Abstract
Objective(s):
Development of new generation of vaccines against leishmaniasis is possible because long-term protection is usually seen after recovery from cutaneous leishmaniasis. ISCOMATRIX is particulate antigen delivery system composed of antigen, cholesterol, phospholipid and saponin. In this study, the role of ISCOMATRIX bilayer composition made by different phase transition temperature (Tc) to induce a type of immune response and protection against leishmaniasis was assessed.
Materials and Methods:
ISCOMATRIX formulations with different bilayer compositions consisting of EPC (Tc <0 °C), DMPC (Tc 23 °C) and DSPC (Tc 54 °C) were prepared. Different ISCOMATRIX formulations were mixed with soluble Leishmania antigens (SLA). BALB/c mice were immunized subcutaneously, three times with 2-week intervals. As criteria for protection, footpads swelling, parasite burden, determination of IgG isotypes and the level of IFN-γ and IL-4 were assessed.
Results:
Although the groups of mice immunized with ISCOMATRIX DMPC or ISCOMATRIX DSPC showed the smallest footpad swelling and least parasite burden compared with the buffer group, the difference was not significant. Moreover, the highest level of IFN- γ and IL-4 was observed in the splenocytes of mice immunized with ISCOMATRIX DMPC or ISCOMATRIX DSPC, respectively. After challenge, the mice immunized with ISCOMATRIX DSPC showed the highest elevation of IgG, IgG1 and IgG2a antibodies (P<0.01) compared with control group. However, our results indicated that ISCOMATRIX EPC, DMPC or DSPC generated a mixed Th1/Th2 response that was not protective.
Conclusion:
Our results showed that the adjuvanticity of prepared ISCOMATRIX doesn’t influence with different phospholipids at least in our mice model.
Keywords: Immune response, ISCOMATRIX, Leishmania major, Transition temperature
Introduction
Leishmaniasis is a public health problem caused by several species of protozoan parasites of the genus Leishmania (1). Leishmania species are intracellular protozoan parasites of macrophage-dendritic cell lineage that cause a spectrum of diseases including cutaneous (CL), mucocutaneous (MCL), diffuse cutaneous (DCL), visceral (VL or kala-azar), post kala-azar dermal leishmaniasis (PKDL) and recidivans (LR) (2). The current strategies of vector or reservoir control cannot be used effectively, so countries prefer an effective preventive vaccine, due to high cost of vector or reservoir control (3, 4), toxicity of drug (5, 6), and increasing parasite resistance (7). Recovery and protection against Leishmania major infection at least in murine model is mainly depend upon induction of a Th1 type of immune response (8), whereas as a general rule, induction of a Th2 response controls extracellular infections (9). Development of an effective anti-Leishmania vaccine is possible due to the fact that long lasting protection against further infection is usually seen after recovery from CL (10) or leishmanization (11). However, despite decades of global efforts due to limited efficacy, drug resistance, and toxicities associated with chemotherapy, there is no vaccine against any form of leishmaniasis (3, 12). Recent studies have documented that efforts to generate leishmania vaccine require to an appropriate adjuvant (13).
Leishmanial proteins such as whole killed parasites, soluble leishmania antigens (SLA), fractionated parasite and purified recombinant antigens with an appropriate adjuvant could give rise to variable levels of protection in animal models (14, 15). In this study SLA as a model antigen for generation of vaccine was used with ISCOMATRIX as a nanoparticulate immunoadjuvant. Immunostimulatory complexes (ISCOMs) are particulate antigen delivery systems composed of saponin, cholesterol, phospholipid, and immunogen, usually protein (16). ISCOMATRIX has essentially the same structure of ISCOMs but without the antigen, and is usually referred to as ISCOMATRIX (also called ISCOMATRIX™, a trademark of ISCOTEC AB). Typically, both ISCOMs and ISCOMATRIX exist as spherical, hollow, rigid, cage-like particles of about 40 nm in diameter with a strong negative charge (17, 18). ISCOMATRIX combines the advantages of a particulate carrier system with the presence of an in-built adjuvant (Quil A) and consequently have been found to be more immunogenic than other colloidal systems such as liposomes and protein micelles (19). A further attraction of ISCOMATRIX formulation is their adjuvant activity related to the Quil A, while increasing its stability, reducing its hemolytic activity, and producing less toxicity. These characteristics gave ISCOMATRIX their ability to induce strong antigen-specific cellular or humoral immune responses against a wide range of antigens in a number of animal model (20, 21). Previous studies have been indicated the influence of different ISCOMATRIX characteristics on the immune responses; such as phospholipid composition, charge and size, interaction of Quil A with antigen presenting cells (APC) (22-24). ISCOMA- TRIX with various lipids for example egg phosphatidylcholine (EPC, Tc <0 °C) and phospholipids having high transition temperature (Tc) such as distearoylphosphatidylcholine (DSPC, Tc 54 °C) can induce different immune responses. Hence, in the present study, ISCOMATRIX consisting from different lipids (DMPC, EPC, DSPC) and SLA as a model antigen were prepared and their role in induction of generated immune responses against leishmaniasis in BALB/C mice were explored.
Materials and Methods
Animals, ethics statement
Female BALB/c mice 6–8 weeks old were provided from Pasteur Institute (Tehran, Iran). The animals were maintained in animal house of Pharmaceutical Research Center of Mashhad University of Medical Sciences under standard conditions of humidity, temperature and light. They were fed with tap water and laboratory pellet chow (Khorasan Javane Co., Mashhad, Iran). The experimental design was performed in accordance with the current ethical norms approved by Mashhad University of Medical Sciences Ethical Committee Acts (Education Office dated March 31, 2010; proposal code 88527), based on the Specific National Ethical Guidelines for Biomedical Research issued by the Research and Technology Deputy of Ministry Of Health and Medicinal Education (MOHME) of Iran.
Parasites, soluble leishmania antigen (SLA) and Quil A
The L. major strain (MRHO/IR/75/ER) used in this experiment was previously used for experimental Leishmania vaccine and leishmanin test in Iran (25, 26). The method of SLA preparation was carried out using the protocol developed with minor modifications (27). Briefly, stationary phase promastigotes were harvested and washed four times in HEPES buffer (HS buffer) (10 mM, pH 7.5) containing 10% sucrose. The number of promastigotes was adjusted to 1.2 × 109/ml in buffer solution containing enzyme inhibitor cocktail (50 μl/ml) (Sigma, St. Louis, MO, USA). The parasites were then lysed by freeze-thaw method followed by probe sonication (dr hielscher, Germany) in an ice bath. The supernatant of the centrifuged lysate parasites was collected, dialyzed against HS buffer solution and sterilized by passage through a 0.22 μm membrane and stored at −70°C until use. Quil A was purchased from Sigma Aldrich. Total SLA concentration was determined using BCA (Bicinchoninicacid) protein assay kit (Thermo Scientific, USA) (28). The antigen was aliquoted and stored at -70°C until use.
Preparation and characterization of ISCOMATRIX
ISCOMATRIX was prepared by hydration of a dispersion of lipid in solid sugar matrices. The ratios of the various component are as following: lipid (EPC, DSPC, DMPC): Quil A: cholesterol of 2:2:1 without SLA, the total lipid concentration in formulation was 6.7 mg/ml. Lipid (8 mg) and cholesterol (4 mg) were dissolved in chloroform in a sterile tube. Having removed the solvent by rotary evaporator (Hettich, Germany, sucrose (200 mg) added to sterile tube and dissolved in a mixture of tert-butanol and water (4 ml, v/v 1:1). Snap freezing of the resulting monophase solution was carried out in nitrogen tank, and was freeze-dried overnight (Hettich, Germany). Four ml of PBS (0.01 M, pH 7.4) and 8 mg of Quil A were then added to hydrate the solid matrices followed by 15 min sonication to facilitate dispersion. The ISCOMATRIX dispersion was subsequently extruded through (400, 200, 100) nm polycarbonate membranes (Avestin, Canada) (34).
Zeta potential and size of formulations were measured using a Zetasizer (Nano-ZS, Malvern Instruments, UK) (29). Particle sizes were reported as the mean±standard deviation and poly dispersity index (PDI) (n=3). Zeta potentials were reported as the means±zeta deviation (n=3). Structure of the ISCOMATRIX was confirmed by transmission electron microscopy (TEM) (30). Briefly, samples were coated onto glow-discharged, carbon coated copper grids and negatively stained with 2% phosphotungstic acid (pH 5.2). After that, the samples were scanned using a Phillips CM100 electron microscope with an acceleration voltage of 100 kV and a magnification of 93,000×.
SDS-PAGE analysis
The polyacrylamide gel electrophoretic analysis (SDS-PAGE) was carried out to evaluate different proteins in SLA. The gel consisted of running gel (10.22%, w/v, acrylamide) and stacking gel (4.78%, w/v, acrylamide) at the thickness of 1 mm. The electrophoresis buffer was 25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3. Electrophoresis was carried out at 140 V constant voltages for 45 min. The same amount of SLA (2.5 or 5 μg) was loaded to each well for different formulations. Then, the gels were stained with silver nitrate for protein detection (31).
Vaccination protocol
Female BALB/c mice (10 per group) were subcutaneously (SC) vaccinated three times in 3-week intervals with one of the following formulations: ISCOMATRIX EPC-SLA (50 μg SLA +50 μl ISCOMATRIX/mouse), ISCOMATRIX DMPC-SLA (50 μg SLA + 50 μl ISCOMATRIX/mouse), ISCOMATRIX DSPC-SLA (50 μg SLA + 50 μl ISCOMATRIX/mouse), SLA (50 μg SLA/50 μl/mouse), buffer (HEPES 10 mM, sucrose 10% w/v, pH 7.4). Just before injection, 50 μg of SLA (1 mg/ml) was mixed with 50 μl ISCOMATRIX formulation.
Challenge with L. major promastigotes
Two weeks after the last booster injection, 1 × 106 late stationary phase L. major promastigotes in 50 μl volume was inoculated SC into the right footpad of immunized and control group of mice. Lesion progression was followed by measuring the thickness of the infected footpad as compared to the thickness of the same footpad prior to infection using a digital caliper (Mitutoyo Measuring Instruments, Japan) at weekly intervals for 8 weeks (32).
Quantitative parasite burden after challenge
Spleens and footpad from mice vaccinated with L. major were harvested in each experimental group. The number of viable L. major parasites in the spleen and footpad of mice was estimated using limiting dilution assay method as described previously (33, 34). Briefly, the mice were sacrificed at week 8 post-challenge; the feet were aseptically removed and homogenized in RPMI 1640 supplemented with 10% v/v heat inactivated FCS (Eurobio, Scandinavie), 2 mM glutamine, 100 units of penicillin per ml, and 100 μg/ml of streptomycin sulfate (RPMI-FCS). The homogenate was diluted with the media in 8 serial 10-fold dilutions and then was placed in each well of flat-bottom 96-well microtiter plates (Nunc, Denmark) containing solid layer of rabbit blood agar in tetraplicate and incubated at 25±1 °C for 7-10 days. The positive and negative wells (presence and absence of motile parasite respectively) were detected using an invert microscope (CETI, UK). The number of viable parasite per spleen and infected footpad was determined by GraphPad Prism software, a statistical method for limiting dilution assay.
Antibody isotype assay
Serum-specific IgG subclasses were determined by a standard enzyme linked immunosorbent assay (ELISA) method. Blood samples were collected from mice before and at week 8 after challenge and the sera were isolated and kept at -20 °C until use. Assessment of anti-SLA IgG total, IgG1 and IgG2a were used to detect bound antibodies (31). Briefly, microtiter plates (Nunc, Denmark) were coated with 50 μl of SLA (10 μg/ml) in PBS buffer (0.01 M, pH 7.3) and serial dilutions of serum at 4 °C for overnight. The plates were treated with HRP-rabbit anti-mouse IgG isotype according to the manufacturer’s instructions (Invitrogen Inc., USA). Optical density (OD) was determined at 450 nm using 630 nm as the reference wavelength.
Cytokine production and determination
ELISA method was used to detect the levels of IL-4 and IFN-γ in supernatant as previously described (31). Briefly, three mice from each group were sacrificed at week 3 after the last booster, at the same time with challenge experiment. The spleens were aseptically removed. Mononuclear cells were isolated using Ficoll-Hypaque (Biogene, Iran) density centrifugation method (32). The cells were washed and resuspended in complete medium (RPMI 1640-FCS) and seeded at 2 × 106/ml in 96-well flat-bottom plates (Nunc, Denmark). Then, the spleen cells were cultured and stimulated with SLA (10 μg/l or as control with medium alone and incubated at 37°C with 5% CO2 for 72 hr. The culture supernatants were collected and the level of IFN-γ and IL-4 were determined using ELISA method according to the manufacturer’s instructions (MabTech, Sweden).
Flowcytometry analysis
Spleenocytes were isolated 2 weeks after the last booster, and stained for intracellular cytokine IFN-γ (anti-IFN-γ–FITC) and IL-4 (anti-IL-4-FITC) according to BD protocols Cytofix/Cytoperm™ plus Fixation/Permeabilization Kit. Briefly, splenocytes (106 cells/ml) in medium containing GolgiPlug™ (1 μl/ml) was stimulated with PMA/ionomycin cocktail (2 μl/ml) for 4 hr at 37 °C. After stimulation, 105 splenocytes were transferred into flow cytometry tubes and washed two times with stain buffer (2% FCS in PBS). Splenocytes were stained with 1 μl anti-CD8a-PE-cy5 antibody and 1 μl anti CD4-PE-cy5 antibody in separate tubes for 30 min at
4 °C. The cells were washed with stain buffer and fixed using Cytofix/Cytoperm™ solution. Fixed cells were washed two times with Perm/Wash™ buffer and then stained with 1 μl anti-IFN-γ- FITC antibody for 30 min at 4 °C. CD4 cells were also stained with 1 μl anti-IL-4-PE antibody. The cells were washed with Perm/Wash™ buffer and suspended in 300 μl stain buffer for flow cytometric analysis Calibur (BD Biosciences, USA).
Statistical analysis
Data were recorded in GraphPad Prism software and analyzed. One-way ANOVA statistical test was performed to assess the significance of the differences among the various groups. The mean and standard deviation of all experiments were determined. In case of significant F value, Tukey–Kramer multiple comparisons test was carried out as a post-test to compare the means in different groups of mice. P≤0.05 was considered to indicate statistical significance.
Results
Characterization of ISCOMATRIX
The mean diameter and charge of formulations calculated by zeta sizer (Malvern, UK). Results are presented in (Table 1). The poly-dispersity index of ISCOMATRIX EPC, ISCOMATRIX DMPC and ISCOMATRIX DSPC was 0.21, 0.28, and 0.29 respectively; indicates that all different formulations are fairly homogenous. Zeta potential of all preparations was negative due to the presence of Quil A (Table 1). Detection of SLA was performed by SDS-PAGE electrophoresis (Figure 1). SDS-PAGE analysis of SLA revealed several protein bands with molecular weight ranges between 10 to 70 KDa. The size of the ISCOMATRIX structures was in the range of 40-80 nm as measured by transmission electron microscopy (Figure 2). TEM was used to confirm the size as well as the morphology of the colloids, the morphology showed hollow cage-like structures, which are considered as the typical morphology of ISCOMATRIX.
Table 1.
Particle size distribution, polydispersity index (PDI) and zeta potential of various ISCOMATRIX formulations (mean±SD, n= 3)
| Formulation | Size (nm) | PDI | Zeta potential (mv) |
|---|---|---|---|
| ISCOMATRIX EPC | 108.4±9.5 | 0.21±0.08 | -13.4 ±5.6 |
| ISCOMATRIX DMPC | 70.2±27 | 0.28±0.05 | -12.8±5.2 |
| ISCOMATRIX DSPC | 110.8±22 | 0.29± 0.07 | -14 ±2.8 |
Figure 1.

SDS-PAGE analysis of soluble Leishmania antigens (SLA) alone and ISCOMATRIX. Lane 1, Low-range protein standard (Sigma, USA); Lane 2, SLA (10 μg)
Figure 2.
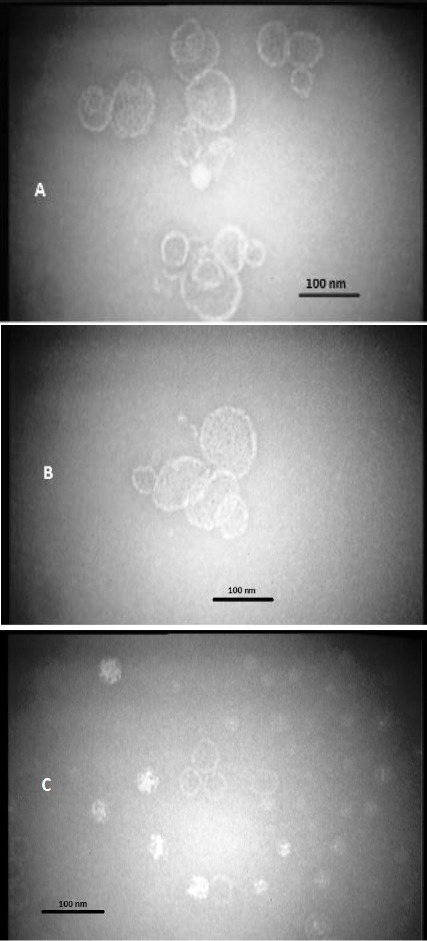
Three pictures of ISCOMATRIX EPC (A), ISCOMATRIX DSPC (B) and ISCOMATRIX DMPC (C) prepared by transmission electron microscopy (TEM)
Challenge results
Lesion development in each mouse was recorded weekly by measurement of footpad thickness (Figure 3). Footpad thickness progressed similarly in all groups of mice up to two weeks after the challenge. The lesion size progressed rapidly in all mice
Figure 3.
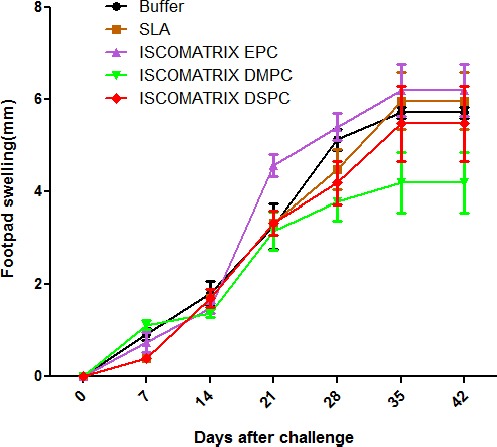
Footpad swelling in BALB/c mice immunized SC, three times in 3-week intervals, with soluble Leishmania antigens (SLA) SLA, ISCOMATRIX EPC, ISCOMATRIX DSPC and ISCOMATRIX DMPC or buffer alone. The footpad thickness of each mouse was measured on both footpads for 42 days. Each point represents the average increase in footpad thickness±SEM (n=7)
Immunized with different formulations from week 3 post infections. Footpad swelling in all groups was progressed continuously and no protection was observed in any group. Totally, the groups of mice immunized with ISCOMATRIX DMPC and ISCOMATRIX DSPC showed the smallest footpad swelling compared with other mice, but there was no significant difference in footpad swelling between the groups of mice immunized with different formulations. In all groups, the footpad swelling reached a plateau after 6 weeks but the disease progressed by metastasis to other organs and some of the mice lost their foot.
Splenic parasite burden after challenge
At day 42 after challenge, the number of viable L. major was quantified in spleen of different mice groups (Figure 4A). Results of splenic parasite burden showed that the group of mice immunized with ISCOMATRIX DMPC had the least parasite, which was significantly (P<0.05) lower than the control group. However, the difference in number of spleen parasites among the groups vaccinated with ISCOMATRIX DSPC or EPC compared with control group were not significant.
Figure 4.
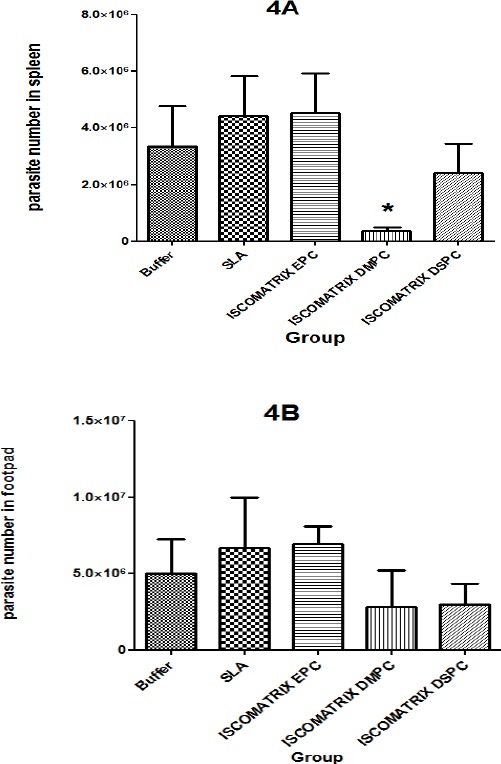
Parasite burden in spleen (4A) and footpad parasite burden (4B) in BALB/c mice. Mice immunized subcutaneously (SC), three times in 3-week intervals with soluble Leishmania antigens (SLA) SLA, ISCOMATRIX EPC, ISCOMATRIX DSPC and ISCOMATRIX DMPC, or buffer alone after challenge with Leishmania major promastigotes. A limiting dilution analysis was performed at week six post infection on the cells isolated from the spleen and foot of individual mice and cultured in tetra plicate in serial 8-fold dilutions. The wells were assessed microscopically for Leishmania major growth, and the number of viable parasite per spleen was determined by GraphPad Prism5 software. The bar represents the average score±SEM (n=3). * denote significant difference from all other formulations
Parasite burden in footpad after challenge
The number of viable L. major was quantified in the infected foot-pad of different groups of mice at day 42 after challenge (Figure 4B). The groups of mice immunized with ISCOMATRIX DMPC or DSPC showed the least parasite burden compared to the buffer group, but there was no significant difference in the number of parasites in the all vaccinated groups compared to the control group (P>0.05).
Antibody response
Serum samples were collected prior and post-challenge, and anti Leishmania IgG isotypes were tested to determine the type of immune response (Figure 5, 6). Before challenge as shown in Figure 5, significantly higher (P<0.0001) level of IgG2a and IgG1 antibodies was seen in the sera of mice immunized with ISCOMATRIX DMPC particularly in serum dilutions of 1:200 and 1:2000, and high level (P<0.0001) of IgG was seen in ISCOMATRIX EPC compared with control group. As shown in Figure 6, after challenge, the mice immunized with ISCOMATRIX DSPC showed the highest level of IgG and IgG2a antibodies (P<0.01) compared with control group. Although there was no significant differences in the level of IgG1 among different vaccinated groups compared to control group.
Figure 5.
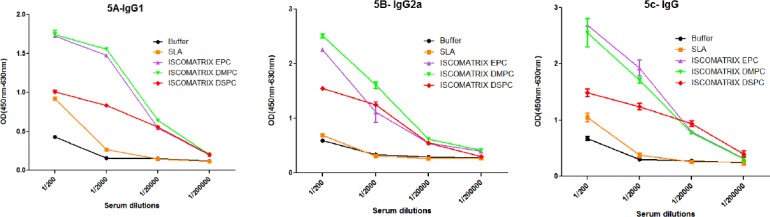
The levels of anti-SLA specific IgG1 (A), IgG2a (B), and IgG (C) antibodies based on mean absorbance in sera of BALB/c mice before challenge. Mice immunized SC, three times in 3-week intervals, with SLA, ISCOMATRIX EPC, ISCOMATRIX DSPC and ISCOMATRIX DMPC, or buffer alone. Blood samples were collected from the mice 2 weeks after the last booster. The assays were performed using ELISA method in triplicate at 200, 2000, 20,000 or 200,000-fold dilution for each serum sample. Values are the mean±SD
Figure 6.
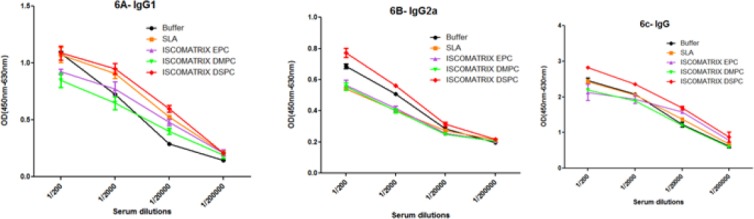
The levels of anti-SLA specific IgG1 (A), IgG2a (B), and IgG (C) antibodies based on mean absorbance in sera of BALB/c mice after challenge. Mice immunized SC, three times in 3-week intervals, with SLA, ISCOMATRIX EPC, ISCOMATRIX DSPC and ISCOMATRIX DMPC, or buffer alone. Blood samples were collected from the mice 6 weeks after challenge. The assays were performed using ELISA method in triplicate at 200, 2000, 20,000 or 200,000-fold dilution for each serum sample. Values are the mean±SD
In vitro cytokine production by splenocytes
In the day before challenge, splenocytes of three immunized mice were isolated and cultured. The results showed that the level of IFN-γ in the supernatant of splenocytes in the group of mice immunized with ISCOMATRIX DMPC was significantly (P<0.001) higher than the other groups. There was significant difference (P<0.01) in the level of IFN-γ among ISCOMATRIX EPC or ISCOMATRIX DSPC compared the buffer group (Figure 7A). The highest amount of IL-4 was detected in the splenocytes of mice immunized with ISCOMATRIX DSPC and ISCOMATRIX DMPC (p < 0.001) compared the buffer group (Figure 7B).
Figure 7.
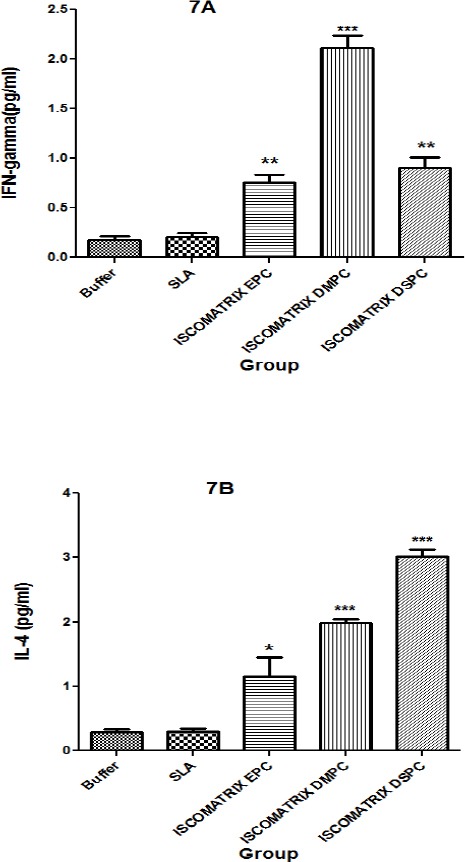
Splenic cell responses of BALB/c mice immunized (SC), Mice immunized three times in 3 weeks intervals, with SLA, ISCOMATRIX EPC, ISCOMATRIX DSPC and ISCOMATRIX DMPC, or buffer alone. Week 2 after the last booster, their spleens were removed and stimulated in vitro with SLA (10 µg/ml). Production of IFN-γ (7A) and IL-4 (7B), were assessed by sandwich ELISA with supernatants removed after 72 h of in vitro incubation. Cells from 3 mice per group were pooled. Each bar represents the mean ± SD of triplicate wells. *** (P< 0.001), ** (P< 0.01) and * (P< 0.05) denote significant difference from buffer and all other formulations.
Flowcytometery results
Splenocytes were isolated at 2 weeks after the last booster injection to determine the antigen-specific T cell responses in different groups of mice. Extracellular staining was used for CD4 and CD8 surface markers and intracellular cytokine staining was used for IFN-γ and IL-4 cytokines followed by flowcytometery analysis. CD4+ and CD8+ cells were studied concomitantly. CD8 and CD4 markers represent the frequency of IFN-γ and IL4 producing cells in Th1 and Th2 population, respectively. The results showed SLA formulation induced a significantly higher level of IFN-γ in CD8+ lymphocytes which represented a higher number of IFN-γ producing cells in CD8+ population in comparison with other groups (Figure 8A). The frequency of CD4+/IFN-γ cells in the group of mice immunized with ISCOMATRIX DMPC was significantly greater than the other groups (Figure 8B), while Flow-cytometric results also showed IL-4 production in CD4+ cells, that implies T cell-dependent humoral immunity, was not induced significantly in all groups when compared with control group (Figure 8C).
Figure 8.
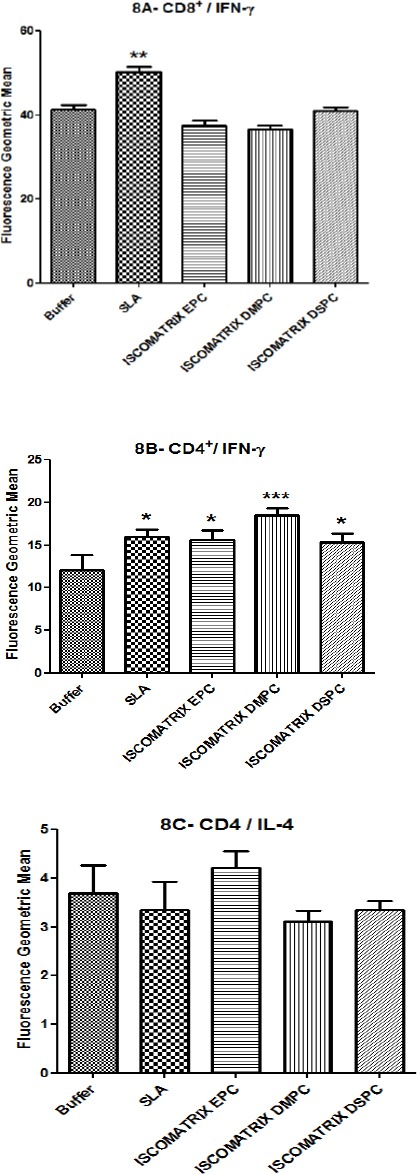
After last booster, splenocytes were isolated and restimulated, then stained for surface CD8, CD4 and intracellular IFN-γ and IL-4. Splenocytes were gated by side vs forward scatter light followed by staining with CD8Pe-cy5 and CD4Pe-cy5. Plots show log fluorescence intensity for IFN-γ- FITC and IL-4-PE. The data indicate the mean±SEM, (n= 3). *** (P<0.001), ** (P<0.01) and * (P<0.05) denote significant difference from buffer and all other formulations
Discussion
The aim of this study was to investigate the effect of phospholipid’s Tc on adjuvanticity of ISCOMATRIX against murine model of leishmaniasis. All ISCOMATRIX formulations were mixed with SLA as a crude antigen and used to immunize mice subcutaneously. The lesion size, splenic parasite burden, evaluation of Th1 cytokine (IFN-γ) and Th2 cytokine (IL-4), and titration of IgG isotypes were carried out to assess the type of generated immune response and extent of protection. The results indicated that different formulations induced a mixed Th1 and Th2 response in mice which were not protective. Protective immunity induced by vaccinations is dependent on the capacity of the vaccine to elicit the appropriate immune response able to control or eliminate the pathogen. Resistance to leishmaniasis is related to a predominant Th1 response with IFN- γ and interleukin-2 (IL-2) production from the antigen-specific CD4+ T lymphocyte population which mediates protective cellular immune responses (33-34). In addition, activation of CD8+ T cell population has been shown to play a critical role in protection after recovery from L. major infection and in effective vaccination against experimental murine leishmaniasis (35). Whereas Th2 cells produce interleukin-4 (IL-4), interleukin-5 (IL-5) and interleukin-10 (IL-10), which augment, humoral immune responses, with uncontrolled development of lesions, metastasis, susceptibility and exacerbation of the disease (36). The cytokine assay results showed that the highest level of IFN-γ production related to ISCOMATRIX DMPC and ISCOMATRIX DSPC (Figure 7A). Moreover, the highest amount of IL-4 was detected in the splenocytes of mice immunized with ISCOMATRIX DSPC and ISCOMATRIX DMPC. It seems that these formulations generated an immune response with a mixed Th1/Th2 response. Therefore, different phospholipids don’t have a significant role in the immune response. Presumably, Quil A as main adjuvant has a dominant effect and determines the final immune response that is a mixed Th1/Th2 response.
ISCOMATRIX vaccines have been shown in numerous studies in animal models to be potent inducers of both T helper (CD4+) and CTL (CD8+) T cell responses to a wide variety of antigens, including naturally occurring immunogens, recombinant proteins, and peptides (37,38). Mice immunized with trans-sialidase-ISCOMATRIX subunit vaccine increased IFN-γ secretion by (CD8+) T lymphocyte (39). Whereas in current study, CD4+ cells were more than CD8+ cells responsible for the secretion of IFN-γ. Vaccination with a recombinant Parasite Surface Antigen 2 from L. major, purified from Escherichia coli and administered in ISCOMs or with Cryptospridium parvum as adjuvant, does not induce protective immunity (40). In another investigation, ISCOMs with gp63, although, could modulate the immune response of vaccinated BALB/c mice preferentially towards a Thl rather than a Th2-type response, induced a partial protection against Leishmania challenge (41). It seems that Quil A is an important factor in determining the type of immune response. Quil A is a heterogeneous mixture of more than 20 components (42). Quil A sub-fractions designated as QH-A, QH-B and QH-C, have been shown to differ in their adjuvant and immunomodulatory properties and also in the toxicity profiles (43). Individual saponins from Quil A such as QS-7, QS-17, QS-18 and QS-21 have also shown different immune stimulating properties. For example, QS-21 was shown to be a potent adjuvant, inducing primarily Th1 immune responses and CTL responses (42). This specification of Quil A can strongly affect the properties such as interaction with antigen presenting cells (APC), activation of CTL and stimulation of T helper (Th) cell subsets. This broad immune response is dependent on the induction of multiple innate and adaptive immune mediators, cellular processes and the interplay between these elements (44, 37).
Conclusion
In summary, the current results showed that EPC, DMPC or DSPC ISCOMATRIX with different Tc, generated a mixed Th1/Th2 immune response that was not protective, despite the activation of large numbers of CD4+ T cells with secreting IFN-γ.
Acknowledgment
The financial support of the Nanotechnology Research Center and Biotechnology Research Center, Mashhad University of Medical Sciences (MUMS) are gratefully acknowledged. This study was part of dissertation of AM that was completed in Nanotechnology Research Center, MUMS, Iran.
References
- 1.World Health Organization. Control of the leishmaniases. World Health Organ. Tech Rep Ser. 2010:1–186. [PubMed] [Google Scholar]
- 2.World Health Organization. Control of the Leishmaniases. Report of a WHO expert committee. Technical report series. 1990;793 [PubMed] [Google Scholar]
- 3.Alvar J, Croft S, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31:244–249. doi: 10.1016/j.vaccine.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 4.Thakur CP, Kumar K. Post Kala-azar dermal leishmaniasis:a neglected aspect of kala-azar control programmes. Ann Trop Med Parasitol. 1992;86:355–359. doi: 10.1080/00034983.1992.11812678. [DOI] [PubMed] [Google Scholar]
- 5.Bryceson A. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop Med Int Health. 2001;6:928–934. doi: 10.1046/j.1365-3156.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 6.Croft SL, Coombs GH. Leishmaniasis current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. Plos Med. 2006;3:659–667. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 9.Grencis RK. Th2-mediated host protective immunity to intestinal nematode infections. Philos Trans R Soc Lond B Biol Sci. 1997;352:1377–1384. doi: 10.1098/rstb.1997.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89:83–88. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- 11.Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, Noazin S, et al. Leishmanization:use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23:3642–3648. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Modabber F, Coler R, Reed SG. Vaccines against Leishmania. In: Modabber F, editor. New Generation Vaccines. New York: E-Publishing Inc; 2009. pp. 273–283. [Google Scholar]
- 13.Mutiso JM, Macharia JC, Mutisya RM, Taracha E. Subcutaneous immunization against Leishmania major infection in mice. Efficacy of formalin-killed promastigotes combined with adjuvants. Rev. Inst. Med. Trop. Sao Paulo. 2012;52:95–100. doi: 10.1590/s0036-46652010000200006. [DOI] [PubMed] [Google Scholar]
- 14.Copland MJ, Rades T, Davies NM, Baird MA. Lipid based particulate formulations for the delivery of antigen. Immunol Cell Bio. 2005;83:97–105. doi: 10.1111/j.1440-1711.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 15.Badiee A, Heravi SV, Khamesipour A, Jaafari MR. Micro/nanoparticle adjuvants for anti leishmanial vaccines:present and future trends. Vaccine. 2013;31:735–749. doi: 10.1016/j.vaccine.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 16.Hong-Xiang S, Yong X, Yi-Ping Y. ISCOMs and ISCOMATRIX TM. Vaccine. 2009;27:4388–4401. doi: 10.1016/j.vaccine.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Rimmelzwaan GF, Osterhaus ADME. In: A novel generation of viral vaccines based on ISCOM matrix. In Vaccine Design, the Subunit and Adjuvant Approach. M. F Powell, M. J Newman., editors. New York: Plenum; 1995. pp. 543–558. [DOI] [PubMed] [Google Scholar]
- 18.Lövgren K, Morein B, Osterhaus A. ISCOM technology-based Matrix M™adjuvant:success in future vaccines relies on formulation. Expert Rev Vaccines. 2011;10:401–403. doi: 10.1586/erv.11.25. [DOI] [PubMed] [Google Scholar]
- 19.Sanders MT, Brown LE, Deliyannis G, Pearse MJ. ISCOM-based vaccines:the Second decade. Immunol Cell Biol. 2005;83:119–128. doi: 10.1111/j.1440-1711.2005.01319.x. [DOI] [PubMed] [Google Scholar]
- 20.Sjölander A, Drane D, Maraskovsky E, Scheerlinck JP, Suhrbier A, Tennent A, et al. Immune responses to ISCOM formulations in animal and primate models. Vaccine. 2001;19:2661–2665. doi: 10.1016/s0264-410x(00)00497-7. [DOI] [PubMed] [Google Scholar]
- 21.Barr IG, Sjolander A, Cox JC. ISCOMs and other saponin based adjuvants. Adv Drug Del Rev. 1998;32:247–271. doi: 10.1016/s0169-409x(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 22.Sjolander A, Cox JC, Barr IG. ISCOMs:an adjuvant with multiple functions. J leukoc Biol. 1998;64:713–723. doi: 10.1002/jlb.64.6.713. [DOI] [PubMed] [Google Scholar]
- 23.Cox JC, Drane D, Suhrbier A. Immunogenic complex and methods relating there to 2000; PCT/AU00/00110 [Google Scholar]
- 24.Behboudi S, Morein B, Vilacres-Eriksson M. In vitro activation of antigen-presenting cells (APC) by defined composition of Quillaja saponaria Molina triterpenoids. Clin Exp Immunol. 1996;105:26–30. doi: 10.1046/j.1365-2249.1996.d01-730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development:a global overview. IJMR. 2006;123:423–438. [PubMed] [Google Scholar]
- 26.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–227. [PubMed] [Google Scholar]
- 27.Bainor A, Chang L, McQuade TJ, Webb B, Gestwicki JE. Bicinchoninicacid (BCA) assay in low volume. Anal Biochem. 2010;410:310–402. doi: 10.1016/j.ab.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Liang MT, Toth I, Davies NM. A novel method for preparing immune stimulating complexes (ISCOMs) by hydration of freeze-dried lipid matrix. Eur J Pharm Biopharm. 2008;68:840–845. doi: 10.1016/j.ejpb.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Jaafari MR, Ghafarian A, Farrokh-Gisour A, Samiei A, Kheiri MT, Mahboudi F, et al. Immune response and protection assay of recombinant major surface glycoprotein of leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine. 2006;24:5708–5717. doi: 10.1016/j.vaccine.2006.04.062. [DOI] [PubMed] [Google Scholar]
- 30.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 31.Badiee A, Jaafari MR, Khamesipour A. Leishmania major:immune response in BALB/c mice immunized with stress-inducible protein 1 encapsulated in liposomes. Exp Parasitol. 2007;115:127–134. doi: 10.1016/j.exppara.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Taswell C. Limiting dilution assays for the determination of immuno competent cell frequencies. 1. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 33.Jaafari MR, Badiee A, Khamesipour A, Samiei A, Soroush D, Kheiri MT, et al. The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine. 2007;25:6107–6117. doi: 10.1016/j.vaccine.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Golali E, Jaafari MR, Khamesipour A, Abbasi A, Saberi Z, Badiee A. Comparison of in vivo Adjuvanticity of Liposomal PO CpG ODN with Liposomal PS CpG ODN:Soluble Leishmania Antigens as a Model. Iran J Basic Med Sci. 2012;15:1032–1045. [PMC free article] [PubMed] [Google Scholar]
- 35.Hejazi H, Tasbihi M, Jaafari M, Badiee A, Pestechian N, Javadi A, et al. The role of liposomal CpG ODN on the course of L. major infection in BALB/C mice. IJP. 2010;5:47–54. [PMC free article] [PubMed] [Google Scholar]
- 36.Vanloubbeeck Y, Jones DE. The immunology of Leishmania infection and the implications for vaccine development. Ann N Y Acad Sci. 2004;1026:267–272. doi: 10.1196/annals.1307.041. [DOI] [PubMed] [Google Scholar]
- 37.Drane D, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev Vaccines. 2007;6:761–772. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]
- 38.Silva A, Mount A, Krstevska K, Pejoski D, Hardy MP, Owczarek C, et al. The combination of ISCOMATRIX adjuvant and TLR agonists induces regression of established solid tumors in vivo. J Immunol. 2015;194:2199–2207. doi: 10.4049/jimmunol.1402228. [DOI] [PubMed] [Google Scholar]
- 39.Bontempi IA, Vicco MH, Cabrera G, Villar SR, González FB, Roggero EA, et al. Efficacy of a trans-sialidase-ISCOMATRIX subunit vaccine candidate to protect against experimental Chagas disease. Vaccine. 2015;33:1274–1283. doi: 10.1016/j.vaccine.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 40.Sjölander A, Baldwin TM, Curtis JM, Bengtsson KL, Handman E. Vaccination with recombinant Parasite Surface Antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine. 1998;16:2077–2084. doi: 10.1016/s0264-410x(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 41.Papadopoulou G, Karagouni E, Dotsika E. ISCOMs vaccine against experimental leishmaniasis. Vaccine. 1998;16:885–892. doi: 10.1016/s0264-410x(97)00308-3. [DOI] [PubMed] [Google Scholar]
- 42.Kensil CR. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 43.Rönnberg B, Fekadu M, Morein B. Adjuvant activity of non-toxic Quillaja saponaria Molina components for use in ISCOM matrix. Vaccine. 1995;13:1375–1382. doi: 10.1016/0264-410x(95)00105-a. [DOI] [PubMed] [Google Scholar]
- 44.Drane D, Pearse M. The ISCOMATRIX adjuvant. In: Schijns VE, O’Hagan DT, editors. Immunopotentiators in Modern Vaccines. Amsterdam; Boston: Elsevier Academic Press; 2006. pp. 191–216. [Google Scholar]


