Abstract
Objective:
We studied the relation of early-life (mean age 25 years) and mid-life (mean age 50 years) cognitive function to early measures of hostile attitudes and effortful coping.
Methods:
In 3,126 black and white men and women (born in 1955–1968) from the Coronary Artery Risk Development in Young Adults Study (CARDIA), we used linear regression to examine the association of hostile attitudes (Cook-Medley questionnaire) and effortful coping assessed at baseline (1985–1986) to cognitive ability measured in 1987 and to a composite cognitive Z score of tests of verbal memory, psychomotor speed, and executive function ascertained in midlife (2010–2011).
Results:
Baseline hostility and effortful coping were prospectively associated with lower cognitive function 25 years later, controlling for age, sex, race, education, long-term exposure to depression, discrimination, negative life events, and baseline cognitive ability. Compared to the lowest quartile, those in the highest quartile of hostility performed 0.21 SD units lower (95% confidence interval [CI] −0.39, −0.02). Those in the highest quartile of effortful coping performed 0.30 SD units lower (95% CI −0.48, −0.12) compared to those in the lowest quartile. Further adjustment for cumulative exposure to cardiovascular risk factors attenuated the association with the cognitive composite Z score for hostility.
Conclusions:
Worse cognition in midlife was independently associated with 2 psychological characteristics measured in young adulthood. This suggests that interventions that promote positive social interactions may have a role in reducing risk of late-age cognitive impairment.
Psychological characteristics can modulate how an individual perceives and responds to stressful experiences.1 Some characteristics, including hostile attitudes, may lower the activation thresholds of the stress response,2 have been recently investigated in relation to cognitive function,3 and are correlated with vascular risk profiles related to cognitive impairment.4 Responses to stress, including effortful coping, defined as the propensity to actively minimize life stressors and difficulties despite repeated barriers to success, may result in persistent physiologic maladaptation to physical and psychological stressors, which may be related to cognitive impairment.5
Both positive1,3,6,7 and null associations between psychological characteristics, including personality traits, and cognitive impairment or neuropathology have been reported in studies of older adults.7–9 However, although personality traits are relatively stable characteristics of an individual, they can change with age and dementing processes.10,11 Studies in younger persons can provide less age or disease-confounded estimates, but evidence on the prospective association of personality traits with midlife cognitive function, when cognitive decline likely begins, is lacking.12
We used data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a large, prospective population-based cohort of black and white men and women followed since 1985–1986 to test the hypothesis that high levels of hostility and effortful coping in early adult life (age < 30 years) would be associated with lower cognitive function in early life and midlife, independent of cardiovascular risk and psychosocial circumstances.
METHODS
Study design, setting, and participants.
CARDIA, described previously,13 aims to study evolution of cardiovascular risk factors and disease in young adults aged 18–30 years at baseline. Briefly, participants sampled (n = 5,115) within balanced strata of age (18–24 years and 25–30 years), race (black and white), and education (high school graduation) were recruited in 1985–1986 in 4 US centers (Birmingham, Alabama; Minneapolis, Minnesota; Chicago, Illinois; and Oakland, California). The cohort has been re-examined at years 2, 5, 7, 10, 15, 20, and 25, with response rates among survivors of 91%, 86%, 81%, 79%, 74%, 72%, and 72%, respectively.
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval was obtained for all examinations from all participating sites and all participants signed informed consent.
Cognitive assessment.
We assessed cognition at the 8th examination (2010–2011) with 3 tests typically used in studies of community-dwelling participants without dementia and that have good distributions in this age group (for instance, no ceiling effect): the Rey Auditory Verbal Learning Test (RAVLT), which assesses verbal memory and retrieval ability14; the Digit Symbol Substitution Test (DSST), for psychomotor speed, attention, and working memory15; and the modified Stroop Test16 interference score for executive function (reversed so that higher score indicated better performance). We computed composite cognitive score by adding sex-specific standardized Z scores (individual value of x − mean of x/SD of x) of the 3 cognitive measures (additional information can be found in the supplementary material on the Neurology® Web site at Neurology.org).
In addition, we made use of a performance test at year 2 (average age 27.1 years, SD 3.6) using the mirror-tracing star test. Participants were asked to draw star diagrams within narrow boundary lines while looking at their hand only as a reflection in a mirror cardboard blocking their direct view (figure e-1). This task encompasses a broad array of cognitive domains including working memory, attention, psychomotor speed, concentration, and executive function, and has been used in studies of people with cognitive impairments.17
Psychological characteristics.
The Cook-Medley scale and John Henryism Scale for Active Coping (JHAC12) were administered at baseline in all participants (year 0, 1985) to measure hostility and effortful coping, respectively, which were previously reported to be associated with cardiovascular outcomes in the CARDIA study,18 are related to stress responses,19,20 and have been used in population-based studies.21 The Hostility scale (range 0–50) measures hostile and suspicious attitudes toward others.22 The JHAC12 scale (range 12–60) measures coping in an effortful manner with chronic and persistent stressors, with high scores representing more effortful coping.23 Additional information on the 2 scales is available in the supplementary material.
Covariates.
We considered potential confounders a priori, based on previous studies.24 Age, sex, and race were recorded and verified at each follow-up. At the 25-year examination, participants reported their highest educational achievement (from grade 1 to 20), smoking habits (previous, current, never), and alcohol consumption (mL/d). We calculated cumulative exposure to cardiovascular risk in adulthood by combining 25-year exposure information on excess body weight, diabetes, and hypertension into a score (range 0–22) described in detail in the supplementary material.
We measured depressive symptoms at the 5-, 10-, 15-, 20-, and 25-year examination with the Center for Epidemiologic Studies Depression scale (CES-D), a 20-item questionnaire that yields scores from 0 to 60.25 We computed cumulative exposure to depression by categorizing at the standard cutpoint of 16 for CES-D scale (yes = 1, no = 0) at each of the 5 follow-ups up to year 20 (score range 0–5).26
We used a validated instrument at years 7, 15, and 25 to inquire about discrimination (being prevented from doing something, hassled, or made to feel inferior) based on sex, race, or socioeconomic status in the following contexts: school, work, home, in public, applying for a job, medical care, the police, or in courts.27 We added up the summary score at each examination (0 = no discrimination, 3 = all types of discrimination in at least one of the above contexts) to obtain a measure of cumulative exposure to discrimination (score range 0–9). Finally, we computed a negative life events score (score range 20–35) combining answers at years 0 and 2 to 20 questions (no = 1; yes = 2) about unequivocal negative life events from the Psychiatric Epidemiology Rating Interview Life Events Scale, including conviction, arrest and imprisonment, job loss, divorce, major injury or illness, and assault, attack, or robbery.28
Analytic sample.
Of the 5,115 participants recruited at study inception in 1985, 273 had died before 2010, 91% participated in the year 2 (1987) examination, and 72% at year 25 (2010–2011), when 3,499 were re-assessed. Of these, 95% (n = 3,319) had complete data for all 3 cognitive outcomes and 92% (n = 3,205) also had data for hostility and effortful coping at baseline. All analyses were carried out in the 3,126 participants with data for all covariates (89% of the sample at year 25) (figure 1). Compared to those included in this study (n = 3,126), those who were not re-assessed at year 25 (n = 1,616) did not differ in age (p = 0.12) or sex distribution (p = 0.13), but had on average 1 year less of education (p = 0.001), were more likely to be black and to smoke (p < 0.001), and had higher baseline hostility (p < 0.001) and effortful coping (p = 0.01) scores. Cognitive function did not differ in those without complete data (n = 193) compared to the analytic sample (p = 0.22).
Figure 1. Derivation of the analytic sample: The Coronary Artery Risk Development in Young Adults Study (1985–2011).

Statistical analysis.
Both Cook-Medley and John Henryism scores had skewed distributions and were separately categorized into sex- and race-specific quartiles. We used analysis of variance and χ2 tests to compare sample characteristics across quartiles, and Spearman rank order correlations at p < 0.05 significance level to estimate correlations between hostility and effortful coping, and the 3 cognitive test scores.
We used multivariable linear regressions to assess the association of midlife (year 25) cognitive function to quartiles of psychological characteristics, with the lowest (best) category as referent. Models were adjusted for age, race, and sex (model 1), and progressively for education and depressive symptoms (CES-D score) measured at year 25 concurrently with the cognitive tests to account for their potential effects on cognitive test performance (model 2). Further adjustment was made for lifelong scores of depressive symptomatology and discrimination (model 3), and for negative life events (at years 0 and 2), smoking habit, alcohol consumption (at year 25), and lifelong scores of cardiovascular risk factors (CVRF) (model 4). We added sex and race interaction terms to models to formally test possible interactions in the associations of hostility and effortful coping with cognitive function (at 0.10 significant level); based on previous evidence,23 we also tested whether the effect of hostility and effortful coping was modified by low education (i.e., less than 12 years) or high discrimination.
To provide further insights into the prospective associations of psychological characteristics with cognitive ability in midlife, we also assessed the relationship between psychological characteristics and mirror-tracing star scores in early adulthood, and subsequently re-ran our main regression models also controlling for this measure of cognitive ability at baseline.
In additional analyses, we explored associations of psychological characteristics to the RAVLT, DSST, and Stroop Test separately, and applied inverse probability of attrition weights (IPCW) using relevant baseline data to explore the robustness of our main analysis to potential bias arising from differential attrition over the 25-year follow-up period.29 We used STATA 12 (STATA Corp, College Station, TX) for all analyses.
RESULTS
Participants were on average 25.1 years of age (3.6 SD) at baseline and 50.1 years of age (3.6 SD) at the year 25 examination when cognitive function was measured. White women had the highest (mean = 1.4, 0.1 SD) and black men the lowest (mean = −1.4, 0.1 SD) education- and age-adjusted cognitive score (p < 0.001).
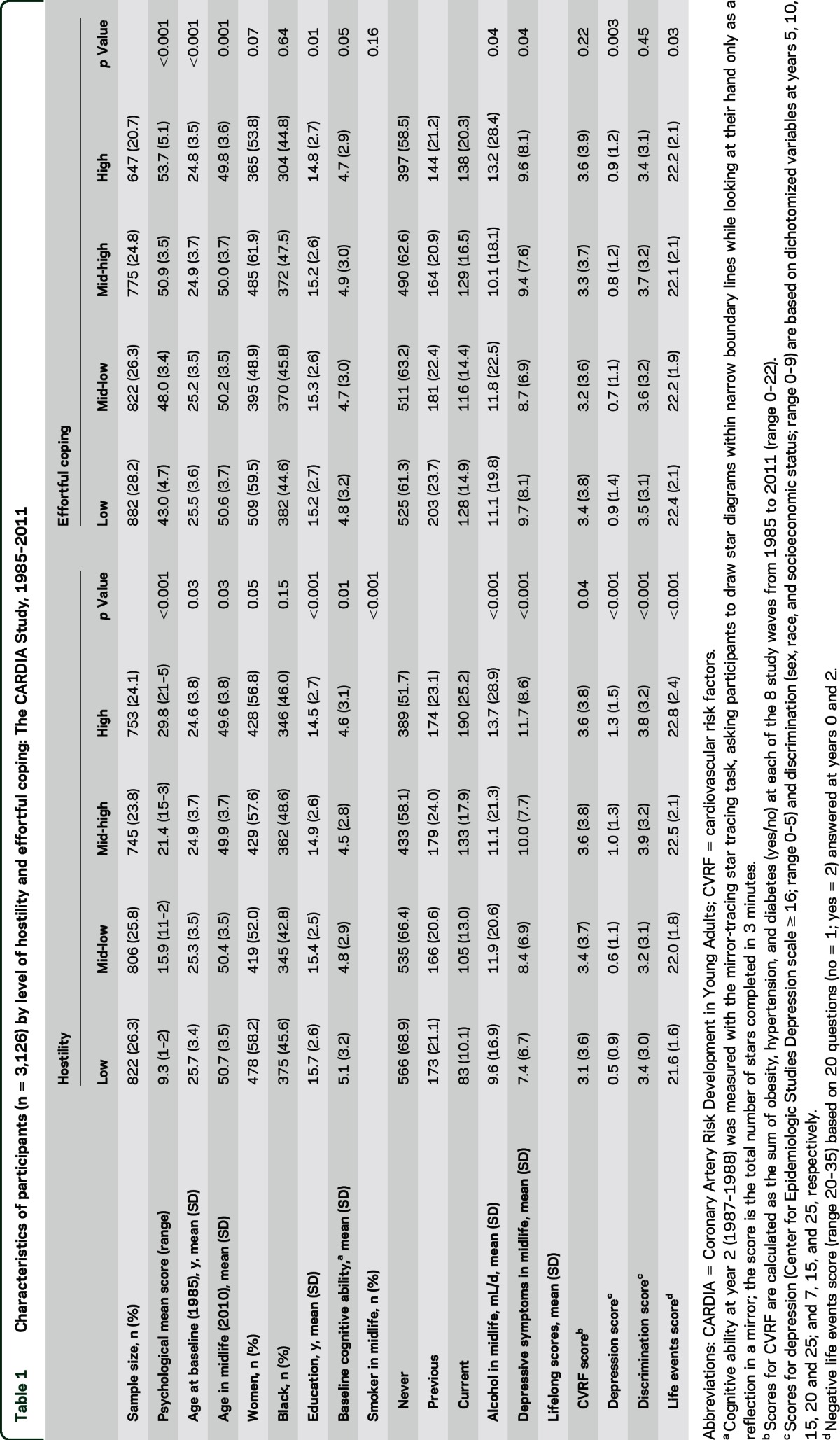
Correlation was modest between hostility and effortful coping (r = 0.14, p < 0.05), and greater among the 3 cognitive test scores (r > 0.40, p < 0.05). Overall, participants with higher hostility and higher effortful coping scores were slightly younger (p < 0.03), less educated (p < 0.01), more likely to report negative life events (p < 0.03), to consume more alcohol (p < 0.01), and to be smokers (p < 0.01) in midlife. Lifelong depressive symptomatology was higher in those in the highest hostility category (p < 0.001) and in the lowest effortful coping category (p < 0.01). Lifelong CVRF (p = 0.04) and discrimination (p < 0.001) scores were higher in those who were more hostile at baseline, but did not differ by level of effortful coping (p > 0.20) (table 1).
Table 1.
Characteristics of participants (n = 3,126) by level of hostility and effortful coping: The CARDIA Study, 1985–2011
Highest level of hostility at baseline was associated with lower cognitive function in midlife independent of demographic characteristics, educational level, and current depression (model 2), and were not confounded by lifelong depression and discrimination (model 3). After addition of negative life events factor, midlife smoking and alcohol habits, and lifelong exposures to CVRF (model 4), the difference in composite cognitive score between those with highest compared to lowest hostility level was attenuated and only borderline statistically significant: −0.18 SD units (95% confidence interval [CI] −0.37 to 0.01) (table 2).
Table 2.
Association between hostility and effortful coping measured in young adulthood (year 0, 1985–1986) with cognitive function assessed in midlife (year 25, 2010–2011) in 3,126 young black and white men and women: The CARDIA Study
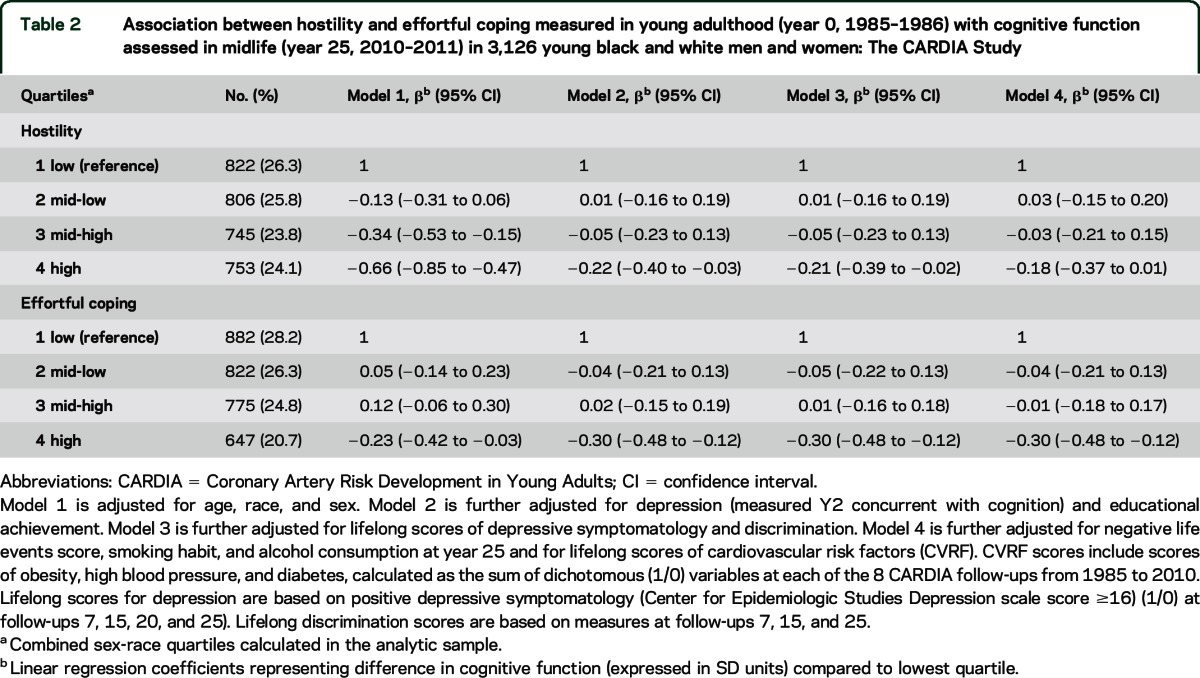
The association between higher effortful coping and lower cognitive function was significant in all models including the fully adjusted one (table 2): the composite cognitive score was 0.30 SD units lower (95% CI −0.48 to −0.12) for those in the highest compared to those in the lowest quartile of effortful coping. In addition, although cognitive score was 0.12 SD units higher (95% CI −0.06 to 0.30) in the third compared to the first quartile of effortful coping (model 1), this association was largely attenuated after adjustment (figure 2 and table 2). In the fully adjusted models, sustained depressive symptoms and exposure to CVRF in adult life (and cognitive ability in young adulthood, below) were all highly significant in our models of both hostility and effortful coping (all p values < 0.001).
Figure 2. Baseline and midlife cognitive ability according to personality scores at baseline (n = 3,126).
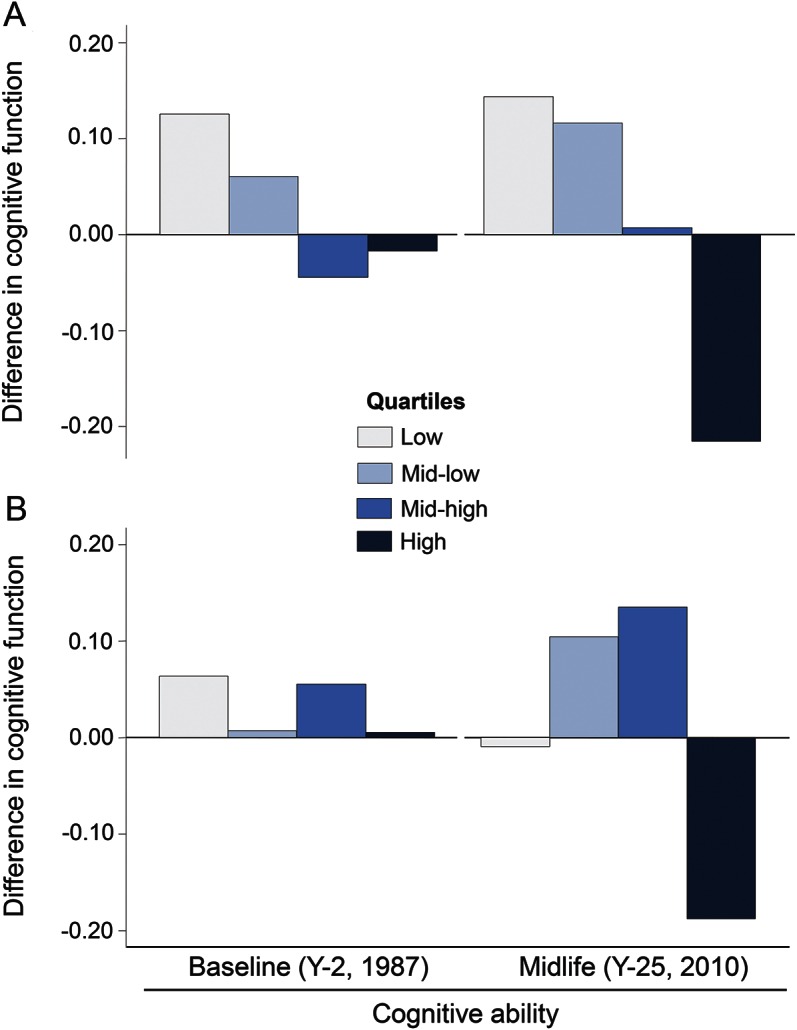
Standardized means (in SD units) of cognitive ability at baseline (mirror-star tracing scores at year 2, 1987–1988) and in midlife (composite cognitive score [sum of Z scores] of tests of memory, psychomotor speed, and executive function at year 25 [2010–2011]) adjusted for sex, race (black, white), and education (years) and age (years) at the time of cognitive assessment, according to sex-race quartiles of hostility (A) and effortful coping (B) scores measured in young adulthood (year 0, 1985–1986); the Coronary Artery Risk Development in Young Adults (CARDIA) study.
The associations of psychological characteristics with cognitive function were not modified by sex, race (all interaction terms p values > 0.20), education (p > 0.13), or discrimination (p > 0.50).
In models adjusted for sex, race, education (years), and age (years) at the time of cognitive assessment, those in the highest compared to those in the lowest quartile of hostility had a lower cognitive score at baseline (−0.14 SD units; 95% CI −0.24 to −0.04), and this difference in cognitive function accrued markedly in midlife (−0.36 SD units; 95% CI −0.54 to −0.18) (figure 2). Effortful coping was not associated with cognitive ability at baseline (all p values across quartiles >0.25) (figure 2). When we further adjusted for baseline cognitive ability, the associations with cognitive function in midlife were attenuated for hostility and nearly identical for effortful coping (table e-1) when compared to the main models (table 2).
Figure 3 shows how psychological characteristics related to RAVLT, DSST, and Stroop Test scores. On average, those in the fourth compared to those in the first quartile of hostility and effortful coping, respectively, recalled 0.16 and 0.30 fewer words (i.e., RAVLT) (p < 0.05), substituted correctly 1.88 and 2.33 fewer symbols (i.e., DSST) (p < 0.05), and had 0.57 and 0.38 higher interference score (i.e., Stroop Test) (p < 0.07), respectively. All these effect sizes were markedly greater than those per 1-year increase in age in the same sample, which were −0.02 (95% CI −0.04 to 0.00), −0.50 (95% CI −0.55 to −0.45), and 0.20 (95% CI 0.10–0.29) for RAVLT, DSST, and Stroop Test score, respectively.
Figure 3. Midlife memory (Rey Auditory Verbal Learning Test [RAVLT]), psychomotor speed (Digit Symbol Substitution Test [DSST]), and executive function (Stroop test) by baseline personality score (n = 3,126).
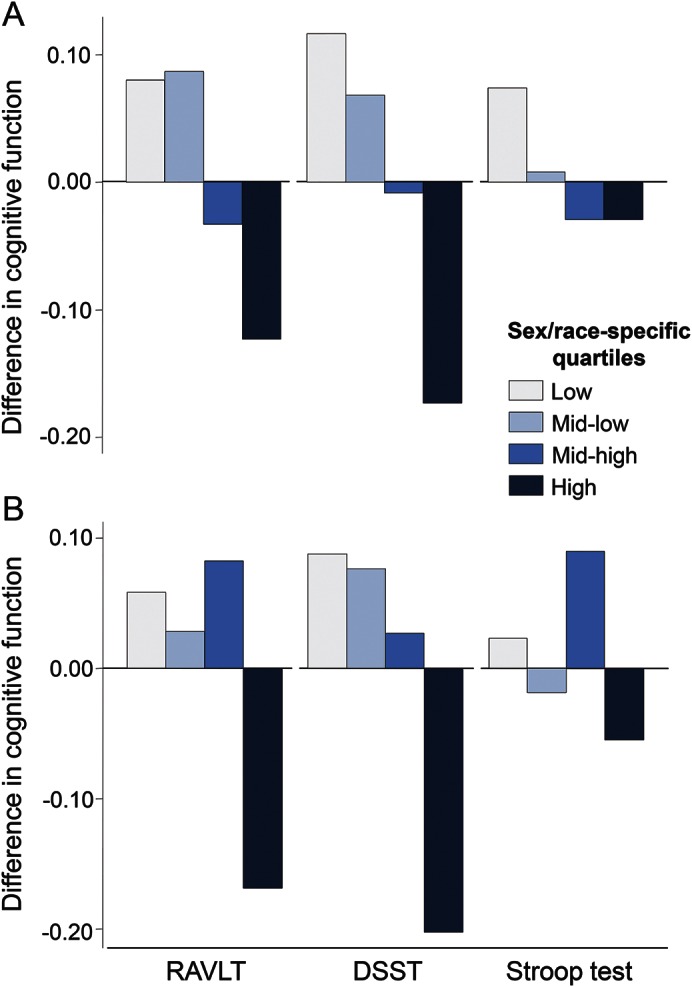
Standardized means (in SD units) of domain-specific cognitive tests in midlife (year 25, 2010–2011) adjusted for age (years), sex, race (black, white), and educational attainment (years), according to quartiles of hostility (A) and effortful coping (B) scores measured in young adulthood (year 0, 1985–1986): The Coronary Artery Risk Development in Young Adults study.
Finally, results were only very slightly attenuated in our sensitivity analysis using marginal structural models weighted for IPCW (table e-2).
DISCUSSION
In this cohort of white and black men and women followed from young adulthood to midlife, those with highest propensity to engage and persist in effortful coping behaviors with life circumstances at year 0 had lower performance on cognitive tests more than 25 years later, independent of sociodemographic characteristics, negative life events, and cumulative exposure to cardiovascular risk factors, depressive symptomatology, discrimination, and cognitive ability at baseline. The same association between higher baseline hostility and lower cognitive function in midlife was attenuated by exposure to cardiovascular risk factors. Associations with cognitive function accrued from young adulthood to midlife, were not modified by sex, race, educational level, or a measure of discrimination, and were robust to a number of alternative models specifications.
Evidence from population-based studies on the associations of psychological characteristics and personality traits with cognitive impairment,6,30 Alzheimer disease (AD),1,31–33 and dementia-related neuropathology1,9,34 remains inconsistent. Higher hostility, which may increase the risk of cognitive impairment through altered stress responses,2 has been reported to be associated with worse cognitive performance in both black and white samples,3 and with markers of neuronal damage,34 but not with global cognitive function or higher risk of AD.7 As outlined earlier, previous studies have typically ascertained hostility in old age, when reverse causality cannot be excluded; age-related subjective memory impairment and mild cognitive impairment have been related to personality changes including higher aggression/hostility.35 Our results extend evidence to midlife, and suggest that sustained depressive symptoms, exposure to CVRF in adult life, and cognitive ability in young adulthood may only in part explain this association.
We found an independent prospective association between high levels of effortful coping with stressors/negative life events and lower cognitive function in midlife. Effortful coping may increase risk of hypertension,36 which, in turn, is associated with cognitive impairment.37 However, adjustment for the vascular risk profile (including cumulative hypertension), and for cognitive ability at baseline, made little difference so there may be a direct effect. The individual propensity to cope actively in response to difficult life situations despite repeated barriers to success may result in persistent physiologic adaptation to physical and psychological stressors, which is also referred to as allostatic load.5 Studies suggest allostatic load may affect the hippocampus, a region implicated in stress response regulation, cognitive functions including memory, and depression.38
Low hostility level was linearly associated with higher cognitive functioning scores in midlife. Interestingly, compared to those with low level (first quartile) of effortful coping, those with moderate effortful coping level (third quartile) had higher midlife cognitive function (figure 2), performing particularly better on the RAVLT (memory) and Stroop interference (executive functioning) tests (figure 3). However, this positive association was progressively attenuated through adjustment for health and socioeducational characteristics (table 2). This seems plausible because moderate effortful coping can improve the response to challenges and demanding situations, which may be associated with healthier lifestyles and higher educational and professional achievements that in turn would benefit cognition.
The observed associations of cognitive test scores with hostility and effortful coping were comparable to (or greater than) those previously reported in the CARDIA Study with respect to cardiovascular health,39 and are similar to the scale of age-related cognitive decline over more than a decade in midlife observed in other cohorts.12 Therefore, the clinical implications of the observed effect sizes may be relevant. Moreover, we explored associations between psychological characteristics and baseline cognitive ability using an indicator (i.e., mirror star tracing test) unlikely prone to ceiling effects due to young age and that encompasses a broad range of cognitive functions comparable to those assessed in midlife.17 This measure of cognitive ability at baseline was also used in our sensitivity analysis to explore potential issues related to directionality (below).
Some limitations of our study are worth noting. We focused on midlife cognitive function and on characteristics measured in young adulthood and some standard measures of personality, including the NEO Personality Inventory, were not available.10 Although comparisons with previous studies conducted in older adults are difficult, our results are not age- or disease-confounded and both hostility and effortful coping have been studied in relation to stress response dysregulation in samples other than CARDIA.19,20 However, their validation as proxies of biological measures of stress response (i.e., cortisol or inflammation) could not be considered. Different from previous studies,32 we did control for reported negative life events, CVRF, and psychosocial factors (including depressive symptoms) measured over time, but residual confounding cannot be excluded. Despite the exceptionally long follow-up between baseline and cognitive assessment in midlife it should be acknowledged that directionality cannot be determined with one measurement of cognitive status. However, associations between psychological characteristics and midlife cognitive function were resistant to adjustment for an indicator of baseline cognitive ability, which could approximate to a model of cognitive change not prone to practice effects.40 Finally, those included in our analyses had lower level of both hostility and effortful coping compared to those who were excluded, which may have led to an underestimation of the true effect in our analysis. However, our results were robust to models that accounted for potential selective participant loss.
We have reported on the prospective association of hostile attitudes and effortful coping measured in young adulthood with cognitive function in midlife, a critical period for primary prevention of cognitive impairment.12 Because each may be modified by interventions that promote positive social interactions,8 they warrant further investigation in prevention studies.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- CARDIA
Coronary Artery Risk Development in Young Adults
- CES-D
Center for Epidemiologic Studies Depression scale
- CI
confidence interval
- CVRF
cardiovascular risk factors
- DSST
Digit Symbol Substitution Test
- IPCW
inverse probability of attrition weights
- JHAC12
John Henryism Scale for Active Coping
- RAVLT
Rey Auditory Verbal Learning Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
E.A. and L.J.L. are the principal authors; they conceived the present study with assistance from K.A.M. E.A. and L.J.L. obtained funding, have the right to publish all data, separate and apart from the guidance of any sponsor of the research, and take responsibility for listing coinvestigators. E.A. and J.Z. planned and carried out the statistical analysis with D.R.J. and had access to all the data and they take responsibility for the accuracy of data management and analysis. E.A. and L.J.L. interpreted the analysis and results with D.R.J. E.A. drafted the manuscript with inputs from L.J.L. and K.A.M. R.A.W., V.G.W., K.Y., and S.S. revised the manuscript for content and helped interpret the results. All authors revised the manuscript for content and approved its final version.
STUDY FUNDING
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). The funding source had no role in the design of the study; analysis and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. This manuscript has been reviewed by CARDIA for scientific content.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology 2003;61:1479–1485. [DOI] [PubMed] [Google Scholar]
- 2.Steptoe A, Marmot M. Burden of psychosocial adversity and vulnerability in middle age: associations with biobehavioral risk factors and quality of life. Psychosom Med 2003;65:1029–1037. [DOI] [PubMed] [Google Scholar]
- 3.Barnes LL, Mendes de Leon CF, Bienias JL, Wilson RS, Everson-Rose SA, Evans DA. Hostility and change in cognitive function over time in older blacks and whites. Psychosom Med 2009;71:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iribarren C, Sidney S, Bild DE, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study: Coronary Artery Risk Development in Young Adults. JAMA 2000;283:2546–2551. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171–179. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Fleischman DA, Myers RA, et al. Premorbid proneness to distress and episodic memory impairment in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2004;75:191–195. [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry 2011;19:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HX, Karp A, Herlitz A, et al. Personality and lifestyle in relation to dementia incidence. Neurology 2009;72:253–259. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med 2007;69:47–53. [DOI] [PubMed] [Google Scholar]
- 10.McCrae RR, Costa PT, Jr, Pedroso de Lima M, et al. Age differences in personality across the adult life span: parallels in five cultures. Dev Psychol 1999;35:466–477. [DOI] [PubMed] [Google Scholar]
- 11.van der Kooij MA, Fantin M, Kraev I, et al. Impaired hippocampal neuroligin-2 function by chronic stress or synthetic peptide treatment is linked to social deficits and increased aggression. Neuropsychopharmacology 2014;39:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 14.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 15.Wechsler D. Administration and Scoring Manual for the Wechsler Adult Intelligence Scale-III. London: Psychological Corporation; 2008. [Google Scholar]
- 16.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–662. [Google Scholar]
- 17.Gabrieli JD, Corkin S, Mickel SF, Growdon JH. Intact acquisition and long-term retention of mirror-tracing skill in Alzheimer's disease and in global amnesia. Behav Neurosci 1993;107:899. [DOI] [PubMed] [Google Scholar]
- 18.Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: the coronary artery risk development in young adults (CARDIA) study. JAMA 2003;290:2138–2148. [DOI] [PubMed] [Google Scholar]
- 19.Ranjit N, Diez-Roux AV, Sanchez B, et al. Association of salivary cortisol circadian pattern with cynical hostility: multi-ethnic study of atherosclerosis. Psychosom Med 2009;71:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt MM, McCallum TJ, Fritsch T. How much striving is too much? John Henryism active coping predicts worse daily cortisol responses for African American but not white female dementia family caregivers. Am J Geriatr Psychiatry 2011;19:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haynes SG, Feinleib M, Levine S, Scotch N, Kannel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham study: II: prevalence of coronary heart disease. Am J Epidemiol 1978;107:384–402. [DOI] [PubMed] [Google Scholar]
- 22.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med 1989;51:46–57. [DOI] [PubMed] [Google Scholar]
- 23.James SA, Hartnett SA, Kalsbeek WD. John Henryism and blood pressure differences among black men. J Behav Med 1983;6:259–278. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Barnes LL, Bennett DA, et al. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology 2005;64:380–382. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D scale: a self-reported depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 26.Mulrow CD, Williams JW, Jr, Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Ann Intern Med 1995;122:913–921. [DOI] [PubMed] [Google Scholar]
- 27.Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. Am J Public Health 1996;86:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: the PERI life events scale. J Health Soc Behav 1978;19:205–229. [PubMed] [Google Scholar]
- 29.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology 2007;68:2085–2092. [DOI] [PubMed] [Google Scholar]
- 31.Archer N, Brown RG, Reeves S, Nicholas H, Boothby H, Lovestone S. Midlife Neuroticism and the age of onset of Alzheimer's disease. Psychol Med 2009;39:665–673. [DOI] [PubMed] [Google Scholar]
- 32.Terracciano A, Sutin AR, An Y, et al. Personality and risk of Alzheimer's disease: new data and meta-analysis. Alzheimers Dementia 2014;10:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson L, Guo X, Duberstein PR, et al. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology 2014;83:1538–1544. [DOI] [PubMed] [Google Scholar]
- 34.Terracciano A, Iacono D, O'Brien RJ, et al. Personality and resilience to Alzheimer's disease neuropathology: a prospective autopsy study. Neurobiol Aging 2013;34:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ausen B, Edman G, Almkvist O, Bogdanovic N. Personality features in subjective cognitive impairment and mild cognitive impairment: early indicators of dementia? Dement Geriatr Cogn Disord 2009;28:528–535. [DOI] [PubMed] [Google Scholar]
- 36.James SA, Strogatz DS, Wing SB, Ramsey DL. Socioeconomic status, John Henryism, and hypertension in blacks and whites. Am J Epidemiol 1987;126:664–673. [DOI] [PubMed] [Google Scholar]
- 37.Hughes TF, Ganguli M. Modifiable midlife risk factors for late-life cognitive impairment and dementia. Curr Psychiatry Rev 2009;5:73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res 2003;28:1735–1742. [DOI] [PubMed] [Google Scholar]
- 39.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol 2013;73:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson RS, Li Y, Bienias L, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychol Aging 2006;21:774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


