Highlights
-
•
Adult cystic breast lymphangioma is an extremely rare condition, especially in the inner upper quadrant of the breast.
-
•
Cystic lymphangiomas of the breast are benign lymphatic malformations.
-
•
Cystic lymphangiomas of the breast are typically located in the upper outer quadrant, the tail of Spence, and subareolar area.
-
•
Surgical excision with histopathology examination provides definitive diagnosis of cystic lymphangioma.
-
•
Complete surgical excision remains the most effective treatment modality for adult cystic lymphangioma of the breast.
Keywords: Breast lymphangioma, Cystic lymphangioma, Adult lymphangioma, Breast mass, Breast cystic lesion
Abstract
Introduction
Adult cystic lymphangioma of the breast is extremely rare, especially in the breast’s upper, inner quadrant. Review of literature is discussed, including etiology, differential diagnosis, workup, and treatment of the disease.
Presentation of case
A 20-year-old female presented with a recurrent left breast cyst. Previous aspirations showed no malignancy. Ultrasonography showed a lobulated anechoic lesion with internal debris and thin septations. The patient then underwent a lumpectomy of the left breast cyst, and pathology showed lymphangioma.
Discussion
Cystic lymphangiomas of the breast are benign lymphatic malformations. Consistent with the main drainage pattern of the breast, cystic lymphangiomas are typically located in the upper, outer quadrant, tail of Spence, and subareolar area. Radiologic evaluation may include ultrasound, mammogram, and MRI. FNA cytology and core biopsy assist in the diagnosis of breast lymphangioma. Although different treatment options exist, complete surgical excision remains the most effective treatment modality for adult cystic lymphangioma of the breast.
Conclusion
Breast cystic lymphangioma is an extremely rare condition, especially in the upper, inner quadrant of the breast. The patient had multiple recurrences of the lesion after fine needle aspirations. Proper index of suspicion, prompt diagnosis, and definitive treatment is necessary to prevent recurrence and complications.
1. Introduction
Adult cystic lymphangioma of the breast is an extremely rare condition with less than 20 cases reported worldwide in the last 40 years. To our knowledge, only one case has been described in the U.S. [1], [2], [3]. Cystic lymphangiomas are benign lymphatic malformations [4], typically found in infants [5], [6]. Diagnosis of cystic lymphangiomas is uncommon in the adult population, as greater than 90% of them are diagnosed prior to 2 years of age [2].
The most common location of breast lymphangiomas, as described in the limited number of case reports, is in the sub-areolar and the upper, outer quadrant of the breast. Most likely, this represents the governing anatomic principle: the majority of lymphatic drainage from the breast is to the ipsilateral axilla [3], [7]. To our knowledge, an isolated location of breast lymphangioma in the upper, inner quadrant of the breast has not been described before. This case report involves the discovery of a rare cystic lymphangioma of the breast in a patient presenting with a history of multiple fine needle aspirations for a cyst located in the upper, inner quadrant of the breast. This work has been reported in line with the CARE criteria [8].
2. Case report
A 20-year-old female was referred to our surgery clinic with a left breast cyst that recurred despite multiple fine needle aspirations by her primary care physician. She had an obvious bulging deformity and mastodynia due to the mass effect. There were no other complaints or systemic symptoms, including fever, chills, or weight loss. The fluid from one of the aspirations was sent for cytological evaluation and was unremarkable, showing no atypia or other discerning neoplastic features.
Clinical examination found a cyst greater than six centimeters located in the upper, inner quadrant of the left breast at the 10 o’clock position, adjacent to the sternal border. There were no skin changes, including erythema, cellulitis, nipple retraction, or breast tenderness. Initial clinical impression was that the lesion represented a cyst, both on clinical examination as well as radiologic studies.
Ultrasound at the palpable area of concern found a 6.1 cm lobulated anechoic lesion with internal debris and several thin partial septations. On color Doppler, there was no flow in the cystic lesion or in the partial septations. Posterior acoustic enhancement was present (Fig. 1).
Fig. 1.
Lobulated cyst with a septated internal wall in the left breast at a 10 o’clock position and middle depth 10 cm from the nipple measuring 6 × 6.1 × 1.9 cm. This lobulated cyst is anechoic with posterior acoustic enhancement.
Lumpectomy was recommended due to the recurrent disease process and the patient’s concern regarding the disfiguring bulge and associated pain. The patient underwent lumpectomy of the left breast cyst with intraoperative ultrasound-guided needle localization. A circumareolar incision in the upper aspect was made using an oncoplastic approach, and a tunnel was made into the upper, inner quadrant. The cyst was intimately associated with the pectoralis muscle at the 10 o’clock position, 10 cm from the nipple. The needle localization wire was then brought into the wound and the cyst was excised in its entirety (Fig. 2).
Fig. 2.
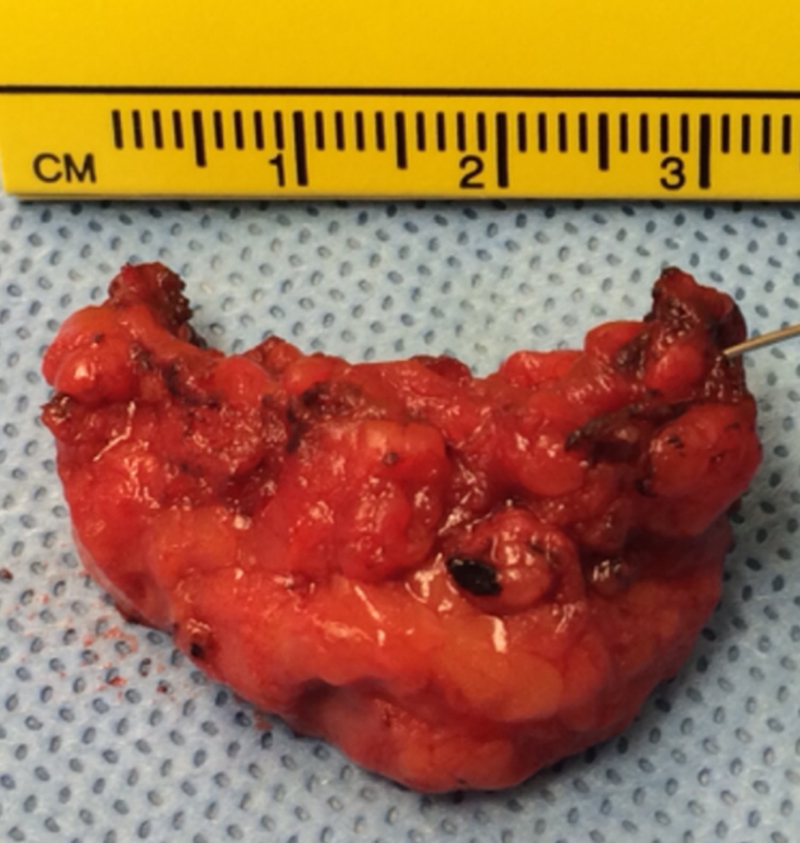
Gross examination of the surgical specimen measuring 3 × 1.5 × 1.5 cm showed a poorly demarcated, multicystic lesion containing clear yellowish fluid.
Pathologic findings and diagnosis included no evidence of a cystic wall on gross inspection and microscopic features supporting diagnosis of lymphangioma (Fig. 3). The patient tolerated the procedure well and was discharged the same day. She was seen in the clinic three weeks after surgery without any signs of postoperative complications.
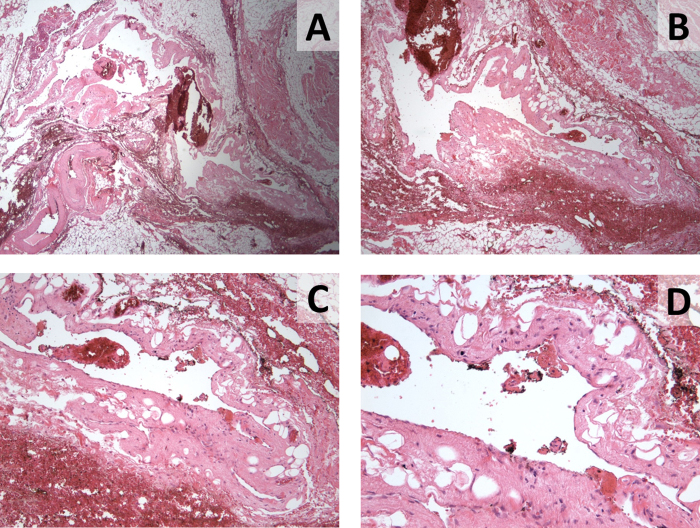
Fig. 3.
Two histologic slides (A and B) displaying fibroadipose tissue with vessels including a dilated cystic lymphatic space lined by attenuated endothelium. Further magnification of Part B better demonstrates the endothelial lining cells with objectives of 40× (C) and 60× (D).
3. Discussions
Lymphangiomas are dilated lymphatics with endothelial linings that may be caused by congenital weakness of the wall, proliferation of lymphatic vessels, and blockage of the lymphatic channels [9]. It has been suggested that lymphangiomas are caused by failure of the sequestrated lymphatic tissue to communicate with the normal lymphatic system during development [4]. Finally, an instigating event, e.g., trauma or infection, can delay proliferation of resting lymphoid cells, resulting in lymphangioma formation in the adult patient [2]. Lymphangiomas are most commonly benign, slow-growing lesions that do not transform into malignancy [4], although malignant degeneration into squamous cell carcinoma in longstanding lymphangioma circumscriptum has been described [1], [5], [10].
There are several subtypes of lymphangiomas that can be clearly distinguished by their pathologic features. Simple lymphangiomas are composed of small capillary, thin-walled vessels. Lymphangioma circumscriptum (capillary lymphangioma) is a cutaneous lymphangioma consisting of small lymphatic channels. Cystic lymphangioma is characterized as spaces lined by endothelial cells, filled with clear lymph fluid. Cavernous lymphangiomas are long standing cavernous lesions with massively dilated cystic spaces lined by endothelial cells separated by connective tissue containing lymphoid aggregates [1], [2], [9].
Cystic lymphangioma is an uncommon congenital lymphatic malformation typically located in the head, neck, and axilla [1], and it is typically diagnosed before 2 years of age [5], [6]. Breast lymphangiomas are typically located in the upper, outer quadrant, the tail of Spence, and subareolar area [9]. This is related to the main drainage pattern of the breast lymphatics toward the tail of Spence and the axilla [11].
Lymphatic drainage of breast originates from breast lobules and flow into the Sappey’s plexus, which is a network of lymphatics within the areola of the nipple. There are three lymphatic drainage pathways from the Sappey’s plexus. The axillary or lateral pathway is on the inferior edge of the pectoralis major and goes to the pectoral group of axillary nodes. The internal mammary pathway passes through the pectoralis major to the contralateral breast, and the retromammary pathway originates from the posterior portion of the breast [12]. The axillary lymph nodes receive more than 75% of the lymph drained from the breast, especially from the lateral quadrants. The remainder of lymph drains to either the parasternal nodes or the opposite breast (medial quadrants) or the inferior phrenic nodes (lower quadrants) [13]. Lymphangioma in the upper, inner quadrant of the breast, as seen in the presented patient, is extremely unusual.
Cystic lymphangiomas are usually asymptomatic [6]. Pain and discomfort may be experienced as they increase in size, as seen cystic lymphangiomas in pregnant or lactating women. Possible complications of cystic lymphangioma include bleeding, infection, and fistula formation [14].
Radiologic evaluation of a palpable lump incudes several modalities. Mammography typically shows a lobulated or round lesion with increased tissue density. Ultrasound is the gold standard to differentiate solid from cystic masses. Differential diagnoses of a cystic lesion on ultrasound include simple cyst, lymphangioma, or lymphocele [11], [14]. Hemangiomas are usually localized, and flow is present on color-flow images. Apocrine cysts and cystic lymphangioma typically appear as hypoechoic to anechoic, well circumscribed mass with linear septations on ultrasound [1], [9]. MRI could be considered preoperatively if there is a concern for a malignancy or the patient is high risk.
FNA cytology and core biopsy assist in the diagnosis of breast lymphangioma by excluding other cystic lesions. The FNA fluid aspirates are typically clear, yellowish, or straw-colored with many lymphocytes; these specimens are usually sparsely cellular. FNA are often nondiagnostic since the architecture of the lesion cannot be evaluated; any blood vessels, fibrous tissue, and fat that are seen in the specimen could be normal tissue. Surgical excision with histopathology examination provides definitive diagnosis of cystic lymphangioma [1].
Complete surgical excision is the treatment of choice. For example, spontaneous resolution of the cystic lymphangioma is unusual [15]. Other treatment options include incision and drainage, sclerotherapy, steroid, radiotherapy, and carbon dioxide laser [5], [6], [7]. Injection sclerotherapy aims to obliterate the lymphatic channels [16]. CO2 laser treatments can provide palliative relief by vaporizing superficial vesicles and sealing the communicating channels to the deeper cisterns [17]. These alternative treatments are associated with high recurrence rates and the scarred tissues make future surgery more difficult. However, they are acceptable adjuvant treatments in cases in poor surgical candidates [9].
4. Conclusions
Breast cystic lymphangioma is an extremely rare condition of the breast, especially in the upper, inner quadrant. Despite its rarity, cystic lymphangioma should be included in the differential diagnosis in patients who present with cystic lesions. Proper index of suspicion, prompt diagnosis, and definitive treatment is necessary to prevent recurrence, complications, and the possibility of malignant degeneration into squamous cell carcinoma in an untreated lesion [5], [10]. Although different treatment options exist, complete surgical excision remains the most effective treatment modality for this rare adult cystic lymphangioma of the breast.
Conflict of interest
None.
Funding
None.
Ethical approval
Not applicable. No research study involved.
Consent
Patient consent obtained on 5/13/2015.
Author contribution
Eve Rusdianto, DO: Writing the paper.
Mary Murray, MD: Surgeon who operated on patient. Contributed in writing the paper.
Jennifer Davis, MD: Breast radiologist. Contributed on the imaging section of the paper.
Anne Caveny, MD: Pathologist. Contributed on the pathology slides and captions.
Guarantor
Eve Rusdianto, DO and Mary Murray, MD.
Contributor Information
Eve Rusdianto, Email: eveline.rusdianto@akrongeneral.org.
Mary Murray, Email: Mary.murray@akrongeneral.org.
Jennifer Davis, Email: Jennifer.Davis@akrongeneral.org.
Anne Caveny, Email: E.AnneCaveny@akrongeneral.org.
References
- 1.Sasi W., Schneider C., Shah R., Ruffles T., Bhagwat P., Mokbel K., Sharma A.K. Recurrent cystic lymphangioma of the breast: case report and literature review. Breast Dis. 2010;31(1):43–47. doi: 10.3233/BD-2009-0287. [DOI] [PubMed] [Google Scholar]
- 2.Kwon S.S., Kim S.J., Kim L., Kim Y.J. Huge cystic lymphangioma involving the entire breast. Ann. Plast. Surg. 2009;62(1):18–21. doi: 10.1097/SAP.0b013e31817e9c2c. [DOI] [PubMed] [Google Scholar]
- 3.Ogun G.O., Oyetunde O., Akang E.E. Cavernous lymphangioma of the breast. World J. Surg. Oncol. 2007;5:69. doi: 10.1186/1477-7819-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn S.Y., Choi H.Y., Park S.H., Jang J. Lymphangioma and lymphangiectasia of the breast mimicking inflammatory breast cancer. J. Ultrasound Med. 2011;30(June (6)):863–865. doi: 10.7863/jum.2011.30.6.863. [DOI] [PubMed] [Google Scholar]
- 5.Waqar S.N., Khan H., Mekan S.F., Kayani N., Raja A.J. Cystic breast lymphangioma. J. Pak. Med. Assoc. 2004;54(October (10)):531–533. [PubMed] [Google Scholar]
- 6.Sa E.J., Choi Y.H. Cystic lymphangioma of the breast. J. Clin. Ultrasound. 1999;27(July–August (6)):351–352. doi: 10.1002/(sici)1097-0096(199907/08)27:6<351::aid-jcu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Chung S.Y., Oh K.K., Kim D.J. Mammographic and sonographic findings of a breast cystic lymphangioma. J. Ultrasound Med. 2003;22(March (3)):307–309. doi: 10.7863/jum.2003.22.3.307. [DOI] [PubMed] [Google Scholar]
- 8.Gagnier J., Kienle G., Altman D.G., Moher D., Sox H., Riley D.S., the CARE group The CARE guidelines: consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2014;67(1):46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Hiremath B., Binu V. Lymphangioma of the breast. BMJ Case Rep. 2014;(March (17)):2014. doi: 10.1136/bcr-2014-203937. pii: bcr2014203937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson G.R., Cox N.H., McLean N.R., Scott D. Squamous cell carcinoma arising within congenital lymphangioma circumscriptum. Br. J. Dermatol. 1993;129:337–339. doi: 10.1111/j.1365-2133.1993.tb11859.x. [DOI] [PubMed] [Google Scholar]
- 11.Min K.W., Jang H.S., Na W., Jang S.M., Jun Y.J., Jang K.S., Paik S.S. Cystic lymphangioma of the breast in an adult woman. Korean J. Pathol. 2008;42:244–246. [Google Scholar]
- 12.H. Knipe, S. Pacifici, et al. Lymphatic Drainage of the Breast. Radiopaedia Blog RSS. Web. 7 July 2015. http://radiopaedia.org/articles/lymphatic-drainage-of-the-breast.
- 13.Anatomy of the Breast Diseases of the Breast. Web. 7 July 2015. http://fitsweb.uchc.edu/student/selectives/Luzietti/Breast_anatomy.htm.
- 14.Balaji R., Ramachandran K. Cystic lymphangioma of the breast: magnetic resonance imaging features. Breast Care (Basel) 2010;5(August (4)):250––252. doi: 10.1159/000319503. Epub 2010 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Guerké L., Baron M., Dessogne P., Callonnec F., d’Anjou J. Cystic lymphangioma of the breast. Breast J. 2005;11(November–December (6)):515–516. doi: 10.1111/j.1075-122X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 16.Okoro P.E., Anyaeze C.M., Ngaikedi C. Recurrent lymphangioma: what are the treatment options? Afr. J. Paediatr. Surg. 2009;6(January–June (1)):44–46. doi: 10.4103/0189-6725.48576. [DOI] [PubMed] [Google Scholar]
- 17.Haas A.F., Narurkar V.A. Recalcitrant breast lymphangioma circumscriptum treated by UltraPulse carbon dioxide laser. Dermatol. Surg. 1998;24(August (8)):893–895. doi: 10.1111/j.1524-4725.1998.tb04269.x. [DOI] [PubMed] [Google Scholar]