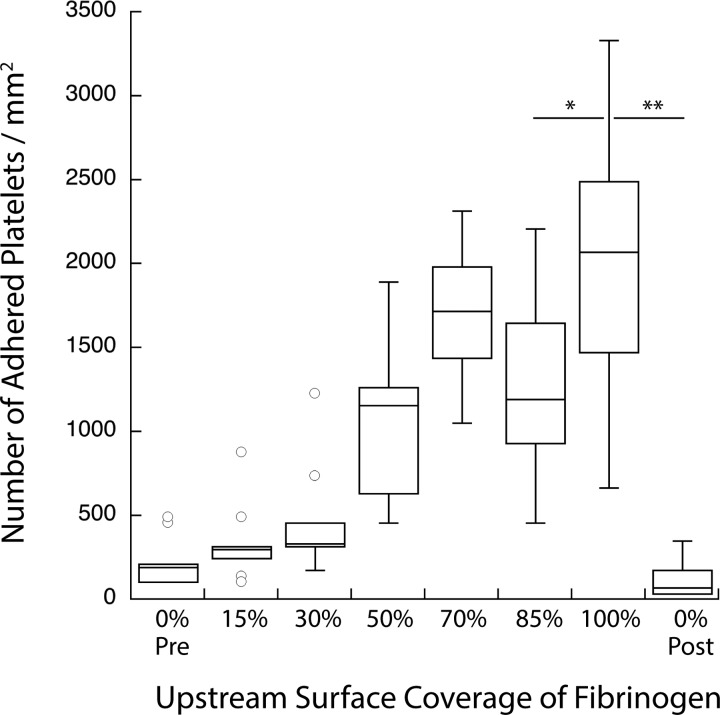
Flow cells were created with varying surface coverage densities of fibrinogen in the upstream priming region. Densities of 15%, 30%, 50%, 75%, 85%, and 100% were used. Negative controls of 0% surface coverage were run both before and after the experiments to ensure that the platelets in the blood sample did not become more active over the course of the experiment. Figure 3 shows that the number of platelets adhered to the downstream capture region increased as the surface density of the immobilized agonist in the upstream priming position is increased. This finding indicates that the elevated chances of platelets contacting the upstream agonist led to a greater number of platelets primed for adhesion downstream. These results demonstrate that precise control over the relative activation state of a platelet population is achievable and detectable using this device.
Fig. 3.
Stimulus-dependent response of platelet activation. Platelet adhesion to a downstream fibrinogen capture region is seen to increase as the density of upstream surface bound fibrinogen increases. Zero percentage pre- and 0% post represent nonprimed controls run before and after all other samples, respectively (**p < 0.0001 and *p < 0.05).
B. Antiplatelet agents
Two classes of antiplatelet agents were examined for their ability to attenuate platelet activation in response to the upstream agonist. The cyclooxygenase (COX) inhibitor ASA (Aspirin®, Bayer) and the two GPIIb/IIIa inhibitors, eptifibatide (Integrilin®, Millennium Pharmaceuticals) and tirofiban (Aggrastat®, Medicure), were selected as they are all nonprodrug candidates, thus could be easily added to the blood prior to perfusion.
1. COX inhibitor
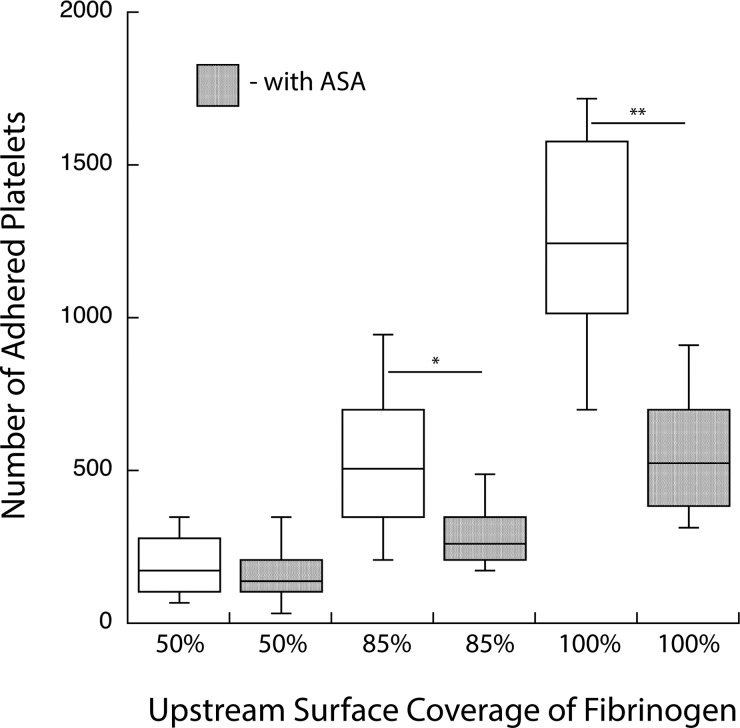
Flow cells were created with 50%, 85%, and 100% coverage of printed fibrinogen in the upstream priming region. Two samples of whole blood were prepared, one untreated, and one treated with 30 μg/ml ASA in whole blood (i.e., the target blood concentration for antiplatelet therapy in adults). Figure 4 shows the platelet activation response to varying surface densities of upstream fibrinogen with and without ASA premixed in the perfusate. While an increasing surface density of priming fibrinogen still resulted in increased platelet adhesion, the relative number of adhered platelets significantly decreased in the presence of ASA. These results illustrate the sensitivity of this assay in assessing the efficacy of antiplatelet agents in response to varying levels of platelet activation.
Fig. 4.
Stimulus-dependent response of platelet activation in the presence of acetylsalicylic acid. ASA is seen to attenuate the platelet activation response. Platelet adhesion to the capture region increases as the density of the upstream priming region increases; however, this rate of increase is diminished in the presence of the recommended therapeutic dose of ASA (**p < 0.0001 and *p < 0.01).
2. GPIIbIIIa inhibitors
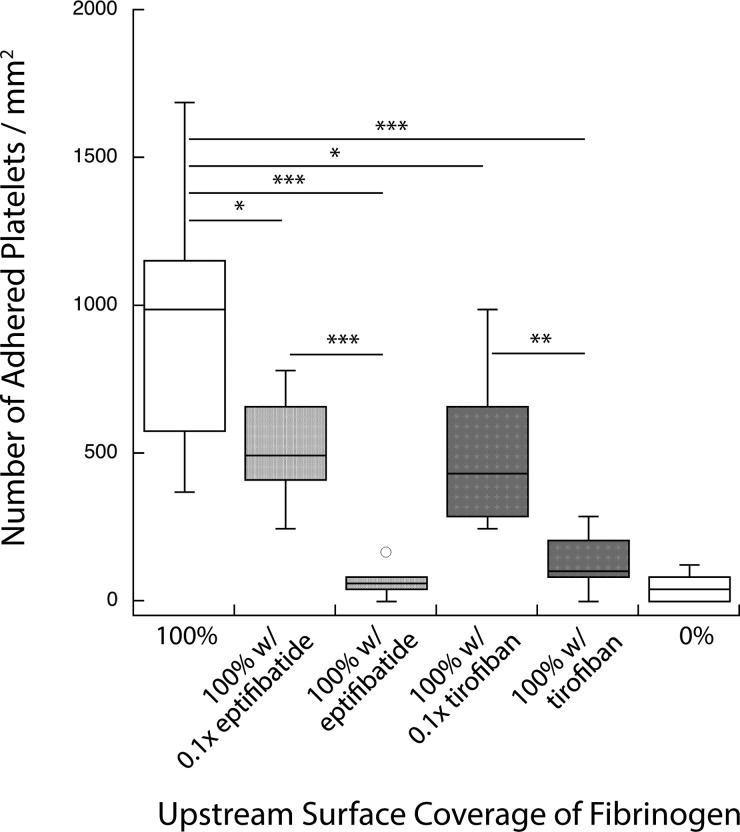
Another set of experiments was used to test a different class of antiplatelet agents, GPIIbIIIa inhibitors. Specifically, eptifibatide (a cyclic heptapeptide) and tirofiban (a nonpeptide inhibitor) were used. Devices were created with 100% surface coverage of fibrinogen in both the priming and capture regions. Whole blood mixed with each of these drugs was used as perfusate. Antiplatelet agents were combined with blood at their respective target therapeutic blood concentrations (3 μg/ml, eptifibatide or 0.33 μg/ml, tirofiban) and also at 1/10 of these target concentrations. Figure 5 shows the results of the GPIIbIIIa inhibitors on downstream platelet adhesion. Both eptifibatide and tirofiban diminished the number of adhered platelets at their target therapeutic concentration and at 1/10 of that level. At the recommended blood concentration of each of these drugs, results were statistically similar to nonprimed controls (i.e., with no agonist present in the upstream region). This result illustrates the ability of the assay to assess antiplatelet therapies in a dose-sensitive manner.
Fig. 5.
Dose-dependent response of platelet activation in the presence of GPIIb/IIIa inhibitors. GPIIb/IIIa inhibitors attenuate the platelet activation response. Both eptifibatide and tirofiban diminish the number of adhered platelets comparable to negative controls at the target therapeutic dose (***p < 0.0001, **p < 0.001, and *p < 0.01).
IV. DISCUSSION
This novel flow assay, which mimics a physiologically relevant blood vessel wall injury to test platelet function and antiplatelet drug efficacy, was made possible by utilizing a dynamic phenomenon by which transient platelet-agonist contacts prime platelets for enhanced activation and adhesion downstream. The flow assay emulates the platelet priming surface of a vascular device or vessel wall injury by presenting a covalently bound platelet-priming agonist to flowing whole blood. A second patch of an immobilized proadhesive protein downstream of the agonist is used to capture primed platelets. In the present study, fibrinogen was used as the protein in the capture region as it has been previously demonstrated to effectively arrest primed platelets;2,11 however, other capture proteins (e.g., collagen or vWF) could easily be incorporated. This assay would not have the required sensitivity without the phenomenon of platelet margination toward flow chamber walls in laminar flow of whole blood. The dimensions of the flow channels were chosen to increase the chances of a platelet interacting with the chamber walls multiple times by exploiting the margination of platelets and the propensity of platelets once marginated to remain near the chamber walls. Numerous in vivo observations and in vitro experiments of whole blood flowing in tubes or channels have established the existence of a near-wall region with few to no red blood cells.31–34 This red-cell depletion zone is characterized by an enhanced concentration of platelets compared to their overall bulk concentration in the blood. The red-cell depleted and platelet enriched zone comprises the layer of fluid within 2–5 μm of the wall, growing thinner as the hematocrit and shear rate increase. Importantly, platelet margination substantially increases (by 50-fold or more) the rate of platelet contacts with the chamber wall.35 Computational models of whole blood, which simulate the motion of collections of red blood cells and platelets suspended in plasma, show the temporal development of the red-cell depletion zone and the subsequent margination of platelets.36–38 These studies also show that the red blood cells along the edge of the red-cell depletion zone form an effective barrier which strongly inhibits the movement of platelets from the near-wall region back into the core flow.36,37 In the context of our flow assay, this implies that most of the platelets that contact the upstream priming region will remain within a few microns of the wall during their transit through the chamber to the downstream capture region, and so have a significant chance of contact with the capture region.
Transient contacts between primed platelets and the capture region result in the arrest of platelets, and the number of arrested platelets has been shown to correlate to the overall activation of the platelet population.39 Other methods such as fluorescence-activated cell sorting analysis of activation-dependent surface markers or β-thromboglobulin plasma levels are more commonly used to assess platelet activation.40–42 Since the fraction of the platelet population that contacts the priming region is rather small (∼2%), it would be difficult to detect such low numbers of active platelets among the whole platelet population using these methods. Thus, the combination of platelet margination in flow and a downstream capture region allows for the self-selection of activated platelets, making the current assay more sensitive than any of the bulk fluid-based platelet activation assays.
The effect of the coagulation cascade was excluded in these experiments by design through the introduction of PPACK, a thrombin inhibitor. Through the elimination of the coagulation response, platelet priming and adhesion could be interrogated on a cell-by-cell basis without the confounding factor of coagulation. If, in the future, it is desired to study particular aspects of the coagulation cascade, it is possible to add back free calcium ions to the blood prior to perfusion and omit the addition of PPACK.
Initially, the surface coverage density of fibrinogen was varied to demonstrate the sensitivity of the assay to increasing levels of platelet priming stimulus. As the percent of surface coverage increased from 0% to 100%, the chance that any given platelet flowing past the priming region would contact a fibrinogen patch also increased. The surface coverage density of fibrinogen was seen to have an effect on the activation state of the platelet population, as increased adhesion was observed in the downstream capture region in a dose dependent manner (Fig. 3). A similar experiment was repeated in which the upstream surface density of bound fibrinogen was varied in the presence of ASA. The results indicate that ASA was able to decrease the activation of the platelet population relative to nontreated controls. Interestingly, platelet priming was still observed to be dependent on the upstream agonist surface density; however, the number of adhered platelets was diminished. ASA inhibits COX, which in turn halts the formation of thromboxane A2 (TXA2). By lowering TXA2 levels, a key stimulus for platelet activation was reduced. Other pathways to activation remained open, however, indicated by a continued (albeit decreased) presence of an active platelet population.
The antiplatelet drugs tirofiban and eptifibatide were also tested for their ability to attenuate platelet activation by surface-bound agonists. These drugs inhibit the GPIIb/IIIa receptor on platelets, which is presumably responsible for platelet priming by upstream fibrinogen. A dose-dependent response was seen when the concentration of each of these drugs was varied. The number of adhered platelets downstream was significantly reduced even when the drugs were used at 1/10 of their recommended dose, and at the recommended dose, adhesion was reduced to levels of the nonprimed control. These results show the ability of this assay to detect the dose-dependent efficacy of antiplatelet agents.
V. CONCLUSION
In summary, these experiments demonstrate the newfound ability to investigate specific platelet priming pathways through the use of an upstream agonist, downstream surface capture assay. The ability of antiplatelet agents to attenuate this response was also shown, suggesting the utility of this flow assay for the screening of current and future antiplatelet therapies. Interpatient variations in drug sensitivity has been a well-documented challenge surrounding this class of drugs; therefore, the ability to detect the minimum effective therapeutic dose on a patient by patient basis would have large impact on the personalized prescription of such pharmaceutics.
ACKNOWLEDGMENTS
The authors would like to thank Andrew Weyrich's lab for providing human blood necessary to complete experiments. This work was supported by the NIH Grant (RO1 HL126864) and the Graduate Research Fellowship at the University of Utah.
References
- 1. Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Borden W. B., Bravata D. M., Dai S., and Fox E. S., Circulation 127, 6 (2013). 10.1161/CIR.0b013e31828124ad [DOI] [Google Scholar]
- 2. Corum L. E. and Hlady V., Biomaterials 33, 1255 (2012). 10.1016/j.biomaterials.2011.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michelson A. D., Platelets ( Academic Press, San Diego, 2013), pp. 519–545. [Google Scholar]
- 4. Kulkarni S. et al. , J. Clin. Invest. 105, 783 (2000). 10.1172/JCI7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ware J. A. and Coller B. S., Williams' Hematology ( McGraw-Hill, USA, 1994), p. 1161. [Google Scholar]
- 6. Blockmans D., Deckmyn H., and Vermylen J., Blood Reviews, edited by Fuster V., Ross R., and Topol E. ( Lippincott-Raven, Philadelphia, 1995), Vol. 9, pp. 143–156. [DOI] [PubMed] [Google Scholar]
- 7. Marguerie G. A., Plow E. F., and Edgington T. S., J. Biol. Chem. 254, 5357 (1979). [PubMed] [Google Scholar]
- 8. Flaumenhaft R., Dilks J. R., Rozenvayn N., Monahan-Earley R. A., Feng D., and Dvorak A. M., Blood 105, 3879 (2005). 10.1182/blood-2004-04-1392 [DOI] [PubMed] [Google Scholar]
- 9. Andrews R. K. and Berndt M. C., Thromb. Res. 114, 447 (2004). 10.1016/j.thromres.2004.07.020 [DOI] [PubMed] [Google Scholar]
- 10. Godo M. N. and Sefton M. V., Biomaterials 20, 1117 (1999). 10.1016/S0142-9612(99)00012-5 [DOI] [PubMed] [Google Scholar]
- 11. Corum L. E., Eichinger C. D., Hsiao T. W., and Hlady V., Langmuir 27, 8316 (2011). 10.1021/la201064d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oprea A. D. and Popescu W. M., Br. J. Anaesth. 111, i3 (2013). 10.1093/bja/aet402 [DOI] [PubMed] [Google Scholar]
- 13. Michelson A. D., Frelinger A. L., and Furman M. I., Am. J. Cardiol. 98, S4 (2006). 10.1016/j.amjcard.2006.09.008 [DOI] [Google Scholar]
- 14. Michelson A. D., Circulation 110, e489 (2004). 10.1161/01.CIR.0000147228.29325.F9 [DOI] [PubMed] [Google Scholar]
- 15. Boldt J., Wolf M., and Mengistu A., Anesth. Analg. 104, 425 (2007). 10.1213/01.ane.0000253484.19070.87 [DOI] [PubMed] [Google Scholar]
- 16. Savion N. and Varon D., Pathophysiol. Haemostasis Thromb. 35, 83 (2006). 10.1159/000093548 [DOI] [PubMed] [Google Scholar]
- 17. Kundo S. K., Heilmann E. J., Sio R., Garcia C., Davidson R. M., and Ostgaard R. A., Seminar in Thrombosis and Hemostasis ( Thieme, Stuttgart, 1995), Vol. 21, pp. 106–113. [DOI] [PubMed] [Google Scholar]
- 18. Favaloro E. J., Haemophilia 7, 170 (2001). 10.1046/j.1365-2516.2001.00486.x [DOI] [PubMed] [Google Scholar]
- 19. Colace T. V., Tormoen G. W., McCarty O. J. T., and Diamond S. L., Annu. Rev. Biomed. Eng. 15, 283 (2013). 10.1146/annurev-bioeng-071812-152406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conant C. G., Schwartz M. A., Beecher J. E., Rudoff R. C., Ionescu-Zanetti C., and Nevill J. T., Biotechnol. Bioeng. 108, 2978 (2011). 10.1002/bit.23243 [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez E., Petrich B. G., Shattil S. J., Ginsberg M. H., Groisman A., and Kasirer-Friede A., Lab Chip 8, 1486 (2008). 10.1039/b804795b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maloney S. F., Brass L. F., and Diamond S. L., Integr. Biol. 2, 183 (2010). 10.1039/b919728a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu S. P., Ringgaard S., Oyre S., Hansen M. S., Rasmus S., and Pedersen E. M., J. Magn. Reson. Imaging 19, 188 (2004). 10.1002/jmri.10441 [DOI] [PubMed] [Google Scholar]
- 24. Holme P. A., Orvim U., Hamers M. J., Solum N. O., Brosstad F. R., Barstad R. M., and Sakariassen K. S., Arterioscler., Thromb., Vasc. Biol. 17, 646 (1997). 10.1161/01.ATV.17.4.646 [DOI] [PubMed] [Google Scholar]
- 25. Whitmore R. L., Rheology of the Circulation ( Pergamon, Oxford/New York, 1968). [Google Scholar]
- 26. Papaioannou T. G., Karatzis E. N., Vavuranakis M., Lekakis J. P., and Stefanadis C., Int. J. Cardiol. 113, 12 (2006). 10.1016/j.ijcard.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 27. Bernard A., Renault J. P., Michel B., Bosshard H. R., and Delamarche E., Adv. Mater. 12, 1067 (2000). [DOI] [Google Scholar]
- 28. Harbers G. M., Emoto K., Greef C., Metzger S. W., Woodward H. N., Mascali J. J., Grainger D. W., and Lochhead M. J., Chem. Mater. 19, 4405 (2007). 10.1021/cm070509u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng C. L., Vancso G. J., and Schönherr H., Adv. Funct. Mater. 16, 1306 (2006). 10.1002/adfm.200500410 [DOI] [Google Scholar]
- 30. Amiji M. and Park K., J. Biomater. Sci. Polym. Ed. 4, 217 (1993). 10.1163/156856293X00537 [DOI] [PubMed] [Google Scholar]
- 31. Tangelder G. J., Teirlinck H. C., Slaaf D. W., and Reneman R. S., Am. J. Physiol. 248, H318 (1985). [DOI] [PubMed] [Google Scholar]
- 32. Aarts P. A., van den Broek S. A., Prins G. W., Kuiken G. D., Sixma J. J., and Heethaar R. M., Arteriosclerosis 8, 819 (1988). 10.1161/01.ATV.8.6.819 [DOI] [PubMed] [Google Scholar]
- 33. Tilles A. W. and Eckstein E. C., Microvasc. Res. 33, 211 (1987). 10.1016/0026-2862(87)90018-5 [DOI] [PubMed] [Google Scholar]
- 34. Eckstein E. C., Tilles A. W., and Millero F. J., Microvasc. Res. 36, 31 (1988). 10.1016/0026-2862(88)90036-2 [DOI] [PubMed] [Google Scholar]
- 35. Turitto V. T. and Baumgartner H. R., Microvasc. Res. 9, 335 (1975). 10.1016/0026-2862(75)90070-9 [DOI] [PubMed] [Google Scholar]
- 36. Crowl L. and Fogelson A. L., J. Fluid Mech. 676, 348 (2011). 10.1017/jfm.2011.54 [DOI] [Google Scholar]
- 37. Zhao H., Shaqfeh E. S. G., and Narsimhan V., Phys. Fluids 24, 11902 (2012). 10.1063/1.3677935 [DOI] [Google Scholar]
- 38. Reasor D. A., Mehrabadi M., Ku D. N., and Aidun C. K., Ann. Biomed. Eng. 41, 238 (2013). 10.1007/s10439-012-0648-7 [DOI] [PubMed] [Google Scholar]
- 39. Savage B., Shattil S. J., and Ruggeri Z. M., J. Biol. Chem. 267, 11300 (1992). [PubMed] [Google Scholar]
- 40. Bonner W. A., Hulett H. R., Sweet R. G., and Herzenberg L. A., Rev. Sci. Instrum. 43, 404 (1972). 10.1063/1.1685647 [DOI] [PubMed] [Google Scholar]
- 41. Shattil S. J., Cunningham M., and Hoxie J. A., Blood 70, 307 (1987). [PubMed] [Google Scholar]
- 42. Ritchie J. L., Alexander H. D., and Rea I. M., Clin. Lab. Haematol. 22, 359 (2000). 10.1046/j.1365-2257.2000.00339.x [DOI] [PubMed] [Google Scholar]