Abstract
Illicium verum, whose extractives can activate the demic acquired immune response, is an expensive medicinal plant. However, the rich extractives in I. verum biomass were seriously wasted for the inefficient extraction and separation processes. In order to further utilize the biomedical resources for the good acquired immune response, the four extractives were obtained by SJYB extraction, and then the immunology moleculars of SJYB extractives were identified and analyzed by GC–MS. The result showed that the first-stage extractives contained 108 components including anethole (40.27%), 4-methoxy-benzaldehyde (4.25%), etc.; the second-stage extractives had 5 components including anethole (84.82%), 2-hydroxy-2-(4-methoxy-phenyl)-n-methyl-acetamide (7.11%), etc.; the third-stage extractives contained one component namely anethole (100%); and the fourth-stage extractives contained 5 components including cyclohexyl-benzene (64.64%), 1-(1-methylethenyl)-3-(1-methylethyl)-benzene (17.17%), etc. The SJYB extractives of I. verum biomass had a main retention time between 10 and 20 min what’s more, the SJYB extractives contained many biomedical moleculars, such as anethole, eucalyptol, [1S-(1α,4aα,10aβ)]-1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid, stigmast-4-en-3-one, γ-sitosterol, and so on. So the functional analytical results suggested that the SJYB extractives of I. verum had a function in activating the acquired immune response and a huge potential in biomedicine.
Keywords: Illicium verum, Extractives, GC–MS, Acquired immune response, Immunogenetic function
1. Introduction
Illicium verum, which originated in southwest China and northeast Vietnam, is an evergreen plant. I. verum fruits have traditionally been used as flavors. Simultaneously, I. verum was also used in medicine for a long time. I. verum has been documented in the book “Herbal Essentials Collection” in 1505’s for the treatment of many fistula and cholera. In 1769, HUANG Gongxiu found that I. verum could heal heavy cold and inveterate cold. It was recorded in the book “Herbal positive” that I. verum could remove teeth mouth disease, detoxify and descend Qi. WANG Fu discovered that I. verum played a role in the treatment of relieving the depressed liver, reinforcing the kidney, and healing beriberi. And I. verum is widely used in many traditional Chinese medicines today. I. verum fruits were diuretic, antibacterial, stimulant, carminative, odontalgic and stomachic (Chopra et al., 1956, Ashraf et al., 2011). I. verum, which was prescribed as an digestive aid which could make the nursing mothers promote breast-milk production, had the anti-bacterial and anti-fungal affection of asthma, bronchitis and dry cough, refreshed the breath, and ensured a good sleep (Cheng and Changli, 2007, Qin et al., 2005, Ashraf et al., 2012). Its essential oil, which contained 75–90% anethole and had the observed estrogenic effect, was useful in providing relief from rheumatism and lower back pain (Wang et al., 2011, Qin et al., 2005, Qureshi et al., 2015). The pharmaceutical ingredients of I. verum began research and analysis since 1948. Kouno et al. reported neolignans and a phenylpropanoid glucoside from Illicium defengpi in 1993 (Kouno et al., 1993). In 1999, Thomas et al. studied novel secoprezizaane sesquiterpenes from North American IIIicium species (Thomas and Schmidt, 1999). Chinese and Japanese scholars all studied some chemical compositions of I. verum biomass (Kenichi et al., 1983, Kenichi et al., 1984, Yoshiyasu et al., 1994). What’s more, Chinese scientists have done extensive researches on the extraction technology, active ingredients identification and utilization of I. verum biomass (Lei and Jun, 2006, Zhi-ke and Shen, 2007, Wenyi et al., 2008, Jiahui et al., 2008, YuLing et al., 2006, Mingdong and Chenghuan, 2011, Nasreen et al., 2015). Especially, after I. verum was eaten, its extractives were dissolved in the blood which could activate the acquired immune response to resist the virus intrusion. Currently, the main biological active ingredients were resin, fatty oil, protein, volatile oil, etc. I. verum biomass was initiated for the production of Tamiflu in 2005. The Independent reported news that the medicinal ingredient of Tamiflu was shikimic acid which came from I. verum (Krämer et al., 2003, Johansson et al., 2005, Hashemi et al., 2015). It made I. verum a famous botanical anti-bird plant which has been planted more than 3.3 × 105 ha in south China. However, rich extractives in I. verum biomass were seriously wasted for the inefficient extraction and separation processes. the four extractives were obtained by SJYB extraction, and then the biomedical molecular of SJYB extractives were identified and analyzed by GC–MS so as to further utilize the biomedical resources.
2. Materials and methods
2.1. Materials
I. verum fruits were collected from Nanning Forest Farm, Guangxi province, P.R. China. The fresh fruits were dried in the indoor air, and about 40 mesh powder was sieved out using AS200 Sieving Instrument (Made in America). Benzene, methanol, ether, petroleum ether, and ethanol (chromatographic grade) were prepared for subsequent experiments. Quantitative filter paper, cotton bag, and cotton thread were all extracted in benzene-ethanol solution for 12 h. The benzene–ethanol solution was mixed according to Vethanol/Vbenzene 4 double. The methanol–ethanol solution was mixed according to Vethanol/Vmethanol 3 double. The ether–ethanol solution was mixed according to Vethanol/Vether 9 double. And the petroleum ether–ethanol solution was mixed according to Vethanol/Vpetroleum ether 1 double.
2.2. Methods
8 pieces of the above powders were weighed, each 10 g (1.0 mg accuracy), and then parceled by using the cotton bag and tied by using cotton thread, and signed. Extraction was gradually carried out by large-caliber Soxhletor and extracted in 800 ml petroleum ether–ethanol, methanol–ethanol, ether–ethanol, and benzene–ethanol solution, respectively. Parallel sample number was 2. Extraction time of petroleum ether–ethanol, methanol–ethanol, ether–ethanol, and benzene–ethanol solution was 5 h, 3 h, 5 h, and 9 h, respectively. Extraction temperature was 85–90 °C. After extraction, four extractive solutions were obtained and dried to 10 ml under the condition of 45 °C and a vacuum of 0.05–0.07 MPa. The petroleum ether–ethanol, methanol–ethanol, ether–ethanol, and benzene–ethanol extractives were obtained, respectively.
2.3. GC–MS analysis
The above extractives were analyzed by online linked gas chromatograph/mass spectrometer(GC–MS), respectively. The GC/MS analysis was carried out on a Aglient 6890 N + 5975C GC–MSTM (Aglient Co., Ltd, USA), which was linked to a mass selective detector. An elastic quartz capillary column DB-5MS (30 m × 250 μm × 0.25 μm) coated with a neutral phase (hewlett–packard-5 cross-linked 5% phenyl methyl silicone) was used. The carrier gas was helium and the injection port temperature was 250 °C. The temperature program of GC began at 50 °C and increased at the rate of 8 °C/min until 250 °C, 5 °C/min until 300 °C was reached, followed by a split injection at a ratio of 15:1. The program of MS was scanned over the 35–335AMU (m/z) respectively, with an ionizing voltage of 70 eV and an ionization current of 150 μA of electron ionization (EI). The flow velocity of helium was 1.2 ml/min. Ion source temperature: 230 °C, quadrupole temperature 150 °C (Mateen et al., 2015).
3. Results and discussion
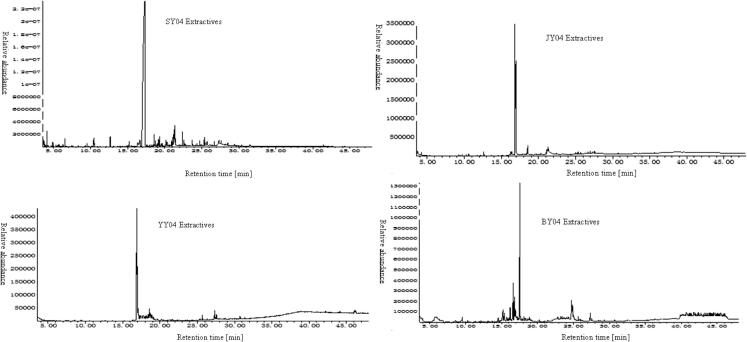
During SJYB extraction, four extractives (petroleum ether–ethanol extractives, methanol-ethanol extractives, ether–ethanol extractives, and benzene-ethanol extractives) were obtained respectively. The total ion chromatograms of four extractives by GC/MS are shown in Fig.1, respectively. Relative content of each component was counted by area normalization. Analyzing the MS data, the NIST standard MS map by computer, open-published books and papers [10–27], then components and their contents were identified.
Figure 1.
Total ion chromatogram of four extractives from Illicium verum Fruit by GC/MS.
3.1. Components of SJYB extractives from I. verum
According to GC/MS result, 108 components were identified on 124 peaks of SY04 extractives from I. verum fruit. The main components were anethole (40.27%), 4-methoxy-benzaldehyde (4.25%), 2-hydroxy-2-(4-methoxy-phenyl)-n-methyl-acetamide (3.53%), spiro [5.5]undecane (1.83%), 2,6-dimethyl-6-(4-methyl-3-pentenyl)-bicyclo[3.1.1]hept-2-ene (1.82%), docosane (1.71%), 2-methyl-naphthalene (1.69%), 4-methoxycinnamaldehyde (1.55%), 4-methoxy-benzoic acid methyl ester (1.44%), 1-(4-methoxyphenyl)-2-propanone (1.29%), 3-methoxyacetophenone (1.24%), caryophyllene (1.24%), 2-[2-pyridyl]-cyclohexanol (1.19%), 1,6,7-trimethyl-naphthalene (1.18%), tetradecane (1.18%), β-bisabolene (1.08%), 1-methoxy-4-(1-methylpropyl)-benzene (1.08%), 4-methoxybenzoic acid, 2,3-dichlorophenyl ester (1.05%), 4-methoxyphenylpropane-2-ol (1.03%), decahydro-4,4,8,9,10-pentamethylnaphthalene (0.95%), cis-β-farnesene (0.94%), and so on. Others were 2,3-dihydro-4,7-dimethyl-1h-indene, 1-(2-butenyl)-2,3-dimethyl-benzene, 1,4,6-trime-thyl-naphthalene, 4-dimethyl (ethenyl)silylbut-1-en-3-yne, [1s-(1α,4aα,10aβ)]-1,2,3,4, 4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid, hexadecane, heptadecane, 2-methyl-1,1’-biphenyl, octadecane, 3,7-dimethyl-1,6-octadien-3-ol, 1,2,3,4-tetrahydro-1,4-dimethyl-naphthalene, 1,6-dimethyl-naphthalene, sulfurous acid butyl dodecyl ester, 1,2,3,4-tetrahydro-1,1,6-trimethyl-naphthalene, 2-methyl-tetradecane, 4-methyl-tridecane, 2,3,6-trimethyl-naphthalene, n-hexadecanoic acid, pentadecane, 1,4-dimethyl-naphthalene, 8-amino-5-fluoro-6-methoxy-2-methylquinoline, 2,6,10-trimethyl-pentadecane, 2,6-dimethyl-undecane, 2,7-dimethyl-naphthalene, octane, (r)-4-methyl-1-(1-methyl-ethyl)-3-cyclohexen-1-ol, (z)-(3,3-dimethylcyclohexy-lidene)-acetaldehyde, 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-cyclohexane, d-limonene, α-farnesene, 1-(1-methylethenyl)-3-(1-methylethyl)-benzene, 1,2-dimethoxy-4-(1-propenyl)-benzene, dibutyl phthalate, 1,4-dimethoxy-2-methyl-benzene, nonadecane, pulegone, isobutyl undecyl phthalic acid ester, nonane, dehydroabietic acid, 2,6,10,14-tetramethyl-hexadecane, eicosane, p-xylene, hexadecanoic acid ethyl ester, linoleic acid ethyl ester, 9,10-dimethylanthracene, heneicosane, 1-methyl-anthracene, ethyl-cyclohexane, 2-methyl-phenanthrene, 2,3-dihydro-4-methyl-1h-indene, eucalyptol, 1-methyl-phenanthrene, 1,1,3-trimethyl-cyclohexane, cis-1,3-dimethyl-cyclohexane, decane, (e)-4,4-dimethyl-2-pentene, 3-methyl-4-(phenylthio)-5h-furan-2-one, stigmast-4-en-3-one, tricosane, 1,2,3-trimethyl-benzene, trans-1,2-dimethyl-cyclohexane, 2-carene, 9-[(4-methoxybenzoyl) oxy]-9-borabicyclo[3.3.1]nonane, (1α,2β,4β)-1,2,4-trimethyl-cycloh-exane, 4-methyl-octane, α-pinene, trans-1,3-dimethyl-cyclohexane, tetrahydro naphthyl methyl carbamate, (+)-3-carene, 1-ethyl-3-methyl-benzene, methyl-cycloheptane, 1-ethyl-2-methyl-cyclo-pentane, ethylbenzene, tetracosane, 2-(2-chlorophenyl)-1h-indole, 4-methoxy-n-[2-(4-methoxyphenyl) ethyl]-benzeneethanamine, 1-adamantan-1-yl-1,3-dihydro-benzoimidazol-2-one, 4-methylene-1-(1-methylethyl)-bicyclo[3.1.0] hexane, trans-1-ethyl-2-methyl-cyclohexane, propyl-cyclohexane, propyl-cyclopentane, 2,6-dimethyl-heptane, hexacosane, 1-methyl-2-propyl-cyclopentane, methabenzthiazuron, γ-sitosterol, and (z)-3,7-dimethyl-1,3,6-octatriene.
5 components were identified on 7 peaks of JY04 extractives from I. verum fruit. The components were anethole (84.82%), 2-hydroxy-2-(4-methoxy-phenyl)-n-methyl-aceta-mide (7.11%), 1-(4-methoxyphenyl)-2-propanone (4.18%), trans-4-methoxycinnamaldehyde (2.05%), and undecane (1.84%).
1 component was identified on many peaks of YY04 extractives from I. verum fruit. The component was anethole (100%).
5 components were identified on 5 peaks of BY04 extractives from I. verum fruit. The components were cyclohexyl-benzene (64.64%), 1-(1-methylethenyl)-3-(1-methyl ethyl)-benzene (17.17%), (3-methylcyclopentyl)-benzene (9.80%), 5,6,7,8,9,10-hexahydro benzocyclo octene (5.38%), 2,7-bis(1,1-dimethylethyl)-naphthalene (3.01%).
3.2. Molecular distribution of SJYB extractives from I. verum
The GC–MS analysis results show the molecular distribution of SJYB extractives from I. verum. The richest components of first-stage extractives (petroleum ether–ethanol extractives) were anethole (40.27%), 4-methoxy-benzaldehyde (4.25%), etc. Relative content of hydrocarbons, alcohols (phenol alcohols), aldehydes/ketones, ethers, acid/esters, and other compounds occupied 45.17%, 3.08%, 0.19%, 40.27%, 5.26%, and 6.03% of petroleum ether–ethanol extractives, respectively. The richest components of second-stage extractives (methanol-ethanol extractives) were anethole (84.82%), 2-hydroxy-2-(4-methoxy-phenyl)-n-methyl-acetamide (7.11%), etc. Relative content of hydrocarbons, alcohols (phenol alcohols), aldehydes/ketones, ethers, acid/esters, and other compounds occupied 3.89%, 0.00%, 4.18%, 84.82%, 0.00%, and 7.11% of methanol–ethanol extractives, respectively. The only component of third-stage extractives (ether–ethanol extractives) was anethole (100%). The richest components of fourth-stage extractives (benzene-ethanol extractives) were cyclohexyl-benzene (64.64%), 1-(1-methylethenyl)-3-(1-methylethyl)-benzene (17.17%), etc. Relative content of hydrocarbons occupied 100%. The results suggested that the first-stage, second-stage and third-stage extractives were suitable to extract anethole, and fourth-stage extractives were suitable to extract hydrocarbons (Khaskheli et al., 2015).
The retention time of each stage extractive of I. verum showed a particular rule. Among the first-stage extractives, the molecules with the retention time of ⩽10 min, ⩽20 min, ⩽30 min, ⩽40 min and ⩾40 min were 2.8%, 71.94%, 24.91%, 0.22% and 0.42%, respectively. Among the second-stage extractives, the molecules with the retention time of ⩽10 min, ⩽20 min, ⩽30 min, ⩽40 min and ⩾40 min were 0.00%, 90.84%, 9.16%, 0.00% and 0.00%, respectively. Among the third-stage extractives, the molecules with the retention time of 16.82–16.95 min were 100%. Among the fourth-stage extractives, the molecules with the retention time of ⩽10 min, ⩽20 min, ⩽30 min, ⩽40 min and ⩾40 min were 0.00%, 96.99%, 3.01%, 0.00% and 0.00% respectively. The results showed that the four extractives of I. verum biomass had a main retention time between 10 and 20 min (Nasreen et al., 2015).
3.3. Resource utilization of SJYB extractives from I. verum
There were many biomedical components in the SJYB extractives of I. verum biomass. Because of its officinal value, α-cedrene and β-cedrene were used for the raw materials of advanced odorants. Squalene, which was used in nutraceutical and pharmaceutical industries, could resist fatigue and strengthen the body’s resistance, protect the liver, and improve human immunity (Kim and Karadeniz, 2012). α-Cadinol, which acted as anti-fungal and as hepatoprotective, was proposed as a possible remedy for drug-resistant tuberculosis (Bueno et al., 2011, Ho et al., 2011, Tung et al., 2011). Anethole, which was widely used as a flavoring substance, had potent antimicrobial properties, against bacteria, yeast, and fungi (De et al., 2002, Camurça-Vasconcelos et al., 2007). In vitro, anethole also had antihelminthic action, haemonchus contortus, and nematicidal activity against the plant nematode meloidogyne javanica in vitro and in pots of cucumber seedlings (Oka et al., 2000, Weiming, 2005). Eucalyptol, which had a fresh camphor-like smell and a spicy, cooling taste, could reduce inflammation and pain, kill leukemia cells in vitro, treat mouthwash and cough suppressant, lower headache on bending, frontal headache, sensitivity of pressure points of trigeminal nerve, nasal obstruction, impairment of general condition, and rhinological secretion (Wanxi et al., 2012a, Wanxi et al., 2012b, Ashraf et al., 2013). [1S-(1α,4aα,10aβ)]-1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid was the active ingredient of skin care which could heal facial peeling[28]. Especially, stigmasta-4,6,22-trien-3β-ol and γ-sitosterol could reduce serum cholesterol and had effect on atherosclerotic lesion development (Gehui et al., 2002, Tabassum et al., 2014). According to the relative content of biomedicine components, the SJYB extractives of I. verum biomass were suitable to extract anethole.
4. Conclusions
The 108, 5, 1 and 5 components were identified on the peaks of SY04, JY04, YY04 and BY04 extractives from I. verum fruit, respectively. The richest components of first-stage extractives were anethole (40.27%), 4-methoxy-benzaldehyde (4.25%), etc. The richest components of second-stage extractives were anethole (84.82%), 2-hydroxy-2-(4-methoxy-phenyl)-n-methyl-acetamide (7.11%), etc. The only component of third-stage extractives was anethole (100%). The richest components of fourth-stage extractives were cyclohexyl-benzene (64.64%), 1-(1-methylethenyl)-3-(1-methylethyl)-benzene (17.17%), etc. And the four extractives of I. verum biomass had a main retention time between 10 and 20 min what’s more, the first-stage, second-stage and third-stage extractives were suitable to extract anethole, and fourth-stage extractives were suitable to extract hydrocarbons.
The functional analytical result suggested that the SJYB extractives of I. verum biomass contained rich immunogenetic function components which had huge potential in biological medicine, especially including anethole, [1S-(1α,4aα,10aβ)]-1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid, stigmast-4-en-3-one and γ-sitosterol, and so on.
Acknowledgments
This work was financially supported by the Project Supported by National Natural Science Foundation of China (No. 31170532), and The National Forestry Technology Popularization Project (No. [2012]37; No. [2012]60; No. [2012]62).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ashraf M.A., Maah M.J., Yusoff I. Study of tin accumulation strategy by Cyperus species in pot experiments. Res. J. Chem. Environ. 2011;15(1):88–98. [Google Scholar]
- Ashraf M.A., Maah M.J., Yusoff I. Assessment of phytoextraction efficiency of naturally grown plant species in the former tin mining catchment. Fresenius Environ. Bull. 2012;21(3):523–533. [Google Scholar]
- Ashraf M.A., Ullah S., Ahmad A., Qureshi A.K., Balkhair K.S., Rehman M.A. Green biocides, a promising technology: current and future applications. J. Sci. Food Agric. 2013;94(3):388–403. doi: 10.1002/jsfa.6371. [DOI] [PubMed] [Google Scholar]
- Bueno J., Escobar P., Martínez J.R., Leal S.M., Stashenko E.E. Composition of three essential oils, and their mammalian cell toxicity and antimycobacterial activity against drug resistant-tuberculosis and nontuberculous mycobacteria strains. Nat. Prod. Commun. 2011;6(11):1743–17438. [PubMed] [Google Scholar]
- Camurça-Vasconcelos A.L., Bevilaqua C.M., Morais S.M., Maciel M.V., Costa C.T., Macedo I.T., Oliveira L.M., Braga R.R., Silva R.A., Vieira L.S. Anthelmintic activity of croton zehntneri and lippia sidoides essential oils. Vet. Parasitology. 2007;148(3–4):288–294. doi: 10.1016/j.vetpar.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Cheng P., Changli H. Processing and exploitation of Star Anise. Agric. Prod. Proc. 2007;6:39–43. [Google Scholar]
- Chopra R.N., Nayar S.L., Chopra I.C., Asolkar L.V., Kakkar K.K. Council of Sci. & Ind. Res.; New Delhi: 1956. Glossary of Indian Medicinal Plants. pp. 12–98. [Google Scholar]
- De M., De A.K., Sen P., Banerjee A.B. Antimicrobial properties of star anise (Illicium verum Hook f) Phytother. Res. 2002;16(1):94–95. doi: 10.1002/ptr.989. [DOI] [PubMed] [Google Scholar]
- Gehui W., Zhanqian S., Liansheng W. Study on preparation and adsorption characteristics of chemically modified barks. Chem. Ind. Forest Prod. 2002;22(2):12–16. [Google Scholar]
- Hashemi S.S.G., Mahmud H.B., Ashraf M.A. Performance of green roofs with respect to water quality and reduction of energy consumption in tropics: a review. Renew. Sustain. Energy Rev. 2015;52(2015):669–679. [Google Scholar]
- Ho C.L., Liao P.C., Wang E.I., Su Y.C. Composition and antifungal activities of the leaf essential oil of Neolitsea parvigemma from Taiwan. Nat. Prod. Commun. 2011;6(9):1357–1360. [PubMed] [Google Scholar]
- Jiahui L., Guoqing L., Huafei Z., Jian K., Weiliang G., Lirong T. Optimization of extraction process for star anise oil. Trans. Chin. Soc. Agric. Eng. 2008;6:254–257. [Google Scholar]
- Johansson L., Lindskog A., Silfversparre G., Cimander C., Nielsen K.F., Lidén G. Shikimic acid production by a modified strain of E. Coli (W3110.shik1) under phosphate-limited and carbon-limited conditions. Biotechnol. Bioeng. 2005;92(5):541–552. doi: 10.1002/bit.20546. [DOI] [PubMed] [Google Scholar]
- Kenichi Y., Tomoko T., Rika S., Hiroyuki M., Shengten L., Hiroshi F. Studies on the constituents of the Plants of Illicium species. II. Structures of phenolic components. Chem. Pharm. Bull. 1983;31(8):2879–2883. [Google Scholar]
- Kenichi Y., Tomoko T., Eiji K. Studies on the constituents of the plants of Illicium specis. III. Structure elucidation of novel photoquinoids, illicinones from Illicium tashiroi Maxim and Illicium arborescens Hayata. Chem. Pharm. Bull. 1984;32(1):11–22. [Google Scholar]
- Khaskheli A.A., Talpur F.N., Ashraf M.A., Cebeci A., Jawaid S., Afridi H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B Enzym. 2015;113:56–61. [Google Scholar]
- Kim S.K., Karadeniz F. Biological importance and applications of squalene and squalane. Adv. Food Nutri. Res. 2012;65:223–233. doi: 10.1016/B978-0-12-416003-3.00014-7. [DOI] [PubMed] [Google Scholar]
- Kouno I., Yanagida K., Shimono S., Shintomi M., Ito Y., Yang C.S. Neolignans and a phenylpropanoid glucoside from Illicium defengpi. Phytochemistry. 1993;32(6):1573–1577. [Google Scholar]
- Krämer M., Bongaerts J., Bovenberg R., Kremer S., Müller U., Orf S., Wubbolts M., Raeven L. Metabolic engineering for microbial production of shikimic acid. Metabolic Eng. 2003;5(4):277–283. doi: 10.1016/j.ymben.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lei Z., Jun H. Study on the extraction of effective ingredients in lllicium verum. J. West Anhui Univ. 2006;2:76–78. [Google Scholar]
- Mateen F., Javed I., Rafique U., Tabassum N., Sarfraz M., Safi S.Z., Yusoff I., Ashraf M.A. New method for the adsorption of organic pollutants using natural zeolite incinerator ash (ZIA) and its application as an environmentally friendly and cost-effective adsorbent. Desalin. Water Treat. 2015;2015:1–9. [Google Scholar]
- Mingdong F., Chenghuan L. Extraction process of star anise oil and its antibacterial and antioxidative effects. Chin. Med. Herald. 2011;34:29–31. [Google Scholar]
- Nasreen S., Rafique U., Ehrman S., Ashraf M.A. Hybrid mesoporous silicates: a distinct aspect to synthesis and application for decontamination of phenols. Saudi J. Biol. Sci. 2015 doi: 10.1016/j.sjbs.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Nacar S., Putievsky E., Ravid U., Yaniv Z., Spiegel Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology. 2000;90(7):710–715. doi: 10.1094/PHYTO.2000.90.7.710. [DOI] [PubMed] [Google Scholar]
- Qin W., Lin J., Qi-biao W. Advances in studies on Illicium verum. J. Cereals Oils. 2005;18(5):18–22. [Google Scholar]
- Qureshi T., Memon N., Memon S.Q., Ashraf M.A. Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin. Water Treat. 2015;2015:1–9. [Google Scholar]
- Tabassum N., Rafique U., Balkhair K.S., Ashraf M.A. Chemodynamics of methyl parathion and ethyl parathion: adsorption models for sustainable agriculture. Biomed. Res. Int. 2014;2014(831989):1–8. doi: 10.1155/2014/831989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt Thomas J. Novel seco-prezizaane sesquiterpenes from North American IIIicium species. J. Nat. Prod. 1999;62(5):684–687. doi: 10.1021/np980382a. [DOI] [PubMed] [Google Scholar]
- Tung Y.T., Huang C.C., Ho S.T. Bioactive phytochemicals of leaf essential oils of Cinnamomum osmophloeum prevent lipopolysaccharide/D-galactosamine (LPS/D-GalN)-induced acute hepatitis in mice. J. Agric. Food Chem. 2011;59(15):8117–8123. doi: 10.1021/jf2018935. [DOI] [PubMed] [Google Scholar]
- Wang G.W., Hu W.T., Huang B.K., Qin L.P. Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011;136(1):10–20. doi: 10.1016/j.jep.2011.04.051. [DOI] [PubMed] [Google Scholar]
- Wanxi P., Lansheng W., Qiu X., Qingding W., Shilong X. TD-GC-MS analysis on thermal release behavior of poplar composite biomaterial under high temperature. J. Comput. Theor. Nanosci. 2012;9(9):1431–1433. [Google Scholar]
- Wanxi P., Fengjuan W., Lansheng W., Qiu X. Crystal structure of 3-(4-bromophenyl)-4-(4-chlorophenylamino) furan-2(5H)-one, C16H11BrClNO2. Z Kristallogr. – New Cryst. Struct. 2012;227(1):61–62. [Google Scholar]
- Weiming Z. Southeast Univ. Press; Nanjing China: 2005. Development and Utilization of Plant Resources. pp. 1–443. [Google Scholar]
- Wenyi K., Yuxin L., Fengge S., Enming D., Saimou L. Extraction technology of the essential oil from Illicium verum. J. Henan Univ. (Med. Sci.) 2008;(2) 6-9+20. [Google Scholar]
- Yoshiyasu F., Naomi S., Yunko H., Mitsuaki K. Prenylated C6–C3 compounds from Illicium tashiroi. Photochemistry. 1994;36(6):1497–1503. [Google Scholar]
- YuLing Z., Huanyang Q., Jubai L., Yanping S. Analysis of volatile compounds released from Illicium verum by solid phase microextraction (SPME) Anal. Test. Technol. Instrum. 2006;1:20–25. [Google Scholar]
- Zhi-ke L., Shen L. The new method for quick extraction and isolation of Illicium verum Hook Oil. J. Fujian Forest. Sci. Technol. 2007;1:5–8. [Google Scholar]