Abstract
In 2008 the US Preventive Services Task Force recommended against screening men ages 75 and older for prostate cancer. Using Medicare Current Beneficiary Survey Access to Care files and linked claims, we compared trends in prostate-specific antigen (PSA) testing rates between men ages 75 and older and men ages 65–74. We estimate that the revised recommendation led to a 7.9-percentage-point decline in annual PSA testing rates over two years among men ages 75 and older. Although 42 percent of men in this age group continue to receive PSA tests, our results highlight the potential of guidelines with negative recommendations to reduce the use of low-value medical care.
Up to two-thirds of prostate tumors detected by prostate-specific antigen (PSA) screening are over-diagnosed—that is, in the absence of screening, the tumor would not have become clinically apparent during the patient’s lifetime.1 Overdiagnosis causes substantial harms, including anxiety, side effects, and increased health care costs.
On August 5, 2008, the US Preventive Services Task Force recommended against screening men ages seventy-five years and older for prostate cancer.2 In developing the recommendation, the Task Force considered the benefits of early detection and the risks of treatment: “The [Task Force] concluded that there is at least moderate certainty that the harms of screening for prostate cancer in men age 75 years or older outweigh the benefits.”2(p188)For men younger than seventy-five, the Task Force maintained its previous recommendation: “The evidence is insufficient to recommend for or against routine screening for prostate cancer.”3
Most medical guidelines recommend services that patients ought to receive. The revision to the Task Force prostate cancer screening recommendation provides an opportunity to examine the impact on practice patterns of a high-profile guideline recommending against receiving a medical service. In this article we evaluate the impact of the revised recommendation on PSA testing rates among men ages seventy-five and older.
A previous study of PSA screening in the Department of Veterans Affairs Pacific Northwest Network found that there was a small decline in screening rates among men ages seventy-five and older after the Task Force published its recommendation.4 It is unclear whether these results reflect the use of PSA in community settings. Physicians practicing in a centralized, government-run system like the Veterans Health Administration may be more compliant with Task Force recommendations than physicians in other settings.
A study using data from the National Health Interview Survey’s Cancer Control Supplement found that there was no change in PSA screening rates among men ages seventy-five and older between 2005 and 2010.5 However, the study used self-reported measures of PSA testing rates. These measures may be inaccurate.6,7 One possible cause of inaccuracy is that many patients might not know that their physician has ordered a PSA screening test. Small surveys of physicians indicate that 20–30 percent of physicians do not always discuss PSA screening with patients prior to ordering tests.8,9 We used claims-based measures of screening rates and thus avoided this inaccuracy.
Study Data And Methods
DATA AND SAMPLE SELECTION
We measured PSA testing rates using the 2006–10 Medicare Current Beneficiary Survey Access to Care files. The Medicare Current Beneficiary Survey is a panel survey that collects data on health care use and access from a stratified, random sample of Medicare beneficiaries. Data from the survey include Medicare claims for respondents enrolled in fee-for-service Medicare. Response rates for the initial rounds of the survey averaged nearly 80 percent during the span of the study.
The sample includes community-dwelling respondents ages sixty-five and older who are continuously enrolled in fee-for-service Medicare for the calendar year. We excluded men who reported being diagnosed with prostate cancer or who had an inpatient, outpatient, or physician office claim that listed a diagnosis code for prostate cancer (International Classification of Diseases, Ninth Revision, or ICD-9, code 185.X). In a sensitivity analysis, we excluded men who did not have at least three years of participation in the survey.
MEASURING PSA TESTING
We measured the receipt of PSA testing annually beginning January 1, 2006. We counted a respondent as having received a PSA test if he had at least one office or outpatient claim that listed Healthcare Common Procedure Coding System (HCPCS) codes 84152, 84153, 84154, or G0103. We measured a respondent’s age at the midpoint of each year to compare annual testing rates by age group (65–74 or 75 and older). For comparison with previous studies, we also measured self-reported PSA testing rates by using the response to the PSA testing question in the Health Status and Functioning module of the Medicare Current Beneficiary Survey.
The claims data do not distinguish between PSA tests used to screen for cancer in asymptomatic men, tests used to diagnosis cancer in men with symptoms consistent with prostate cancer, and tests used to evaluate therapeutic options for men with benign prostatic hyperplasia. In a sensitivity analysis, we excluded men with ICD-9 codes for urinary tract disease (500–580) and male genital disorders (600–608), who might have received a PSA test as part of a diagnostic workup.
STATISTICAL ANALYSIS
We used a difference-in-differences analysis to estimate the impact of the revised Task Force recommendation on PSA testing rates. A difference-in-differences analysis compares the change in a treatment group—in this case, men ages 75 and older—to the change in a control group—here, men ages 65–74.
We estimated a probit regression in which receiving a PSA test was the dependent variable. The independent variables were age group (65–74, ≥75), period (2006 and 2007 versus 2009 and 2010), an interaction between age group and period, race (white, black, or other), self-reported health status (excellent, very good, or good versus fair or poor), marital status (married versus other), educational attainment (college degree versus no degree), annual income (≥$25,000 versus <$25,000), and census region. The unit of observation was the person-year.
We excluded data from 2008 from the regression analysis because the Task Force released its revised prostate cancer screening recommendation in the middle of that year. We weighted the regressions using the survey weights and clustered standard errors at the level of the respondent.
We used the coefficients from the regression model to calculate the marginal effect of each variable on the likelihood that a man received a PSA test. We performed the analysis using the statistical software Stata, version 11.0.
In sensitivity analyses, we examined the impact of including additional income categories and Elixhauser comorbidity counts10 calculated from inpatient, outpatient, and physician office claims. We also performed a falsification test: We reestimated the model using the receipt of a cholesterol test as the dependent variable (HCPCS codes 80061, 82465, 83718, or 84478). We would not expect cholesterol testing rates to decline among men ages seventy-five and older following the release of the revised US Preventive Services Task Force prostate cancer screening recommendation. A finding to the contrary would suggest that there was another factor in 2008 that changed the use of preventive services among older men.
Study Results
There were 14,926 person-year observations for 7,418 unique respondents. Respondents were followed for a maximum of four years. On average, the proportion of the sample in one year that also was represented in the following year was 62 percent.
Annual PSA testing rates among men ages 75 and older were more or less unchanged between 2006 and 2007, but they began declining in 2008 (Exhibit 1). Between 2006 and 2010, PSA testing rates declined by 5.3 percentage points. Among men ages 65–74, testing rates moved in the opposite direction, increasing by 2.4 percentage points between 2006 and 2010.
EXHIBIT 1. Prostate-Specific Antigen (PSA) Testing Rates Among Medicare Beneficiaries, By Age Group, 2006–10.
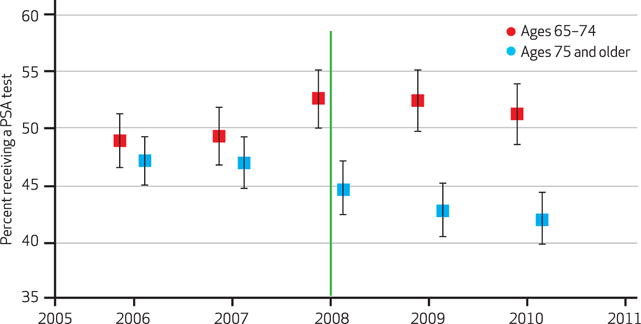
SOURCE Authors’ analysis of Medicare Current Beneficiary Survey Access to Care files. NOTES The sample includes community-dwelling, fee-for-service Medicare beneficiaries not diagnosed with prostate cancer. Error bars represent 95 percent confidence intervals. The green line indicates the release of the screening guidelines by the US Preventive Services Task Force in 2008.
The demographic, health, and socioeconomic characteristics of the sample were qualitatively similar in the pre period (2006–07) and the post period (2009–10). However, some of the differences were significant (Exhibit 2).
EXHIBIT 2.
Characteristics Of The Sample, Study Of Prostate-Specific Antigen Testing Among Medicare Beneficiaries, By Age Group And Year, Selected Years 2006–10
| Period
|
p value | ||
|---|---|---|---|
| 2006–07 | 2009–10 | ||
| AGES 65–74 | |||
|
| |||
| Respondents (no.) | 2,021 | 1,679 | —a |
| Mean age (years) | 69 | 69 | 0.850 |
| White | 90% | 88% | 0.171 |
| Black | 6 | 7 | 0.089 |
| Fair or poor health | 20 | 17 | 0.044 |
| Married | 77 | 74 | 0.038 |
| College degree | 25 | 30 | 0.004 |
| Income ≥$25,000 | 65 | 68 | 0.015 |
|
| |||
| AGES ≥75 | |||
|
| |||
| Respondents (no.) | 2,400 | 2,228 | —a |
| Mean age (years) | 80 | 81 | 0.008 |
| White | 90% | 91% | 0.880 |
| Black | 5 | 5 | 0.899 |
| Fair or poor health | 22 | 21 | 0.566 |
| Married | 70 | 69 | 0.198 |
| College degree | 23 | 27 | 0.002 |
| Income ≥$25,000 | 56 | 61 | <0.001 |
SOURCE Authors’ analysis of data from the 2006, 2007, 2009, and 2010 Medicare Current Beneficiary Surveys.
NOTE We used t-tests and t-tests for proportions to assess the significance of differences between periods.
Not applicable.
Exhibit 3 displays estimates of the impact of respondents’ characteristics on the probability of receiving a PSA test. For example, the results indicate that annual PSA testing rates are 4.4 percentage points lower among men ages 75 and older in poor or fair health compared to men in good, very good, or excellent health.
EXHIBIT 3.
Estimates Of The Impact Of Beneficiary Characteristics On The Probability Of Receiving A Prostate-Specific Antigen (PSA) Test
| Characteristic | Change in probability of receiving PSA test | Standard error |
|---|---|---|
| Whitea | −5.0 | 2.8**** |
| Blacka | −6.8 | 3.6**** |
|
| ||
| Fair or poor health | −4.4 | 1.3**** |
| Married | 5.5 | 1.3**** |
|
| ||
| College degree | 7.5 | 1.4* |
| Income ≥$25,000 | 7.8 | 1.2**** |
|
| ||
| Age ≥75 | −0.9 | 1.5 |
| Post periodb | 2.4 | 1.6 |
| Age ≥75×(post period) | −7.9 | 2.0**** |
SOURCE Authors’ analysis of data from the Medicare Current Beneficiary Surveys, 2006, 2007, 2009, and 2010.
NOTES The probabilities are from a probit regression with standard errors clustered at the level of the beneficiary. The regression also included controls for census region (not shown).
Other is the omitted category.
The post period is 2009–10. The pre period is 2006–07.
p < 0:001
The estimated effect of the revised Task Force recommendation on PSA testing rates, the difference-in-differences estimator, is based on the interaction between age group (ages 75 and older) and the period following the release of the revised Task Force recommendation (2009 and 2010). Assuming that without the revised Task Force recommendation, testing rates among men ages 75 and older would have mirrored trends among men ages 65–74, we estimate that the recommendation led to a 7.9-percentage-point decline in PSA testing rates among men in the older age group (p < 0:001).
We performed a number of sensitivity analyses, as described in the Study Data And Methods section. The results of these analyses are provided in Appendix Exhibit 1.11 The difference-in-differences estimates were all significant and qualitatively similar to the baseline estimate of −7.9 percentage points.
As noted above, we reestimated the baseline model using the receipt of a cholesterol test as the dependent variable. The full results of the analysis are shown in Appendix Exhibit 1.11 The difference-in-differences estimator was small (−1.2) and not significant (p = 0.54). This finding increases our confidence that the revised Task Force recommendation, rather than some unobserved factor, caused PSA testing rates to decline in men ages 75 and older.
Self-reported PSA testing rates among men ages 75 and older were similar between the sample used in the baseline analysis and an expanded sample that included respondents in Medicare managed care plans (Appendix Exhibits 2 and 3).11 Rates were much higher than rates calculated from claims data and did not increase or decrease monotonically over time.
Among men ages 75 and older, self-reported PSA testing rates were 71.8 percent in 2006 and 67.9 percent in 2010. Among men ages 65–74, rates were 69.8 percent in 2006 and 67.9 percent in 2010.
Discussion
Our analysis indicates that the revised US Preventive Services Task Force prostate cancer screening recommendation led to a 7.9-percentage-point decline in PSA testing rates among men ages seventy-five and older in 2009 and 2010. The change is significant, and thus it is unlikely to be due to random fluctuations in testing rates over time. However, more than 40 percent of men ages seventy-five and older continue to receive PSA tests.
If the Task Force recommendation had an impact on screening rates, then we would expect to see a decrease in the incidence of early-stage prostate tumors, which are primarily detected by screening, among men ages seventy-five and older. This is indeed what happened.12
The number of men ages seventy-five and older diagnosed with early-stage tumors began declining immediately after the release of the revised Task Force recommendation. Taken together, declines in testing rates and in the incidence of early-stage tumors provide strong evidence that the revised recommendation led to a decrease in screening rates.
Like claims-based PSA testing rates, self-reported testing rates decreased between 2006 and 2010 among men ages seventy-five and older. However, the magnitude of the decrease was small. Prior studies indicate that self-reported PSA testing rates have poor accuracy.6–9 In all likelihood, claims-based measures provide a more reliable guide to changes in screening rates over time.
The results of several randomized controlled trials of PSA screening were published in March 2009.13,14 These may have affected screening rates, although neither study produced definitive results.15 It seems implausible that testing rates among men ages 65–74 and men ages 75 and older would have moved in opposite directions in response to the trial findings.
Although Task Force recommendations have the imprimatur of the federal government, they are not necessarily the most influential set of cancer screening guidelines.16 Guidelines from the American Cancer Society,17 the American Urological Association,18 and other groups advise against screening men with a life expectancy of less than ten years but do not recommend age-based cutoffs. The existence of multiple and conflicting guidelines might limit the impact of a particular guideline on practice patterns.16
Policy Implications
The 2008 revision to the Task Force prostate cancer screening recommendation was intended to reduce the risk of overdiagnosis and the overuse of PSA testing among older men with limited life expectancy.19–21 Shared decision making is often promoted as the ideal for reducing the use of screening tests that are unlikely to increase life expectancy. However, shared decision making has not diffused widely into routine clinical practice.7,22,23
In a sample of previously screened men ages fifty and older, only 6 percent had plans to discontinue prostate cancer screening.24 However, in another study, many men discontinued screening after experiencing a serious health event such as acute myocardial infarction.25
Age-delimited screening guidelines provide a clear, easy-to-implement recommendation for discontinuing screening. They are widely used in Europe. The limitation is that age is only a rough proxy for remaining life expectancy.
Most guidelines contain only affirmative recommendations, stating the services that patients are advised to receive. Task Force recommendations for prostate cancer and other screening services are unique in that they include recommendations advising against receiving certain services.
Recent revisions, particularly the 2009 revision to the breast cancer screening recommendation, have sparked heated debate among clinicians and in the popular news media. With the enactment of the Affordable Care Act, the recommendations carry greater weight because insurers are required to cover services recommended by the Task Force.
Our study confirms that PSA testing rates declined after the Task Force recommended against screening men ages seventy-five and older in 2008. Although 40 percent of older men continue to receive PSA tests, a major change in behavior occurred—and it was accomplished without coercion, regulation, or changes in coverage policies. Thus, our study highlights the potential of guidelines that include negative recommendations to reduce the use of services that are harmful, unnecessary, or of low value.
Acknowledgments
David Howard’s work was supported by an Interagency Personnel Agreement with the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Biographies

David H. Howard is an associate professor at Emory University.
In this month’s Health Affairs, David Howard and coauthors report on their study of prostate-specific antigen (PSA) testing in men ages 75 and older after the US Preventive Services Task Force recommended against such screening in 2008. Using Medicare data and insurance claims, the authors found a 7.9-percentage-point reduction in annual PSA testing rates over two years among men in that age group. Although 42 percent of these older men continue to receive PSA tests, the drop highlights the potential of guidelines with negative recommendations to reduce the use of low-value medical care.
Howard is an associate professor in the Department of Health Policy and Management and the Department of Economics at Emory University. He is also a core member of the Cancer Control and Population Sciences Program, Winship Cancer Institute, Emory University School of Medicine. His current areas of research include the impact of patients’ life expectancies on the use of cancer screening and the responsiveness of practice patterns to comparative effectiveness research. He received a doctorate in health policy from Harvard University.

Florence K. Tangka is a health economist at the Centers for Disease Control and Prevention.
Florence Tangka is a health economist at the Centers for Disease Control and Prevention (CDC) and an adjunct professor in the Department of Behavioral Sciences and Health Education, Emory University. She also serves as a peer-review board member at the National Center for Chronic Disease Prevention and Health Promotion, a member of the National Colorectal Cancer Roundtable, and a member of the CDC’s Health Economic Research Group. Tangka earned a master’s degree in agricultural economics from Rutgers University and a doctorate in food and resource economics from the University of Florida.

Gery P. Guy is a health economist at the Centers for Disease Control and Prevention.
Gery Guy is a health economist in the Division of Cancer Prevention and Control at the CDC, where he conducts health economics research focusing on the economic burden of cancer survivorship, prevalence and correlates of the use of indoor tanning devices, economic evaluation of cancer registry operations, modeling the health and economic impact of indoor tanning polices, and an examination of the costs and cost-effectiveness of a national colorectal cancer screening program.
Guy is also a contract researcher in the Department of Health Policy and Management, Rollins School of Public Health, Emory University, and a prevention effectiveness postdoctoral fellow in the CDC Division of Cancer Prevention and Control. He earned a master’s degree in public health and a doctorate in health services research and health policy from Emory University.

Donatus U. Ekwueme is the senior health economist in the Division of Cancer Prevention and Control, Centers for Disease Control and Prevention.
Donatus Ekwueme is the senior health economist in the Division of Cancer Prevention and Control at the CDC, where he provides leadership and direction for applied economics research projects and policy analysis on the burden of cancer disease in the United States and other countries. Ekwueme conducts economic research on international and domestic public health issues in several national centers within the CDC, using various modeling approaches to understand the interplay between economics, epidemiology, human behavior, and the prevention and control of cancer. He also is an adjunct professor at the Morehouse School of Medicine. He received a doctorate in economics from Wayne State University.

Joseph Lipscomb is a professor of health policy and management at Emory University.
Joseph Lipscomb is a professor of health policy and management and the Georgia Cancer Coalition Distinguished Cancer Scholar at the Rollins School of Public Health, Emory University. He is also associate director of population sciences at the university’s Winship Cancer Institute. Lipscomb has published widely on various topics in health economics and outcomes research, including on patient-reported outcomes assessment, quality-of-care evaluation and improvement, and the theory and practice of cost-effectiveness analysis. Recently, he investigated the quality of cancer care in rural areas of the country, including regions of the state of Georgia. He earned a doctorate in economics from the University of North Carolina at Chapel Hill.
Footnotes
This research was presented at the annual meeting of the American Society of Health Economists, Minneapolis, Minnesota, June 2012.
Contributor Information
David H. Howard, Email: david.howard@emory.edu, Associate professor in the Department of Health Policy and Management and the Department of Economics at Emory University, in Atlanta, Georgia.
Florence K. Tangka, Health economist at the Centers for Disease Control and Prevention (CDC), in Atlanta
Gery P. Guy, Health economist in the Division of Cancer Prevention and Control at the CDC
Donatus U. Ekwueme, Senior health economist in the Division of Cancer Prevention and Control at the CDC
Joseph Lipscomb, Professor of health policy and management at the Rollins School of Public Health, Emory University.
NOTES
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Screening for prostate cancer: recommendation and rationale. Ann Intern Med. 2002;137(11):915. doi: 10.7326/0003-4819-137-11-200212030-00013. [DOI] [PubMed] [Google Scholar]
- 4.Zeliadt SB, Hoffman RM, Etzioni R, Gore JL, Kessler LG, Lin DW. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103(6):520–3. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 5.Prasad SM, Drazer MW, Huo D, Hu JC, Eggener SE. 2008 US Preventive Services Task Force recommendations and prostate cancer screening rates. JAMA. 2012;307(16):1692–4. doi: 10.1001/jama.2012.534. [DOI] [PubMed] [Google Scholar]
- 6.Hall HI, Van Den Eeden SK, Tolsma DD, Rardin K, Thompson T, Hughes Sinclair A, et al. Testing for prostate and colorectal cancer: comparison of self-report and medical record audit. Prev Med. 2004;39(1):27–35. doi: 10.1016/j.ypmed.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Chan EC, Vernon SW, Ahn C, Greisinger A. Do men know that they have had a prostate-specific antigen test? Accuracy of self-reports of testing at 2 sites. Am J Public Health. 2004;94(8):1336–8. doi: 10.2105/ajph.94.8.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra CE, Jacobs SE, Holmes JH, Shea JA. Are physicians discussing prostate cancer screening with their patients and why or why not? A pilot study. J Gen Intern Med. 2007;22(7):901–7. doi: 10.1007/s11606-007-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linder SK, Hawley ST, Cooper CP, Scholl LE, Jibaja-Weiss M, Volk RJ. Primary care physicians’ reported use of pre-screening discussions for prostate cancer screening: a cross-sectional survey. BMC Family Pract. 2009;10:19. doi: 10.1186/1471-2296-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 11.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 12.Howard DH. Declines in prostate cancer incidence after changes in screening recommendations. Arch Intern Med. 2012;172(16):1267–8. doi: 10.1001/archinternmed.2012.2768. [DOI] [PubMed] [Google Scholar]
- 13.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 15.Barry MJ. Screening for prostate cancer—the controversy that refuses to die. N Engl J Med. 2009;360(13):1351–4. doi: 10.1056/NEJMe0901166. [DOI] [PubMed] [Google Scholar]
- 16.Han PK, Klabunde CN, Breen N, Yuan G, Grauman A, Davis WW, et al. Multiple clinical practice guidelines for breast and cervical cancer screening: perceptions of US primary care physicians. Med Care. 2011;49(2):139–48. doi: 10.1097/MLR.0b013e318202858e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 18.American Urological Association. Prostate-specific antigen best practice statement: 2009 update [Internet] Linthicum (MD): AUA; 2009. [cited 2013 Feb 20]. Available from: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. [Google Scholar]
- 19.Kerfoot BP, Holmberg EF, Lawler EV, Krupat E, Conlin PR. Practitioner-level determinants of inappropriate prostate-specific antigen screening. Arch Intern Med. 2007;167(13):1367–72. doi: 10.1001/archinte.167.13.1367. [DOI] [PubMed] [Google Scholar]
- 20.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–42. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 21.Sima CS, Panageas KS, Schrag D. Cancer screening among patients with advanced cancer. JAMA. 2010;304(14):1584–91. doi: 10.1001/jama.2010.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman RM, Couper MP, Zikmund-Fisher BJ, Levin CA, McNaughton-Collins M, Helitzer DL, et al. Prostate cancer screening decisions: results from the National Survey of Medical Decisions (DECISIONS study) Arch Intern Med. 2009;169(17):1611–8. doi: 10.1001/archinternmed.2009.262. [DOI] [PubMed] [Google Scholar]
- 23.Guerra CE, Gimotty PA, Shea JA, Pagán JA, Schwartz JS, Armstrong K. Effect of guidelines on primary care physician use of PSA screening: results from the Community Tracking Study Physician Survey. Med Decis Making. 2008;28(5):681–9. doi: 10.1177/0272989X08315243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis CL, Couper MP, Levin CA, Pignone MP, Zikmund-Fisher BJ. Plans to stop cancer screening tests among adults who recently considered screening. J Gen Intern Med. 2010;25(8):859–64. doi: 10.1007/s11606-010-1346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard DH, Huang Y. Serious health events and discontinuation of routine cancer screening. Med Decis Making. 2012;32(4):627–35. doi: 10.1177/0272989X11434600. [DOI] [PubMed] [Google Scholar]


