Abstract
Objective(s):
Dependence and tolerance to opioid analgesics are major problems limiting their clinical application. α-Terpineol is a monoterpenoid alcohol with neuroprotective effects which is found in several medicinal plants such as Myrtus communis, Laurus nobilis, and Stachys byzantina. It has been shown that some of these medicinal plants such as S. byzantina attenuate dependence and tolerance to morphine. Since α-terpineol is one of the bioactive phytochemical constituent of these medicinal plants, the present study was conducted to investigate the effects of α-terpineol on morphine-induced dependence and tolerance in mice.
Materials and Methods:
The mice were rendered dependent or tolerant to morphine by a 3-day administration schedule. The hot-plate test and naloxone-induced withdrawal syndrome were used to evaluate tolerance and dependence on morphine, respectively. To investigate a possible role for nitric oxide (NO) in the protective effect of α-terpineol, the NO synthase inhibitor, L-N(G)-nitroarginine methyl ester (L-NAME) and NO precursor, L-arginine, were used.
Results:
Administration of α-terpineol (5, 10, and 20 mg/kg, IP) significantly decreased the number of jumps in morphine dependent animals. Moreover, α-terpineol (20 and 40 mg/kg, IP) attenuated tolerance to the analgesic effect of morphine. The inhibitory effects of α-terpineol on morphine-induced dependence and tolerance were enhanced by pretreatment with L-NAME (10 mg/kg, IP). However, L-arginine (300 mg/kg, IP) antagonized the protective effects of α-terpineol on dependence and tolerance to morphine.
Conclusion:
These findings indicate that α-terpineol prevents the development of dependence and tolerance to morphine probably through the influence on NO production.
Keywords: α-Terpineol, Dependence, Monoterpenoid, Morphine, Nitric oxide, Tolerance
Introduction
Dependence and tolerance to opioid analgesics are major problems limiting the clinical application of these drugs (1). Many attempts have been carried out to resolve this problem but there is still the need for efficacious solution. In this regard, various natural compounds, medicinal herbs, and their active constituents have been investigated for possible inhibitory effect on opioid dependence and tolerance (2). Several studies have shown that herbal extracts or essential oils may be beneficial in the prevention of dependence and tolerance to opioid analgesics (2-4).
One of the most biologically active and best studied herbal compounds, are monoterpenoids. Monoterpenoids are considered as a substructure of an important naturally occurring organic compound named terpenes (5). They have a wide variety of structures but generally consist of two isoprene units. They are volatile oils with highly distinctive aromas and flavors (5, 6). More than 90% of the essential oils of medicinal plants consist of monoterpenoids (5). Monoterpenoids have been extensively studied for their potentially therapeutic effects (6-8). Amongst monoterpenoids, α-terpineol is emerging as a unique molecule with outstanding therapeutic effects. Several pharmacological properties including antioxidant (9, 10), anticonvulsant (11), and antinociceptive (7, 12, 13) effects have been attributed to α-terpineol. Analytical studies have shown that α-terpineol is the major constituent of several medicinal plants such as Eucalyptus spp. (14, 15), Myrtus communis (16-18), Laurus nobilis (19), and Stachys byzantina (20). In addition, it has been reported that some of these medicinal plants such as S. byzantina afford protection against morphine-induced dependence and tolerance (21). Since α-terpineol is one of the active constituent of S. byzantina, it can be hypothesized that the preventive effects of S. byzantina on morphine-induced dependence and tolerance may arise from the neuroprotective effects of α-terpineol.
Moreover, several lines of evidence indicate that nitric oxide (NO) system might be involved in the development of dependence and tolerance to opioid analgesics (22, 23). In support of this view, various NO synthase (NOS) inhibitors have been used to attenuate morphine-induced dependence and tolerance (22-24). In this regard, it has been reported that some of the pharmacological effects of α-terpineol is exerts through the inhibition of NO production (12).
Based on these evidences, the present study was carried out in order to clarify the effects of α-terpineol on the development and the expression of dependence and tolerance to morphine in mice. Also, the involvement of NO system in the preventive effects of α-terpineol on morphine-induced dependence and tolerance was evaluated through the use of NOS inhibitor, L-N(G)-nitroarginine methyl ester (L-NAME) and NO precursor, L-arginine.
Materials and Methods
Animals
Adult male NMRI mice weighing 20-30 g were used. The animals were kept at 23±2 °C on a 12 hours light/dark cycle. Commercial food pellets and tap water were freely available at all times except during the experiments. The study was performed in accordance with the guidelines of Shahid Beheshti University of Medical Sciences for laboratory animal care and use.
Drugs
The following drugs were used in this study: α-terpineol (Sigma-Aldrich), morphine sulfate (Temad, Iran), naloxone hydrochloride (Inresa Arzneimittel GmbH, Germany), L-N(G)-nitroarginine methyl ester (Sigma-Aldrich), L-arginine (Sigma-Aldrich). Drugs were dissolved in normal saline. α-Terpineol was suspended in 0.8% (v/v) Tween 80. All compounds were prepared freshly and administered intraperitoneally (IP) in a volume of 0.1 ml/20 g body weight. Control animals received the same volume of normal saline + Tween 80 as the vehicle.
Induction of dependence and tolerance
In order to induction of morphine dependence and tolerance in mice, morphine was injected intraperitoneally three times daily at 9 am (50 mg/kg), 1 pm (50 mg/kg), and 5 pm (75 mg/kg) for 3 consecutive days. The dose of 75 mg/kg at 5 pm was administered to prevent overnight withdrawal syndrome in mice. One more dose of morphine (50 mg/kg, IP in morphine-dependent and 10 mg/kg, IP in morphine-tolerant mice) was administered at 9 am on the fourth day (25). Straub-tail reaction and hyperactivity were seen after the injection of morphine in mice. Weight loss and death were recorded during the administration of morphine.
Evaluation of the effect of α-terpineol on morphine dependence
The animals subjected to morphine dependence were randomly allocated into 7 groups (each consisting of 7 mice): Group 1 (control) received vehicle (normal saline + Tween 80). Groups 2, 3, and 4, received different single doses of α-terpineol (5, 10, and 20 mg/kg, IP) 30 min before the injection of naloxone on the fourth day. These groups of animals (2 to 4) were used to evaluate the effect of α-terpineol on the expression of morphine dependence. Groups 5, 6, and 7, received different doses of α-terpineol (5, 10, and 20 mg/kg, IP) three times daily, 30 min before each dose of morphine on days 1 to 3. These three groups (5 to 7) were considered to evaluate the effect of α-terpineol on the induction of morphine dependence.
Two hours after the administration of the last dose of morphine (50 mg/kg) on the fourth day, a single dose of naloxone (2 mg/kg, IP), the selective antagonist of opioid receptors, was injected to the animals (groups 1 to 7) to precipitate withdrawal syndrome. After the injection of naloxone, mice were immediately placed individually in a Plexiglas box and the number of jumps, as the sign of morphine withdrawal syndrome, was recorded for 30 min (25).
Evaluation of the effect of α-terpineol on morphine tolerance
The animals subjected to morphine tolerance were randomly allocated into 7 groups (each consisting of 7 mice): Group 8 (control) received vehicle (normal saline + Tween 80). Groups 9, 10, and 11 received α-terpineol (10, 20, and 40 mg/kg, IP) 30 min before each dose of morphine during 3 days. These groups of animals (9 to 11) were considered to evaluate the effects of α-terpineol on the development of tolerance to analgesic effects of morphine. Groups 12, 13, and 14 received different single doses of α-terpineol (10, 20, and 40 mg/kg, IP) 30 min before the injection of morphine (10 mg/kg, IP) on the fourth day. These groups of mice (12 to 14) were considered to assess the effect of α-terpineol on the expression of tolerance to morphine. Moreover, the analgesic effect of α-terpineol (10, 20, and 40 mg/kg, IP) was evaluated in 3 more groups of mice to determine whether or not, α-terpineol possesses antinociceptive effect on its own.
In order to assess tolerance to analgesic effects of morphine, the hot-plate test was used. Thirty minutes after the injection of morphine (10 mg/kg, IP) at the beginning (day 1) and the end (day 4) of treatment, the mice in groups 8 to 14 were placed on the hot-plate (55±1 °C) and the latency of nociceptive responses (licking or flicking of the limbs or jumping) were measured. A cut-off time of 40 sec was selected to prevent tissue damage (26).
Evaluation of the role of NO in morphine dependence and tolerance
In order to assess the possible role of NO in the preventive effects of α-terpineol on morphine-induced dependence and tolerance, NO synthase inhibitor, L-N(G)-nitroarginine methyl ester (L-NAME) and NO precursor, L-arginine, were used. Animals were divided into 4 groups (n=7) and were then rendered tolerant to (groups 15 and 16) or dependent on (groups 17 and 18) morphine by a 3-day administration schedule. Thirty min before the injection of α-terpineol (20 mg/kg, IP), L-NAME (10 mg/kg, IP) was given to the animals in groups 15 and 17, and L-arginine (300 mg/kg, IP) was given to the animals in groups 16 and 18.
To further examine whether L-NAME or L-arginine afford protection against morphine-induced tolerance or physical dependence, in an additional set of experiments, the mice subjected to dependence or tolerance to morphine received L-NAME (10 mg/kg, IP) or L-arginine (300 mg/kg, IP) in the absence of α-terpineol.
Evaluation of locomotor activity
The effect of α-terpineol on the locomotor activity of the animals was assessed using open-field test. The apparatus, made of white wood, had a floor of 100 × 100 cm divided by red lines into 25 squares of 20 × 20 cm. The walls (30 cm high) were also painted in white. Thirty min after the injection of α-terpineol (5, 10, and 20 mg/kg, IP), the animals were placed in the center of the open-field and their behavior was observed for 10 min. The total number of squares crossed by each mouse (total locomotion) and the numbers of rearings (standing on two hind paws without touching the walls) and leanings (one or two paws in contact with the wall) were recorded (27).
Statistical analysis
The data were expressed as mean values±SEM for each group (n=7) and tested with analysis of variance (ANOVA) followed by the multiple comparison test of Tukey. Differences between means were considered significant if P<0.05.
Results
Effects of α-terpineol on morphine dependence
Administration of naloxone (2 mg/kg, IP) two hours after the injection of the last dose of morphine (50 mg/kg, IP) on the fourth day, precipitated the jumping behavior as the major sign of withdrawal syndrome in control group (Figures 1 and 2). This indicated that repeated administration of morphine has induced physical dependence in mice.
Figure 1.
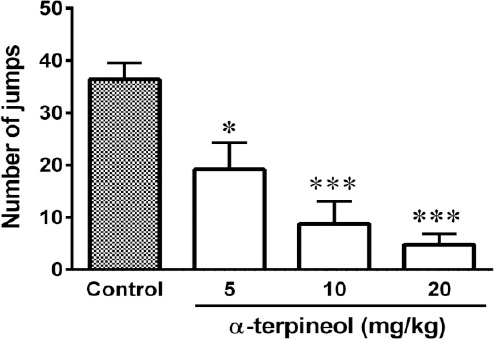
The effect of α-terpineol on the expression of morphine dependence in mice. The animals received morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. Two hours after the administration of the last dose of morphine (50 mg/kg, IP) on day 4, naloxone (2 mg/kg, IP) was injected to the animals. α-Terpineol and control were administered 30 min before the injection of naloxone. Control: Normal saline + Tween 80; Values are expressed as mean±SEM for 7 mice; *P<0.05, ***P<0.001, as compared to control
Figure 2.
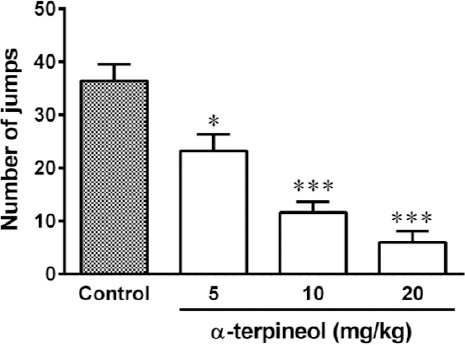
The effects of α-terpineol on the induction of morphine dependence in mice. The animals received morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. α-Terpineol and control were administered 30 min before each dose of morphine on days 1 to 3. Two hours after the administration of the last dose of morphine (50 mg/kg, IP) on day 4, naloxone (2 mg/kg, IP) was injected to the animals. Control: Normal saline + Tween 80; Values are expressed as mean±SEM for 7 mice; *P<0.05, ***P<0.001, as compared to control
Administration of different single doses of α-terpineol (5, 10, and 20 mg/kg, IP) 30 min before the injection of naloxone (2 mg/kg, IP) on the fourth day, significantly decreased the number of jumps in morphine dependent mice (Figure 1). Moreover, repeated administration of α-terpineol (5, 10, and 20 mg/kg, IP) 30 min before each injection of morphine during the days 1 to 3, significantly decreased the number of jumps in morphine dependent mice (Figure 2). Loss of weight (5-10%) occurred with chronic administration of morphine sulfate.
Effects of α-terpineol on morphine tolerance
Administration of a single dose of morphine (10 mg/kg, IP) significantly prolonged the hot-plate latency in mice at the beginning of the experiment (day 1), while following repeated administration of morphine during 3 days, tolerance to morphine developed and the analgesic effect of morphine abolished (P<0.001, Figures 3 and 4). But, administration of α-terpineol (20 and 40 mg/kg, IP) 30 min before each dose of morphine during 3 days, significantly attenuated the development of morphine tolerance (Figure 3). The administration of single doses of α-terpineol (10, 20, and 40 mg/kg, IP) 30 min before the injection of morphine (10 mg/kg, IP) on day 4, could not restore analgesic effect of morphine (Figure 4). The attenuating effect of α-terpineol on the development of morphine tolerance was not attributed to the analgesic effects of α-terpineol, because none of doses of α-terpineol used in this study (5, 10, 20, and 40 mg/kg), produced any antinociceptive effects on their own (Figure 5).
Figure 3.
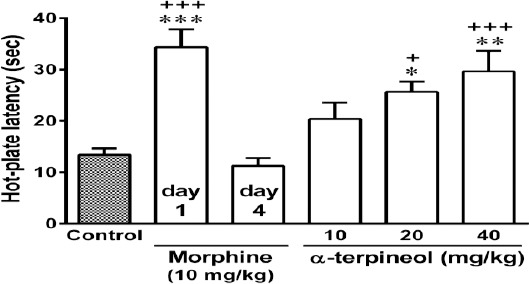
The effects of α-terpineol on the induction of morphine tolerance in mice. First, an analgesic dose of morphine (10 mg/kg, IP) was injected in mice (day 1). Then, the animals were rendered tolerant to morphine by the injection of morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. α-Terpineol and control were administered 30 min before each dose of morphine on days 1 to 3. Thirty minutes after the injection of morphine (10 mg/kg, IP) on day 4, the hot-plate test was performed. Control: Normal saline + Tween 80; Values are expressed as mean±SEM for 7 mice; *P<0.05, **P<0.01, ***P<0.001 (compared to control); +P<0.05, +++P<0.001 (compared to morphine on day 4)
Figure 4.
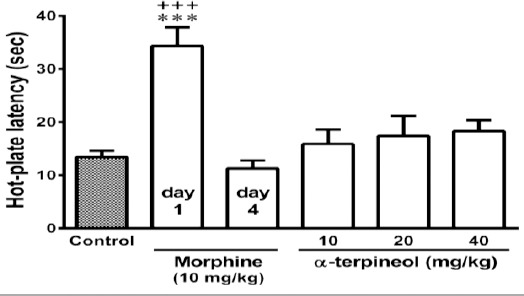
The effects of α-terpineol on the expression of tolerance to analgesic effects of morphine in mice. First, an analgesic dose of morphine (10 mg/kg, IP) was injected in mice (day 1). Then, the animals were rendered tolerant to morphine by the injection of morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. Thirty minutes after the injection of the last dose of morphine (10 mg/kg, IP) on day 4, the hot-plate test was performed. α-Terpineol and control were administered 30 min before the injection of the last dose of morphine (10 mg/kg, IP) on day 4. Control: Normal saline + Tween 80; Values are expressed as mean±SEM for 7 mice; ***P<0.001 (compared to control); +++P<0.001 (compared to morphine on day 4)
Figure 5.
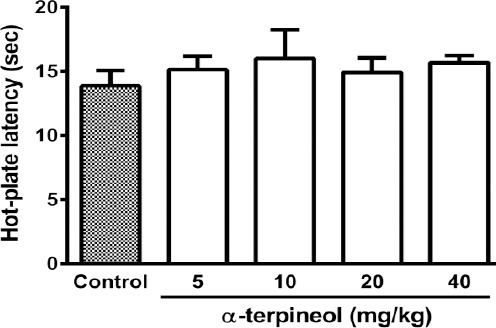
The effects of α-terpineol on the nociceptive responses of mice in hot-plate test. Thirty min after the injection of α-terpineol, animals were placed on the hot-plate and the latency of nociceptive response was recorded. Control: Normal saline+ Tween 80; Values are expressed as mean±SEM for 7 mice Control: Normal saline + Tween 80; Values are expressed as mean±SEM for 7 mice
Effect of L-NAME and L-arginine on the preventive effects of α-terpineol on morphine-induced tolerance and dependence
Pretreatment of animals with L-arginine (300 mg/kg, IP) for 3 days antagonized the attenuating effect of α-terpineol on morphine tolerance (Figure 6). However, the administration of L-NAME (10 mg/kg, IP) before α-terpineol (20 mg/kg, IP) during the 3-day administration schedule did not have any effect on the inhibitory effect of α-terpineol on the development of tolerance to analgesic effect of morphine (Figure 6). In addition, the attenuating effect of α-terpineol on the development of morphine withdrawal syndrome following the naloxone injection was enhanced by the administration of L-NAME (10 mg/kg, IP) 30 min before each injection of α-terpineol (20 mg/kg, IP) during 3 days. However, the difference between two groups was not statistically significant (P=0.49). Pretreatment of mice with L-arginine (300 mg/kg, IP) significantly abolished the inhibitory effect of α-terpineol on the development of morphine dependence (Figure 7). Furthermore, the results showed that pretreatment of animals with L-NAME (10 mg/kg, IP) or L-arginine (300 mg/kg, IP) did not attenuate the development of dependence and tolerance to morphine on their own (Figure 8).
Figure 6.
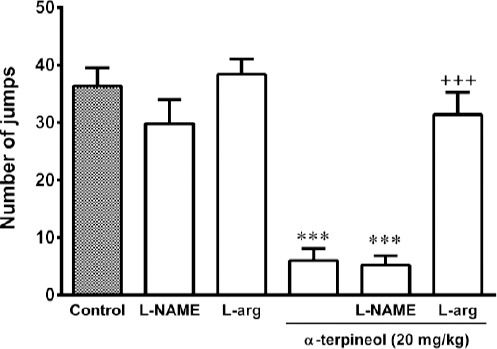
The effects of L-NAME and L-arginine on the inhibitory effects of α-terpineol on morphine dependence in mice. The animals were rendered dependent on morphine by the injection of morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. α-Terpineol (20 mg/kg, IP) and control were administered 30 min before each dose of morphine during 3 days. L-NAME (10 mg/kg, IP) and L-arginine (300 mg/kg, IP) were injected 30 min before α-terpineol. Two hours after the administration of the last dose of morphine (50 mg/kg, IP) on day 4, naloxone (2 mg/kg, IP) was injected to the animals. Control: Normal saline + Tween 80; L-arg: L-arginine; Values are expressed as mean±SEM for 7 mice; ***P<0.001 (compared to control); +++P<0.001 (compared to α-terpineol alone)
Figure 7.
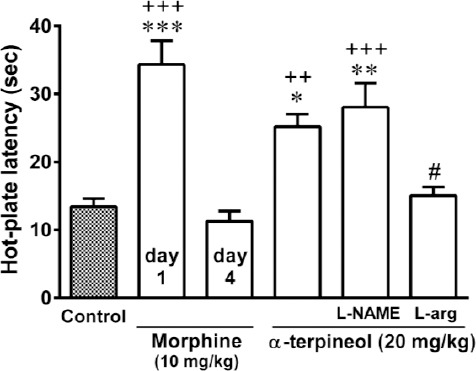
The effects of L-NAME and L-arginine on the inhibitory effects of α-terpineol on morphine tolerance in mice. The animals were rendered tolerant to morphine by the injection of morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. α-Terpineol (20 mg/kg, IP) and control were administered 30 min before each dose of morphine during 3 days. L-NAME (10 mg/kg, IP) and L-arginine (300 mg/kg, IP) were injected 30 min before α-terpineol. Thirty minutes after the injection of the single dose of morphine (10 mg/kg, IP) on day 4, the hot-plate test was performed. Control: Normal saline + Tween 80; L-arg: L-arginine; Values are expressed as mean±SEM for 7 mice; *P<0.05, **P<0.01, ***P<0.001 (compared to control); ++P<0.01, +++P<0.001 (compared to morphine on day 4); #P<0.05 (compared to α-terpineol alone)
Figure 8.
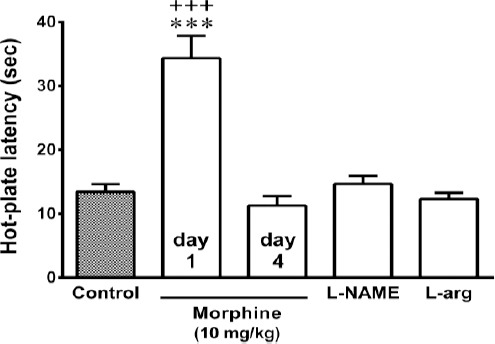
The effects of L-NAME and L-arginine on morphine tolerance in mice. The animals were rendered tolerant to morphine by the injection of morphine 3 times daily (50, 50, and 75 mg/kg, IP) for 3 days. L-NAME (10 mg/kg, IP) and L-arginine (300 mg/kg, IP) were administered 30 min before each dose of morphine during 3 days. Thirty minutes after the injection of the single dose of morphine (10 mg/kg, IP) on day 4, the hot-plate test was performed. Control: Normal saline; L-arg: L-arginine; Values are expressed as mean±SEM for 7 mice; ***P<0.001 (compared to control); +++P<0.001 (compared to morphine on day 4)
Effects of α-terpineol on locomotor activity
In the open-field test, α-terpineol (5, 10, and 20 mg/kg) did not have any effect on the total locomotion and numbers of leanings and rearings in mice (Table 1).
Table 1.
The effect of α-terpineol on leaning, rearing, and total locomotion of mice in open-field test
| Treatment | Rearing | Leaning | Total locomotion |
|---|---|---|---|
| Control | 13.1±7.7 | 43.9±3.6 | 230.6±14.3 |
| α-Terpineol (5 mg/kg, IP) | 14.8±6.7 | 35.8±6.3 | 209.6±18.3 |
| α-Terpineol (10 mg/kg, IP) | 15.5±2.8 | 30.5±4.2 | 250.5±10.3 |
| α-Terpineol (20 mg/kg, IP) | 11.1±2.8 | 36.3±3.9 | 219.4±14.9 |
Control: Normal saline + Tween 80; Values are expressed as mean±SEM for 7 mice
Discussion
The results of this study clearly showed that α-terpineol inhibited the induction of dependence on morphine and attenuated the signs of withdrawal syndrome in morphine-dependent mice. In addition, α-terpineol prevented the development of tolerance to analgesic effects of morphine. The present study also provided evidence for the involvement of NO in the inhibitory effects of α-terpineol on morphine-induced dependence and tolerance.
In consistent with many studies (24, 25, 28), the results obtained from this study showed that repeated administration of morphine during a 3-day treatment schedule, induced physical dependence in mice so that the injection of naloxone elicited morphine withdrawal manifestations (Figures 1 and 2). The results also showed that repeated administration of morphine over the course of 3 days resulted in a progressive decrease in the pain threshold in mice which reflects development of tolerance to the analgesic effect of morphine (Figures 3 and 4).
The results of the present study showed that the pretreatment of animal with either single or repeated doses of α-terpineol, prevented dependence on morphine and attenuated the signs of withdrawal syndrome in a dose-dependently manner (Figures 1 and 2). On the other hand, repeated administration of α-terpineol (20 and 40 mg/kg) before each dose of morphine during 3 days, dose-dependently prolonged the hot-plate latency in mice (Figure 3). This finding indicates that α-terpineol can prevent the induction of tolerance to the analgesic effect of morphine.
As shown in Figure 5, α-terpineol alone did not have any antinociceptive effect. This finding indicates that the inhibitory effect of α-terpineol on the induction of morphine tolerance is not attributed to the analgesic effects of α-terpineol. In confirmation of this finding, our results showed that the administration of different single doses of α-terpineol before the injection of the last dose of morphine on the fourth day could not restore the analgesic effect of morphine (Figure 4). It should be noted that the dosing regimen used in the present study is considerably lower than that used in previous studies of α-terpineol. There have been reports of antinociceptive effects of α-terpineol at doses higher than those used in the present study (7, 12, 13). What is certain is that repeated (but not single) administration of non-analgesic doses of α-terpineol might be efficacious against development of morphine tolerance.
Tolerance to opioid drugs, which result in the loss of their analgesic effects, necessitate the use of higher dose of the opioid agent for equivalent analgesic effect. This may also lead to dependence on opioid drug and further complicate the process of pain management (1). Several mechanisms have been suggested to be involved in the induction and development of tolerance and dependence to opioids (29). It is becoming increasingly evident that the production of NO and the activity of NOS enzyme contribute in the development of morphine dependence and tolerance. In support of this view, it has been well established that L-NAME, as a non-selective NOS inhibitor (23), 7-nitroindazole, the selective inhibitor of neuronal NOS (30), and aminoguanidine, the selective inhibitor of inducible NOS (31), attenuate the development of morphine tolerance and dependence. Several naturally occurring organic compounds with inhibitory action on NOS enzyme have been also used to attenuate dependence and tolerance to morphine and their beneficial effects have been well established. For example, it has been reported that Nigella sativa oil and its major constituent, thymoquinone, attenuated tolerance and dependence to morphine and tramadol through inhibiting the activity of NOS enzyme (24, 32).
Consistent with these findings, the results obtained from the present study showed that the inhibition of NOS enzyme by L-NAME potentiated the attenuating effect of α-terpineol on morphine tolerance, however the effect was not statistically significant (P=0.49). Conversely, pretreatment of animals with L-arginine, the only metabolic precursor for NO biosynthesis, significantly antagonized the preventive effect of α-terpineol against morphine dependence and tolerance. Our results also showed that the administration of L-NAME or L-arginine could not diminish morphine-induced dependence and tolerance in the absence of α-terpineol. These results were obtained from repeated administration of non-analgesic doses of L-NAME or L-arginine. Some evidence indicates that L-NAME at doses higher than those used in the present study may prevent the development of tolerance to the analgesic effect of morphine. For example it has been reported that L-NAME at the doses of 20 to 200 mg/kg, IP, given in single or repeated doses, produced a significant decrease of naloxone-precipitated withdrawal jumping (33, 34). However, L-NAME at such a high doses significantly increases the pain threshold on nociceptive assay (35, 36). Collectively, these observations provide evidence implicating the contribution of NO in the inhibitory effects of α-terpineol on the development of morphine dependence and tolerance. These results are consistent with the findings of the previous studies indicating the inhibitory effect of α-terpineol on the production of NO (12).
α-Terpineol and its parent medicinal plants including Eucalyptus spp., M. communis, L. nobilis, and S. byzantine are amongst well-studied antioxidant compounds (37-42). Several studies have shown that oxidative stress might be involved in the induction and development of dependence on and tolerance to opioid analgesics (22, 23). In support of this view, various antioxidant agents have been used to attenuate morphine-induced dependence and tolerance. For example, it has been reported that N. sativa oil and its major constituent, thymoquinone, inhibit tolerance to and dependence on morphine through the inhibition of oxidative stress (24, 32). Consistent with these findings, there are some evidence indicate that α-terpineol functions as a potent antioxidant and radical scavenger (9, 10). It is thus suggested that the antioxidant activity of α-terpineol, may play a role in the attenuation of morphine dependence and tolerance. However, this effect needs to be assessed in further investigations.
The results obtained from open-field test showed that α-terpineol did not have any effect on the numbers of leanings, rearings, and total locomotion in mice (Table 1). This precludes the possibility that the impairment of locomotor activity by α-terpineol may have had some influence on behavioral responses of animals in both jumping and hot-plate tests.
Conclusion
Based on the results of this study, it can be concluded that α-terpineol can attenuate dependence on and tolerance to morphine in mice. These effects of α-terpineol may be mediated through the inhibition of NO over-production. The current study provides evidence for the effectiveness of applying α-terpineol to prevent tolerance to the analgesic effects of morphine and to attenuate the acute signs of morphine withdrawal syndrome.
Conflict of interest
The authors have declared that there is no conflict of interest.
Acknowledgment
The present article is financially supported by “Research Department of the School of Medicine, Shahid Beheshti University of Medical Sciences” (Grant No. 13/1851).
References
- 1.Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, et al. Koda-Kimble and Young’s Applied Therapeutics:The Clinical Use of Drugs. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2013. [Google Scholar]
- 2.Tabatabai SM, Dashti S, Doosti F, Hosseinzadeh H. Phytotherapy of opioid dependence and withdrawal syndrome:a review. Phytother Res. 2014;28:811–830. doi: 10.1002/ptr.5073. [DOI] [PubMed] [Google Scholar]
- 3.Shokraviyan M, Miladi-Gorji H, Vaezi GH. Voluntary and forced exercises prevent the development of tolerance to analgesic effects of morphine in rats. Iran J Basic Med Sci. 2014;17:271–277. [PMC free article] [PubMed] [Google Scholar]
- 4.Adnan LH, Bakar NH, Mohamad N. Opioid dependence and substitution therapy:thymoquinone as potential novel supplement therapy for better outcome for methadone maintenance therapy substitution therapy. Iran J Basic Med Sci. 2014;17:926–928. [PMC free article] [PubMed] [Google Scholar]
- 5.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils- a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 6.Aprotosoaie AC, Hăncianu M, Costache II, Miron A. Linalool:a review on a key odorant molecule with valuable biological properties. Flavour Fragr J. 2014;29:193–219. [Google Scholar]
- 7.Guimarães AG, Quintans JSS, Quintans-Júnior LJ. Monoterpenes with analgesic activity- A systematic review. Phytother Res. 2013;27:1–15. doi: 10.1002/ptr.4686. [DOI] [PubMed] [Google Scholar]
- 8.Dobetsberger C, Buchbauer G. Actions of essential oils on the central nervous system:An updated review. Flavour Fragr J. 2011;26:300–316. [Google Scholar]
- 9.El-Ghorab A, El-Massry KF, Shibamoto T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian Corn Silk (Zea mays L.) J Agric Food Chem. 2007;55:9124–9127. doi: 10.1021/jf071646e. [DOI] [PubMed] [Google Scholar]
- 10.Bicas JL, Neri-Numa IA, Ruiz ALTG, De Carvalho JE, Pastore GM. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol. 2011;49:1610–1615. doi: 10.1016/j.fct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 11.de Sousa DP, Quintans L, de Almeida RN. Evolution of the anticonvulsant activity of α-terpineol. Pharm Biol. 2007;45:69–70. [Google Scholar]
- 12.de Oliveira MG, Marques RB, de Santana MF, Santos AB, Brito FA, Barreto EO, et al. α-Terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin Pharmacol Toxicol. 2012;111:120–125. doi: 10.1111/j.1742-7843.2012.00875.x. [DOI] [PubMed] [Google Scholar]
- 13.Quintans-Júnior LJ, Oliveira MG, Santana MF, Santana MT, Guimarães AG, Siqueira JS, et al. α-Terpineol reduces nociceptive behavior in mice. Pharm Biol. 2011;49:583–586. doi: 10.3109/13880209.2010.529616. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi-Nasrabadi M, Nazarian S, Farahani H, Fallah Koohbijari GR, Ahmadi F, Batooli H. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus largiflorens F. Muell. Int J Food Prop. 2013;16:369–381. [Google Scholar]
- 15.Gilles M, Zhao J, An M, Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010;119:731–737. [Google Scholar]
- 16.Ben Hsouna A, Hamdi N, Miladi R, Abdelkafi S. Myrtus communis essential oil:chemical composition and antimicrobial activities against food spoilage pathogens. Chem Biodivers. 2014;11:571–580. doi: 10.1002/cbdv.201300153. [DOI] [PubMed] [Google Scholar]
- 17.Salehi Surmaghi MH, Amin G, Shakibafar A, Azadi B. Unexpected volatile compounds of myrtus communis L. fruit rind growing in Iran. Int J Biol Pharm Res. 2014;5:428–431. [Google Scholar]
- 18.Khan M, Al-Mansour MA, Mousa AA, Alkhathlan HZ. Compositional characteristics of the essential oil of Myrtus communis grown in the central part of Saudi Arabia. J Essent Oil Res. 2014;26:13–18. [Google Scholar]
- 19.Abu-Dahab R, Kasabri V, Afifi FU. Evaluation of the volatile oil composition and antiproliferative activity of Laurus nobilis L. (Lauraceae) on breast cancer cell line models. Rec Nat Prod. 2014;8:136–147. [Google Scholar]
- 20.Mostafavi H, Mousavi SH, Zalaghi A, Delsouzi R. Chemical composition of essential oil of Stachys byzantina from North-West Iran. J Essent Oil Bear Plant. 2013;16:334–337. [Google Scholar]
- 21.Hosseinzadeh H, Dowlati S, Etemad L. Effects of Stachys byzantina C. Koch aerial parts aqueous extract on morphine dependence and tolerance in mice. Pharmacologyonline. 2008;2:614–617. [Google Scholar]
- 22.Özek M, Üresin Y, Güngör M. Comparison of the effects of specific and nonspecific inhibition of nitric oxide synthase on morphine analgesia, tolerance and dependence in mice. Life Sci. 2003;72:1943–1951. doi: 10.1016/s0024-3205(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Zaher AO, Mostafa MG, Farghaly HSM, Hamdy MM, Abdel-Hady RH. Role of oxidative stress and inducible nitric oxide synthase in morphine-induced tolerance and dependence in mice. Effect of alpha-lipoic acid. Behav Brain Res. 2013;247:17–26. doi: 10.1016/j.bbr.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Zaher AO, Abdel-Rahman MS, ELwasei FM. Blockade of nitric oxide over-production and oxidative stress by Nigella sativa oil attenuates morphine-induced tolerance and dependence in mice. Neurochem Res. 2010;35:1557–1565. doi: 10.1007/s11064-010-0215-2. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinzadeh H, Parvardeh S, Masoudi A, Moghimi M, Mahboobifard F. Attenuation of morphine tolerance and dependence by thymoquinone in mice. Avicenna J Phytomed. 2016;6:55–66. [PMC free article] [PubMed] [Google Scholar]
- 26.Parvardeh S, Nassiri Asl M, Niapoor M, Hosseinzadeh H. Antinociceptive, anti-inflammatory and acute toxicity effects of Pistacia Vera L. gum extract in mice and rat. J Med Plant. 2002;4:58–67. [Google Scholar]
- 27.Parvardeh S, Hosseinzadeh H. Hypnotic and muscle relaxant activity of thymoquinone, the major active constituent of Nigella sativa seeds, and its effects on locomotor activity and motor coordination in mice. J Med Plant. 2003;4:17–26. [Google Scholar]
- 28.Imenshahidi M, Hosseinzadeh H, Es’haghian A. Effects of ethosuximide on morphine tolerance and dependence in mice. Pharmacologyonline. 2007;2:287–299. [Google Scholar]
- 29.Kandel ER, Schwartz JH. Principles of Neural Science. 5th ed. McGraw-Hill; 2013. [Google Scholar]
- 30.Vaupel DB, Kimes AS, London ED. Further in vivo studies on attenuating morphine withdrawal:isoform-selective nitric oxide synthase inhibitors differ in efficacy. Eur J Pharmacol. 1997;324:11–20. doi: 10.1016/s0014-2999(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Zaher AO, Hamdy MM, Aly SA, Abdel-Hady RH, Abdel-Rahman S. Attenuation of morphine tolerance and dependence by aminoguanidine in mice. Eur J Pharmacol. 2006;540:60–66. doi: 10.1016/j.ejphar.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Zaher AO, Mostafa MG, Farghly HM, Hamdy MM, Omran GA, Al-Shaibani NK. Inhibition of brain oxidative stress and inducible nitric oxide synthase expression by thymoquinone attenuates the development of morphine tolerance and dependence in mice. Eur J Pharmacol. 2013;702:62–70. doi: 10.1016/j.ejphar.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Majeed NH, Przewłocka B, Machelska H, Przewłocki R. Inhibition of nitric oxide synthase attenuates the development of morphine tolerance and dependence in mice. Neuropharmacology. 1994;33:189–192. doi: 10.1016/0028-3908(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 34.Cappendijk SL, de Vries R, Dzoljic MR. Inhibitory effect of nitric oxide (NO) synthase inhibitors on naloxone-precipitated withdrawal syndrome in morphine-dependent mice. Neurosci Lett. 1993;162:97–100. doi: 10.1016/0304-3940(93)90569-7. [DOI] [PubMed] [Google Scholar]
- 35.Özek M, Üresin Y, Güngör M. Comparison of the effects of specific and nonspecific inhibition of nitric oxide synthase on morphine analgesia, tolerance and dependence in mice. Life Sci. 2003;72:1943–1951. doi: 10.1016/s0024-3205(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 36.Homayoun H, Khavandgar S, Namiranian K, Dehpour AR. The effect of cyclosporin A on morphine tolerance and dependence:involvement of L-arginine/nitric oxide pathway. Eur J Pharmacol. 2002;452:67–75. doi: 10.1016/s0014-2999(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 37.Vuong QV, Hirun S, Chuen TL, Goldsmith CD, Munro B, Bowyer MC, et al. Physicochemical, antioxidant and anti-cancer activity of a Eucalyptus robusta (Sm.). leaf aqueous extract. Indust Crop Prod. 2015;64:167–174. [Google Scholar]
- 38.Al-Sayed E, El-Naga RN. Protective role of ellagitannins from Eucalyptus citriodora against ethanol-induced gastric ulcer in rats:Impact on oxidative stress, inflammation and calcitonin-gene related peptide. Phytomedicine. 2015;22:5–15. doi: 10.1016/j.phymed.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Goudjil MB, Ladjel S, Bencheikh SE, Zighmi S, Hamada D. Study of the chemical composition, antibacterial and antioxidant activities of the essential oil extracted from the leaves of Algerian Laurus nobilis Lauraceae. J Chem Pharm Res. 2015;7:379–385. [Google Scholar]
- 40.Cherrat L, Espina L, Bakkali M, García-Gonzalo D, Pagán R, Laglaoui A. Chemical composition and antioxidant properties of Laurus nobilis L. and Myrtus communis L. essential oils from Morocco and evaluation of their antimicrobial activity acting alone or in combined processes for food preservation. J Sci Food Agric. 2014;94:1197–1204. doi: 10.1002/jsfa.6397. [DOI] [PubMed] [Google Scholar]
- 41.El SN, Karagozlu N, Karakaya S, Sahın S. Antioxidant and antimicrobial activities of essential oils extracted from Laurus nobilis L. leaves by using solvent-free microwave and hydrodistillation. Food Nutr Sci. 2014;5:97–106. [Google Scholar]
- 42.Asnaashari S, Delazar A, Alipour SS, Nahar L, Williams AS, Pasdaran A, et al. Chemical composition, free-radical-scavenging and insecticidal activities of the aerial parts of Stachys byzantina. Arch Biol Sci. 2010;62:653–662. [Google Scholar]


