Abstract
The present study is characterized toward thespesone isolation from Thespesia populnea (Malvaceae). Subsequently it was modified and characterized to study its effect on diabetes related symptoms. The complex is administered to diabetes induced mice with the doses of 5, 10 and 20 mg/kg, p.o. and the effect of complex on the level of body weight, lipid profile and blood glucose was studied after 22 days. The results have indicated that diabetic mice show a significant (p < 0.01) decrease in the level of serum triglyceride, plasma glucose and increase in body weight. Hence the present investigation reveals that newly synthesized complex is useful in the management of Type-II diabetes mellitus because of its ability to reduce insulin resistance.
Keywords: Thespesia populnea, Vanadium complex, Insulin resistance, Diabetes
1. Introduction
Diabetes is the most common disease as far as metabolic disorder is concerned, its incidence in the year 2010 was 210 million and by 2025 it is proposed to increase to 300 million (Patel et al., 2013). Diabetes mellitus is divided into two types, Type-I and Type-II. Type-II diabetes, however, is also called non-insulin dependent diabetes mellitus, it develops on the root of impaired insulin secretion or increased insulin resistance (Kuzuya et al., 2002). The use of synthetic drugs might be connected with unnecessary side effects, including hypoglycemia and cell death, which are effective in increasing insulin secretion (Giorgino et al., 2006). Hence, there is an increased scope to develop more safe, effective and cheap methods for its treatment. It is a body’s condition where tissues turn resistant to insulin, which results in an evident reduction of glucose metabolism in response to insulin. Recent studies also suggest that insulin resistance results from complex interactions between genetic and environmental factors and is associated with common diseases such as Type-II diabetes, hypertension, obesity and coronary artery disease (Pei et al., 2012, Grundy, 2007). Nowadays researchers are focusing on target based treatment. Piroxicam proliferative activated receptors, glucagon like peptide hormone β3-Adenoreceptor agonist, DPP-IV inhibitors, α-lipoic acid, liver selective glucocorticoid antagonist, PTB-1B inhibitors, glycogen synthase kinase involved in glycogen metabolism, fructose-1, 6-biphosphate as a therapeutic target for Type-II diabetes are the new approaches in the treatment of diabetes mellitus (Patel et al., 2013).
Vanadium is a trace element which is essential for living organisms (Miroslaw et al., 2013). The insulin-like properties of Vanadium provide standard diet which has acknowledged cationic type vanadyl into its anionic type vanadate, which is concentrated in cells. So, vanadyl sulfate used as a choice for the treatment of management of diabetes is linked to their hyperinsulinemic, anti-hyperglycemic and anti hyperlipidemic effects (Ali, 2012). In traditional systems, plants are proven to be a good source of herbal medicine for the treatment of diabetes mellitus. Advances in different areas of plant investigation initiated from extraction procedures then isolation, identification, confirmation, design and utilization of bio-assay for efficacy testing, dosage form design, and study of pharmacodynamic, pharmacokinetic, toxicological and pharmacological mode of action (Banerjee and Mitra, 2012). Thespesia populnea belonging to the family Malvaceae has been recommended as an anti-diabetic agent in Ayurvedic Pharmacopoeia of India (Anonymous, 2006). The various experimental findings reveal that T. populnea elicited a wide spectrum of activities mainly Antibacterial activity (Gaind and Bapna, 1967), Anti-Hepatotoxic activity (Shirwaiikar et al., 1995), Antisteroidogenic activity (Kavimani et al., 1999), Cytotoxicity and superoxide anion generation (Inbaraj et al., 1999), Antinociceptive and anti-inflammatory activities (Vasudevan et al., 2007), Anti-psoriatic activity (Shrivastav et al., 2009), Wound healing activity (Nagappa and Binu, 2001), Dermatitis (Hausen et al., 1997), Anti-oxidant activity (Ilavarasan et al., 2003), Alzheimer’s Disease (Vasudevan and Parle, 2006), Antidiabetic activity (Parthasarathy et al., 2009), Synergistic activity (Vasudevan and Sivakumar, 2009), Hypoglycemic, anti-diabetic and anti-ulcer activities (Jayakumari et al., 2003), Immunomodulatory activity (Gaikwad and Mohan, 2011), Anti-inflammatory activity (Shah and Alagawadi, 2011), α-Amylase Inhibitory Activity (Sangeetha and Vedasree, 2012), antiulcer activity (Patil et al., 2010), Antioxidant and anti inflammatory activities (Sarma et al., 2011), Memory-Enhancing activity (Vasudevan and Parle, 2007).
In view of modern studies of phytochemistry, the modified complex of isolated phytochemical constituent exhibits better efficacy and activity. Therefore, the existing research work is aimed at identifying the combined effect of isolated compound thespesone (plant source) and Vanadium (mineral source) on diabetic experimental mice.
2. Experimental
2.1. Plant collection
The dry bark of T. populnea was collected from a nearby area of Pune, Maharashtra, India. The plant material is authenticated from the Botanical Survey of India with voucher specimen conserved under reference number BSI/WRC/Herbarium/2011.
2.1.1. Extraction of bark and isolation of compound
The air dried finely powdered bark was extracted by soxhlet extraction process with 90% hexane, then isolation of the sesquiterpene by using column chromatography with solvent system. The yield was found to be 10% after recrystallization with ethanol.
2.1.2. General procedure for synthesis of its metal complex
The isolated compound forms a complex with Vanadium in alkaline condition (Sodium acetate 30 mg) with methanolic solutions of Vanadyl sulfate and the respective ligand in 1:2 (metal:ligand). The reaction mixture was stirred for 6 h at room temperature. The precipitated metal complex was collected by filtration, washed with cold methanol to get pure complex.
2.2. Characterization of isolated thespesone and modified metal complex
2.2.1. Infrared spectroscopy (IR)
Infrared spectra were recorded using KBr pellets on a Shimadzu 8400S infrared spectrophotometer to know the functional groups present in thespesone and Vanadium complex at the laboratory of Modern College of Pharmacy, Pune (Donald et al., 2007, Sharma, 1980).
2.2.2. Electronic absorption spectroscopy
Electronic absorption spectra were recorded on a Shimadzu 1701 UV–VIS spectrophotometer using a matched pair of 1 cm2 quartz cells at the laboratory of Modern College of Pharmacy, Pune (Donald et al., 2007, Sharma, 1980).
2.2.3. Nuclear magnetic resonance spectroscopy
Proton NMR spectra were measured on a Perkin Elmer instrument Chemistry Department, University of Pune, operating at 300 MHz in CDCl3 solvent (Donald et al., 2007, Sharma, 1980).
2.2.4. Mass spectroscopy (MS)
The MS analysis of isolated compound was carried out on a API 4000 (Triple Quadruple) using direct Infusion technique in the split mode with scan Type: Q1 MS (Q1) and Ion Spray Voltage is 4200 (Donald et al., 2007, Sharma, 1980).
2.2.5. Electron paramagnetic resonance spectroscopy (EPR)
The electron spin resonance studies of all the Vanadium complexes were recorded at 77 K on a Varian X-band spectrometer at the Regional Sophisticated Instruments Center (RSIC), Indian Institute of Technology (IIT) Pavai, Mumbai, India.
2.3. Animals and experimental protocols
The study protocol was approved in accordance with the ethical recommendations of animal experimentation protocol (Certificate no: MCP/IAEC/46/2011). The animals had free access to standard pellet (Amrut feeds, Bombay), with water supplied under strict hygienic conditions. The Swiss albino mice weighing 20–30 g were acclimatized for seven days under standard husbandry conditions i.e. room temperature of 25 °C; relative humidity 45–55% of the animal facility at the Modern college of Pharmacy, Pune, Maharashtra, India.
2.4. Acute toxicity study (OECD, 2001)
The acute toxicity study for complex was performed using albino mice followed by OECD 2001 guidelines. The animals were fasted over-night prior to the experiment and maintained under standard conditions.
2.5. Induction of diabetes
To induce diabetes in mice, a single intramuscular injection of Dexamethasone at dose 1 mg/kg/day (Cadila Healthcare Ltd., India) was given to overnight fasted mice from day 1 to 7.
2.6. Dexamethasone induced insulin resistance in mice (Shalam et al., 2006, Gholap and Kar, 2005).
The overnight fasted animals were divided into groups (n = 6) as follows, Animals of Group-I received 1% gum acacia (1 ml/kg/day, p.o.) from day 1 to 22. Animals of Group-II received Dexamethasone (1 mg/kg/day, i.m.) from day 1 to 22. Animals of Group-III, IV, V, VI, VII, VIII received Dexamethasone (1 mg/kg/day, i.m.) from day 1 to 7 and Ketoconazole (24 mg/kg/day, p.o), Pioglitazone (2 mg/kg/day, p.o), LVa complex (5 mg/kg/day, p.o.), LVa complex (10 mg/kg/day, p.o.), LVa complex (20 mg/kg/day, p.o), Va (0.2 mM/kg/day, p.o.) from day 8 to 22, respectively.
2.6.1. Biochemical parameters
Diabetic animals were weighed before and after drug treatment. On the 22nd day blood was withdrawn by puncturing retro-orbital plexus by using fine glass capillary and collected and further it was used for the estimation of glucose and triglyceride. Biochemical estimation of plasma glucose (GOD/POD Method) and serum triglyceride (GPO/POD Method) was done by using standard diagnostic kits.
2.7. Result interpretation by statistical analysis
The results are expressed as mean ± SEM and statistically analyzed by Analysis of Variance (ANOVA) followed by Dunnett test, with level of significance set at p < 0.05, **p < 0.01.
3. Result and discussion
3.1. Characterization of isolated thespesone and modified metal complex
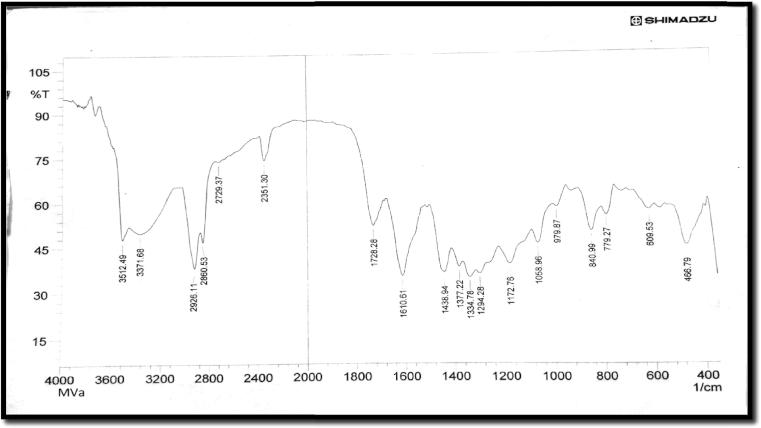
The compound (Thespesone) molecular formula is C15H14O4. The Infrared spectrum of synthesized Vanadium complex seen at different frequencies like OH at 3410.26 and C C at 1610.61 and the spectra of Vanadium complex are given in Fig. 1. The Electronic absorption spectra of compound were carried out by using DMSO solvent, λmax was found to be 289. The NMR signal of the CH2 group appears at δ ∼1.70 of B ring and 2.38 of A ring. δ ∼3.24 of B ring, δ ∼4.69 and δ ∼4.57 in C ring. The CH3 group appears at δ ∼1.48 of C ring, signal of the CH group in C ring appears at δ ∼4.28 and aromatic proton of A ring appears at δ ∼6.94 and Mass Spectroscopy analysis of isolated compound shows at m/z 258.2 it confirms the identity structure of Thespesone (Donald et al., 2007, Sharma, 1980).
Figure 1.
Infrared spectra of synthesized Vanadium complex.
The EPR spectra of the Vanadium complex provided information on metal ion environment and the electron delocalization (Table 1). The X-band EPR spectra of Vanadium complex, were recorded in DMSO glass at 77 K. The four well-resolved metal hyperfines in the low field region are indicative of monomeric and Vanadium complex which gives conformation about the complex.
Table 1.
Electron paramagnetic resonance parameters (at 77 K in DMSO glass) for Vanadium complexes (at 300 K). LVa–Thespesone–Vanadium complex.
| Compound | g‖ | g⊥ | A | F (cm−1) |
|---|---|---|---|---|
| LVa | 1.99 | 1.98 | 190 | 106 |
3.2. Acute toxicity study
From acute toxicity study it was found that the Vanadium complex was found safe up to a dose of 100 mg/kg, orally.
3.3. Dexamethasone induced insulin resistance in mice
In the adipocyte tissue glucocorticoids induce the ob gene expression within 24 h then followed by multifaceted metabolic changes resulting in fall of body weight, decline in food consumption, usually accompanied by diabetes and the development of insulin resistance along with increased blood glucose and triglyceride level. Different mechanisms for corticosteroids have been advocated for the increase in glucose level, one of them being insulin resistance caused by the altered binding of insulin receptors (Shalam et al., 2006, Gholap and Kar, 2005). Ketoconazole is an anti-glucocorticoid drug mainly, which inhibits the synthesis of cortisol and it prevents insulin resistance activity of dexamethasone (Storlien et al., 1992).
In the skeletal muscle, glucocorticoids reduce the rate of basal and insulin-stimulated glucose uptake, although the chronic accumulation of glycogen is improved. Glucocorticoid-induced insulin resistance is credited mainly to a post-receptor failing of insulin action, but this does not decrease the insulin-sensitive glucose transporter isoform GLUT 4 mRNA or GLUT 4 abundance in muscle membranes (Thomas et al., 1998).
The development of non insulin dependent diabetes mellitus was characterized by increased insulin resistance and failure of normal β-cell activity resulting in a slow decrease in insulin secretion (Talchai et al., 2009, Tanaka et al., 2011). Vanadium is an essential element which converted cationic type vanadyl into its anionic type vanadate in cells. Vanadate as a potent inhibitor of Na+–K+ ATPase increases the release of insulin from the pancreatic β-cells (Crans et al., 1995) and stimulates GTUT 4 so that glucose transport across the cell increases (Brichard and Henquin, 1995).
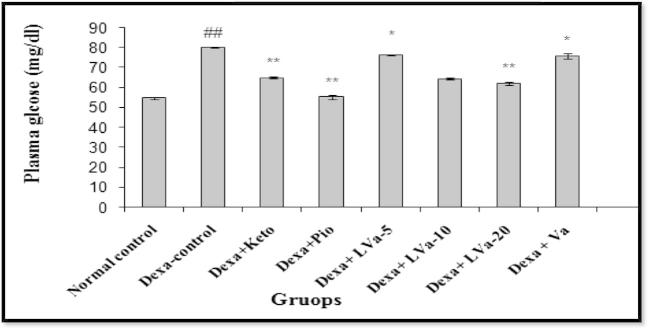
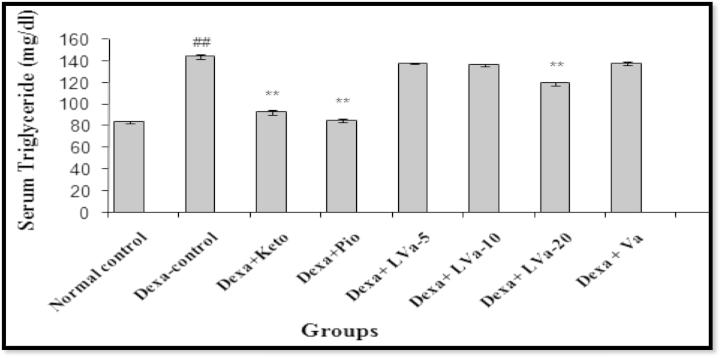
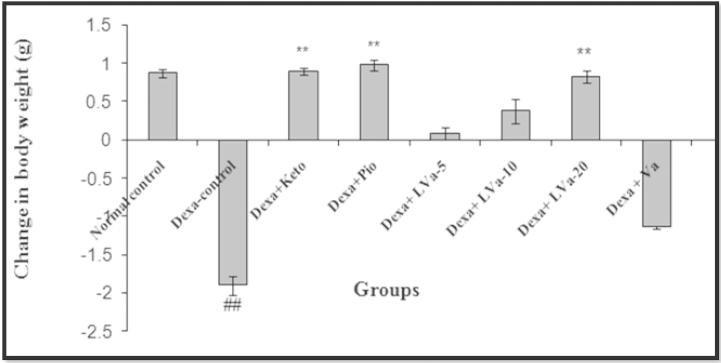
In the present study, dexamethasone administered for 22 days produced significant increase in blood glucose and triglyceride level. The treatment with Keto, Pio and different doses of Vanadium complex prevented the rise in plasma glucose due to dexamethasone (Figure 3, Figure 4). The anti-hyperglycemic activity of Vanadium complex in dexamethasone treated animals appears to be mediated by decreasing the peripheral insulin resistance or by suppressing enzymes involved in hepatic gluconeogenesis or by stimulating glucose uptake in the peripheral tissues (Gholap and Kar, 2005). There was a decrease in relative body weight of the diabetic mice compared to control mice (Fig. 2). Treatment of diabetic mice with Vanadium complex increases the reduced body weight by altering gluconeogenesis, glycolysis prevention, lipolysis and causing an imbalance in lipid metabolism. The plant T. populnea is also reported to have hepatoprotective activity. Thus in the present study the antihyperlipidemic activity of the Vanadium complex in dexamethasone treated animal is likely to be mediated by decreasing the lipolysis due to its antioxidant and hepatoprotective activities (Ghosh and Bhattacharya, 2004), also plays a role in the stimulatory action of Vanadium complex on glucose uptake.
Figure 3.
Effect of Vanadium complex on plasma glucose in Dexamethasone induced insulin resistance. Values are expressed as mean ± SEM. (n = 6), ANOVA followed by Dunnett test. ##p < 0.01 when compared with control; *p < 05, **p < 0.01 when compared with Dexa-control.
Figure 4.
Effect of Vanadium complex on serum triglyceride in Dexamethasone induced insulin resistance. Values are expressed as mean ± SEM. (n = 6), ANOVA followed by Dunnett test. ##p < 0.01 when compared with control; *p < 05, **p < 0.01 when compared with Dexa-control.
Figure 2.
Effect of Vanadium complex on the body weight in Dexamethasone induced insulin resistance. Values are expressed as mean ± SEM. (n = 6), ANOVA followed by Dunnett test. ##p < 0.01 when compared with control; *p < 05, **p < 0.01 when compared with Dexa-control.
4. Conclusions
In the results of the present study, it was observed that the Vanadium complex found may prove to be useful in the treatment of diabetes mellitus probably by overcoming the insulin resistance. Further, Vanadium complex also prevents the progressive increase in glucose and triglyceride level and decrease in body weight caused by dexamethasone. Complex may significantly increase glucose uptake, which indicates that there was boost in the insulin sensitivity.
Conflicts of interest
There is no conflict of interest relevant to this article.
Acknowledgements
The authors wish to thank to Dean of Pune University and Principal, Modern College of Pharmacy, Pune. Dr. P.D. Chaudhari, Director JNTU, Hyderabad and Regional Sophisticated Instruments Center (RSIC) and University of Pune, India for providing technical support, facility for the study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ali W.S. Antihyperglycemic effect of chicory leaves and Vanadium consumption on diabetic experimental rats. World J. Dairy Food Sci. 2012;7(2):167–173. [Google Scholar]
- Anonymous, 2006. Ayurvedic Pharmacopoeia of India. Part 1, vol. 5, pp. 63–64.
- Banerjee S., Mitra A. Changing landscape of herbal medicine: technology attributing renaissance. Int. J. Pharm. Pharm. Sci. 2012;4(1):47–52. [Google Scholar]
- Brichard S.M., Henquin J.C. Role of Vanadium in management of diabetes. TiPS. 1995;16:265–270. doi: 10.1016/s0165-6147(00)89043-4. [DOI] [PubMed] [Google Scholar]
- Crans D.C., Mahroof-Tahir M., Keramidas A.D. Vanadium chemistry and biochemistry of relevance for use of Vanadium complexes as anti-diabetic agents. Mol. Cell. Biochem. 1995;153:17–24. doi: 10.1007/BF01075914. [DOI] [PubMed] [Google Scholar]
- Donald, L. Pavia, Gary, M. Lampman, 2007. Textbook of Spectroscopical Analysis. Indian ed., pp. 26–478.
- Gaikwad S.B., Mohan G.K. Immunomodulatory activity of methanolic extract of T. populnea leaves in Westar albino rats. Asian J. Pharm. Clin. Res. 2011;4(4):99–101. [Google Scholar]
- Gaind K.N., Bapna S.C. Antibacterial activity of T. populnea. Indian J. Pharm. 1967;29:8–9. [Google Scholar]
- Gholap S., Kar A. Gymnemic acids from Gymnema sylvestre potentially regulates dexamethasone-induced hyperglycemia in mice. Pharm. Biol. 2005;43:192–195. [Google Scholar]
- Ghosh T., Bhattacharya K. Preliminary study on the anti-implantation activity of compound from the extract of seeds of T. populnea. Indian J. Pharm. 2004;36:288–291. [Google Scholar]
- Giorgino F., Laviola L., Leonardini A., Natalicchio A. GLP-1: a new approach for Type-II diabetes therapy. Diabetes Res. Clin. Pract. 2006;74:152–155. [Google Scholar]
- Grundy S.M. Metabolic syndrome: a multiplex cardiovascular risk actor. J. Clin. Endocrinol. Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- Hausen B.M., Knight T.E., Milbrodt M. T. populnea Dermatitis. Am. J. Contact Dermatitis. 1997;8:225–228. [PubMed] [Google Scholar]
- Ilavarasan R., Vasudevan M., Anbazhagan S., Venukataraman S. Anti-oxidant activity of T. populnea bark extracts against carbon tetrachloride induced liver injury in rats. J. Ethnopharmacol. 2003;87:227–230. doi: 10.1016/s0378-8741(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Inbaraj J.J., Gandhidasan R., Murugesan R. Cytotoxicity and superoxide anion generation by some naturally occurring quinine. Free Radical Biol. Med. 1999;26:1072–1078. doi: 10.1016/s0891-5849(98)00318-9. [DOI] [PubMed] [Google Scholar]
- Jayakumari S., Rajkumar M., Joanofarc J., Srinivasa R.G., Sadish K.S., Umadevi S.K. Hypoglycaemic, anti-diabetic and anti-ulcer screening of Thespesia populnea Linn. Nat. Prod. Sci. 2003;9(3):167–169. [Google Scholar]
- Kavimani S., Ilango R., Karpagam S., Suryaprabha K., Jaykar B. Antisteroidogenic activity of floral extract of T. populnea in mouse ovary. Indian J. Exp. Biol. 1999;37:1241–1242. [PubMed] [Google Scholar]
- Kuzuya T., Nakagawa S., Satoh J., Kanazawa Y., Iwamoto Y., Kobayashi M. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res. Clin. Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Miroslaw K., Francik R., Kowalska J., Gryboa R., Blusz M., Kwiatek W.M. Effects of Vanadium complexes supplementation on V, Fe, Cu, Zn, Mn, Ca and K concentration in STZ diabetic rat’s spleen. Acta Pol. Pharm. Drug Res. 2013;70(1):71–77. [PubMed] [Google Scholar]
- Nagappa A.N., Binu C. Wound healing activity of the aqueous extract of T. populnea fruit. Fitoterapia. 2001;72:503–506. doi: 10.1016/s0367-326x(01)00275-1. [DOI] [PubMed] [Google Scholar]
- OECD, 2001. Guidelines for Testing of Chemicals 425. Acute Oral Toxicity-Up-and-Down Procedure, pp. 1–26.
- Parthasarathy, Ilavarasan R.R., Karrunakaran C.M. Anti-diabetic activity of T. populnea bark and leaf extract against streptozotocin induced diabetic rats. Int. J. Pharm. Tech. Res. 2009;1(4):1069–1072. [Google Scholar]
- Patel K.P., Joshi H.M., Mujumdar F.D., Patel V.J. Newer approaches in the treatment of diabetes mellitus. NHL J. Med. Sci. 2013;2(1):6–11. [Google Scholar]
- Patil P.H., Patil J.Y., Mahale J.N., Patel J.B., Surana S.J. Evaluation of antiulcer activity of the terpenoid fraction from the leaves of T. populnea (L) (Malvaceae) in albino rats. Res. J. Pharm. Biol. Chem. Sci. 2010;1(4):495–513. [Google Scholar]
- Pei D., Dai J., Kuang Y., Wang H., Ren L., Shao J., et al. Effect of influenza A virus non-structural protein 1 (NS1) on a mouse model of diabetes mellitus induced by Streptozotocin. Biochem. Biophys. Res. Commun. 2012;419:120–125. doi: 10.1016/j.bbrc.2012.01.146. [DOI] [PubMed] [Google Scholar]
- Sangeetha R., Vedasree N. In vitro α-amylase inhibitory activity of the leaves of T. populnea. ISRN Pharmacol. 2012:1–4. doi: 10.5402/2012/515634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D.S., Venkata A., Babu S., Krishna K.R. Antioxidant and anti inflammatory activities of T. populnea linn. Int. J. Res. Pharm. Chem. 2011;1(3):674–676. [Google Scholar]
- Shah A.S., Alagawadi K.R. Anti-inflammatory, analgesic and antipyretic properties of T. populnea Soland ex. Correa seed extracts and its fractions in animal models. J. Ethno Pharmacol. 2011;137:1504–1509. doi: 10.1016/j.jep.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Shalam M.D., Harish M.S., Farhana S.A. Prevention of dexamethasone and fructose-induced insulin resistance in rats by SH-01D, a herbal preparation. Indian J. Pharmacol. 2006;38:419–422. [Google Scholar]
- Sharma, Y.R., 1980. Elementary Organic Spectroscopy. first ed., pp. 69–339.
- Shirwaiikar A., Vasanth K.A., Krishnanand B.R., Sreenivasan K.K. Chemical instigation and anti-hepatotoxic activity of T. populnea. Int. J. Pharmacognosy. 1995;33(4):305–310. [Google Scholar]
- Shrivastav S., Sindhu R.K., Sanjeev Kumar., Pradeep Kumar. Anti-psoriatic and phytochemical evaluation of T. populnea bark extracts. Int. J. Pharm. Pharm. Sci. 2009;1(1):176–185. [Google Scholar]
- Storlien L.H., Kusunoki M., Cooney G.J. Antiglucocorticoid treatment ameliorates high-fat feeding induced insulin resistance in rats. Diabetologia. 1992;35 doi: 10.2337/diab.44.6.718. A98–A98. [DOI] [PubMed] [Google Scholar]
- Talchai C., Lin H.V., Kitamura T., Accili D. Genetic and biochemical pathways of beta-cell failure in Type-II diabetes. Diabetes Obes. Metab. 2009;11(4):38–45. doi: 10.1111/j.1463-1326.2009.01115.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Shimaya A., Kiso T., Kuramochi T., Shimokawa T., Shibasaski M. Enhanced insulin secretion and sensitization in diabetic mice on chronic treatment with a transient receptor potential vanilloid 1 antagonist. Life Sci. 2011;88:559–563. doi: 10.1016/j.lfs.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Thomas D.M., Udagawa N., Hards D.K., Quinn J.M.W., Moseley J.M., Findlay D.M., Best J.D. Insulin receptor expression in primary and cultured osteoclast-like cells. Bone. 1998;23(3):181–186. doi: 10.1016/s8756-3282(98)00095-7. [DOI] [PubMed] [Google Scholar]
- Vasudevan M., Parle M. Pharmacological action of T. populnea relevant to Alzheimer’s disease. Phytomedicine. 2006;13:677–687. doi: 10.1016/j.phymed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Vasudevan M., Parle M. Memory-enhancing activity of T. populnea in rats. Pharm. Biol. 2007;45(4):267–273. [Google Scholar]
- Vasudevan, Sivakumar T. Synergistic activity of methanolic extract of T. populnea (Malvaceae) flowers with oxytetracycline. Bangladesh J. Pharmacol. 2009;4:13–16. [Google Scholar]
- Vasudevan M., Gunnam K.K., Parle M. Antinociceptive and anti-inflammatory effect of T. populnea Bark extract. J. Ethnopharmacol. 2007;109:264–270. doi: 10.1016/j.jep.2006.07.025. [DOI] [PubMed] [Google Scholar]