Abstract
Objective(s):
This study was performed to investigate the effects of mucosal acidification on mRNA expression and protein synthesis of cystathionine gamma lyase (CSE), cystathionine beta synthase (CBS), and mucosal release of H2S in gastric mucosa in rats.
Materials and Methods:
Thirty-two rats were randomly assigned into 4 groups (8 in each), including: the control group, HCl (10 mM) treated group, HCl (100 mM) treated group, and one group to study the effect of Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME). Anesthetized rats underwent tracheostomy and midline laparotomy. Ninety min after the instillation of neutral or acidic solutions, animals were sacrificed and the gastric mucosa was collected to measure the H2S concentration by ELISA method and to quantify mRNA expression of CSE and CBS by quantitative real-time PCR (qRT-PCR). Protein synthesis was also detected by Western blot method.
Results:
Mucosal acidification with 10 and 100 HCl, significantly increased mucosal levels of H2S (P<0.01 and P<0.001) and mRNA (P<0.01 and P<0.001) and protein expressions of CSE (P<0.01 and P<0.001). L-NAME treatment reversed H2S release to control level.
Conclusion:
Our findings indicated that mucosal acidification with HCl increased mucosal release of H2S through upregulation of CSE gene and its protein expression. This effect is mainly mediated through the involvement of nitric oxide.
Keywords: Cystathionine gamma lyase H2S, Mucosal acidification, Rat
Introduction
A growing body of evidence has shown that endogenous signaling gaseous molecules including nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) regulate physiologic functions in the mammalian tissues (1). Under physiologic conditions, mucosal defensive barriers in stomach protect the gastric mucosa against offensive factors such as gastric acid and pepsin. Recently, it has been revealed that H2S protects and maintains the gastric mucosal integrity (2, 3) against physiologic factors including hydrochloric acid (HCI) and pepsin through stimulating mucus and bicarbonate (HCO3-) secretion as well as inhibiting gastric acid secretion (4-6). It has been well documented that H2S promotes the gastric mucosal barrier against gastric ulcers induced by variousirritants such as ischemia/reperfusion (7, 8), ethanol (9), non-steroidal anti-inflammatory drugs (NSAIDs) (10), and water immersion restraint stress (11), by increasing the gastric mucosal blood flow, stimulating the HCO3- secretion (4), reducing the plasma levels of pro-inflammatory cytokines (8), increasing prostaglandin synthesis (5), and reducing the production of reactive oxygen species (5).
Moreover, endogenous and exogenous H2S have been shown to stimulate duodenal secretion of HCO3- in rats (4). Mucosal acidification stimulates HCO3- secretion concomitant with increase in PGE2 and NO production, and these responses are mitigated by production, and these responses are mitigated by propargylglycine (PAG), a selective cystathionine gamma-lyase (CSE) inhibitor, implying that endogenous H2S is involved in acid-induced HCO3- secretion in the rat duodenum (4). Additionally, pharmacologically inhibition of the endogenous production of H2S by PAG has been reported to worsen the acid-induced duodenal damage, showing that H2S stimulates HCO3- secretion in the duodenum (12). Recently, it has been suggested that exogenous but not endogenous H2S stimulates HCO3- secretion in the rat stomach through the involvement of NO, prostaglandins, and calcitonin gen related peptide neurons (13).
To our knowledge, there is no report about the effect of mucosal acidification on H2Srelease in rats’ gastric mucosa. Therefore, the aim of present study was to evaluate the effect of mucosal acidification with HCl (10 and 100 mM) on mucosal H2S release, as well as on gene and protein expressions of two key enzymes, cystathionine beta synthase (CBS) and CSE, which are associated with endogenous generation of H2S and the involvement of NO on this effect.
IVS9+459 T/C (rs2280883) with susceptibility to lung cancer in a population from the South of Iran.
Materials and Methods
Animals
Male Wistar rats (200-250 g) were purchased from the animal house of Ahvaz Jundishapur University of Medical Sciences. The animals were fed on conventional diet and had free access to tap water. They were maintained under standard conditions of humidity, temperature (22±2 °C), and 12 hr light/dark cycle. The animals were deprived of food, but not water, overnight before intervention. All experiments were carried out in accordance with the regulations set by ethics committee of Ahvaz Jundishapur University of Medical Sciences (PRC154).
Animal grouping and experimental procedures
Adult male Wistar rats were randomly assigned into 4 groups (8 in each), including: the control group, HCl (10 mM) treated group, HCl (100 mM) group, and one group for evaluation of the NO effect. To investigate the involvement of NO in mediating the effect of HCI on mucosal H2S release, one group of rats received a single dose of Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME, 10 mg/kg, IP) concomitant with instilling acidic saline solution (100 mM, 37 °C) (14).
Under a mixture of ketamine and xylazine anesthesia (60+15 mg/kg, IP) (14), animals underwent tracheostomy and midline laparotomy. A catheter was inserted in the stomach through duodenum for gastric distention and gastric washout. Depth of anesthesia was checked throughout the experiment by the pedal withdrawal (toe pinch) reflex, every 30 min. If the pedal withdrawal reflex was observed, a supplemental dose of ketamine+xylazine (1/3 of the initial dose) was administered to maintain adequate anesthesia. Animal body temperature was measured with a rectal thermometer and maintained at 37 °C, using a homoeothermic blanket control system (Harvard, UK). At the beginning of each experiment, the lumen of the stomach was gently rinsed with isotonic saline (at 37 °C, pH 7) until gastric washout was clear. Thirty minutes after surgical operation, 1 ml of neutral isotonic saline (at 37 °C, pH 7) was instilled into stomach of control group and 1 ml of acidic solution (10 or 100 mM, at 37 °C) were instilled into stomach in HCl treated groups, and 15 min later the gastric content was washed and a new volume of neutral isotonic saline or HCI was instilled through duodenal catheter into the stomach, and repeated until end of experiment.
Ninety min after the instillation of neutral or acidic isotonic saline (after six consecutive instillation of neutral or acidic solutions), animals were sacrificed by an overdose of anesthetics, their stomach was removed, opened along the greater curvature, rinsed with physiological saline, and pinned out in ice-cold saline. Two samples of gastric mucosal tissue were quickly excised and snap-frozen in liquid nitrogen. One sample was used for measuring the mRNA expression and protein synthesis of CSE and CBS by qRT-PCR, and another sample was used for determining mucosal H2S release by an enzyme-linked immubosorbent assay (ELISA) kit (ABIN771902, antibodies-online, USA).
RNA extraction and cDNA synthesis
The total RNA was extracted from the frozen tissue samples using RNeasy Plus Mini Kit (Qiagen, USA). The purity and concentration of the extracted RNA was determined spectrophotometrically, at 260 and 280 nm wavelengths (Eppendorf, BioPhotometer Plus, Germany). The cDNA was synthesized from 1 microgram of the total RNA using QuantiTect reverse transcription Kit (Qiagen, USA) according to the manufacturer´s instruction.
Quantitative real-time PCR
The mRNA levels of the CBS, CSE, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were measured by qRT-PCR using step-one systems (Applied Biosystems, USA). The specific primers (Bioneer, South Korea) for measurement of CBS, CSE, and GAPDH were used and the lengths for amplified products were as follows: GAPDH (Forward: 5´-TGCTGGTGCT-
GAGTATGTCGTG-3 and reverse: 5´-CGGA-GATGATGACCCTTTTGG-3´, 101 bp); CSE (Forward: 5´-TGTTGTCATGGGCTTAGTG-3´ and reverse: 5´-CCATCCCATTCCTGAAGTG-3, 167 bp); and CBS (Forward: 5´-CTGTGAAGGGCTATCGCTGC-3´ and reverse: 5´-CTGGCATTGCGGTACTGGTC-3´, 205 bp). All PCR amplifications were performed in duplicate reactions and in final volume of 20 µl, containing 2 μl cDNA, 50 nm of specific primers, and 10 µl of master mix SYBR green (2× SYBR® Green PCR Master Mix; Applied Biosystems®; USA), using the following protocol: incubation at 95 °C for 10 min to activate DNA Taq polymerase and 40 two-step cycles with denaturation at 95 °C for 20 sec, and annealing/-extension at 60 °C for 1 min. In addition, the no-template negative control (H2O) was routinely run in every PCR. The melting curve was examined at the end of amplification process to ensure the specificity of the PCR products. The purity of each amplicon for each reaction was further confirmed by agarose gel electrophoresis. Expression levels of CBS and CSE genes were normalized against GAPDH expression (internal calibrator for equal RNA template loading and normalization). To determine the relative quantifica-tion of gene expression, comparative cycle of threshold (Ct) method with arithmetic formulae (2−ΔΔCt) was used.
Protein extraction
Frozen mucosal tissue was extracted using radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail (complete mini, Roche, Indianapolis, IN, USA). To analyze the protein fraction, proteins extracted using RIPA buffer, from gastric mucosal samples, were resuspended in 1% SDS. The total recovery and integrity of these fractions were determined by Bradford assay and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE).
Western blot analysis
Mucosal proteins were separated by SDS-PAGE on 10% acrylamide gels and were transferred onto a nitrocellulose membrane. The membranes were blocked by 5% non-fat dry milk dissolved in tris-buffered saline with 0.1% Tween 20 (TBST, pH 7.6) for 6 hr, and then incubated overnight at 4 °C with anti-CBS antibody (mouse monoclonal, dilution 1:200; Santa Cruz Biotechnology; SC-133154), anti-CSE antibody (rabbit polyclonal, dilution 1:150; Santa Cruz Biotechnology; SC-135203), anti-eNOS antibody (rabbit polyclonal, dilution 1:250; ab66127) or anti-beta Actin antibody (mouse monoclonal, dilution 1:5000; Abcam [ab20272], USA). After 5 washes with TBST, membranes were incubated with a rabbit polyclonal secondary antibody to mouse IgG HRP; dilution 1:7000), for 90 min at room temperature. Labelled proteins were detected using a chemiluminescence Western blotting system. The expression of studied proteins was semi-quantified by Image J analysis software and the values were normalized to β-actin.
Determination of mucosal H2S levels
To investigate the effect of mucosal acidification with 10 and 100 mM HCl on the mucosal H2S production/release, we performed ELISA using commercial ELISA Kit (ABIN771902, antibodies-online, USA) according to the manufacturer’s instructions.
Chemicals
Nitrocellulose membrane and L-NAME were purchased from Sigma Co, USA. HCl and NaCl were purchased from Merck Co. (Germany).
Statistical analysis
Data are shown as mean ± SEM. Statistical analysis was performed by one-way ANOVA and followed by Tukey’s Post-hoc test. Significance was set at a P<0.05 level.
Results
Effect of mucosal acidification on mucosal H2S release and the involvement of NO
Based on ELISA data (Figure 1), mucosal release of H2S, in response to mucosal acidification with 10 and 100 mM HCl, was significantly increased compared to the control (P<0.01 and P<0.001, respectively); this stimulatory effect was prevented by L-NAME.
Figure 1.
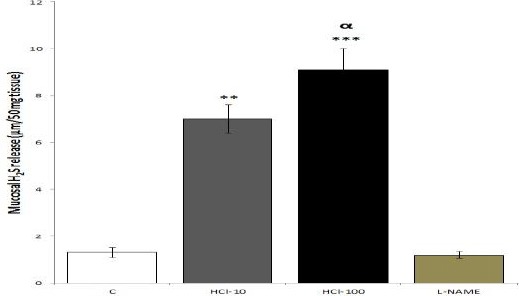
Effects of mucosal acidfication on the gastric mucosal release of H2S. C: control; HCl-(10 or 100): mucosal acidification with 10 or 100 mM HCl for 90 min; L-NAME: one group of rats received a single dose of L-NAME (10 mg/kg, IP) accompanied with instilling acidic saline solution (pH 1, at 37 °C). The results of ELISA showed that mucosal acidification increased mucosal release of H2S; L-NAME inhibited this increase. **P<0.01 and ***P<0.001 versus control group. αP<0.05 significant increase compared to 10 mM HCl-treated group. Data are expressed as mean±SEM
Effect of mucosal acidification on mucosal mRNA and protein expressions of CSE
As illustrated in Figure 2, qRT-PCR data showed that the mRNA expression of CSE in groups treated with 10 and 100 mM HCI, was significantly higher than the control rats (P<0.01 and P<0.001, respectively). According to the Western blot (Figure 3), protein expression of CSE was significantly increased in response to mucosal acidification with 10 and 100 mM HCl compared with the control rats, (P<0.01 and P<0.001, respectively).
Figure 2.
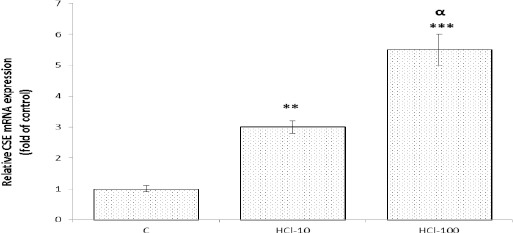
Effect of mucosal acidification on mRNA expression of cystathionine gamma lyase (CSE) in gastric mucosa. The resulta of qRT-PCR showed that mucosal acidification with 10 and 100 mM HCl, upregulated gene expression of CSE in gastric mucosa. **P<0.01 and ***P<0.001 versus control rats. αP<0.05 significant increase compared to 10 mM HCl-treated group. Data are expressed as mean±SEM
Figure 3.
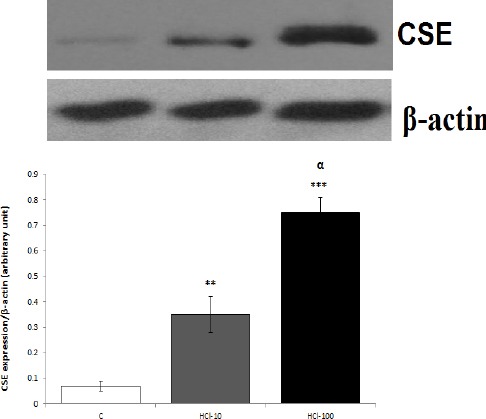
Effect of mucosal acidification on protein expression of cystathionine gamma lyase (CSE) in gastric mucosa. Western blot results showed that mucosal acidification with 10 and 100 mM HCl upregulated protein expression of CSE in gastric mucosa. **P<0.01 and ***P<0.01 versus control rats. αP<0.05 significant increase compared to 10 mM HCl-treated group. Data are expressed as mean±SEM
Effect of mucosal acidification on mucosal mRNA and protein expressions of CBS
As demonstrated in Figures 4 and 5, mRNA and protein of CBS were expressed in gastric mucosa of the control and HCl-treated rats; qRT-PCR and Western blot showed no significant differences between the control and HCl-treated rats.
Figure 4.
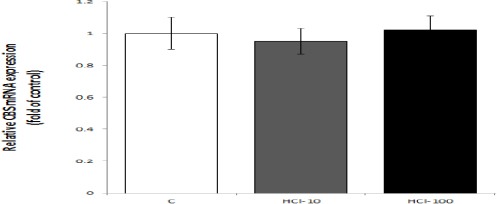
Effects of mucosal acidification with 10 and 100 mM HCl on mRNA expression of cystathionine beta synthase (CBS) in gastric mucosa. The results of qRT-PCR showed that the level of CBS mRNA expression in control and experimental groups was equal. Data are expressed as mean±SEM
Figure 5.
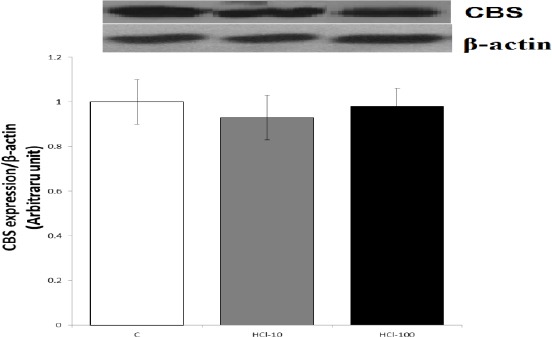
Effects of mucosal acidification with 10 and 100 mM HCl on protein expression of cystathionine beta synthase (CBS) in gastric mucosa. of the results of Western blot (B) showed that the level of protein expression of CBS in control and experimental groups was equal. Data are expressed as mean±SEM
Discussion
The findings of the present study indicated that: 1. mucosal acidification with 10 and 100 mM HCl, increased mucosal H2S release and up-regulated mRNA and protein expressions of CSE in the gastric mucosa of rats; 2. the increased level of H2S release in response to mucosal acidification with 100 mM HCl was reversed to control level by L-NAME treatment, as the inhibitor of nitric oxide synthase.
The present study showed, for the first time, that the mucosal H2S release was significantly increased in response to mucosal acidification with 10 and 100 mM HCl. The results also demonstrated that any decrease in intra-gastric pH stimulates mucosal H2S synthesis. As previously mentioned, duodenal acidification with 100 mM HCl has been shown to stimulate HCO3- secretion concomitant with increase in PGE2 and NO production, and these responses were mitigated by PAG, a selective CSE inhibitor, implying that endogenous H2S is involved in acid-induced HCO3- secretion in the rat duodenum (4). Overall, these findings showed that mucosal acidification with HCl in addition to duodenum, increased H2S release in gastric mucosa of rats. L-NAME treatment prevented H2S response to mucosal acidification; therefore, NO might be involved in this response.
The current results showed that CBS and CSE are expressed in gastric mucosa of rat. Consistent with these results, it has been indicated that, although both CBS and CSE are expressed in the gastric mucosa in rat, the main responsible enzyme for H2S production is CSE (10). Based on the evidence, CBS is a constitutive enzyme that its expression and activity are not affected by acetyl salicylic acid or ethanol (10, 15). Our results also were in agreement with previous findings (10, 15), that mRNA and protein expressions of CBS are not changed by mucosal acidification of the stomach with 10 and 100 mM HCl. Therefore, the rate of CBS production in gastric mucosa is independent of the changes in intra-gastric pH; which confirms that CBS is a constitutive enzyme.
Based on quantitative real-time PCR, mRNA expression of CSE is increased in response to mucosal acidification with 10 and 100 mM HCl; so, increased production of H2S in response to mucosal acidification is largely mediated through up-regulating mRNA expression of CSE.
We could reveal that protein expression of CSE is increased in response to mucosal acidification; so, the mucosal acidification with HCl, in addition to up-regulation of the CSE gene expression, increases its protein expression. In this regard, the up-regulation of protein expression of CSE in response to mucosal acidification with HCl is another involved mechanism which cause to mucosal increase of H2S.
The results also showed that the expression of mRNA and protein of CSE as well as mucosal release of H2S in response to 100 mM HCl were significantly higher than 10 mM HCl. Recently, it has been shown that when the acid output was stimulated by gastric distention with 15% alcoholic solution, these responses were stronger than when it was excited by the same volume of isotonic saline (14). Alcoholic solution (15%) has been indicated to increase the permeability of mucous layer (16). Therefore, the mucosal H2S release and CSE protein as well as mRNA expressions were higher when the mucus layer was exposed to higher content of HCl, as shown by the present study, or when its permeability was increased, as shown by previous report (14).
What is the physiological significance of the present results? Based on the results of the present study, under physiologic condition any decrease in intra-gastric pH is along with increased mucosal H2S production, while under non-physiologic situations such as NSAIDs-induced gastritis, the increased acid output was not associated with H2S release, as shown by Fiorucci et al (10).
Conclusion
Our findings showed that mucosal acidification increased mucosal H2S production through up-regulating mRNA and protein expression of CSE in rat gastric mucosa; this response is mediated by the involvement of NO.
Acknowledgment
The authors would like to gratefully acknowledge the financial support of the Vice Chancellor of Research Affairs of Ahvaz Jundishapur University of Medical Sciences (Grant No. PRC-154), Ahvaz, Iran. The results described in this paper were part of PhD thesis of Ali Veisi.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- 1.Farrugia G, Szurszewski JH. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147:303–313. doi: 10.1053/j.gastro.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace JL. Hydrogen sulfide:a rescue molecule for mucosal defence and repair. Dig Dis Sci. 2012;57:1432–1434. doi: 10.1007/s10620-012-2119-2. [DOI] [PubMed] [Google Scholar]
- 3.Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and-hydrogen sulfide inthe mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules. 2015;20:9099–9123. doi: 10.3390/molecules20059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, et al. Stimulation of duodenal HCO3ࢤ secretion by hydrogen sulphide in rats:Relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiol. 2011;201:117–126. doi: 10.1111/j.1748-1716.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 5.Magierowski M, Jasnos K, Kwiecien S, Brzozowski T. [Role of hydrogen sulfide in the physiology of gastrointestinal tract and in the mechanism of gastroprotection] Postepy Hig Med Dosw (Online) 2012;67:150–156. doi: 10.5604/17322693.1038356. [DOI] [PubMed] [Google Scholar]
- 6.Mard SA, Askari H, Neisi N, Veisi A. Antisecretory effect of hydrogen sulfide on gastric acid secretion and the involvement of nitric oxide. Biomed Res Int. 2014;2014:480921. doi: 10.1155/2014/480921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, et al. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Mard SA, Neisi N, Solgi G, Hassanpour M, Darbor M, Maleki M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig Dis Sci. 2012;57:1496–1503. doi: 10.1007/s10620-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros JV, Bezerra VH, Gomes AS, Barbosa AL, Lima-Júnior RC, Soares PM, et al. Hydrogen sulfide prevents ethanol-induced gastric damage in mice:role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 2009;330:764–770. doi: 10.1124/jpet.109.152801. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 11.Lou LX, Geng B, Du JB, Tang CS. Hydrogen sulphide-induced hypothermia attenuates stress-related ulceration in rats. Clin Exp Pharmacol Physiol. 2008;35:223–228. doi: 10.1111/j.1440-1681.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi K, Aihara E, Kimura M, Dogishi K, Hara T, Hayashi S. Gas mediators involved in modulating duodenal HCO3-secretion. Cur Med Chem. 2012;19:43–54. doi: 10.2174/092986712803413962. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi K, Ise F, Takahashi K, Aihara E, Hayashi S. H2S-induced HCO3 (-) secretion in the rat stomach-Involvement of nitric oxide, prostaglandins, and capsaicin-sensitive sensory neurons. Nitric Oxide. 2015;30(46):157–64. doi: 10.1016/j.niox.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Mard SA, Veisi A, Ahangarpour A, Gharib-Naseri MK. Gastric acid induces mucosal H2S release in rats by upregulating mRNA and protein expression of cystathionine gamma lyase. J Physiol Sci. 2015;65:545–554. doi: 10.1007/s12576-015-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medeiros JV, Soares PM, Castro Brito GA, Souza MH. Immunohistochemical approach reveals localization of cystathionine-gamma-lyase and cystathionine-beta-synthetase in ethanol-induced gastric mucosa damage in mice. Arq Gastroenterol. 2013;50:157–160. doi: 10.1590/s0004-28032013000200027. [DOI] [PubMed] [Google Scholar]
- 16.Tarnawski A, Hollander D, Stachura J, Krause WJ, Gergely H. Prostaglandin protection of the gastric mucosa against alcohol injury-a dynamic time-related process:role of the mucosal proliferative zone. Gastroenterology. 1985;88:334–352. doi: 10.1016/s0016-5085(85)80188-8. [DOI] [PubMed] [Google Scholar]


