Abstract
Objective(s):
Immunosuppressive property of mesenchymal stem cells (MSCs) has great attraction in regenerative medicine especially when dealing with tissue damage involving immune reactions. The most attractive tissue sources of MSCs used in clinical applications are bone marrow (BM), adipose tissue (AT), and Wharton’s jelly (WJ) of human umbilical cord. The current study has compared immunomodulatory properties of human BM, AT, and WJ-MSCs.
Materials and Methods:
Three different types of human MSCs were isolated, cultured, and characterized by flow cytometry and differentiation potentials. The MSCs were co-cultured with allogeneic phytohemagglutinin (PHA) activated peripheral blood mononuclear cells (PBMCs). The proliferation of PBMCs was assessed by flow cytometry of carboxyfluorescein succinimidyl ester (CFSE) stained cells and compared to each other and to the growth of PBMCs in the absence of MSCs. Additionally, the growth suppression was indirectly assessed by using the transwell culture system.
Results:
The proliferation of PBMCs reduced to 6.2, 7 and 15.4- fold in cultures with AT-MSCs, WJ-MSCs, and BM-MSCs, respectively, compared to the PHA-activated cells. When the growth suppression was indirectly assessed by using the transwell culture system, it was revealed that AT-MSCs, WJ-MSCs, and BM-MSCs caused growth reduction in PBMCs to 3, 8, and 8 -fold, respectively, compared to the PHA-activated cells.
Conclusion:
These data collectively conclude that the immunomodulatory effects of MSCs, which may mostly carry out through direct cell to cell contact, are different between various sources. Accordingly results of this study may contribute to the application of these cells in cell therapy and regenerative medicine.
Keywords: Adipose tissue, Bone marrow, Immunosuppression, Mesenchymal stem cell (MSC), Regenerative medicine, Wharton’s jelly
Introduction
Mesenchymal stem cells (MSCs) are one of the most interesting types of adult stem cells that are isolated, cultured, manipulated ex vivo, and have the ability of self-renewal and differentiation into various mesodermal cell lineages (1-3).
Since the first description of MSCs by Friedenstein et al in 1976 as the clonal and plastic adherent cells, the interest in MSCs rapidly grew with expanding knowledge about their exceptional characteristics and usefulness in the cell therapy applications (4, 5). Several clinical case reports (6) have concluded the successful treatment of bone and cartilage defects, vascular ischemia and coronary artery disease, and of chronic skin wounds upon local administration of MSCs to sites of injury. The injected cells were well tolerated and some spectacular healing results were obtained (7). These results indicated that the immunosuppressive effects should be considered whenever MSC transplantation takes place.
Human MSCs may participate in cell therapy protocols by producing a broad range of mediators such as transforming growth factor-β, hepatocyte growth factor (HGF), and nitric oxide (8, 9). Intravenous administration of murine MSCs improves the outcome of neural (10) and lung (11) injury in experimental animal models primarily through paracrine effects and a shift from the production of pro-inflammatory to anti-inflammatory cytokines at the site of injury.
In addition, several studies have shown the immunosuppressive effect of MSCs through mechanisms identified for inhibition of proliferation and differentiation of immune cells (12).
The primary source of MSCs in adult individuals is bone marrow (BM), where they are immersed in the stroma at a low frequency. In humans, there is one MSC per 34,000 nucleated cells (8). Although present in very low numbers, MSCs are easily isolated from BM and are capable of substantial proliferation and expansion in culture. However, due to the possibility of donor morbidity, high degree of viral exposure, and invasiveness of procedures, the need to identify alternative sources to provide MSCs with immunomodulatory properties has emerged (2).
MSCs in adult individuals are also isolated and efficiently expanded from other body tissues such as adipose tissue (AT), which is derived from the embryonic mesenchyme, represent a rich source of MSCs, and provides an abundant and accessible source of adult stem cells with minimal patient discomfort (13-16). A recently reported alternative tissue source of MSCs is the connective tissue Wharton’s jelly (WJ) of human umbilical cord. These cells have the potential to be expanded, and can be obtained by a less invasive method, without posing harm to the mother or infant. Moreover, it is a biological waste and can be used as an abundant source of MSCs (3, 14, 17, 18).
In this study, we compared the growth suppression effect of MSCs derived from adult human BM, AT, and WJ on peripheral blood mononuclear cells (PBMCs).
Materials and Methods
Isolation and culture of human BM, AT, and WJ-derived SCs BM-derived MSCs
Human MSCs were obtained from 5 ml BM aspirates from the iliac crest of normal donors within the age range of 19–45 years. They were donors of BM to a related patient after obtaining approval of the Ethics Committee, Shiraz University of Medical Sciences, Shiraz, Iran. Written informed consent was also obtained allowing analysis of the clinical data and testing mentioned in this study. Each sample of aspirate was diluted 1:1 with DMEM-low glucose (Invitrogen, Merelbeke, Belgium) and layered over about 5 ml of Ficoll (Lymphoprep; Oslo, Norway). The isolation method was according to a previously reported method (19) by some modifications, which will be stated completely. After centrifugation at 939 g for 20 min, the mononuclear cell layer was removed from the interface. Cells were suspended in DMEM and centrifuged at 338 g for 15 min then resuspended in basal DMEM medium containing 10% fetal calf serum (Invitrogen, Merelbeke, Belgium), 1% penicillin (Invitrogen, Merelbeke, Belgium), 1% streptomycin (Invitrogen, Merelbeke, Belgium), and 2 mM glutamine (Invitrogen, Merelbeke, Belgium). The cells were seeded at a density of 80.000/cm2 in 25 cm2 T-flasks and maintained at 37 °C in an atmosphere of 5% CO2. After 4 days, the non-adherent cells were removed and the media was changed every 3 days. In order to expand MSCs, the adhered monolayer was detached with trypsin-EDTA (Invitrogen, Merelbeke, Belgium) for 5 min at 37 °C, after 14 days for the first passage and every 4–5 days for successive passages. During in vitro passaging, the cells were seeded at a density of 5-10×103 cells/cm2 and expanded for several passages until they no longer reached confluence.
AT-derived MSCs
AT-MSCs were extracted from breast ATs of healthy human adult donors with cosmetic mammoplasty surgery after obtaining the fully informed consent as described previously (20, 21). Briefly, fragments of ATs washed with phosphate buffered saline (PBS), minced and digested with 0.2% collagenase type I (GIBCO, USA) at 37 °C. The resulting cell suspension was centrifuged and the pellet including the adherent stromal cells was put on a Ficoll gradient (Biosera, UK) to separate the stromal vascular fraction (SVF). The separated cells were resuspended in DMEM culture medium (GIBCO, USA) containing 10% fetal bovine serum (GIBCO, USA) and 1% penicillin/streptomycin (Biosera, UK). Non-adherent cells were discarded after 2 to 3 days of culture. The adherent cells were cultured and harvested on passage 3 for experiments. The resulting cells were subjected to immunophenotyping by flow cytometry.
WJ-derived MSCs
Umbilical cords from healthy infants were transferred to our laboratory within 4–24 hr after delivery. Informed consents were taken from the infants’ parents. The umbilical cords were washed and flushed with PBS containing 1% penicillin/-streptomycin. A longitudinal section was made through the umbilical vein and the endothelial cells were scratched and discarded. The umbilical arteries were removed and the rest was cut into pieces. Each piece was put into a 100 mm petri dish and cultured in the presence of α-MEM containing 10% FCS, 0.1% L-glutamine, and 0.1% penicillin/streptomycin for 8–10 days. Upon confluency, the cells were passaged.
Characterization of human MSCs
At each passage, the cells were counted and analyzed for viability by trypan blue staining analysis. Flow cytometric analysis and functional ability of differentiation into osteocyte and adipocyte were achieved in response to specific culture conditions.
Flow cytometric analysis
The identification of adherent cells was performed by flow cytometric analysis. At the third passage, the cells were detached from the culture flasks with trypsin-EDTA and counted. About 1×106 cells were incubated on ice for 30 min with goat serum, resuspended in PBS and pelleted by centrifugation. Subsequently, the cells were stained for 30 min at 4 °C with fluorescein isothiocyanate (FITC)-coupled or phycoerythrin (PE)-conjugated CD34, CD45, CD90, CD73, CD80, and HLA-class II. Isotype-matched mouse monoclonal antibodies (BD-pharmingen, USA) were used to rule out non-specific staining of the cells. The labeled cells were thoroughly washed with PBS and analyzed on an FACSCalibur machine (BD Biosciences, USA) using the Cell quest as data acquisition software. The WinMDI 2.8 software was used for the graphical presentation of data.
Differentiation ability of MSCs
The potential of AT-MSCs to differentiate into chondrogenic, osteogenic, and adipogenic lineages was examined as described before (20, 21). Briefly, 1×105 AT-MSCs were used for differentiation when the cultures were 60–80% confluent by using appropriate differentiation kits (STEMPRO Differentiation Kit, GIBCO, USA). After 2 weeks the cells were stained with alizarin red S, safranin, and Oil Red O for evaluating osteogenic, chondrogenic, and adipogenic differentiation, respectively (Merck, Germany).
The potentials of the BM-MSCs and WJ-MSCs to differentiate into osteogenic and adipogenic lineages were examined. For osteogenic differentiation, the 4th-passage cells were treated with osteogenic medium for three weeks. Osteogenic medium consisted of DMEM supplemented with 10-8 M/l dexamethasone (Sigma-Aldrich, St. Louis, USA), 10 mM/l glycerol phosphate (Sigma-Aldrich, St. Louis, USA), 3.7 g/l sodium bicarbonate (Sigma-Aldrich, St. Louis, USA), and 0.05 g/l ascorbic acid (Sigma-Aldrich, St. Louis, USA). Osteogenesis was assessed by alizarin red S staining. To induce adipogenic differentiation, the 4th-passage cells were treated with an adipogenic medium for 3 weeks. Adipogenic medium consisted of DMEM supplemented with 1 M/L hydrocortisone (Sigma-Aldrich, St. Louis, USA), 0.05 g/l ascorbic acid, 0.05 g/l indomethacin (Sigma-Aldrich, St. Louis, USA), and 10-6 M/l dexamethasone. Adipogenesis was assessed by Oil Red O staining. Medium changes were performed twice weekly for the two assays.
Antiproliferative properties of human MSCs
Peripheral blood sample was collected from a healthy donor and PBMCs were prepared by density gradient centrifugation using Ficoll-hypaque media and were labeled with 5 nM CFSE dye (Invitrogen Molecular probe, USA). Then cells were incubated at 37ºC for 10 min; 2 ml fetal bovine serum (FBS) (Gibco, USA) was added to the cells and centrifuged at 1800 rpm for 10 min. Afterward, RPMI culture media with 2% FBS was added to the cell pellet and centrifuged again at 1800 rpm for 10 min. Next CFSE labeled PBMCs were counted and added into wells at a concentration of 1×106 cells per well. 2×105 MSCs were plated in culture plates 24 hr prior to co-culture with PBMCs. In this way, the ratio of MSC to PBMCs were estimated 1:5. PBMCs were stimulated with PHA (Gibco, UK) and the effect of MSCs on lymphocyte proliferation was studied in both direct and indirect (using 0.4 µm Transwell plates, corning, USA) culture system after 3, 5, and 7 days post culture by flow cytometry and compared to PHA-activated PBMCs. All experiments were carried out with 3 AT, 2 WJ, and 2 BM samples.
Results
MSCs isolated from BM, AT, and WJ exhibit mesenchymal characteristics Human BM-MSCs
Using the same previous approach, single cell-derived colonies of MSC-like cells which were rapidly grown and exhibiting homogeneous morphology were selected for culture expansion. BM cells rapidly generated a confluent layer of cells possessing an elongated and fibroblastic shape.
Their surface phenotype was determined by flowcytometry; also, we have characterized a population of cells resident within human BM cultures that were negative for the major histocompatibility complex (MHC) class II, and CD80 (B7-1) costimulators for T lymphocyte activation. Furthermore, no cells expressed the hematopoietic markers CD45 and CD34. However, they were positive for the MSC-candidate markers CD90 and CD73 (Figure 1A).
Figure 1.
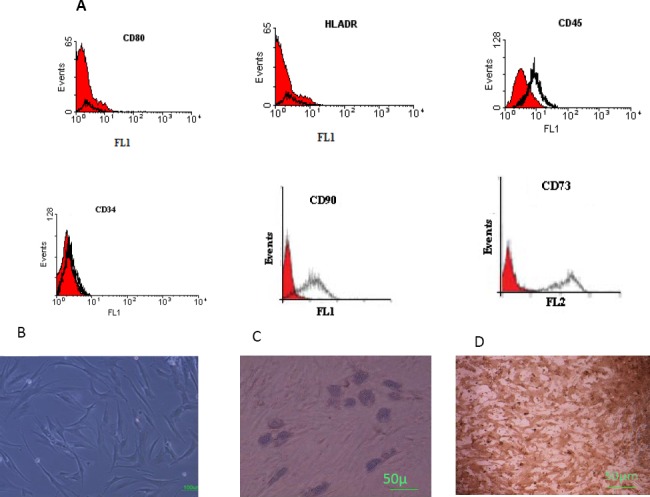
Immunophenotyping and differentiation of human bone marrow derived MSCs. A. The cells were negative for CD80, HLA-DR, CD45, and CD34 (the black histogram shows the profile of the isotype control), but were positive for CD90 and CD73 (the shaded area shows the profile of the negative control). B. Human BM-MSCs before differentiation C. The adipose droplet in differentiated cells after incubating with adipogenic media (10x) D. Osteogenic differentiation was positive for alizarin red staining (10x)
Lipid droplets in differentiated adipocytes were located by Oil Red O-staining. Likewise, alizarin red S staining confirmed the presence of calcium deposits in osteocytes, whereas undifferentiated MSCs were negative in staining procedure (Figures 1B and C). These results indicate that the isolated cells have the basic properties of MSCs.
Human AT-MSCs
AT-MSCs emerged with a spindle-shaped appearance in the culture as depicted in Figure 2A. The cells were characterized as MSCs through differentiation into a variety of lineages. Results of osteocyte differentiation demonstrated the accumulation of calcium in the culture (Figure 2B). Differentiation to chondrocytes caused morphological changes in AT-MSCs into rounder and cuboidal shapes as shown in Figure 2C. Differentiation to adipocytes caused the formation of well-formed lipid vesicles in AT-MSCs as presented in Figure 2D. The flow cytometry analysis for stem cell-specific markers revealed that AT-MSCs express CD44 and CD73 (higher than 98% of all cells). They were negative (higher than 90% of all ASCs) for the expressions of MHC II, CD80, CD45, and CD34 (Figure 2E).
Figure 2.
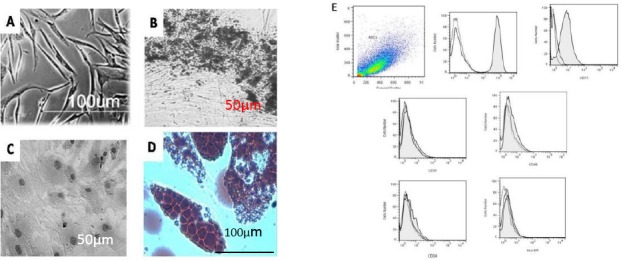
AT-MSCs with a spindle-shaped appearance in culture before treating for differentiation (A). These cells emerged in culture with the ability to differentiate into osteocytes (B), chondrocytes (C), and adipocytes (D). The flow cytometric characteristics of AT-MSCs (E). Results are representative of two independent experiments. Filled histograms represent the specific markers (CDs) and the unfilled were isotype control (40X).
Human WJ-MSCs
WJ-derived cells were seen as spindle-shaped cells in the culture. Figure 3 shows the phenotypic and differentiation characteristics of these cells. WJ-MSCs were positive for the expression of CD90, CD73, CD44, CD105, and CD106, but were negative for the expression of CD34 and CD144 (Figure 3A). Adipose vacuoles and calcium deposition in culture showed the ability of WJ-MSCs for differentiation (Figure 3B and C).
Figure 3.
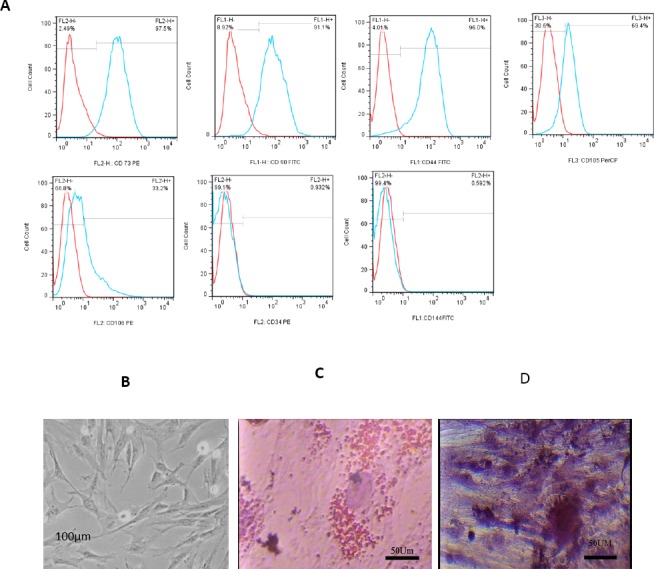
A. The phenothypic characteristics of WJ-MSCs by flow cytomety. B. Undifferentiated WJ-MSCs. C and D. Differentiation ability of WJ-MSCs to adipocytes and osteocytes, respectively
Antiproliferative effects of human BM-MSC, AT-MSC, and WJ-MSC on PBMCs
In order to show the inhibitory effects of the extracted cells, three different types of MSCs were co-cultured with normal allogeneic human PHA-activated PBMCs. The proliferation of PBMCs was assessed by flow cytometry of CFSE stained cells and compared to each other and to the growth of PBMCs in the absence of MSCs, 3, 5, and 7 days post co-culture. Based on the results, data of days 3 and 7 were compared between different groups.
As depicted in Figure 4, the proliferation of PBMCs reduced to 6.2, 7, and 15.4- fold (74%, 76%, and 83% growth suppression, respectively) in cultures with AT-MSCs, WJ-MSCs, and BM-MSCs, respectively, compared to the PHA-activated cells at day 3 post culture. The mean±SEM frequencies of dividing cells were 14.8±5.6, 12.6±3.4, 5.7±0.4, and 88.3 for AT-MSCs, WJ-MSCs, BM-MSCs, and non-co-cultured cells, respectively. When the growth suppression was indirectly assessed by using transwell culture system, it is revealed that AT-MSCs, WJ-MSCs, and BM-MSCs caused growth reduction in PBMCs to 3, 8, and 8-fold (59%, 77%, and 77% growth suppression), respectively, lower than PHA-activated cells. The mean±SEM frequencies of dividing cells were 29±6.4, 11± 6.4, and 11±7.6 for AT-MSCs, WJ-MSCs, and BM-MSCs, respectively (Figure 4).
Figure 4.
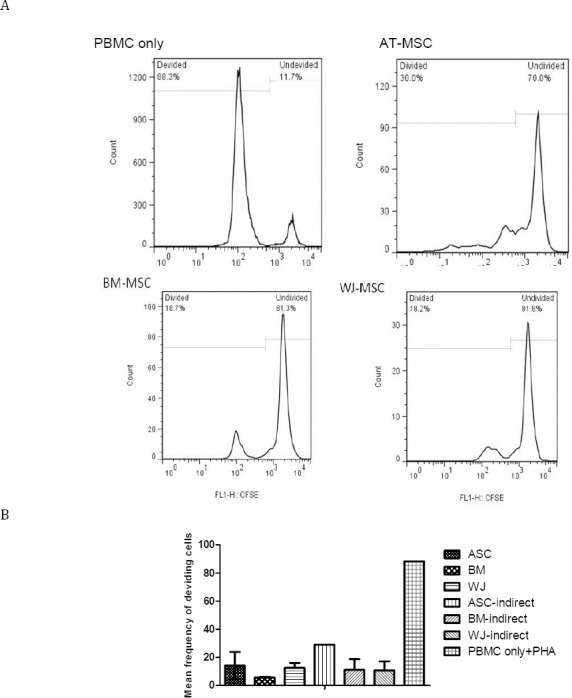
Growth suppression of PBMCs after coculture with AT, BM, and WJ-MSCs. (A) Typical representation of flow cytometry plots showing growth suppression of PBMCs in coculture with MSCs after 3 days. (B) Growth suppression of PBMCs in coculture with MSCs based on the mean frequency of dividing PBMCs
No significant difference was found between the data of days 3 and 5, but results of day 7 were different. At day 7 the growth suppression effect of all types of MSCs was lower than that of day 3 because it showed 60%, 49%, and 84% reduction 7 days post culture with AT-, WJ-, and BM-MSCs, respectively, compared to day 3. Similar effects were seen in indirect cultures for AT- and WJ-, but not BM-MSC because 27% and 4% reduction in growth suppression effect of AT- and WJ-MSC was observed compared to day 3.
Discussion
The specific characteristics of MSCs, including their extensive proliferative potential and the ability to differentiate into various cell types, like bone, fat, and cartilage, make them an attractive tool in regenerative medicine. Apparently, these cells might be simply isolated from various tissues and expanded in culture in large numbers, which gives the opportunity to create tissue-engineered constructs for re-introducing them into patients (22). Further interest in the clinical application of MSCs has been raised by the observation that these cells are strongly immunosuppressive in vitro and in vivo.
MSCs exist in almost all tissues, including BM, fat, muscle, tooth root, hair follicles, placenta, dermis, umbilical cord, WJ, lung, liver, and spleen (1, 13). To date, human BM represents the major source of MSC. The MSCs derived from BM have been applied in cell-based therapies, including the treatment of osteogenesis imperfecta, intracoronary transplantation in patients with acute myocardial infarction (23-25), and the treatment of graft-versus-host disease after allogeneic BM transplantation (26). Although BM has been represented as the main available and well-accepted source of MSCs, alternative sources such as AT and WJ of human umbilical cord may potentially be used in tissue injuries (16, 27).
BM-, AT-, and WJ-MSCs have initially shown similarities in their immunomodulatory effects, but additional studies showed various disparities (28, 29). Amable PR et al reported several differences between WJ-, BM-, and AT-MSCs including a higher proliferation potential and higher production of chemokines, pro-inflammatory proteins, and growth factors in WJ-MSC. They showed that AT-MSCs are better sources for pro-angiogenic factors such as VEGF and extracellular matrix components and metalloproteinases (30). AT-MSCs showed a stronger inhibitory effect on CD4+ and CD8+ T cells. In contrast to BM- and AT-MSCs, umbilical cord matrix-MSCs did not influence the activation or lymphoblast characteristics of B cells (31).
Here, we compared the growth suppression effects of BM-, AT-, and WJ-MSCs on PBMCs. Initial studies on MSCs showed that extracted cells from all three sources have the specific mesenchymal characteristics such as expression of surface markers and differentiation abilities.
Additionally, based on the results of this study, all kinds of MSCs showed suppression effects on the proliferation of PBMCs. When the growth suppression was compared between different types of MSCs, BM-MSCs and then WJ-MSCs showed higher growth suppression effects on PBMCs compared to AT-MSCs. Indirect culture experiments using the transwell system showed similar results because using AT-ASCs had the least effect on growth suppression of PBMCs compared to the culture supernatants of WJ- and BM-MSCs. When we compared the direct and indirect cultures of each MSC to itself, it was revealed that the presence of AT and BM-MSCs, thus cell to cell contact, was more important than the secretary effects of these cells. On day 7, the influence of AT-MSCs compared to other sources was similar to day 3, but it seems that the effects of all types of MSCs were generally diminished during the time.
Bochev et al indicated that AT-MSCs inhibited antibody production in PBMCs to a much greater extent than BM-MSCs (32). Furthermore, they demonstrated that AT-MSCs are more capable of suppressing the differentiation of blood monocytes into dendritic cells (DCs) and the expression of costimulatory molecules in mature monocyte-derived DCs (33). Results of our study on the underlying effect of AT- and BM-MSCs on the proliferation of PBMCs were inconsistent with these reports because we showed that BM-MSCs had a higher suppression effect than AT-MSCs which may be related to the source of AT because they used liposuction, but our AT-MSCs originated from breast tissue. Thus, MSCs originating from different sources of adipose may not have similar immunomodulatory effects.
The assessment of immune-suppression caused by BM-MSCs and WJ-MSCs showed that although both MSCs distort the cytokine profile from a pro-inflammatory to an anti-inflammatory phenotype, BM-MSCs, but not WJ-MSCs increase the levels of immune suppressive cytokine IL-10 and modulate the level of pro-inflammatory cytokine IL-2. Interestingly, indoleamine 2,3-dioxygenase (IDO) activity was higher in BM-MSCs than WJ-MSCs when both cells were treated with interferon gamma (IFNγ). Also, BM-MSCs secreted higher levels of prostaglandin E2 (PGE2) in comparison with WJ-MSCs (29). These differences in secretion pattern of MSCs and disparity in expressing distinct molecules (34) may reflect why MSCs from different sources show specific growth suppression effects. Our results of direct cultures are consistent with these data because we also observed the higher suppression effect of BM-MSCs compared to WJ-MSCs. In contrast, the supernatant of WJ-MSCs was more effective than BM-MSCs for reducing the proliferation of PBMCs.
Conclusion
Based on the present investigation, it is concluded that direct or cell-cell contact and indirect immunomodulatory effects of MSCs are different. Additionally, MSCs from various sources and even different parts of the body have special characteristics, which undoubtedly impact their properties and effects. Results of this study may contribute to the application of adult stem cells in regenerative medicine.
Acknowledgment
The authors thank patients and all participants for their kind contribution in this project. Also, we are grateful to Nooshafarin Chenari, Ahmad Hosseini, Mahsa Sani and Parisa Soveid for doing the laboratory experiments. This work was supported by grants from Shiraz University of Medical Sciences, Shiraz Institute for Cancer Research [ICR-100-504] and Transplant Research Center [8172].
References
- 1.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review:mesenchymal stem cells:their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 2.Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Gorskaja UF, Julagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 5.Ankrum J, Karp JM. Mesenchymal stem cell therapy:Two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells:cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- 7.Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment:surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nardi NB, Meirelles SD. Mesenchymal stem cells:isolation, in vitro expansion and characterization. HEP. 2006;174:249–282. [PubMed] [Google Scholar]
- 9.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anerg. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-c activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues:superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 14.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 15.Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504–509. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- 16.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue:implications for cell-based therapies. Cell Tissue Res. 2008;332:415–426. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells:A source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 18.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 19.Ayatollahi M, Soleimani M, Geramizadeh B, Imanieh MH. Insulin-like growth factor1 (IGF-I) improves hepatic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35:1169–1176. doi: 10.1042/CBI20110016. [DOI] [PubMed] [Google Scholar]
- 20.Razmkhah M, Abedi N, Hosseini A, Imani MT, Talei AR, Ghaderi A. Induction of T regulatory subsets from Naïve CD4+T cells after exposure to breast cancer adipose derived stem cells. Iran J Immunol. 2015;12:1–15. [PubMed] [Google Scholar]
- 21.Rezaeifard S, Razmkhah M, Robati M, Momtahan M, Ghaderi A. Adipose derived stem cells isolated from omentum:a novel source of chemokines for ovarian cancer growth. J Cancer Res Ther. 2014;10:159–164. doi: 10.4103/0973-1482.131451. [DOI] [PubMed] [Google Scholar]
- 22.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc:a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 23.Shang Q, Wang Z, Liu W, Shi Y, Cui L, Cao Y. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg. 2001;12:586–593. doi: 10.1097/00001665-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 25.Noort WA, Kruisselbrink AB, In’t Anker PS, Kruger M, van Bezooijen RL, de Paus RA, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 27.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Hematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 28.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 29.Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther. 2014;5:53. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4:125. doi: 10.1186/scrt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochev I, Elmadjian G, Kyurkchiev D, Tzvetanov L, Altankova I, Tivchev P, et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int. 2008;32:384–393. doi: 10.1016/j.cellbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Najar M, Raicevic G, Jebbawi F, De Bruyn C, Meuleman N, Bron D, et al. Characterization and functionality of the CD200-CD200R system during mesenchymal stromal cell interactions with T-lymphocytes. Immunol Lett. 2012;146:50–56. doi: 10.1016/j.imlet.2012.04.017. [DOI] [PubMed] [Google Scholar]


