Abstract
Imbalanced immune responses against fetus alloantigens can lead to abnormality in pregnancy. Interleukin-10 (IL-10) plays key roles in regulation of immune responses against self and foreign antigens to induce tolerance to these antigens. Therefore, alteration in expression of IL-10 during pregnancy may result in several pathologic conditions such as preterm labor. IL-10 leads to a normal pregnancy via several molecular mechanisms including development of tolerogenic dendritic cells, T regulatory lymphocytes and activation of the JAK1/STAT3 pathway in the target cells. This review has collected recent data regarding the status of IL-10 expression during term and preterm deliveries and also its molecular mechanisms that lead to a normal pregnancy.
Keywords: IL-10, Preterm delivery, Term delivery
Introduction
It has been documented that pregnancy is a unique immunological state with both immune response and tolerance against fetus antigens (1). Previous studies demonstrated that the imbalanced immune responses against fetus antigens can result in abnormality in pregnancy. Cytokines as important immune molecules, play crucial roles in regulation of immune responses against microbes and foreign antigens including graft and fetus antigens (2). Interleukin-10 (IL-10), as a main anti-inflammatory cytokine, significantly participates in regulation of immune responses against self and foreign antigens in the case of tolerance to these antigens (3-5). Therefore, alteration in expression of this cytokine during pregnancy may lead to several pathologic conditions such as preterm labor (PTL) (6, 7). PTL is the common disorder in the pregnancy and is defined as birth before the 37th week of gestation (8). Previous studies revealed that PTL can be associated with inflammation: hence, regarding the important roles played by IL-10 in the suppression of inflammation, it appears that this cytokine can play key roles in the pathogenesis of PTL. Therefore, the main aim of this review article is to present the status and function of IL-10 in the pathogenesis of PTL.
Preterm labor
PTL is a birth that occurs before the 37th week of gestation and as described in the previous section the complication is a common disorder in the pregnancy occurring in approximately 10% of deliveries (9). In the normal human fetus, several organ systems mature between 34 and 37 weeks, and the fetus reaches adequate maturity by the end of this period (9, 10). The preterm birth is associated with several complications leading to mortality and morbidity such as neurological, respiratory, gastrointestinal and metabolic problems, hematologic disorders, and infection (10). Based on the fact that the etiology and the main responsible mechanisms that lead to preterm birth are unknown, several hypotheses are in process worldwide.
IL-10, structure and intracellular signaling
IL-10 is categorized as an anti-inflammatory cytokine and is produced by several cells including activated macrophages, T regulatory and Th2 lymphocytes and so on (11). The main function of this cytokine is to suppress Th1, Th2 and B lymphocytes, NK cells, macrophages, and dendritic cells inflammatory functions (12, 13). Sp1 and Sp3 (two transcription factors) regulate IL-10 expression. 1q31-1q32 is the location of the IL-10 gene (approximately 5.2 kB), which consists of five exons (14), and encodes a protein of 178 amino acids called pro-IL-10 (13). Pro-IL-10 activation is done by cleavage of a signal peptide of 18 amino acids (13). IL-10 performs its actions via its corresponded receptor (IL-10R) (15). It has been demonstrated that IL-10R consists of α and β chains and is categorized as a type II cytokine receptor (15). Several intracellular signaling pathways will be activated following IL-10/IL-10R α and β chain interaction by phosphorylation of JAK1 (Janus Kinase-1) and TYK2 (Tyrosine Kinase-2), respectively, as intracellular protein kinases (16). In a positive feedback, activated JAK1 and TYK2 phosphorylate Y446 and Y496 positions of IL-10R α chain (17). The phosphorylation is a place for binding and phosphorylation of signal transducer and activator of transcription 3 (STAT3) tyrosine residues (18). Homodimerized STAT3 is translocated into the nucleus and recognizes STAT-binding element (SBE) regions at the promoters of several genes including IL-10, anti-apoptotic, cell-cycle-progression, suppressor of cytokine signaling 3 (SOCS3) genes, and so on (19). Several other pathways such as phosphoinositide-3 kinase (PI3K) (20) and Mitogen-Activated Protein Kinase (p38/MAPK) (21) are also regulated by IL-10/IL-10R interaction.
IL-10 and delivery
Based on the aforementioned information, it appears that IL-10 may significantly participate in the outcome of pregnancy. Interestingly, previous investigations approved the hypothesis. For instance, it is documented that IL-10 suppresses the production and function of pro-inflammatory cytokines including IL-12, IFN-γ, IL-1, and so on (22), and numerous studies have reported its expression at the maternal-fetal interface (23-25). It was also demonstrated that, under effects of progesterone, IL-10 serum levels are elevated, which results in suppression and activation of Th1 and Th2-type cytokines, respectively (i.e., IL-10 and IL-4). Robertson et al also showed that IL-10 mRNA and protein is up-regulated in gestational tissues in normal pregnancy. Interestingly, they have concluded that up-regulation of IL-10 can be considered as a critical factor for resistance to preterm labor (26). It has been demonstrated that LPS could induce preterm labor in some cases (27). Experimental studies have shown that exogenous IL-10 inhibits LPS-induced preterm labor (28, 29). A study by Gotsch et al revealed that in 41–57 days (third phase) after gestation, the concentration of IL-10 was increased by high levels of 17β-estradiol (30). Up-regulation of IL-10 and 17β-estradiol leads to the suppression of dendritic cells (DCs) and consequently inhibits the stimulation of T lymphocytes by DCs (30), which is associated with a normal pregnancy. Additionally, another study demonstrated that decreased number of peripheral blood mononuclear leukocytes that produce IL-10, leads to recurrent preterm births during the second trimester (31). It has also been documented that preterm delivery during mid-trimester is associated with unchanged levels of IL-10 in amniotic fluid, where the concentration of IL-10 needs to be increased (32). Our previous study also revealed that serum levels of IL-10 were not differed in preterm in comparison to term neonates (33). The results have been confirmed by Gotsch et al who reported that IL-10 is expressed in high concentrations in the amniotic fluid of normal term women (30). The investigators also showed that spontaneous parturition in either term or preterm gestation is associated with elevated concentrations of IL-10 in amniotic fluid (30). Increased amniotic fluid concentrations of IL-10 during intra-amniotic infection/inflammation have also been reported by Gotsch and colleagues (30). Moreover, another research demonstrated that preterm neonates compared to term neonates, produce higher and lower inflammatory and anti-inflammatory cytokines in response to specific bacteria, respectively (34). Therefore, it may result in uncontrolled inflammatory response, which is associated with preterm labor (34). Researchers have evaluated the expression profiles of IL-10 and cyclo-oxygenase-2 (COX-2), as an enzyme for producing prostanoids (prostaglandins, prostacyclin, and thromboxane), which are the potential inducers of delivery (35). They have reported that IL-10 significantly regulates the expression of COX-2 and consequently prostaglandins, hence, the authors concluded that IL-10 plays important roles in countering inflammation that is produced in preterm labor (35). Although, the aforementioned studies have demonstrated that the IL-10 levels were lower in preterm vs. term delivery, some investigations reported that IL-10 levels were high in preterm delivery associated with infection. For example, it was shown that the cord blood levels of IL-10 were significantly increased in infected versus non-infected mothers (36). Another study demonstrated that the cord blood IL-10 levels are increased during intrauterine infection (37). Based on these results, it may be concluded that up-regulation of IL-10 in the infected preterm delivery is a normal response of the immune system to regulate the infection-dependent inflammation. Accordingly, Ferguson et al reported that IL-10 is significantly associated with preterm delivery. For example, it has been demonstrated that IL-10 levels are positively associated with elevated odds ratio of placental-mediated preterm birth (38). It may be related to the infections that have not been examined in the evaluated preterm neonates. According to the data
presented here, it appears that IL-10 plays significant roles in the induction of an appropriate pregnancy because its expression is up-regulated in the normal pregnancy, while its production is disrupted during preterm labor. Several documents approve this hypothesis. For example, it has been established that surfactant protein A (SP-A) is produced in the fetus to provide signals for the onset of parturition (39). Interestingly, the study revealed that SP-A suppresses preterm delivery via TLR2-dependent IL-10 production (39). Indoleamine 2,3-dioxygenase (IDO) is an enzyme for catabolism of tryptophan, which is crucial for T lymphocyte activation and proliferation (40). IDO regulate maternal immune responses against fetus alloantigens via suppression of maternal T lymphocytes and also up-regulation of anti-inflammatory cytokines such as IL-10 (40). Additionally, it has been evidenced that tolerogenic DCs plays key roles in the induction of maternal immune tolerance to fetus alloantigens (41). Interestingly, IL-10 is a potential factor that stimulates the production of tolerogenic DCs (42), hence, it can induce a successful pregnancy. Previous investigations identified that regulatory T lymphocytes also significantly participate in induction of maternal immune tolerance to fetus alloantigens and subsequently a successful pregnancy (43). IL-10 is not only produced by regulatory T lymphocytes, but also leads to the development of these cells (43).
IL-10 binding to its corresponded receptor (IL-10R) leads to activation of the IL-10/JAK1/STAT3 cascade and subsequently phosphorylation of STAT3. The phosphorylation leads to the production of STAT3 homodimer (STAT3/STAT3) and its translocation to the nucleus can trigger the expression of the target gene (see the previous section), which participates in the induction of a successful pregnancy (44–46). The molecular mechanisms played by IL-10 in pregnancy are summarized in Figure 1.
Figure 1.
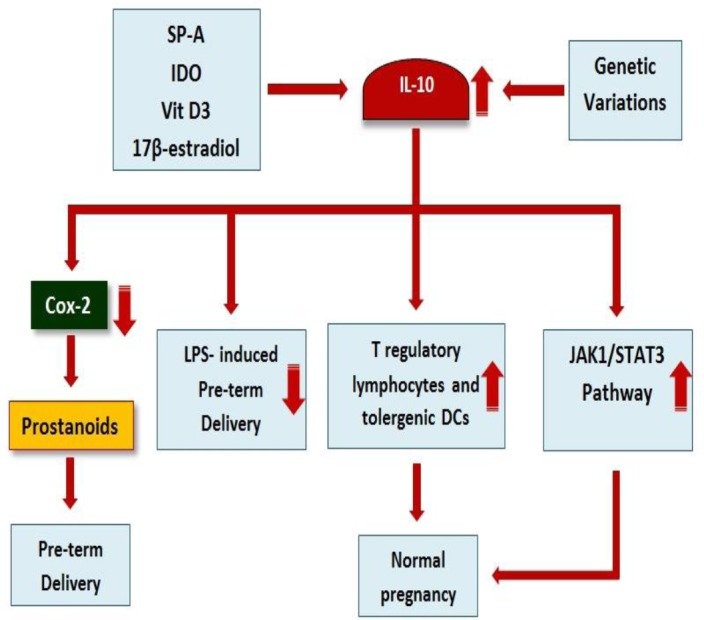
Roles of interleukin-10 (IL-10) in determination of pregnancy. As mentioned in the figure, SP-A, 17β-esteradiol, IDO vitamin D3, and IL-10 genetic variations can alter expression of IL-10. IL-10 inhibits preterm delivery using several mechanisms including development of T regulatory lymphocytes and tolerogenic DCs, activation of the JAK1/STAT3 pathway, down-regulation of COX-2, and also inhibition of LPS-induced preterm delivery
Interestingly, genetic research revealed that the genetic variations in the IL-10 gene are also associated with preterm delivery. It has also been evidenced that the polymorphisms within IL-10 gene are associated with its expression during pregnancy (47, 48). For instance, it was shown that IL-10 (rs1800896) polymorphism is associated with gram-negative infections in preterm labor (49). The relation between the polymorphisms within the promoter region of IL-10 and cervical insufficiency have been reported by Warren and colleagues (50). Another study also revealed that IL-10 (-1082A) polymorphism is significantly associated with genitourinary infections and/or inflammation (51). Another study has shown that IL10 (-1082)*G plays crucial roles in less than 29 weeks extreme preterm delivery (52). A study on Australian population revealed that IL-10 82A/-819T/-592A haplotype is associated with preterm delivery (53). Interestingly the study demonstrated that this haplotype is more prevalent in women with preterm premature rupture of membranes (53).
Contrastly, one study was unable to find a relation between IL-10 polymorphisms and preterm delivery (54). Another study was also unable to find an association between the polymorphisms within IL-10 gene (IL10 -1082 G>A, IL10 -819 C>T, and IL10 -592 C>A) and spontaneous preterm delivery (55). A study on Austrian population demonstrated that IL-10 -1082 G/A single nucleotide polymorphism was not associated with preterm delivery (56). No association between this polymorphism and preterm delivery was also demonstrated by Brazilian researchers (57). It appears that further studies are essential to complete our knowledge regarding the roles of genetic variations in the induction of preterm delivery.
Conclusion
Due to the aforementioned results, it appears that IL-10 plays key roles in the induction of a normal delivery via development of tolerogenic DCs, T regulatory lymphocytes, and up-regulation of STAT3 target genes. Up-regulation of this anti-inflammatory cytokine leads to suppression of NK cells and T lymphocytes against their fetus alloantigens. The factors that alter the expression of IL-10, including infections and genetic variations within IL-10 gene, could determine the outcome of pregnancy. Additionally, it has also been demonstrated that preterm delivery is associated with several short and long-term health problems. It may be hypothesized that alteration in expression of IL-10 is also associated with the incidence of the complications. Moreover, IL-10 can decline the pathologic effects of inflammation that is prevalent in the preterm delivery. Therefore, it appears that regulation of IL-10 expression during normal pregnancy is cautiously regulated.
Acknowledgment
This project was supported by a grant from the Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
References
- 1.Arababadi MK, Pourfathollah AA, Jafarzadeh A, Hassanshahi G. Decreased expression of CCR5 on the NK cells in occult HBV infected patients. LabMed. 2010;41:735–738. [Google Scholar]
- 2.Arababadi MK, Nasiri Ahmadabadi B, Kennedy D. Current information on the immunologic status of occult hepatitis B infection. Transfusion. 2012;52:1819–1826. doi: 10.1111/j.1537-2995.2012.03575.x. [DOI] [PubMed] [Google Scholar]
- 3.Seeram NP, Zhang Y, Henning SM, Lee R, Niu Y, Lin G, et al. Pistachio skin phenolics are destroyed by bleaching resulting in reduced antioxidative capacities. J Agric Food Chem. 2006;54:7036–7040. doi: 10.1021/jf0614948. [DOI] [PubMed] [Google Scholar]
- 4.Karimabad MN, Arababadi MK, Hakimizadeh E, Daredori HY, Nazari M, Hassanshahi G, et al. Is the IL-10 promoter polymorphism at position -592 associated with immune system-related diseases? Inflammation. 2013;36:35–41. doi: 10.1007/s10753-012-9517-7. [DOI] [PubMed] [Google Scholar]
- 5.Arababadi MK, Mosavi R, Khorramdelazad H, Yaghini N, Zarandi ER, Araste M, et al. Cytokine patterns after therapy with Avonex(R), Rebif(R), Betaferon(R) and CinnoVex in relapsing-remitting multiple sclerosis in Iranian patients. Biomark Med. 2010;4:755–759. doi: 10.2217/bmm.10.81. [DOI] [PubMed] [Google Scholar]
- 6.Arababadi MK, Pourfathollah AA, Jafarzadeh AA, Hassanshahi G. Serum levels of Interleukin (IL)-10 and IL-17A in occult HBV infected south-east Iranian patients. Hepat Mon. 2010;10:31–35. [PMC free article] [PubMed] [Google Scholar]
- 7.del Rio L, Barbera-Cremades M, Navarro JA, Buendia AJ, Cuello F, Ortega N, et al. IFN-gamma expression in placenta is associated to resistance to Chlamydia abortus after intragastric infection. Microb Pathog. 2013;56:1–7. doi: 10.1016/j.micpath.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Chang HH, Reich BJ, Miranda ML. Time-to-event analysis of fine particle air pollution and preterm birth:results from North Carolina 2001-2005. Americ J Epidem. 2012;175(2):91–8. doi: 10.1093/aje/kwr403. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell-Lewis D, Engebretson SP, Chen J, Lamster IB, Papapanou PN. Periodontal infections and pre-term birth:early findings from a cohort of young minority women in New York. Eur J Oral Sci. 2001;109:34–39. doi: 10.1034/j.1600-0722.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki E, Starfield B. Prevention of low birth weight and pre-term birth:literature review and suggestions for research policy. Milbank Mem Fund Q Health Soc. 1977;56:339–361. [PubMed] [Google Scholar]
- 11.Groux H, Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmun. 2003;20:281–285. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 12.Scapini P, Lamagna C, Hu Y, Lee K, Tang Q, Defranco AL, et al. B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc Natl Acad Sci U S A. 2011;108:E823–832. doi: 10.1073/pnas.1107913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Howell WM, Rose-Zerilli MJ. Interleukin-10 polymorphisms, cancer susceptibility and prognosis. Fam Cancer. 2006;5:143–149. doi: 10.1007/s10689-005-0072-3. [DOI] [PubMed] [Google Scholar]
- 15.Shah N, Kammermeier J, Elawad M, Glocker E-O. Interleukin-10 and interleukin-10-receptor defects in inflammatory bowel disease. Curr Allergy Asthma Rep. 2012;12:373–379. doi: 10.1007/s11882-012-0286-z. [DOI] [PubMed] [Google Scholar]
- 16.Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M, et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–3770. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 18.Gaba A, Grivennikov SI, Do MV, Stumpo DJ, Blackshear PJ, Karin M. Cutting edge:IL-10-mediated tristetraprolin induction is part of a feedback loop that controls macrophage STAT3 activation and cytokine production. J Immunol. 2012;189:2089–2093. doi: 10.4049/jimmunol.1201126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi H, Sanada T, Minoda Y, Yoshimura A. Regulation of cytokine and toll-like receptor signaling by SOCS family genes. Nihon Rinsho Japan J Clin Med. 2004;62:2189. [PubMed] [Google Scholar]
- 20.Strle K, Zhou J-H, Broussard SR, Venters HD, Johnson RW, Freund GG, et al. IL-10 promotes survival of microglia without activating Akt. J Neuroimmunol. 2002;122:9–19. doi: 10.1016/s0165-5728(01)00444-1. [DOI] [PubMed] [Google Scholar]
- 21.Bebien M, Hensler ME, Davanture S, Hsu L-C, Karin M, Park JM, et al. The pore-forming toxin βhemolysin/cytolysin triggers p38 MAPK-dependent IL-10 production in macrophages and inhibits innate immunity. PLoS Pathog. 2012;8:e1002812. doi: 10.1371/journal.ppat.1002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakimi H, Zare-Bidaki M, Zainodini N, Assar S, Arababadi MK. Significant roles played by IL-10 in chlamydia infections. Inflammation. 2014;37:818–823. doi: 10.1007/s10753-013-9801-1. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Mosmann MT, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface Synthesis, maternal-fetal oTh-tcat, interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 24.Bennett WA, Lagoo-Deenadayalan S, Whitworth NS, Stopple JA, Barber WH, Hale E, et al. First-trimester human, inflammatory cvebia, the carfi-ir, cytokine network of pregnancy. Am J Reprod Immunol. 1999;41:70–78. doi: 10.1111/j.1600-0897.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett W, Lagoo-Deenadayalan S, Whitworth N, Brackin M, Hale E, Cowan B. Expression and production of interleukin-10 by human trophoblast:relationship to pregnancy immunotolerance. Biol Med:Official J Societ Invest Early Pregn. 1997;3:190–198. [PubMed] [Google Scholar]
- 26.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–4896. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- 27.Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM. Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PloS One. 2013;8:e56161. doi: 10.1371/journal.pone.0056161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera DL, Olister SM, Liu X, Thompson JH, Zhang XJ, Pennline K, et al. Interleukin-10 attenuates experimental fetal growth restriction and demise. FASEB J. 1998;12:189–197. doi: 10.1096/fasebj.12.2.189. [DOI] [PubMed] [Google Scholar]
- 29.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN, Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxininduced preterm birth in a rat model. Obstet Gynecol. 2001;98:476–480. doi: 10.1016/s0029-7844(01)01424-7. [DOI] [PubMed] [Google Scholar]
- 30.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term:a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elovitz MA, Mrinalini C. The use of progestational agents for preterm birth:lessons from a mouse model. Am J Obstet Gynecol. 2006;195:1004–1010. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, et al. Mid-trimester amniotic fluid interleukins (IL-1β, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo. 2011;25:141–148. [PubMed] [Google Scholar]
- 33.Arababadi MK, Aminzadeh F, Hassanshahi G, Khoramdelazad H, Karimabad MN, zarandi ER, et al. Cytokines in Preterm Delivery. Lab Med. 2012;43:131–134. [Google Scholar]
- 34.Tatad AF, Nesin M, Peoples J, Cheung S, Lin H, Sison C, et al. Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology. 2007;94:8–15. doi: 10.1159/000112541. [DOI] [PubMed] [Google Scholar]
- 35.Neurath AR, Strick N, Li YY. Cells transfected with human interleukin 6 cDNA acquire binding sites for the hepatitis B virus envelope protein. J Exp Med. 1992;176:1561–1569. doi: 10.1084/jem.176.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achary K, Mandal N, Mishra S, Sarangi S, Kar S, Satapathy A, et al. Maternal filarial infection:association of anti-sheath antibody responses with plasma levels of IFN-γ and IL-10. Parasitology. 2013;140:598–603. doi: 10.1017/S0031182012002144. [DOI] [PubMed] [Google Scholar]
- 37.Weissenbacher T, Laubender RP, Witkin SS, Gingelmaier A, Schiessl B, Kainer F, et al. Diagnostic biomarkers of pro-inflammatory immune-mediated preterm birth. Arch Gynecol Obstet. 2013;287:673–685. doi: 10.1007/s00404-012-2629-3. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am J Reprod Immunol. 2014;72:326–336. doi: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal V, Smart K, Jilling T, Hirsch E. Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2. PloS One. 2013;8:e63990. doi: 10.1371/journal.pone.0063990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.do Prado KM, Correa-Silva S, Oliveira LG, Camara NOS, Ono É, Sandri S, et al. Indoleamine 2, 3-dioxygenase (IDO) activity in placental compartments of renal-transplanted pregnant women. Am J Reprod Immunol. 2014;72:45–56. doi: 10.1111/aji.12233. [DOI] [PubMed] [Google Scholar]
- 41.Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol. 2014;8:196. doi: 10.3389/fimmu.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang A, Fu J, Ning B, Li D, Sun N, Wei W, et al. Tolerogenic dendritic cells generated with IL-10/TGF-1 relieve immune thrombocytopenia in mice. Thromb Res. 2013;132(1):63–68. doi: 10.1016/j.thromres.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy:A new challenging role for regulatory T cells. Placenta. 2014;35:241–248. doi: 10.1016/j.placenta.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Teng CB, Diao HL, Ma H, Cong J, Yu H, Ma XH, et al. Signal transducer and activator of transcription 3 (Stat3) expression and activation in rat uterus during early pregnancy. Reproduction. 2004;128:197–205. doi: 10.1530/rep.1.00053. [DOI] [PubMed] [Google Scholar]
- 45.Ladyman S, Grattan D. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145:3704–3711. doi: 10.1210/en.2004-0338. [DOI] [PubMed] [Google Scholar]
- 46.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response:recent developments and future challenges. Brief Funct Genomics. 2013;12:489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menon R, Fortunato SJ, Velez Edwards DR, Williams SM. Association of genetic variants, ethnicity and preterm birth with amniotic fluid cytokine concentrations. Ann Hum Genet. 2010;74:165–183. doi: 10.1111/j.1469-1809.2010.00562.x. [DOI] [PubMed] [Google Scholar]
- 48.Simhan HN, Ryckman KK, Williams SM, Krohn MA. Genetic regulation of cervical antiinflammatory cytokine concentrations during pregnancy. Am J Obstet Gynecol. 2008;199(163):e1–e11. doi: 10.1016/j.ajog.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 49.Abu-Maziad A, Schaa K, Bell EF, Dagle JM, Cooper M, Marazita ML, et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr Res. 2010;68:323–329. doi: 10.1203/PDR.0b013e3181e6a068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren JE, Nelson LM, Stoddard GJ, Esplin MS, Varner MW, Silver RM. Polymorphisms in the promoter region of the interleukin-10 (IL-10) gene in women with cervical insufficiency. Am J Obstet Gynecol. 2009;201(372):e1–e5. doi: 10.1016/j.ajog.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Speer EM, Gentile DA, Zeevi A, Pillage G, Huo D, Skoner DP. Role of single nucleotide polymorphisms of cytokine genes in spontaneous preterm delivery. Hum Immunol. 2006;67:915–923. doi: 10.1016/j.humimm.2006.08.291. [DOI] [PubMed] [Google Scholar]
- 52.Kerk J, Dördelmann M, Bartels DB, Brinkhaus M-J, Dammann CE, Dörk T, et al. Multiplex measurement of cytokine/receptor gene polymorphisms and interaction between interleukin-10 (-1082) genotype and chorioamnionitis in extreme preterm delivery. J Soc Gynecol Invest. 2006;13:350–356. doi: 10.1016/j.jsgi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, et al. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women:Risk of preterm birth. Am J Obstet Gynecol. 2004;191:2056–2067. doi: 10.1016/j.ajog.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Sugita N, Kobayashi T, Kikuchi A, Shimada Y, Hirano E, Sasahara J, et al. Immunoregulatory gene polymorphisms in Japanese women with preterm births and periodontitis. J Reprod Immunol. 2012;93:94–101. doi: 10.1016/j.jri.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Moura E, Mattar R, de Souza E, Torloni MR, Gonçalves-Primo A, Daher S. Inflammatory cytokine gene polymorphisms and spontaneous preterm birth. J Reprod Immunol. 2009;80:115–121. doi: 10.1016/j.jri.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Stonek F, Metzenbauer M, Hafner E, Philipp K, Tempfer C. Interleukin-10− 1082 G/A promoter polymorphism and pregnancy complications:results of a prospective cohort study in 1,616 pregnant women. Acta Obstet Gynecol Scand. 2008;87:430–433. doi: 10.1080/00016340801995657. [DOI] [PubMed] [Google Scholar]
- 57.Mattar R, de Souza E, Daher S. Preterm delivery and cytokine gene polymorphisms. J Reprod Med. 2006;51:317–320. [PubMed] [Google Scholar]


