Abstract
Objective:
To determine the relation between markers of kidney disease—estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (UACR)—with cerebral blood flow (CBF) and white matter volume (WMV) in hypertensive adults.
Methods:
We used baseline data collected from 665 nondiabetic hypertensive adults aged ≥50 years participating in the Systolic Blood Pressure Intervention Trial (SPRINT). We used arterial spin labeling to measure CBF and structural 3T images to segment tissue into normal and abnormal WMV. We used quantile regression to estimate the association between eGFR and UACR with CBF and abnormal WMV, adjusting for sociodemographic and clinical characteristics.
Results:
There were 218 participants (33%) with eGFR <60 mL/min/1.73 m2 and 146 participants (22%) with UACR ≥30 mg/g. Reduced eGFR was independently associated with higher adjusted median CBF, but not with abnormal WMV. Conversely, in adjusted analyses, there was a linear independent association between UACR and larger abnormal WMV, but not with CBF. Compared to participants with neither marker of CKD (eGFR ≥60 mL/min/1.73 m2 and UACR <30 mg/g), median CBF was 5.03 mL/100 g/min higher (95% confidence interval [CI] 0.78, 9.29) and abnormal WMV was 0.63 cm3 larger (95% CI 0.08, 1.17) among participants with both markers of CKD (eGFR <60 mL/min/1.73 m2 and UACR ≥30 mg/g).
Conclusions:
Among nondiabetic hypertensive adults, reduced eGFR was associated with higher CBF and higher UACR was associated with larger abnormal WMV.
Chronic kidney disease (CKD) affects 12% of US adults and is associated with an increased risk of stroke.1,2 In addition, CKD is associated with a large burden of white matter disease, presumed to reflect ischemic injury.3–6 The brain and kidney both have high resting demand and tightly autoregulated blood flow across a range of perfusion pressures. CKD is associated with vascular remodeling, which could impair regulation of local cerebral blood flow (CBF).7,8 White matter may be particularly susceptible to ischemic injury with disruption in CBF autoregulation due to its blood supply.9 Newer imaging techniques, such as arterial spin labeling (ASL), offer the ability to quantify CBF without administration of contrast, and may provide insight into the mechanisms linking CKD with cerebrovascular disease.
CKD is characterized by a reduction in glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 or by the presence of albuminuria. These complementary markers of kidney disease are independently associated with cardiovascular mortality, and appear to have different implications with respect to risk for cerebrovascular disease. Albuminuria is independently associated with ischemic and hemorrhagic stroke, whereas reduced estimated GFR (eGFR) is principally linked to an elevated risk of ischemic stroke.2 The physiologic mechanisms underlying these differences in stroke risk have not been elucidated. In cross-sectional studies, albuminuria and reduced eGFR have each been associated with white matter disease.3–6 However, it remains unclear whether albuminuria and reduced eGFR are independent markers of white matter disease, as some previous studies did not account for both CKD markers simultaneously, and all included patients with diabetes, which itself is a risk factor for white matter disease.
Using baseline neuroimaging data from nondiabetic hypertensive adults participating in the Systolic Blood Pressure Intervention Trial (SPRINT), we sought to characterize the relations between eGFR and albuminuria with CBF and abnormal white matter volume (WMV), a marker of cerebral white matter disease.
The study is listed on clinicaltrials.gov (NCT01206062)
METHODS
Study design and recruitment.
SPRINT is a randomized clinical trial testing whether reducing systolic blood pressure to <120 mm Hg will reduce cardiovascular events as compared to a systolic blood pressure target of <140 mm Hg. The study design and methods have been described previously.10 Between November 2010 and March 2013, SPRINT recruited 9,361 participants age ≥50 years with hypertension. Eligible participants had a systolic blood pressure between 130 to 180 mm Hg, depending on the number of antihypertensive medications they were receiving during screening, and at least 1 of 4 cardiovascular risk factors: the presence of clinical or subclinical cardiovascular disease (CVD), an eGFR 20–59 mL/min/1.73 m2, a Framingham 10-year risk score ≥15%, or age ≥75 years. Major exclusion criteria included diabetes mellitus, history of stroke, cardiovascular event or procedure in the prior 3 months, symptomatic heart failure in the past 6 months or left ventricular ejection fraction <35%, proteinuria >1 g/d or the equivalent based on spot urine measurement, polycystic kidney disease, recent immunosuppression, coexisting disease likely to affect survival, and organ transplant.
A subset of SPRINT participants were recruited for the SPRINT Memory and Cognition in Decreased Hypertension Magnetic Resonance Imaging (SPRINT MIND MRI) substudy. In 2012, additional participants were recruited through the ancillary study MIND the Kidneys.
Participants from 11 clinical centers in SPRINT were screened for MRI eligibility. Exclusion criteria included claustrophobia or an MRI-incompatible metal or electrical device implant.
A total of 779 participants completed a baseline MRI. Of these, 28 were excluded for poor image quality, 38 for having the MRI scan performed >90 days after randomization, 28 for missing eGFR or urine albumin to creatinine ratio (UACR) measurements, 8 for missing other covariates, and 12 for self-report of diabetes. Thus the analytic cohort included 665 participants.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at all clinical sites approved the study protocol and all participants signed informed consent.
MRI protocol.
We conducted the standardized MRI protocol on 3.0T scanners and included sagittal 3D fluid-attenuated inversion recovery (FLAIR), T2-weighted, and T1-weighted sequences with whole brain coverage, and an axial ASL perfusion sequence (appendix e-1 on the Neurology® Web site at Neurology.org). The MRI scanners were from 3 manufacturers (Siemens, Munich, Germany; Philips, Best, the Netherlands; GE Healthcare, Cleveland, OH) and had identical field strength. All scanners ran similar pulse sequences except for ASL, for which 2 manufacturers (10 centers) utilized a 2D pseudocontinuous ASL and one utilized a 3D pseudocontinuous ASL sequence.
The University of Pennsylvania managed MRI quality control. Each field center performed quarterly phantom scans for the evaluation of scanner stability and image distortion using Alzheimer's Disease Neuroimaging Initiative (3D imaging sequences) and Function Bioinformatics Research Network (physiologic sequences) phantoms. MRI scanner performance across the clinical centers was stable over the duration of the study.
We first preprocessed T1 scans to correct intensity inhomogeneities.11 We then partitioned the brain into 148 anatomical regions of interest (ROIs) by applying a multi-atlas consensus-based label fusion method on atlas ROIs aligned to subject space through nonlinear registration.12 These ROIs were collapsed into 10 larger ROIs for this analysis, as well as CSF. White matter lesions were segmented to further characterize the brain tissue in ROIs as normal and abnormal, with abnormal WMV the outcome of interest. We used a supervised learning-based multimodal segmentation technique, which has been previously validated using expert-defined data.13 The method uses a support vector machine classifier that is trained on a set of expert-defined abnormal white matter lesions, and the model is used to classify abnormal WMV in new scans. For algorithm training purposes, abnormal WMV was operationally defined as a non-mass lesion having fast spin-echo FLAIR signal intensity greater than that of normal gray matter in a vascular distribution. We applied a standard quality control procedure, consisting of visual inspection of final segmentations for a randomly selected subset of subjects, as well as automated outlier detection on quantitative results to ensure the quality of the final data.
We transformed ASL maps into CBF maps, with the values at each voxel in the map representing mean blood flow in mL/100 g/min.14 The CBF maps were then registered to the Jakob atlas so that mean CBF could be calculated for whole brain and within individual ROIs.
Assessment of kidney disease.
We measured serum creatinine, urine albumin, and urine creatinine using samples collected at the baseline visit. Serum and urine creatinine were measured using a method traceable to isotope dilution mass spectrometry. Urine creatinine was measured with the Siemens ProSpec nephelometric analyzer. We calculated the eGFR with the Chronic Kidney Disease Epidemiology Collaboration equation, and categorized participants as ≥60 mL/min/1.73 m2 or <60 mL/min/1.73 m2 based on clinical practice guidelines.15 We calculated the UACR from a spot urine sample and analyzed UACR as a continuous variable after log transformation, based on previous studies demonstrating a linear association between UACR and stroke risk.2,16 In complementary analyses, we categorized eGFR as ≥90, 60–89, 45–59, and <45 mL/min/1.73 m2, and we categorized UACR as <30 and ≥30 mg/g.17
Covariates.
At the baseline visit, participants completed questionnaires ascertaining sociodemographic information, medical and family history, and health behaviors. Height, weight, and blood pressure were recorded by trained study personnel. We defined CVD as self-report or clinical evidence of coronary artery disease or peripheral arterial disease.10 We categorized smoking status as current, former, or never smokers. We categorized alcohol use as nondrinker (<1 drink per month), light drinker (1–2 drinks per week), moderate drinker (≥3 drinks per week but <1 drink per day), or heavy drinker (≥1 drink per day). We measured lipids using fasting blood specimens. We calculated the 10-year Framingham risk score using age, sex, total cholesterol, high-density lipoprotein cholesterol, smoking status, systolic blood pressure, and use of blood pressure medications.
Statistical analysis.
We expressed continuous variables as mean ± SD or median (interquartile range [IQR]), and compared these using t tests or Wilcoxon rank-sum tests as appropriate. We expressed categorical variables as proportions and compared them using χ2 tests or Fisher exact tests for variables with low cell frequencies. We used quantile (median) regression to model the relation between eGFR or UACR with CBF and abnormal WMV. We used quantile regression because (1) the outcome distributions, particularly for abnormal WMV, were highly skewed and thus not amenable to transformation; and (2) effect estimates are computed on the original scale of measurement, and so are more readily interpretable. We fit quantile regression models using the quantreg package for the R Statistical Computing Environment, using bootstrap resampling (5,000 replicates) to estimate standard errors for model coefficients.18,19 For each of the MRI outcomes, we adjusted for intracranial volume, age (mean-centered and then modeled as a quadratic polynomial), sex, race/ethnicity, education, smoking, alcohol consumption, history of CVD, systolic blood pressure, diastolic blood pressure, use of angiotensin-converting enzyme (ACE) inhibitors, use of angiotensin II antagonists, and MRI scanner type. The eGFR models were additionally adjusted for UACR, and the UACR models were additionally adjusted for eGFR. The primary CBF analysis utilized whole brain CBF. Secondary analyses assessed CBF in 10 ROIs, correcting for multiple comparisons using the false discovery rate.20 These analyses expressed differences in CBF for eGFR and albuminuria groups as a percentage relative to CBF in the referent group within each ROI.
Next, we assessed the joint association between eGFR and albuminuria with CBF and WMV. For these analyses, we categorized patients into 4 strata: eGFR ≥60 and UACR <30 mg/g (the referent group), eGFR <60 and UACR <30 mg/g, eGFR ≥60 and UACR ≥30 mg/g, and eGFR <60 and UACR ≥30 mg/g. Unless otherwise mentioned, all analyses were performed using either SAS v9.4 (Cary, NC) or the R Statistical Computing Environment.
RESULTS
Participant characteristics.
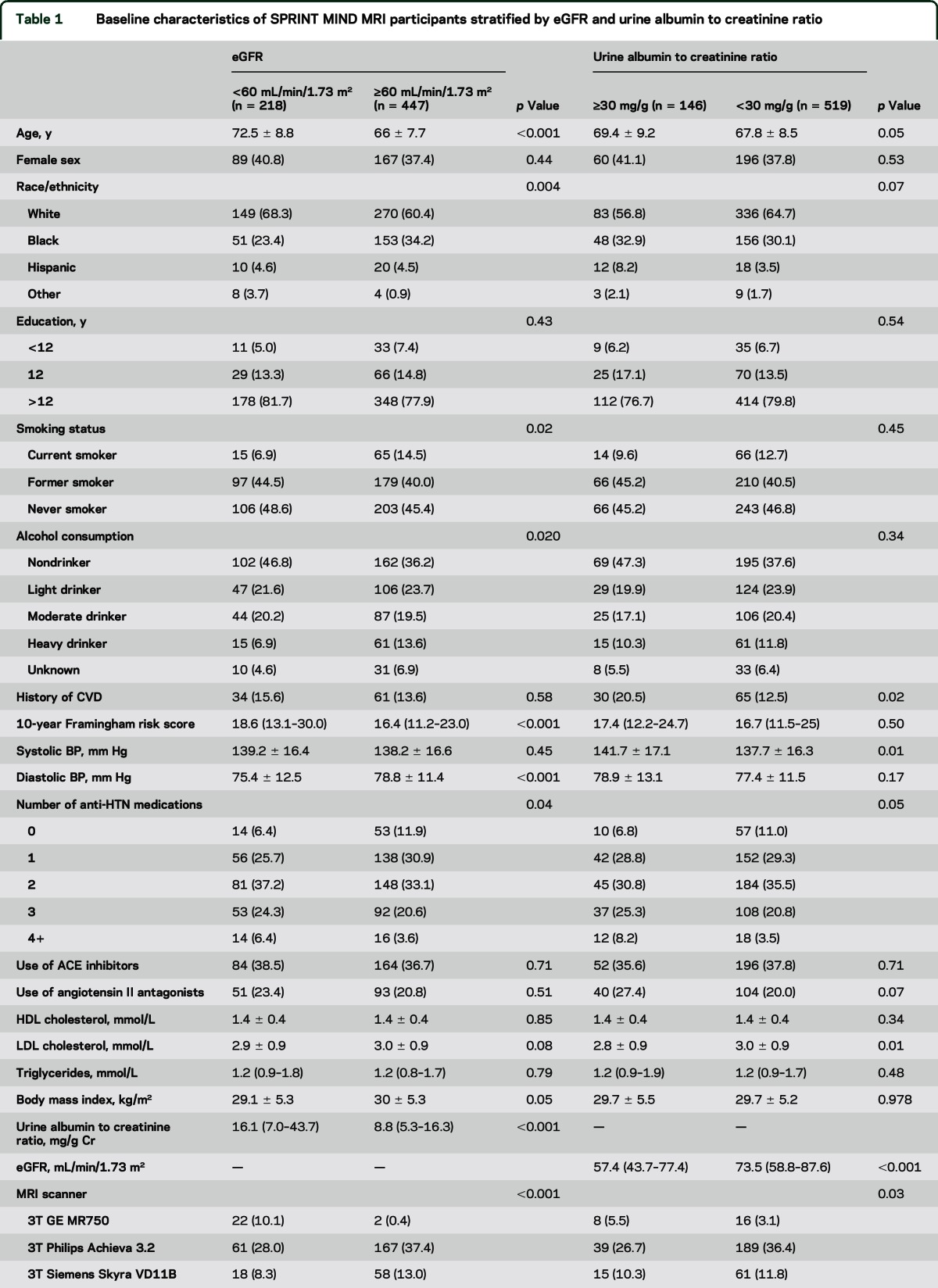
Of the 665 participants in the analytic cohort, there were 218 participants (32.3%) with eGFR <60 mL/min/1.73 m2. Of these 218 participants, 75 had an eGFR <45 mL/min/1.73 m2. There were 146 participants (22%) with albuminuria. Of these 146 participants, 127 had a UACR 30,299 mg/g, and 19 had a UACR ≥300 mg/g. Participant characteristics stratified by eGFR and albuminuria status are shown in table 1. Participants in the cohort had a median CBF of 44.6 mL/100 g/min (IQR 37.0–53.7) and median abnormal WMV of 0.76 cm3 (IQR 0.25–2.11).
Table 1.
Baseline characteristics of SPRINT MIND MRI participants stratified by eGFR and urine albumin to creatinine ratio
Relation of eGFR with CBF and abnormal WMV.
Median CBF adjusted for MRI scanner was 1.85 mL/100 g/min (95% confidence interval [CI] −0.14, 3.83), higher among participants with eGFR <60 mL/min/1.73 m2 vs ≥ 60 mL/min/1.73 m2. After additional adjustment for UACR, age, sex, race/ethnicity, education, smoking, alcohol consumption, history of CVD, systolic blood pressure, diastolic blood pressure, use of ACE inhibitors, and use of angiotensin II antagonists, median CBF remained higher for eGFR <60 mL/min/1.73 m2 (vs ≥60 mL/min/1.73 m2) but this did not reach statistical significance (table 2). To assess for threshold effects, we then evaluated CBF in 4 eGFR categories. Compared to participants with eGFR ≥90 mL/min/1.73 m2, median CBF was not significantly different among participants with eGFR 45–89 mL/min/1.73 m2, but was 4.83 mL/100 g/min higher (95% CI 0.56, 9.10) among participants with eGFR <45 mL/min/1.73 m2 (table 2).
Table 2.
Adjusted associations of eGFR and albuminuria with cerebral blood flow and abnormal white matter volume
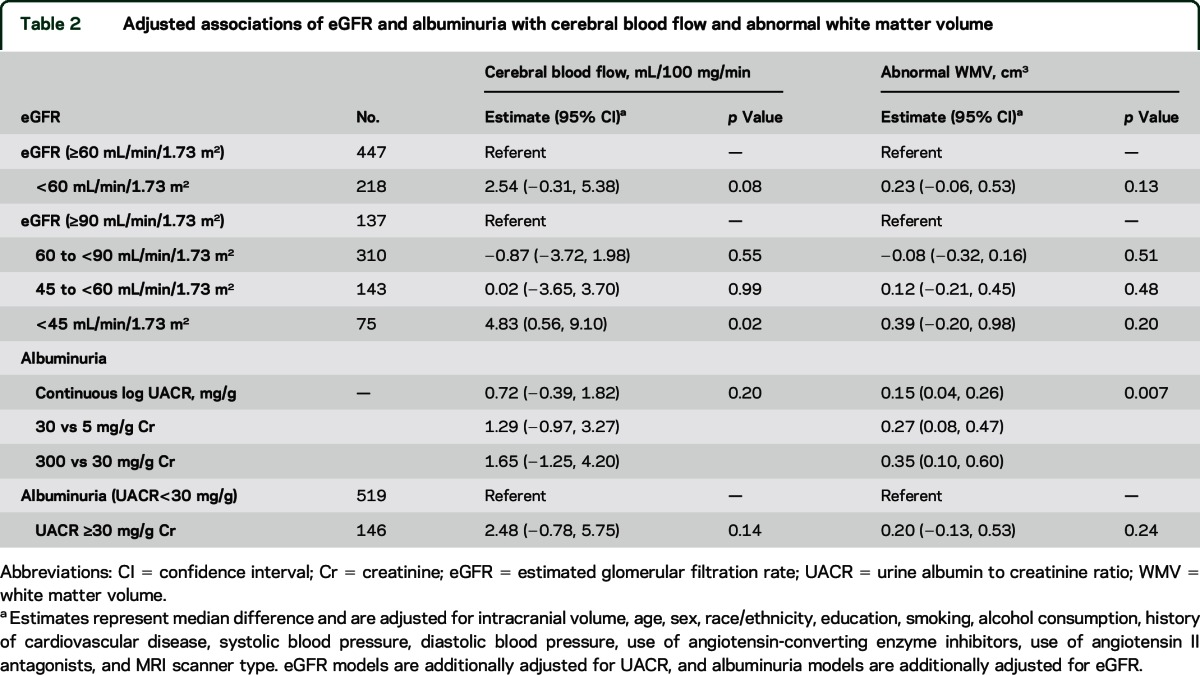
Median abnormal WMV adjusted for MRI scanner was 0.48 cm3 (95% CI 0.21, 0.75) higher among participants with eGFR <60 mL/min/1.73 m2 vs ≥ 60 mL/min/1.73 m2. After adjustment for the same set of covariates, there was no significant difference in abnormal WMV between participants with eGFR <60 mL/min/1.73 m2 vs ≥ 60 mL/min/1.73 m2 (table 2). The results were similar when we stratified participants into additional eGFR categories.
Relation of albuminuria with CBF and abnormal WMV.
Median CBF adjusted for MRI scanner was 0.73 mL/100 g/min (95% CI −0.22, 1.67) higher with each SD increase in log(UACR). After additional adjustment for eGFR, age, sex, race/ethnicity, education, smoking, alcohol consumption, history of CVD, systolic blood pressure, diastolic blood pressure, use of ACE inhibitors, and use of angiotensin II antagonists, UACR was not significantly associated with CBF (table 2). Similarly, there were no significant differences in CBF when we stratified participants as UACR <30 and ≥30 mg/g.
Conversely, in analyses adjusted for MRI scanner and intracranial volume, UACR was linearly associated with larger abnormal WMV. This result remained significant after additional adjustment for eGFR and other covariates (table 2).
CBF in white matter and gray matter.
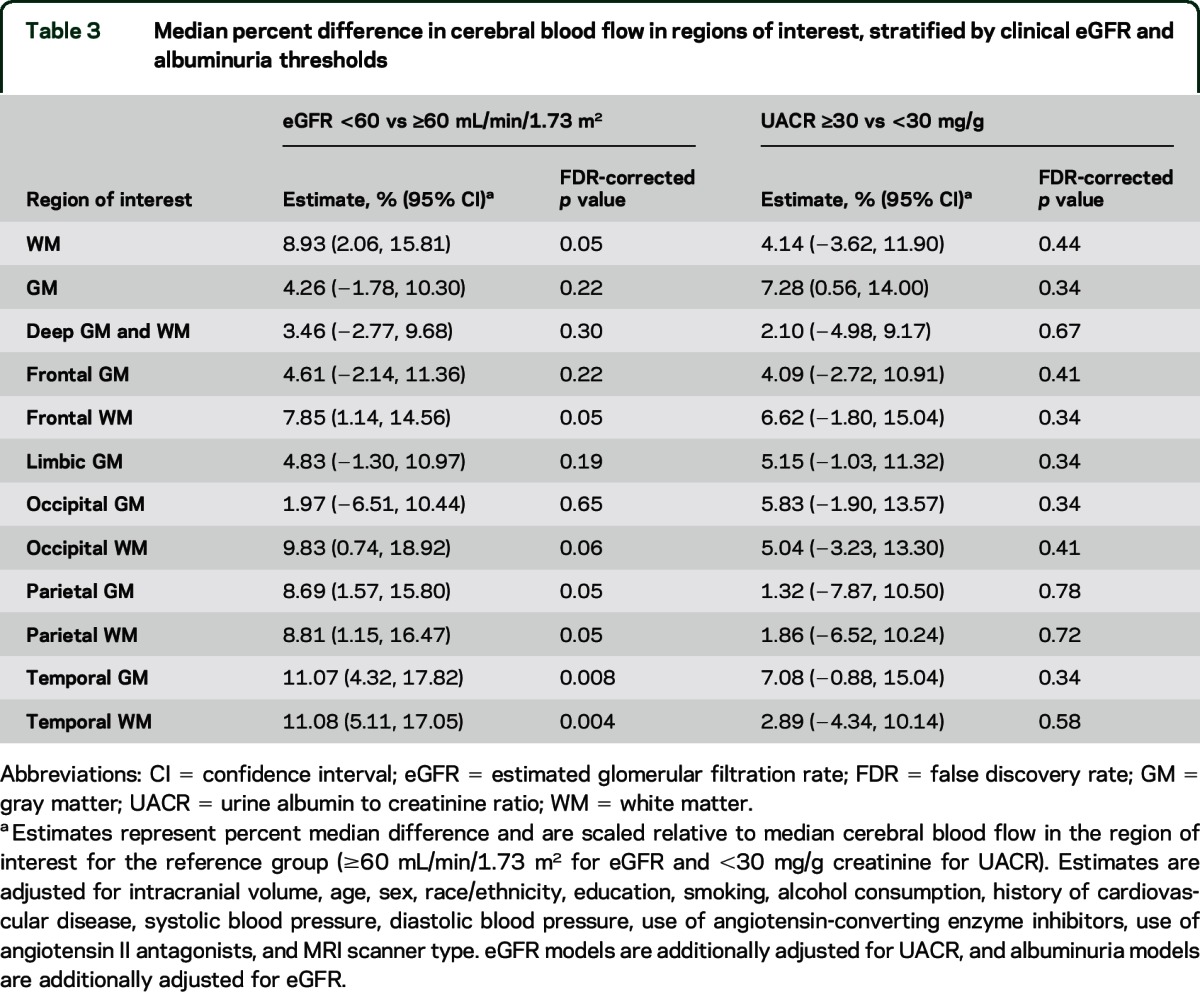
The percentage difference in median CBF was significantly higher for participants with eGFR <60 mL/min/1.73 m2 vs ≥60 mL/min/1.73 m2 in 6 of the 12 ROIs evaluated (table 3). Relatively larger differences in CBF were noted in white matter regions and in temporal gray matter. Conversely, there were no significant differences in CBF in any ROIs according to albuminuria status.
Table 3.
Median percent difference in cerebral blood flow in regions of interest, stratified by clinical eGFR and albuminuria thresholds
Joint effects of eGFR and albuminuria.
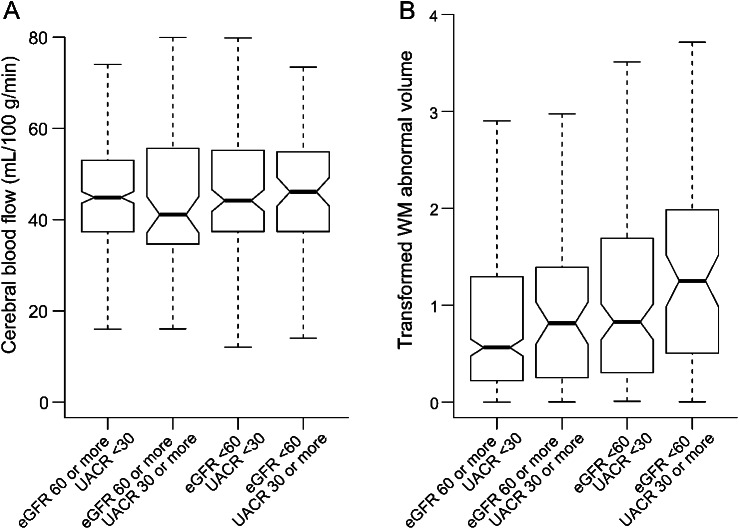
Compared to participants without CKD (eGFR ≥60 mL/min/1.73 m2 and UACR <30 mg/g), unadjusted median CBF was not significantly different among participants with one or both markers of CKD (figure). In adjusted analyses, median CBF was higher among participants with albuminuria alone or reduced eGFR alone, reaching statistical significance for participants with both markers of CKD (5.03 mL/100 g/min, 95% CI 0.78, 9.29, table 4). Consistent with additive effects of reduced eGFR and UACR, a test for statistical interaction was not significant (p = 0.97).
Figure. Unadjusted median cerebral blood flow (A) and abnormal white matter volume (B) in estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (UACR) strata.
White matter (WM) abnormal volumes (in cm3) are displayed on a transformed axis, based on an inverse hyperbolic sine transformation (x) = log (x + [x2 + 1] 0.5). Notches on boxplot represent median ± (interquartile range/n1/2), and approximately provide a 95% confidence interval for the median. Dashed lines represent minimum and maximum values.
Table 4.
Joint association of eGFR and albuminuria with cerebral blood flow and abnormal white matter volume
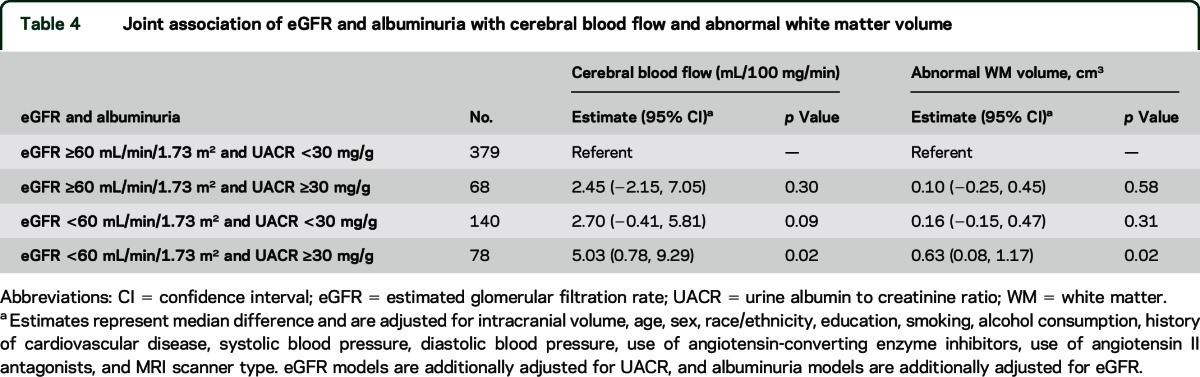
In unadjusted analyses, abnormal WMV was larger among participants with one or both markers of CKD compared to participants without CKD (figure). A similar pattern to CBF was seen after adjustment, with incrementally larger abnormal WMV for participants with albuminuria alone or reduced eGFR alone, reaching statistical significance for participants with both markers of CKD (0.63 cm3, 95% CI 0.08, 1.17, table 4). There was no evidence of statistical interaction between reduced eGFR and UACR (p value 0.27).
DISCUSSION
This study, in a large cohort of nondiabetic hypertensive adults, has several important findings. First, reduced eGFR was associated with higher CBF, independent of UACR and other confounders, whereas UACR was not independently associated with CBF after controlling for eGFR. Second, UACR was linearly associated with larger abnormal WMV, independent of eGFR and other confounders, whereas reduced eGFR was not independently associated with abnormal WMV after controlling for UACR.
In the current study, higher CBF among participants with reduced eGFR was not explained by differences in blood pressure, the main determinant of cerebral perfusion pressure, or other clinical characteristics. The relative difference in CBF was more pronounced in white matter, which has lower basal CBF and may be more vulnerable to ischemia under conditions of hemodynamic stress than gray matter regions. Related to this observation, elevated CBF was apparent at a higher eGFR threshold in white matter (eGFR <60 mL/min/1.73 m2) than for total CBF (eGFR <45 mL/min/1.73 m2). Previous small studies have assessed CBF with SPECT in patients receiving hemodialysis, and have reported both higher21,22 and lower23 CBF compared to patients with normal kidney function. A recent population-based cohort study that utilized phase-contrast imaging to assess CBF found that lower eGFR was associated with lower CBF.24 Differences in the method of assessing CBF or in cohort characteristics such as the prevalence of CKD, which was higher in the current study, might explain the difference in findings. Alternatively, a nonlinear association between eGFR and CBF may exist that was not detected due to the distribution of eGFR in each cohort.
Elevated CBF may be a marker of impaired autoregulation. When autoregulatory capacity is lost, CBF changes linearly with blood pressure. This may predispose patients to hyperperfusion or hypoperfusion, particularly in arterial watersheds.25 Alternatively, higher CBF might reflect higher metabolic demand, development of collateral circulation, or reduced arterial oxygen content related to anemia. Although the current study is not able to directly distinguish between these possibilities, several other lines of evidence support a link between reduced eGFR and impaired autoregulation.26–28
Albuminuria, but not eGFR, was independently associated with abnormal WMV. This finding confirms and extends previous cross-sectional studies in several ways.5,6,29,30 First, prior studies had limited power due to the small numbers of participants with eGFR <45 mL/min/1.73 m2. Other studies excluded individuals with serum creatinine concentrations >2 mg/dL (approximately an eGFR <45 mL/min/1.73 m2).29 In addition, it was not clear from earlier studies whether the relation between albuminuria and abnormal WMV was independent of diabetes. This is important because diabetes is associated with white matter injury, which may have both vascular and nonvascular origins.31,32
Albuminuria is increasingly recognized as a marker of not only abnormal glomerular capillary permeability, but of systemic endothelial dysfunction. Endothelial dysfunction in the glomerular capillaries leads to loss of serum proteins into the urine; a similar process in the brain microvasculature might lead to escape of serum proteins into brain perivascular spaces, causing white matter injury.33
This study has several limitations. First, clinical trial participants may not be representative of the general population. However, hypertension is a common clinical condition and SPRINT had few exclusions. Second, we classified participants based on a single measurement of serum creatinine and UACR. Serum creatinine–based estimates of GFR may misclassify individuals with low muscle mass. However, misclassification would be expected to bias the results towards the null. Third, anemia may affect the quantification of CBF using ASL.34 Hematocrit was not assessed in SPRINT; however, the degree of anemia typically present in patients with CKD35 is not likely to fully explain the CBF differences found here. Fourth, while our study sample was larger than many previous studies, we had limited power to assess the joint relationship of reduced eGFR and albuminuria. Thus, we cannot exclude the possibility that reduced eGFR and albuminuria have additive, independent effects on CBF and abnormal WMV. Finally, because this is a cross-sectional study, we were unable to determine whether reduced eGFR and albuminuria might be causes of abnormal CBF and WMV, or markers. This study also has several strengths, including the large racially and geographically diverse study population, concurrent assessment of eGFR and albuminuria to characterize kidney disease, and quantitative assessment of CBF and WMV.
We found that reduced eGFR was associated with higher CBF and albuminuria was associated with larger abnormal WMV. While the interrelationship among kidney disease, kidney disease markers, and brain structure and function is multifaceted, these findings provide insight into the hemodynamic and structural changes within the brain that may contribute to stroke risk in patients with kidney disease.
Supplementary Material
GLOSSARY
- ACE
angiotensin-converting enzyme
- ASL
arterial spin labeling
- CBF
cerebral blood flow
- CI
confidence interval
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- FLAIR
fluid-attenuated inversion recovery
- GFR
glomerular filtration rate
- IQR
interquartile range
- ROI
region of interest
- SPRINT
Systolic Blood Pressure Intervention Trial
- UACR
urine albumin to creatinine ratio
- WMV
white matter volume
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: SPRINT Study Research Group, Paul Whelton, Karen C. Johnson, Lawrence Fine, Joni Snyder, Diane Bild, Denise Bonds, Nakela Cook, Jeffrey Cutler, Susan Czajkowski, Lawrence Fine, Peter Kaufmann, Paul Kimmel, Lenore Launer, Claudia Moy, William Riley, Laurie Ryan, Joni Snyder, Song Yang, Alberta Bee, Alan J Lerner, Mahboob Rahman, Carolyn Still, Alans Wiggers, Jackson T Wright, Jr, Renee Dancie, William Cushman, Barry Wall, Linda Nichols, Robert Burns, Jennifer Martindale-Adams, Dan Berlowitz, Elizabeth Clark, Sandy Walsh, Terry Geraci, Carol Huff, Linda Shaw, Suzanne Oparil, Cora E. Lewis, Virginia Bradley, David Calhoun, Stephen Glasser, Kim Jenkins, Tom Ramsey, Alfred K. Cheung, Srinivasan Beddhu, Gordon Chelune, Jeffrey Childs, Lisa Gren, Anne Randall, Michael Rocco, David Goff, Carlos Rodriguez, Laura Coker, Amret Hawfield, Joseph Yeboah, Lenore Crago, John Summerson, Anita Hege, David Reboussin, Jeff Williamson, Walter Ambrosius, William Applegate, Greg Evans, Capri Foy, Barry Freedman, Dalane Kitzman, Nick Pajewski, Steve Rapp, Scott Rushing, Neel Shah, Mara Vitolins, Lynne Wagenknecht, Valerie Wilson, Kaycee M. Sink, Brenda Craven, Tim Craven, Katelyn Garcia, Sarah Gaussoin, Laura Lovato, Jill Newman, Bobby Amoroso, Jason Griffin, Darrin Harris, Mark King, Kathy Lane, Debbie Steinberg, Donna Ashford, Loretta Cloud, Debbie Felton, Marjorie Howard, Pamela Nance, Letitia Perdue, Nicole Puccinelli-Ortega, Laurie Russell, Jennifer Walker, Nancy Woolard, Anthony A. Killeen, Anna M. Lukkari, Robert Ringer, Brandi Dillard, Stuart Warren, Mike Sather, James Pontzer, Zach Taylor, Elsayed Z Soliman, Zhu-Ming Zhang, Yabing Li, Chuck Campbell, Susan Hensley, Julie Hu, Lisa Keasler, Mary Barr, Tonya Taylor, R. Nick Bryan, Christos Davatzikos, Ilya Nasarallah, Lisa Desiderio, Mark Elliott, Ari Borthakur, Harsha Battapady, Guray Erus, Alex Smith, Ze Wang, Jimit Doshi, Raymond Townsend, Debbie Cohen, Yonghong Huan, Mark Duckworth, Virginia Ford, Kelly Sexton, Jackson T. Wright, Jr, Alan Lerner, Mahboob Rahman, Carolyn Still, Alberta Bee, Debra Lee Stokes, Shonte Smith, Jeffrey Sunshine, Mark Clampitt, Seth Smith, Brian Welch, Manus Donahue, Alex Dagley, Dave Pennell, Chris Cannistraci, Kristin Merkle, Julie Lewis, Mohammed Sika, Clinton Wright, Mohammad Sabati, Edward Campuzano, Hector Martin, Andrea Roman, Jesus Cruz, Natalya Nagornaya, Laura Coker, Anita Hege, Joseph Maldjian, Sandra Kaminsky, Debra Fuller, Youngkoo Jung, Suzanne Oparil, Beth Lewis, Virginia Wadley, Kim Jenkins, Tom Ramsey, William Evanochko, Glenn Roberson, Trina Corbitt, William Fisher, Cathy Clements, Daniel Weiner, Andrew Wells, Amanda Civiletto, Gerard P. Aurigemma, Noelle Bodkin, Alex Norbash, Margaret Lavoye, Andrew Ellison, Ronald Killiany, Osama Sakai, Alfred Cheung, Walter Ambrosius, Dan Berlowitz, William Cushman, Suzanne Oparil, Jackson T. Wright, Jr, David Goff, Kaycee Sink, Jeff Williamson, George Thomas, Martin Schreiber, Jr, Sankar Dass Navaneethan, John Hickner, Michael Lioudis, Susan Marczewski, Jennifer Maraschky, Martha Colman, Andrea Ababy, Mahboob Rahman, Paul Draws, Denise Kresevic, Pratibha P. Raghavendra, Scott Ober, Ronda Mourad, Lisa Tucker, Bill Schwing, John Sedor, Edward J. Horwitz, Jeffery Schellling, Lisa Humbert, Wendy Tutolo, Suzanne White, Robin Hughes, Jackson T. Wright, Jr, Mirela Dobre, Carolyn H. Still, Alberta Bee, Monique Williams, Udayan Bhatt, Anil Agarwal, Melissa Brown, Nicole Ford, Cynthia Stratton, Jody Baxter, Alicia A. Lykins, Alison McKinley Neal, Leena Hirmath, Osei Kwame, William F. Miser, Colleen Sagrilla, Jan Johnston, Amber Anaya, Kelly Rogers, Donald Ebersbacher, Lucy Long, Beth Bednarchik, Alan Wiggers, Lucy Long, Adrian Schnall, Jonathan Smith, Lori Peysha, Carla Harwell, Pinkie Ellington, Mary Ann Banerji, Pranav Ghody, Melissa Vahudeh Rambaud, Raymond Townsend, Debbie Cohen, Yonghong Huan, Mark Duckworth, Virginia Ford, Juliet Leshner, Vander Veen Sarah, Crystal Gadegbeku, Avi Gillespie, Sandra Amoroso, Zoe Pfeffer, Jiang He, Jing Chen, Eva Lustigova, Jackie Dolan, Lanie Sansing, Erin Malone, Bernadette Borja, Marie Krousel-Wood, Richard Deichmann, Patricia Ronney, Susan Muery, Donnalee Trapani, Matt Diamond, Laura Mulloy, Heather Anderson, Emily Stone, Walida Walker, Charlene Weathers, Andrew McWilliams, Michael Dulin, Lindsay Kuhn, Susan Standridge, Kelry Preston, Susan Norton, Silena Gaines, Ali A. Rizvi, Andrew W. Sides, Matthew M. Hix, Philip Hutchinson, Joseph Espiritu, Mark Feinglos, Eugene Kovalik, Georgianne Gedon-Lipscomb, Kathryn Evans, MaryAnn Mason, Ronna Zimmer, Mary Furst, James Powell, Paul Bolin, Junhong Zhang, Presley Phelps, Connie Garris-Sutton, Beatrice Atkinson, Winifred Bryant, Gabriele Contreras, Maritza Suarez, Ivonne Schulman, Don Koggan, Jackie Vassallo, Gloria Peruyera, Michael Rocco, Amret Hawfield, Cassandra Bethea, Laura Gilliam, Carolyn Pedley, Geraldine Zurek, Miriam Baird, Mary Martha Smoak, Julie Williams, Samantha Rogers, Lindsay Gordon, Jessica McCorkle-Doomy, Erin Kennedy, Jonathan Adams, Dana Chamberlain, Ramon Lopez, Juris Janavs, Arlene Chapman, Allen Dollar, Olubunmi Williams, Yoosun Han, William Haley, Peter Fitzpatrick, Joseph Blackshear, Brian Shapiro, Ashley Johnson, Heath Gonzalez, Anna Harrell, Jermain Robinson, Leonardo Tamariz, Ivonne Schulman, Jennifer Denizard, Dhurga Krishnamoorthy, Frank Greenway, Timothy Church, Chelsea Hendrick, Aimee Yoches, Leighanne Sones, Markee Baltazar, Priscilla Pemu, Connie Jones, Derrick Akpalu, Laura Dember, Denise Soares, Jerry Yee, Kausik Umanath, Naima Ogletree, Schawana Thaxton, Karen Campana, Dayna Sheldon, Krista MacArthur, J. Brent Muhlestein, Nathan Allred, Brian Clements, Ritesh Dhar, Kent Meredith, Viet Le, Edward Miner, James Orford, Erik R. Riessen, Becca Ballantyne, Ben Chisum, Kevin Johnson, Dixie Peeler, Glenn Chertow, Manju Tamura, Tara Chang, Kevin Erickson, Jenny Shen, Randall S. Stafford, Gregory Zaharchuk, Margareth Del Cid, Michelle Dentinger, Jennifer Sabino, Rukmani Sahay, Ekaterina Katie Telminova, Daniel E. Weiner, Mark Sarnak, Lily Chan, Amanda Civiletto, Alyson Heath, Amy Kantor, Priyanka Jain, Bethany Kirkpatrick, Andrew Well, Barry Yuen, Michel Chonchol, Beverly Farmer, Heather Farmer, Carol Greenwald, Mikaela Malaczewski, James Lash, Anna Porter, Ana Ricardo, Robert T. Rosman, Janet Cohan, Nieves Lopez Barrera, Daniel Meslar, Patricia Meslar, Margaret Molly Conroy, Mark Unruh, Rachel Hess, Manisha Jhamb, Holly Thomas, Pam Fazio, Elle Klixbull, Melissa Komlos-Weimer, LeeAnne Mandich, Tina Vita, Robert Toto, Peter Van Buren, Julia Inrig, Martha Cruz, Tammy Lightfoot, Nancy Wang, Lori Webster, Srinivasan Beddhu, Kalani Raphael, Barry Stults, Tahir Zaman, Debra Simmons, Tooran Lavasani, Rebecca Filipowicz, Guo Wei, Gracie Mary Miller, Jenice Harerra, Jeff Christensen, Ajay Giri, Xiaorui Chen, Natalie Anderton, Arianna Jensen, Julia Lewis, Anna Burgner, Jamie P. Dwyer, Gerald Schulman, Terri Herrud, Ewanda Leavell, Tiffany McCray, Edwina McNeil-Simaan, Munmun Poudel, Malia Reed, Mohammed Sika, Delia Woods, Janice L. Zirkenbach, Dominic S. Raj, Scott Cohen, Samir Patel, Manuel Velasquez, Roshni S. Bastian, Maria Wing, Akshay Roy-Chaudhury, Thomas Depner, Lorien Dalyrymple, George Kaysen, Susan Anderson, Srinivasan Beddhu, John Nord, Debra Simmons, Gracie Mary Miller, Jenice Harerra, Ajay Giri, Joachim H. Ix, Leonard Goldenstein, Cynthia M. Miracle, Nketi Forbang, Maja Mircic, Brenda Thomas, Tiffany Tran, Anjay Rastogi, Mihae Kim, Mohamad Rashid, Bianca Lizarraga, Amy Hocza, Kristine Sarmosyan, Jason Norris, Tushar Sharma, Amanda Chioy, Eric Bernard, Eleanore Cabrera, Christina Lopez, Susana Nunez, Joseph Riad, Suzanne Schweitzer, Siran Sirop, Sarah Thomas, Lauren Wada, Holly Kramer, Vinod Bansal, Corliss E. Taylor, Mark S. Segal, Karen L. Hall, Amir Kazory, Lesa Gilbert, Linda Owens, Danielle Poulton, Elaine Whidden, Caroline Blaum, Jocelyn Jo Wiggins, Tanya Gure, Linda Nyquist, Eileen Robinson, Nauman Qureshi, Karen Ferguson, Sumrah Haider, Mandy James, Christy Jones, Kim Renfroe, April Seay, Carrie Weigart, Denyse Thornley-Brown, Dana Rizik, Bari Cotton, Meredith Fitz-Gerald, Tiffany Grimes, Carolyn Johnson, Sara Kennedy, Chanel Mason, Lesa Rosato-Burson, Robin Willingham, David Calhoun, Eric Judd, Tonya Breaux-Shropshire, Felice Cook, Julia Medina, James Lewis, Roman Brantley, John Brouilette, Jeffrey Glaze, Stephanie Hall, Nancy Hiott, David Tharpe, Spencer Boddy, Catherine Mack, Karen C. Johnson, Catherine Womack, Beate Griffin, Carol Hendrix, Karen Johnson, Lisa Jones, Chelsea Towers, Henry Punzi, Kathy Cassidy, Kristin Schumacher, Carmen Irizarry, Ilma Colon, Pedro Colon-Ortiz, Pedro Colon-Hernandez, Merari Carrasquillo, Nivea Vazquez, Miguel Sosa-Padilla, Alex Cintron-Pinero, Mayra Ayala, Olga Pacheco, Catalina Rivera, Irma Sotomayor-Gonzalez, Jamie Claudio, Jose Lazaro, Migdalia Arce, Lourdes Heres, Alba Perez, Jose Tavarez-Valle, Ferlinda Arocho, Mercedes Torres, Melvaliz Vazquez, Gerard P. Aurigemma, Rebecca Takis-Smith, Julia Andrieni, Noelle Bodkin, Kiran Chaudhary, Paula Hu, John Kostis, Nora Cosgrove, Denise Bankowski, Monica Boleyn, Laurie Casazza, Victoria Giresi, Tosha Patel, Erin Squindo, Yan Wu, Marion Wofford, Michael Flessner, Cathy Adair, Jordan Asher, Debbie Loope, Rita Cobb, Reiner Venegas, Thomas Bigger, Daniel Donovan, Carlos Lopez-Jimenez, Amilcar Tirado, Thomas Bigger, Asqual Getaneh, Rocky Tang, Sabrina Durant, Thomas Bigger, Mathew Maurer, Sergio Teruya, Stephen Helmke, Julissa Alvarez, Ruth Campbell, Roberto Pisoni, Rachel Sturdivant, Caroline Counts, Vickie Hunt, Lori Spillers, Donald Brautigam, Timothy Kitchen, Timothy Gorman, Jessica Sayers, Sarah Button, June Chiarot, Rosemary Fischer, Melissa Lyon, Maria Resnick, Karen Servilla, Darlene Vigil, Terry Barrett, Mary Meyers, Mary Ellen Sweeney, Rebecca Johnson, Susan McConnell, Khadijeh Shahid Salles, Francoise Watson, Maxine Maher, Cheryl Schenk, Laura Whittington, Stephen Swartz, Paul Conlin, Jonathan Williams, George Alexis, Helen Gomes, Rebecca Lamkin, Clive Rosendorff, Stephen Atlas, Nisharahmed Kherada, Waddy Gonzalez, Samih Barcham, Joseph John, Shakaib Rehman, Jan Basile, Michael Kagan, Hadi Baig, Deborah Ham, Mohammed Saklayen, Jason Yap, Carol Miller, Helen Neff, Saib Gappy, Arathi Raman, Shiva Rau, Vicki Berchou, Elizabeth Jones, Erin Olgren, Sithiporn Sastrasinh, Jessica Fiore, Marianne Kutza, Ronald Shorr, Anthony Bavry, Rattana Mount, Mark Segal, Jeremy Thoms, Denise Waddell, Helen Dunn, Jessica Hunter, Susan Stinson, Addison Taylor, Jeffery Bates, Jennifer Cowart, Shawn Ragbir, Catherine Anderson, Charlyne Wright, Kent Kirchner, Jodi Stubbs, Ardell Hinton, Anita Kaye Spencer, Santosh Sharma, Thomas Wiegmann, Archana Goel, Amina Khan, Virginia Savin, Smita Mehta, Michelle Krause, Debra Simmons, Karl Straub, Kate Dishongh, Barry Wall, Jaya Adabala, Richard Childress, William Cushman, Geeta Gyamlani, Atossa Niakan, Cathy Thompson, Janelle Moody, Jeffrey Whittle, Gary Barnas, Dawn Wolfgram, Heidi Cortese, Christianne Roumie, Adriana Hung, Kurt Niesner, Jennifer Wharton, Lois Katz, George Brock, Elizabeth Richardson, Troy Dixon, Joanne Holland, Athena Zias, Christine Spiller, James Felicetta, Penelope Baker, Shakaib Rehman, Penny Carrel, Suzanne Watnick, Jessica Weiss, Tera Johnston, Jacqueline Walczyk, Stephen Giddings, Andrew Klein, Caroline Rowe, Linda Conwill, Kristin Vargo, Kristi Waidmann, Vasilios Papademetriou, Jean Pierre Elkhoury, Susan Amodeo, Mary Bloom, Barbara Gregory, Katherine Hare, Dalia Goldfarb-Waysman, Richard Treger, Karen Knibloe, Areef Ishani, Yelena Slinin, Christine Olney, Paolo Fanti, Shweta Bansal, Monica Dunnam, Christopher Dyer, Lih-Lan Hu, and Perla Zarate-Abbott
AUTHOR CONTRIBUTIONS
Dr. Kurella Tamura designed the study, obtained funding, analyzed the data, and drafted the manuscript. Dr. Pajewski analyzed and interpreted the data. Dr. Bryan contributed to acquisition, analysis, and interpretation of data. Dr. Weiner contributed to acquisition of data and critical revision of the manuscript for important intellectual content. Dr. Diamond contributed to critical revision of the manuscript for important intellectual content. Dr. Van Buren contributed to critical revision of the manuscript for important intellectual content. Dr. Taylor contributed to critical revision of the manuscript for important intellectual content. Dr. Beddhu contributed to acquisition of data and critical revision of the manuscript for important intellectual content. Dr. Rosendorff contributed to critical revision of the manuscript for important intellectual content. Dr. Jahanian contributed to acquisition of the data and critical revision of the manuscript for important intellectual content. Dr. Zaharchuk contributed to acquisition, analysis, and interpretation of data.
STUDY FUNDING
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the NIH, including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part by resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the US Department of Veterans Affairs, or the US government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list. ClinicalTrials.gov Identifier: NCT01206062. MIND the Kidneys is funded by R01 DK092241 from the National Institute of Diabetes and Digestive and Kidney Diseases. Also supported by the following CTSAs funded by NCATS: CWRU: UL1TR000439; OSU: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston: UL1RR025771; Stanford: UL1TR000093; Tufts: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; UT Southwestern: 9U54TR000017-06; University of Utah: UL1TR00010505; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1 TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337; COBRE Award NIGMS; SPRINT.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ 2010;341:c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoodi BK, Yatsuya H, Matsushita K, et al. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke 2014;45:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke 2007;38:3121–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke 2008;39:55–61. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 2009;53:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman DS, Mosley TH, Jr, Bailey KR, Jack CR, Jr, Schwartz GL, Turner ST. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci 2008;271:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 2006;69:350–357. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008;105:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-atlas skull-stripping. Acad Radiol 2013;20:1566–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doshi J, Erus G, Ou Y, Davatzikos C. Ensemble-based medical image labelling via sampling morphological appearance manifolds. Presented at the MICCAI Challenge Workshop on Segmentation: Algorithms, Theory and Applications (“SATA”); Nagoya, Japan; September 22–26, 2013.
- 13.Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. International Conference on Medical Image Computing and Computer-assisted Intervention 2008;11:620–627. [DOI] [PubMed]
- 14.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of asl data analysis using an asl data processing toolbox: Asltbx. Magn Reson Imaging 2008;26:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar MI, O'Meara ES, Seliger S, et al. Albuminuria, the risk of incident stroke and stroke types in older adults. Neurology 2010;75:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes: Chronic Kidney Disease Guideline Development Workgroup M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 18.Koenker R. Quantreg: Quantile Regression R Package Version 5.05. Vienna: R Foundation; 2013. [Google Scholar]
- 19.R: A Language and Environment for Statistical Computing. Vienna: R Foundation; 2013. [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995;57:289–300. [Google Scholar]
- 21.Vorstrup S, Lass P, Waldemar G, et al. Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab 1992;12:745–749. [DOI] [PubMed] [Google Scholar]
- 22.Hirakata H, Yao H, Osato S, et al. CBF and oxygen metabolism in hemodialysis patients: effects of anemia correction with recombinant human EPO. Am J Physiol 1992;262:F737–F743. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas G, Fazekas F, Schmidt R, et al. Pattern of cerebral blood flow and cognition in patients undergoing chronic haemodialysis treatment. Nucl Med Commun 1996;17:603–608. [DOI] [PubMed] [Google Scholar]
- 24.Sedaghat S, Vernooij MW, Loehrer E, et al. Kidney function, cerebral blood flow: The Rotterdam Study. J Am Soc Nephrol Epub 2015 Aug 6. [DOI] [PMC free article] [PubMed]
- 25.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990;2:161–192. [PubMed] [Google Scholar]
- 26.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 2013;22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner DE, Tighiouart H, Levey AS, et al. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol 2007;18:960–966. [DOI] [PubMed] [Google Scholar]
- 28.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int 2006;69:266–271. [DOI] [PubMed] [Google Scholar]
- 29.Sink KM, Divers J, Whitlow CT, et al. Cerebral structural changes in diabetic kidney disease: African American-Diabetes Heart Study MIND. Diabetes care 2015;38:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akoudad S, Sedaghat S, Hofman A, et al. Kidney function and cerebral small vessel disease in the general population. Int J Stroke 2015;10:603–608. [DOI] [PubMed] [Google Scholar]
- 31.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnea-Goraly N, Raman M, Mazaika P, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care 2014;37:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopman DS. Invited commentary: albuminuria and microvascular disease of the brain-a shared pathophysiology. Am J Epidemiol 2010;171:287–289. author reply 290–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (t1) of blood at 3.0 tesla. Magn Reson Med 2004;52:679–682. [DOI] [PubMed] [Google Scholar]
- 35.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the third national health and nutrition examination survey (1988-1994). Arch Intern Med 2002;162:1401–1408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.