Highlights
-
•
Gastric cancer after sleeve gastrectomy has been previously reported, completion gastrectomy is the recommended treatment.
-
•
No direct relation has been established between sleeve gastrectomy and the development of gastric cancer.
-
•
A minimally invasive approach should always be attempted to minimize the stress of surgery.
-
•
The robotic system allows for complex multi-organ resections.
Keywords: Gastric cancer, Sleeve gastrectomy, Robotic gastrectomy, Novo gastric adenocarcinoma, Gastric cancer in transplant patient
Abstract
Introduction
It has been reported in the literature that upper gastrointestinal malignancies after bariatric surgery are mostly gastro-esophageal, although it is not clear whether bariatric surgery represents a risk factor for the development of esophageal and/or gastric cancer. We report a case of a de novo gastric adenocarcinoma occurring in a transplant patient 1 year after a laparoscopic sleeve gastrectomy.
Presentation of case
A 44 year-old woman with a BMI of 38 kg/m2, hypertension, type 1 diabetes mellitus, multiple malignancies and a pancreas transplant underwent laparoscopic sleeve gastrectomy. The patient presented with intense dysphagias during the follow up. Studies were performed and the diagnoses of grade 2/3 adenocarcinoma were made. The patient underwent a robotic assisted total gastrectomy with a roux-en-y intracorporeal esophagojejunostomy. The procedure resulted in multiple metastasic lymph nodes, focal and transmural invasions to multiple organs with a tumor free margin resection. The patient presented with a postoperative pleural effusion, with no further complications.
Discussion
The diagnosis of gastroesophageal cancer after bariatric surgery is usually late since these patients have common upper gastrointestinal symptoms related to the procedure that could delay the diagnosis. De novo gastric cancer after sleeve gastrectomy has only been reported in one instance, in contrast with other bariatric surgery procedures.
Conclusions
No direct relation has been established between sleeve gastrectomy and the development of gastric cancer. Robotic procedures allow for complex multiorgan resections, while preserving the benefits of minimally invasive surgery.
1. Introduction
The relationship between a high body mass index (BMI) and malignancies has been well established as obesity is associated with an increased susceptibility to gastric and esophageal adenocarcinoma [1], [2], [3]. It is not clear whether bariatric surgery may represent a risk factor for the development of esophageal and/or gastric cancer [4]. Sleeve gastrectomy (SG) has become one of the most popular surgical procedures for weight loss in obese patients due to its safety, technical simplicity, resolution of co-morbidities and exceptional weight loss outcomes [5]. In the past 20 years there have been few studies reporting malignancies after bariatric surgery [3], [4], with only 2 reported cases of esophago-gastric adenocarcinoma after sleeve gastrectomy [6], [7].
The aim of this study is to report a case of de novo gastric adenocarcinoma occurring in a transplant patient 1 year after sleeve gastrectomy.
2. Case report
A 44 year-old woman with a BMI of 38 kg/m2 and a past medical hisroty of hypertension, type 1 diabetes mellitus, hyperlipidemia, bipolar disorder, sleep apnea, vaginal dysplasia, grade III vulvar intraepithelial neoplasia, anal condilomatosis and of heavy tobacco use; past surgical history of a pancreas transplant performed 4 years prior underwent laparoscopic sleeve gastrectomy (LSG). As part of the work up, a swallow contrast study showed grade 1 esophagitis. The patient underwent LSG and an incidental hiatal hernia was repaired without complications. Specimen was negative for histopathology changes and negative for Helicobacter pylori (pathology report: fundic type gastric mucosa and muscularis propia with negative for significant histopathologic changes).
Patient presented with an uneventful follow-up at 1, 3, 6, and 8 months. At the 8 month follow-up %EBWL was 52%, also presented with mild dysphagia to solids; an esophagogastroduodenoscopy (EGD) was performed that reported inflammation of the gastric and esophageal mucosa with no strictures. The patient was discharged with proton pump inhibitor medication.
The patient returned to the hospital 3 weeks after with worsening symptomatology, extreme dysphagia to solids and liquid intolerance. A second EGD and fluoroscopy study were performed that showed a tight stricture, 10 cm from the gastroesophageal junction (Fig. 1). Endoscopic dilation of the area and random biopsies were performed. The pathology examination showed moderately differentiated grade 2/3 adenocarcinoma and positive inmunostain to HER2/NEU. A CT scan was performed for stratification that showed a thickened gastric sleeve wall with no other metastatic nodules.
Fig. 1.
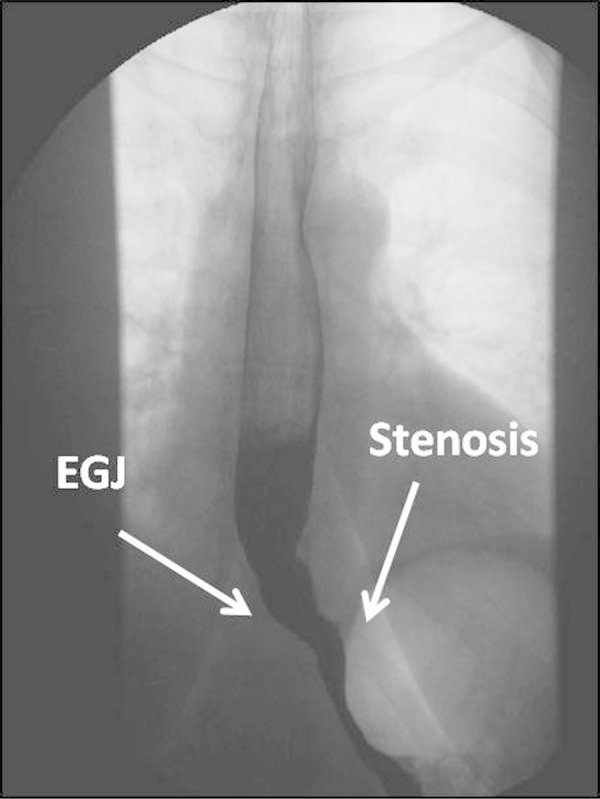
Esophagus fluoroscopy identifying gastric stricture (EGJ = Esophago-gastric junction).
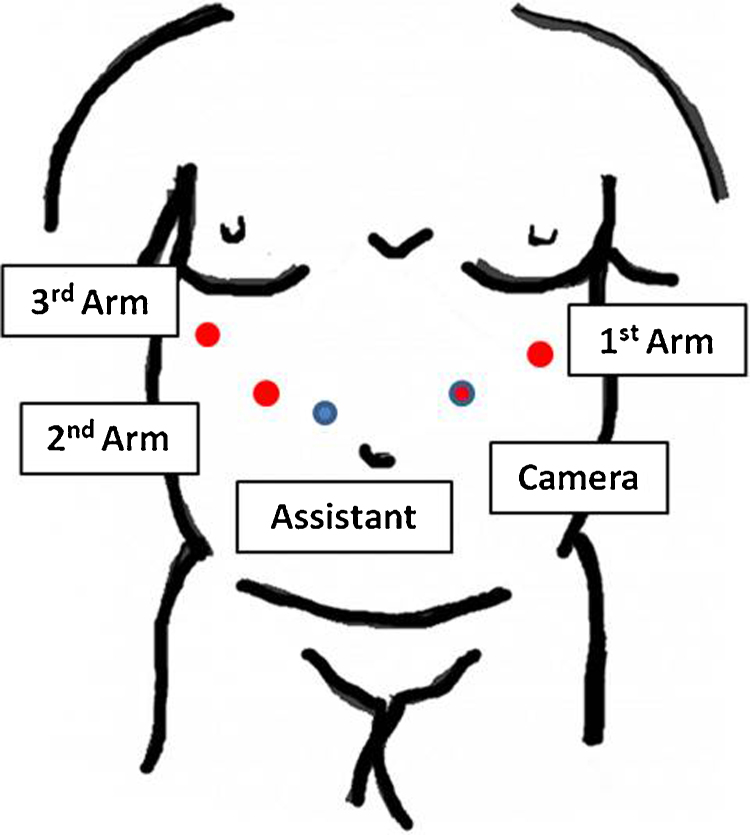
The patient underwent a minimally invasive approach, a 12 mm trocar for the robotic camera was placed left to the umbilicus with mid clavicle line, 8 mm robotic trocar for the 1st arm was placed subcostal following the left anterior axilar line, the 2nd and 3rd arm trocars was placed in the subcostal area following the right mid clavicular line and anterior axilary line, respectively, for the assistant port the trocar was placed 5 cm medially of the 2nd arm, the robot cart was docked from the head of the patient (Fig. 2). During the operation a rigid stomach was found with dysplastic changes in the gastric esophageal junction and thickening of the distal esophagus (Fig. 3). The tumor was invading transmurally into the perigastric adipose tissue and focally into the visceral peritoneum, pancreatic stromal tissue, transverse colon and distal esophagus. Robot-assisted total gastrectomy with a Roux-en-Y intracorporeal esophagojejunostomy, omentectomy, distal esophagectomy, distal splenopancretectomy, tangential colon resection with a feeding jejunostomy were all performed (Figs. 4 A–D and 5 A–D).
Fig. 2.
Trocar placement.
Fig. 3.
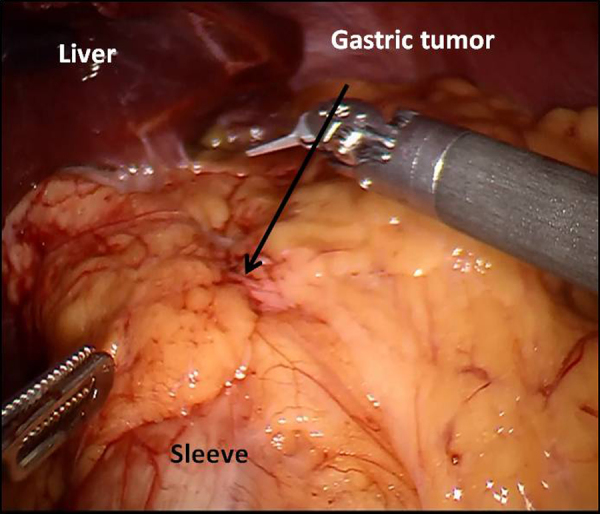
Intraoperative view of the gastric sleeve.
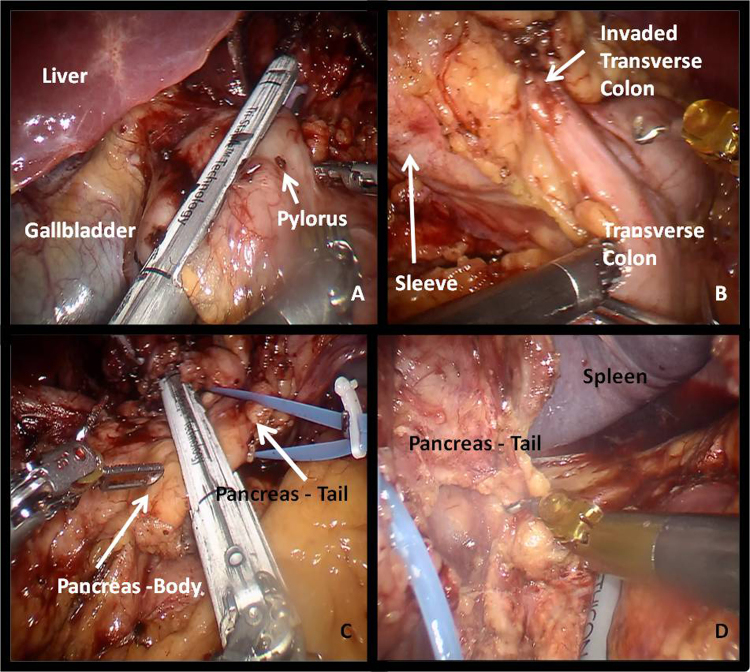
Fig. 4.
Intraoperative view of transection at 1st portion of duodenum (A), transverse colon invasion (B), transection of distal pancreas (C) and M-block disection of distal pancreas (D).
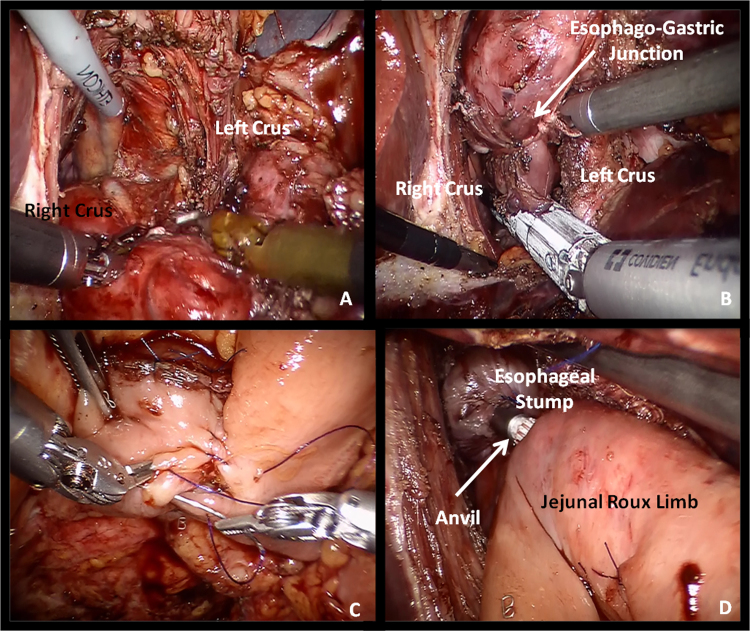
Fig. 5.
Intraoperative view of mediastinal dissection of distal esophagus (A), transection of distal esophagus (B), jejuno-jejunostomy (C) and esophago-jejunostomy with circular stapler (D).
The histologic examination revealed poorly differentiated and diffused type grade 3 gastric adenocarcinoma (pT4b, pN3a). The resection margins were tumor free, while there were metastasis to 2/2 lesser curvature lymph nodes, 2/2 perigastric lymph nodes, 1/1 splenic lymph nodes and 4/4 esophageal pedicle lymph nodes.
The operation was completed within 360 min, with an approximate blood loss of 200 ml. The patient was transferred to the surgical intensive care unit (SICU) for monitoring. The patient presented with a postoperative bibasal pleural effusion while in the SICU requiring chest tube drainage, with no further complications. The patient was tolerating diet and was discharge on postoperative day 14th. Patient is currently doing well at 8 month follow-up, with no sign of recurrences. Patient completed chemotherapy using Herceptin.
3. Discussion
Gastroesophageal cancer following a bariatric procedure has been previously reported in the literature [3], [4], [5], [6]. The diagnosis of gastroesophageal cancer following bariatric surgery is usually late since these patients present with common upper gastrointestinal symptoms related to the procedure such as oral intolerance, nausea and vomiting.
To our knowledge this is the third reported case of de novo gastric cancer after LSG; nonetheless, it appears to be the bariatric procedure least related to cancer when compared with other bariatric procedures. Previous reports showed a case of adenocarcinoma of the lower esophageal sphincter (T2N1Mx) diagnosed 4 month after SG in a patient that did not undergo a preoperative EGD evaluation [5] and a second case of signet-ring cell adenocarcinoma of the body, antrum and pylorus of the stomach with lymph nodes metastasis (T4N1Mx) diagnosed 4 years after SG [6].
In our case, the cancer was aggressive and locally invasive, involving continuous organs and lymph nodes. The patient had several known risk factors for cancer. She was morbidly obese, a heavy cigarette smoker, immunosuppressed post pancreatic transplant and presented other neoplasm (grade III vulvar intraepithelial neoplasia, anal condilomatosis). In consideration of the patient's transplant history and immune suppression therapy, laparoscopic sleeve gastrectomy was the procedure chosen for the metabolic and restrictive changes, avoiding the malabsortion component of alternative procedures that can jeopardize the outcome of the transplant by adjusting the immunosuppressive medication [8].
The increase in minimally invasive surgical skills provided by the robotic system allows for complex multiorgan resections while preserving the benefits of the minimally invasive surgical approach.
Despite the reported occurrences of upper gastrointestinal cancer following bariatric surgery, the incidence continues to be very low to suggest implementing a specific screening prior to SG. Although previous reports suggest performing EGD’s routinely [9], there is not enough data to support the need for further screening. In our practice EGD’s are not performed routinely, unless patients present with suspicious symptoms. Patients do undergo routinely Upper GI swallow test pre-operatively with the aim of detecting asymptomatic hiatal hernias.
4. Conclusion
No direct relation has been established between sleeve gastrectomy and the development of gastric cancer. We believe it is important to report this occurrence to better understand the nature or relation of bariatric surgery and upper gastrointestinal cancer. Further studies are needed in order to draw definitive conclusions regarding screening and the role of the preoperative EGD.
Conflicts of interest
Dr. Giulianotti has a consultant agreement with Covidien. The Department of Surgery at the University of Illinois has a proctoring agreement with Intuitive Surgical. Dr. Giulianotti as faculty included in this agreement provides proctoring services but is not remunerated. Financial benefits from this proctoring agreement are directly paid to the University of Illinois, Department of Surgery. Intuitive Surgical has provided a grant to the University of Illinois, Division of General, Minimally Invasive and Robotic Surgery. Dr. Giulianotti is the Chief of the Division and has no personal financial benefits resulting from this grant. None of the other authors have potential conflicts of interest to declare.
Funding
No sponsor involvement.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
Mario Masrur: contributed on study concept. Mario Masrur and Luis Fernando Gonzalez Ciccarelli contributed on data collection, analysis and interpretation and wrote the manuscript. Fernando Elli and Pier Giuloanotti: acting surgeons, data analysis and interpretation, reviewed and commented manuscript.
Guarantor
Pier C. Giulianotti.
References
- 1.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Abnet C.C., Freedman N.D., Hollenbeck A.R., Fraumeni J.F., Leitzmann M., Schatzkin A. A prospective study of BMI and risk of esophageal and gastric adenocarcinoma. Eur. J. Cancer. 2008;44(3):465–471. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scozzari G., Trapani R., Toppino M., Morino M. Esophagogastric cancer after bariatric surgery: systematic review of the literature. Surg. Obes. Relat. Dis. 2013;9:133–142. doi: 10.1016/j.soard.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Orlando G., Pilone V., Vitiello A., Gervasi R., Lerose M., Silecchia G. Gastric cancer following baritric surgery: a review. Surg. Laparosc. Endosc. Percutan. Tech. 2014;24(5):400–405. doi: 10.1097/SLE.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal R. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg. Obes. Relat. Dis. 2012;8:8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Scheepers A.F., Schoon E., Nienhuijs S.W. Esophageal carcinoma after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2011;7:11–12. doi: 10.1016/j.soard.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Angrisani L., Santonicola A., Iovino P. Gastric cancer: a de novo diagnosis after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2014;10:186–187. doi: 10.1016/j.soard.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Elli F., Masrur M., Guilianotti P.C. Robotic sleeve gastrectomy after liver transplantation. Surg. Obes. Relat. Dis. 2013;9:20–22. doi: 10.1016/j.soard.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Praveenraj P., Gomes R., Kumar S., Senthilnathan P., Parathasarathi R., Rajapandian S. Diagnostic yield and clinical implications of preoperative upper gastrointestinal endoscopy in morbidly obese patient undergoing bariatric surgery. J. Laparoendosc. Adv. Surg. Tech. 2015;25(8):465–469. doi: 10.1089/lap.2015.0041. [DOI] [PubMed] [Google Scholar]