Abstract
Objective(s):
Berberine is one of the main alkaloids and it has been proven to have different pharmacological effects including inhibition of cell cycle and progression of apoptosis in various cancerous cells; however, its effects on cancer metastasis are not well known. Cancer cells obtain the ability to change their chemokine system and convert into metastatic cells. In this study, we examined the effect of berberine on breast cancer cell migration and its probable interaction with the chemokine system in cancer cells.
Materials and Methods:
The MCF-7 breast cancer cell line was cultured, and then, treated with berberine (10, 20, 40 and 80 μg/ml) for 24 hr. MTT assay was used in order to determine the cytotoxic effect of berberine on MCF-7 breast cancer cells. Wound healing assay was applied to determine the inhibitory effect of berberine on cell migration. Moreover, real-time quantitative PCR analysis of selected chemokine receptors was performed to determine the probable molecular mechanism underlying the effect of berberine on breast cancer cell migration.
Results:
The results of wound healing assay revealed that berberine decreases cell migration. Moreover, we found that the mRNA levels of some chemokine receptors were reduced after berberine treatment, and this may be the underlying mechanism for decreased cell migration.
Conclusion:
Our results indicate that berberine might be a potential preventive biofactor for human breast cancer metastasis by targeting chemokine receptor genes.
Keywords: Anticancer agents, Breast cancer, Berberine, Chemokine receptors, Metastasis
Introduction
Breast cancer is the most common cancer among women worldwide and the second leading cause of cancer-related mortality in developed countries. Most breast cancer-related deaths are not the result of primary tumor growth but are rather caused by the metastasis of cancer to other organs (1). Metastasis is the last stage in the progression of cancer during which cancer cells detach from the primary tumor, migrate into blood vessels, disseminate throughout the body, and ultimately seeding in distant organs and give rise to new tumors. Several studies have shown that chemokines and chemokine receptors play a key role in cancer metastasis. Chemokines are expressed in specific organs and their interactions with the correspondent receptors which are expressed on tumor cells induce
directed cell migration (2). The extent of this response triggered by chemokines depends on the amount of chemokine receptors expressed on the plasma membrane (3). Chemokine receptors may potentially facilitate tumor dissemination via activation of key survival pathways such as MEK1/2 and PI3/AKT (4). Chemokine receptors belong to a subfamily of G protein-coupled receptors and they are named based on the chemokine ligands to which they bind. So, they are classified into four groups of (I) CXC receptors (CXCR1, 2, 3, 4 and 5), (II) CC receptors (CCR1, 2, 3, 4, 5, 6, 7, 8, and 9); (III) CX3C and (IV) the receptor of XC. It has been suggested that interactions of some specific chemokine receptors with their ligands play important roles in many of the critical steps of the metastatic process (5). Among different classes of chemokine receptors,
CXCR4 was found to be the most common chemokine receptor overexpressed in different human malignancies including breast cancer. It was revealed that CXCR4 and its ligand (CXCL12) have a key role in initiating and regulating tumor cell migration and metastasis (6). Moreover, it was revealed that the chemokine receptors CCR1, CCR6, CCR9, and CXCR1 are often overexpressed in cancer cells and are involved in cancer metastasis (7-10). Regarding the important role of chemokine receptors in cancer metastasis, scientists focus on developing new drugs to target them. Berberine is an isoquinoline derivative alkaloid with antioxidant and anti-cancer activities. Although pharmacological effects of berberine have been previously reported, recently, special attention was paid to its bioactivities including antioxidant, anti-inflammatory anti-diabetic, neuroprotective effects and its ability as a natural drug for breast cancer treatment (11). Moreover, It was shown that berberine is effective in inhibition of cell proliferation and promotion of apoptosis in different cancerous cells (12). Unlike anti-cancer growth effects of berberine, very little is known about the effect of berberine on cancer cell migration and metastasis. Therefore, in this study, we examined the effect of berberine on migration and invasion of breast cancer cells. Moreover, to clarify the underlying mechanism of berberine effect on migration of breast cancer cells, the expression levels of some selected chemokine genes were analyzed by qPCR. Specific chemokine receptors namely, CCR1, CCR6, CCR9, CXCR1, and CXCR4 were selected since they were previously reported to be associated with cancer metastasis (8). The present study aimed to demonstrate berberine effects against the metastasis of breast cancer cells.
Materials and Methods
Cell culture
MCF-7 human breast cancer cell line was cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and antibiotics (1% penicillin- streptomycin 10000 units/ml) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. All cell culture reagents were purchased from Sigma.
Cell viability assay
MTT assay was performed to evaluate the cytotoxic effect of berberine on MCF-7 breast cancer cells (7). Briefly, the cells were seeded at a density of 1.2×104 cells/ml and incubated with berberine (Sigma) at 0, 10, 20, 40, 80 and 160 µM for 24 hr. Thereafter, the medium was changed and cells were incubated with MTT (Sigma) solution (0.5 mg/ml) for 4 hr. The number of viable cells is directly proportional to the production of a purple dye, after solubilization with isopropanol which can be measured spectrophotometrically (λ= 545 nm). The percentage of the viable cells was calculated using the following equation: (mean OD of treated cells/mean OD of control cells)×100. Control groups were treated with media containing 0.1% DMSO in all experiments.
Wound-healing Assay
For the wound-healing migration assay, cells were seeded in 6-well plates (8). When cells were confluent, scratch was made with a 200 µl sterile pipette tip, generating a cell-free area of approximately 1 mm in width. Cellular debris removal was done by gentle washing with culture medium and the photos of the wounds (0 hr) were taken. Thereafter, medium was replaced by culture medium with different concentrations of berberine (0, 10, 20, 40, and 80 µM) and the cells were allowed to migrate for 24 hr. At the end of migration experiment, another set of photos was taken, from the same regions. The gap size was analyzed using Image-J 1.45 software. In order to assess migration ability of cultured cells, cell-free areas of
the scratches at 24 hr post-wounding were subtracted from the area of the scratches (0 hr)and calculated as a percentage of untreated (0 hr) cultures. The experiment was carried out in triplicates.
RNA extraction and real-time PCR
Total RNAs were isolated from cells using TriPure isolation reagent (Roche, Germany) based on the manufacturer’s instructions. Extracted RNA was treated with DNase I enzyme. One microgram of RNA from each sample was used for cDNA synthesis by reverse transcriptase. RNA samples were incubated at 65°C for 5 min with 1 μl OligodT (0.5 μg/μl) at a final volume of 12 μl, and then, chilled on ice and mixed with 4 μl 5X buffer, 2 μl dNTPs (10 mM), 0.5 μl Ribolock, and 1 μl M-MLV-RTase. After incubation at 42°C for 60 min, and at 70°C for 10 min, the mixture was transcribed into cDNA. Synthesized cDNAs were diluted at 1:4 ratio and 2 µl cDNA of each sample was used for real-time PCR in a 20 µl reaction mixture with 10 µl of 2X SYBR Green PCR Mastermix (Parstous, Iran) and 1 μl of specific primer pair. The housekeeping gene, β-actin was used to normalize target gene expression. The primer sequences are presented in Table 1.
Table 1.
Description of the designed primers
| Gene | Primer sequence | Product size | |
|---|---|---|---|
| β-actin | Sense | 5’-GCTCAGGAGGAGCAAT-3’ | 187 |
| Antisense | 5’-GGCATCCACGAAACTAC-3’ | ||
| CCR1 | Sense | 5’-CACGGACAAAGTCCCTTGG-3’ | 134 |
| Antisense | 5’-CAAAGGCCCTCTCGTTCAC-3’ | ||
| CCR6 | Sense | 5’-GCTAGTGTTTCCTCTCATTTCC-3’ | 132 |
| Antisense | 5’-ACCTGTTCTGCCATTGTCC-3’ | ||
| CCR9 | Sense | 5’-TGATTGGCTCTTGACTGTGATG-3’ | 108 |
| Antisense | 5’-GAGGCAGCGGCATTGATG-3’ | ||
| CXCR1 | Sense | 5’-CATCAGTGTGGACCGTTACC-3’ | 120 |
| Antisense | 5’-GGCAGGGACAGATTCATAGAC-3’ | ||
| CXCR4 | Sense | 5’-ATCCCTGCCCTCCTGCTGACTATTC-3’ | 232 |
| Antisense | 5’-GAGGGCCTTGCGCTTCTGGTG-3’ |
Each plate was run at 95°C for 10 min, then 40 cycles of 95°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec. All real-time RT-PCRs were performed in duplicate, and relative mRNA of each target gene was determined by using the formula 2-ΔCT (CT, cycle threshold) where ΔCT=CT (target gene) − CT (β-actin). The comparative expression level of each target gene between different samples was calculated by 2-ΔΔCT. In addition, melting curves were used to determine non-specific amplification.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism, version 5.0. The significance of difference among the experimental groups and controls was assessed by one-way ANOVA and Post hoc Tukey test. The results are presented as mean±standard deviation (SD). P<0.05 represented significant differences.
Results
Berberine decreased the proliferation of human breast cancer cells
In this study, using MTT assay, we determined the cytotoxicity of berberine by treating MCF-7 cells with various concentrations berberine for 24 hr. The results from MTT assay revealed that growth retardation was correlated with increased concentrations of berberine in a concentration-dependent manner (Figure 1). Cell viability was 99%, 89%, 81%, 79%, and 64% for 10, 20, 40, 80, and 160 μM berberine, respectively.
Figure 1.
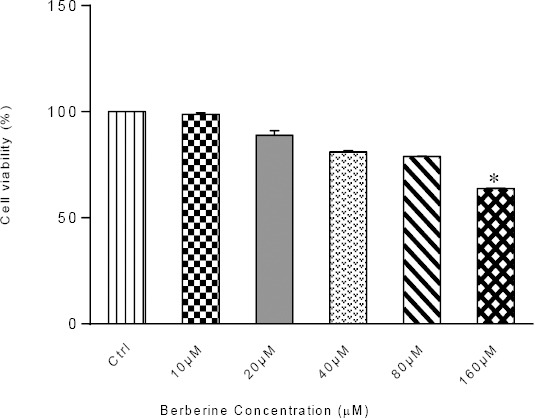
Viable MCF-7 cells after 24 hr-exposure to various concentrations of berberine. Results are represented as mean± SD; n=3, (*P< 0.05 compared to control)
Berberine reduced wound-healing migration of breast cancer cells
Here, we checked whether berberine inhibits cell migration in breast cancer cells. For this purpose, MCF-7 breast cancer cells were subjected to the cell migration assay after 24 hr-treatment with various concentrations of berberine (0, 10, 20, 40 and 80 μM), which were relatively non-toxic for the cells (Figure 2A). Treatment of the cells with berberine resulted in a concentration-dependent reduction in the cell migration capacity of MCF-7 cells as compared to control (Figure 2B).
Figure 2.
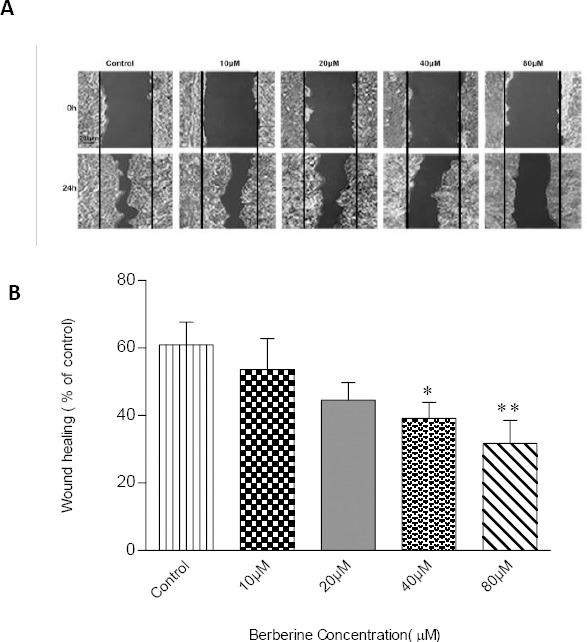
Effect of different concentrations of berberine on cell migration as examined by wound-healing assay. (A) MCF-7 cells grew to 90% confluent and were scratched by a sterile 200 μl pipette tip. The ability of cells to migrate into the scratch area was monitored by photographing the same spot with an inverted microscope equipped with a digital camera, 0 and 24 hr after scratch. Wound-healing images show the extent of healing and black lines indicate the wound edge. Scale bar: 200 μm. (B) Statistical analysis of the migration image results. The graph represents the mean ± SD for at least three independent experiments. * and ** donate P <0.05 and P <0.01, respectively, as compared to the control
Berberine decreased the expression of chemokine receptors in breast cancer cells
After observing the decrease in cell migration, we were interested to investigate whether alternations of chemokine receptors expression at mRNA level occurred in MCF-7 cells following berberine treatment. Therefore, qPCR was performed to determine the expression of selected chemokine receptor genes. As shown in Figure 3, 24 hr-incubation with berberine caused no significant effect on the expression of CCR1(A); however, CCR6 was significantly down-regulated following treatment with berberine 40 μM (B). A similar effect was also observed for CCR9 gene expression following treatment with all concentrations of berberine except 10 μM (Figure 3C). Moreover, CXCR1 and CXCR4 were significantly down-regulated following treatment with all concentrations of berberine (Figures 3D and E). These data suggested that the inhibition of chemokine receptors expression in the presence of berberine may resulted in inhibition of MCF-7 breast cancer cell migration.
Figure 3.
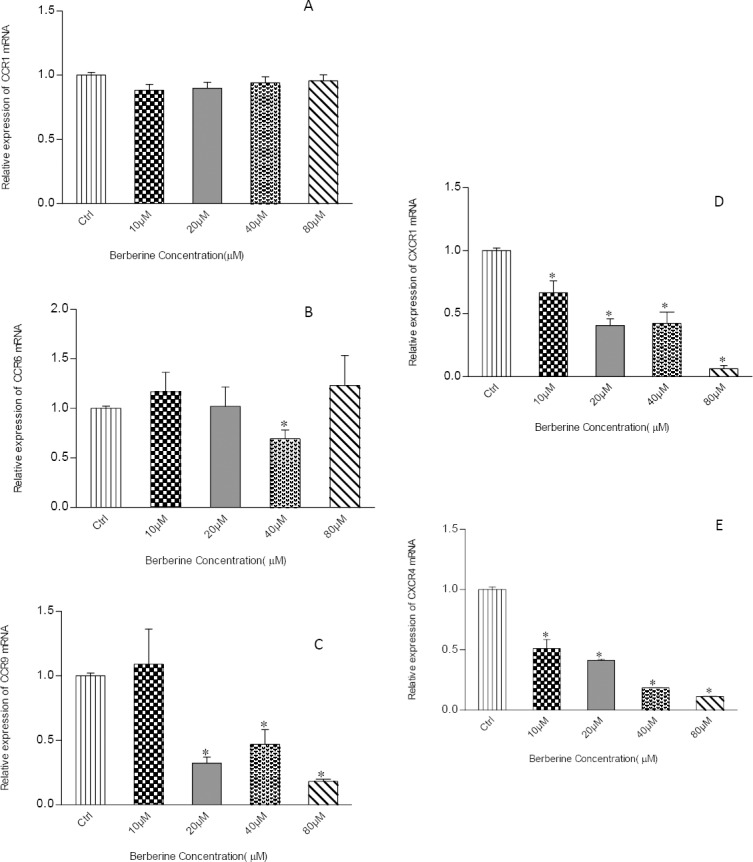
Effects of berberine on the gene expression of chemokine receptors. MCF7 cells were treated with berberine at concentrations of 0, 10, 20, 40 and 80 μM for 24 hr, then, subjected to real-time PCR analysis for determination of alterations in gene expression of selected chemokine receptors including CCR1 (A), CCR6 (B), CCR9 (C), CXCR1 (D), and CXCR4 (E). Data were presented as mean±SD from three independent experiments. Results were statistically evaluated by using one-way ANOVA with Post hoc test. *P<0.05 was considered significant as compared to control
Discussion
The anti-cancer effects of berberine against many different types of human cancers have been well documented (13). In this study, our results revealed that berberine decreased the percentage of viable MCF-7 breast cancer cells in a concentration-dependent manner which is in agreement with previous studies (14). However, there was no available information regarding the effect of berberine on migration and invasion of MCF-7 breast cancer cells and associated signaling pathways. In this study, the results of wound-healing assay indicated that berberine decreased the migration and invasion of viable MCF-7 breast cancer cells and these effects were concentration-dependent. Cancer cells motility and metastasis are multifactorial processes involving different mechanisms (15). Chemokine receptors play an important role in the induction of directed cell migration (3). Therefore, to discover underlying mechanisms involved in decreased cell migration in response to berberine, we also examined the level of chemokine receptors gene expression after berberine treatment in breast cancer cells.
CC chemokine receptor 1 (CCR1) plays a critical role in the recruitment of leukocytes to the site of inflammation (16). Knockdown of CCR1 resulted in a significantly reduced invasive ability of hepatocellular carcinoma cells and human non-small cell lung cancer cell (7). The potential role of CCR1 in breast cancer cells metastasis has not been studied yet. The result of our study indicated that berberine did not change the expression level of CCR1 in breast cancer cells.
CCR6, another chemokine receptor is closely related to disease stages when up-regulated and its down-regulation has been proposed as one of the strategies to stop metastasis (8). According to the results of our study, CCR6 showed a statistically significant down-regulation when breast cancer cells were treated with berberine 40 μM.
CCR9, another chemokine receptor associated with invasiveness and metastasis of tumors (9) impaired the migration and invasive potential of prostate cancer cells when its expression was blocked, indicating that these responses were dependent on chemokine and chemokine–receptor interactions (17). To our knowledge, for the first time, we demonstrated decreased expression of CCR9 in breast cancer cells treated with berberine. Decreased expression of CCR9 may be the answer to why migration potential of breast cancer cells decreased after berberine treatment.
Among the genes over-expressed in the breast cancer stem cell (CSCs) population, CXCR1, a receptor that binds to the pro-inflammatory chemokines of IL-8/CXCL8 and CXCL6, appeared to have an important role in growth and metastasis of breast CSCs (18). It was shown that CXCR1 blockade resulted in apoptotic induction, retarded tumor growth and reduced systemic metastasis of breast cancer in NOD/SCID mice (10). Human colon cancer liver metastases can be inhibited by small molecule antagonists of CXCR1 (19). Using wound-healing assay, we demonstrated that treatment with berberine resulted in decreased cell migration in breast cancer cells. We also demonstrated a decrease in the expression of CXCR1 in response to berberine. Consistent with these findings, we suggested that berberine may affect migration of breast cancer cells through down-regulation of CXCR1.
CXCR4 is the most common chemokine receptor expressed in tumor cells, and plays a vital role in the migration, invasion, and prognosis of breast cancer (20).
Furthermore, it was demonstrated that targeted down-regulation of CXCR4 expression in breast cancer cell line via different strategies like RNA interference (RNAi) or CXCR4 antagonists, significantly decreased metastasis of breast cancer cells (21). It has also been reported that CXCL12-CXCR4 axis stimulates the natural selection of breast cancer cell metastasis (22). CXCR4 receptors ligands stimulate small GTPases and induce reorganization of the actin cytoskeleton, which indicates that these receptors might be involved in the migration and, perhaps, metastasis of tumor cells (23). In the present study, decreased migration of breast cancer cell line following berberine treatment was observed with decreased expression of CXCR4. These observations may offer an understanding of the molecular mechanisms that lead to decreased breast cancer migration following berberine treatment.
The activation of NF-κB occurs as a consequence of activation of chemokine receptors. It has also been shown that tumor angiogenesis, growth and metastasis are facilitated by the transcription factor of NF-κB which modulates chemokine genes (23). Treatment of melanoma cells with caffeic acid phenethyl ester, an inhibitor of NF-κB, resulted in an inhibitory effect on cell migration (24). Furthermore, previous experiments on MCF-7 breast cancer cells showed that berberine triggers apoptotic processes through formation of P53 and ROS, over-expression of p21 and inhibition of NF-κB and Bcl-2 expression (25). These observations support the hypothesis that down-regulation of NF-κB target genes as a consequence of decreased chemokine receptors expression, may contribute to berberine-induced inhibition of cell migration.
Moreover, it is interesting to know that berberine can inhibit Ras/MAPK and PI3K pathways, which play a major role in promoting gene expression and might be excessively activated in cancer cells (13). PI3K signaling can also be activated by the G-protein-coupled chemokine receptor CXCR4, which seems to be the principal chemokine receptor expressed on cancer cells (26). Therefore, chemokine-induced activation of PI3K may allow for enhanced survival and metastasis of cancer cells. Moreover, it was revealed that dysregulation of the CXCR4-CXCL12 axis in melanoma, activates cell cycle progression and migration via stimulation of MAPK pathway (27). A better understanding of the gene networks and signaling pathways regulated by berberine will definitely enable us to have a better understanding of breast cancer pathogenesis and therapy.
Most cancer-related deaths are not the result of primary tumor growth but are rather caused by the spread of cancer to other organs; hence, therapies designed to minimize metastasis are greatly needed. Targeting chemokine receptors is one of procedures used to reach this goal. For this purpose, neutralizing antibodies, small molecule antagonists, peptide-derived inhibitors and siRNA nanoparticles have been used (28). Despite extensive pre-clinical literature, there has been limited knowledge on cancer clinical trials because safety and efficacy of each of these strategies in the treatment of patients is currently unknown.
Identification of a natural molecule that can specifically target cancer cells with minimal or no toxicity to normal cells would be of great benefit and berberine can be one of promising natural compounds to inhibit the proliferation of cancer cells and to alter key markers involved in development and progress of cancer, directly or indirectly.
Conclusion
The significant finding of the current study is that treatment of MCF-7 breast cancer cells with berberine decreased cell migration and invasion. Moreover, down-regulation of key chemokine receptors in MCF-7 breast cancer cells may probably represent one of the mechanisms of anti-metastatic effect of berberine. These data may be helpful for future use of berberine in clinical trials for cancer chemotherapy.
Acknowledgment
Thanks to Mojgan Fazli and Piraste Norouzifor for providing technical support. This work was supported in part by a grant (grant no. 9247) from Shahroud University and also a grant, (grant no. 710) from ACECR, Mashhad Branch.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Naderi-Meshkin H, Bahrami AR, Bidkhori HR, Mirahmadi M, Ahmadiankia N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell biol Int. 2015;39:23–34. doi: 10.1002/cbin.10378. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Baruch A. Organ selectivity in metastasis:regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 4.Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13:1561–1568. doi: 10.3748/wjg.v13.i10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papatheodorou H, Papanastasiou AD, Sirinian C, Scopa C, Kalofonos HP, Leotsinidis M, et al. Expression patterns of SDF1/CXCR4 in human invasive breast carcinoma and adjacent normal stroma:Correlation with tumor clinicopathological parameters and patient survival. Pathol Res Pract. 2014;210:662–667. doi: 10.1016/j.prp.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Wang CL, Sun BS, Tang Y, Zhuang HQ, Cao WZ. CCR1 knockdown suppresses human non-small cell lung cancer cell invasion. J Cancer Res Clin Oncol. 2009;135:695–701. doi: 10.1007/s00432-008-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Ke F, Xu Z, Liu Z, Zhang L, Yan S, et al. CCR6 Is a Prognostic Marker for Overall Survival in Patients with Colorectal Cancer, and Its Overexpression Enhances Metastasis In vivo. PLoS One. 2014;9:e101137. doi: 10.1371/journal.pone.0101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson EL, Singh R, Singh S, Johnson-Holiday CM, Grizzle WE, Partridge EE, et al. CCL25-CCR9 interaction modulates ovarian cancer cell migration, metalloproteinase expression, and invasion. World J Surg Oncol. 2010;8:62. doi: 10.1186/1477-7819-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moghaddam HK, Baluchnejadmojarad T, Roghani M, Khaksari M, Norouzi P, Ahooie M, et al. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol Neurobiol. 2014;49:820–826. doi: 10.1007/s12035-013-8559-7. [DOI] [PubMed] [Google Scholar]
- 12.Refaat A, Abdelhamed S, Yagita H, Inoue H, Yokoyama S, Hayakawa Y, et al. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol Lett. 2013;6:840–844. doi: 10.3892/ol.2013.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbarzadeh Kaboli P, Rahmat A, Ismail P, Ling KH. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur J Pharmacol. 2014;740:584, 595. doi: 10.1016/j.ejphar.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Patil JB, Kim J, Jayaprakasha GK. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur J Pharmacol. 2010;645:70–78. doi: 10.1016/j.ejphar.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Shibata T, Matsuo Y, Shamoto T, Hirokawa T, Tsuboi K, Takahashi H, et al. Girdin, a regulator of cell motility, is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep. 2013;29:2127–2132. doi: 10.3892/or.2013.2406. [DOI] [PubMed] [Google Scholar]
- 16.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Singh UP, Stiles JK, Grizzle WE, Lillard JW., Jr Expression and functional role of CCR9 in prostate cancer cell migration and invasion. Clin Cancer Res. 2004;10:8743–8750. doi: 10.1158/1078-0432.CCR-04-0266. [DOI] [PubMed] [Google Scholar]
- 18.Jiang QF, Wu TT, Yang JY, Dong CR, Wang N, Liu XH, et al. 17beta-estradiol promotes the invasion and migration of nuclear estrogen receptor-negative breast cancer cells through cross-talk between GPER1 and CXCR1. J Steroid Biochem Mol Biol. 2013;138:314–324. doi: 10.1016/j.jsbmb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011;300:180–188. doi: 10.1016/j.canlet.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Ni C, Chen W, Wu P, Wang Z, Yin J, et al. Expression of CXCR4 and breast cancer prognosis:a systematic review and meta-analysis. BMC Cancer. 2014;14:49. doi: 10.1186/1471-2407-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling X, Spaeth E, Chen Y, Shi Y, Zhang W, Schober W, et al. The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo. PLoS One. 2013;8:e58426. doi: 10.1371/journal.pone.0058426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S, et al. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol 2014. 35:7765–7773. doi: 10.1007/s13277-014-1816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh T, Vaid M, Katiyar N, Sharma S, Katiyar SK. Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase-2, prostaglandin E(2) and prostaglandin E(2) receptors. Carcinogenesis. 2011;32:86–92. doi: 10.1093/carcin/bgq215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Tillhon M, Guaman Ortiz LM, Lombardi P, Scovassi AI. Berberine:new perspectives for old remedies. Biochem Pharmacol. 2012;84:1260–1267. doi: 10.1016/j.bcp.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell B, Leone D, Feller K, Menon S, Bondzie P, Yang S, et al. Protein expression of the chemokine receptor CXCR4 and its ligand CXCL12 in primary cutaneous melanoma-biomarkers of potential utility? Hum Patho. 2014;45:2094–3000. doi: 10.1016/j.humpath.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Natur. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]


