Abstract
Introduction
Early diffuse cutaneous systemic sclerosis (dcSSc) is characterized by rapid changes of skin and internal organs. Our objective was to develop a composite response index in dcSSc (abbreviated CRISS) for use in randomized controlled trial (RCT.
Methods
We developed 150 paper patient profiles with standardized clinical outcome elements (core set items) using patients with dcSSc. Forty scleroderma experts rated 20 patient profiles each and assessed whether each patient had improved or not over a period of 1 year. Using profiles where raters reached a consensus on whether the patients were improved vs. not (79% of profiles examined), we fit logistic regression models where the binary outcome referred to whether the patient was improved or not, and the change in the core set items from baseline to follow-up were entered as covariates. We tested the final index in a previously completed RCT.
Results
Sixteen of 31 core items were included in the patient profiles after a consensus meeting and review of test characteristics of patient-level data. The logistic regression model that included the following core set items: changes in the Rodnan skin score, forced vital capacity (FVC)% predicted, patient and physician global assessments, and HAQ-DI over 1 year had sensitivity of 0.982 (95%CI: 0.981–0.983), specificity of 0.931 (95% CI: 0.930–0.932), and had the highest face validity. Subjects with a significant decline in renal or cardiopulmonary involvement were classified as not improved, regardless of improvements in other core items. The index was able to differentiate the effect of methotrexate from placebo in a 1-year RCT (p< 0.05).
Conclusion
We have developed CRISS that is appropriate for use as an outcome assessment in RCT of early dcSSc.
Background
Systemic sclerosis (scleroderma, SSc) is one of the most life-threatening rheumatic diseases (1, 2), and is associated with substantial morbidity and many detrimental effects on health-related quality of life (3). In recent years, progress has been made in the development and validation of outcome measures and refinement of trial methodology in SSc (4–7). These advances were paralleled by an increased understanding of the pathogenesis of SSc (8) and development of potential targeted therapies (9). The Modified Rodnan Skin Score, a measure of skin thickness (6), has been used as the primary outcome measure in clinical trials of diffuse cutaneous SSc (dcSSc). However, the complexity and heterogeneity of the disease mandate a composite response measure that captures multiple organ involvement and patient-reported outcomes.
An accepted, validated, composite response index in dcSSc could substantially facilitate drug development and clinical research. Compared to individual outcome measures, a composite index has the potential to be more responsive to change (10–12), improve assessment of therapeutic interventions, and facilitate the comparison of responses across trials. Regulatory and funding agencies would then have greater confidence in proposals for interventions.
Our objective was to develop a Composite Response Index in Systemic Sclerosis (abbreviated CRISS) for use in clinical trials.
Patients and Methods
The index was developed using well-accepted expert consensus (13) and data-driven approaches (Figure 1), including the American College of Rheumatology standards for the development of response criteria (14). Details are included in the Supplementary material. The basic process was as follows: i) We conducted a consensus exercise to select domains and outcome measures (core items) for potential inclusion in the composite response index. ii) We then tested the psychometric properties of the core items in a longitudinal cohort of patients followed over 1 year to assess the items’ feasibility, reliability, validity, and sensitivity to change. iii) We developed a set of 150 patient profiles based on the data generated from the cohort study (and using the core items). Forty scleroderma experts were invited to classify each patient profile as improved or not improved. iv) We performed statistical reduction of the data to a minimum number of domains and core items, which retained the maximally responsive index and was acceptable to the experts (face validity). v) We then tested the ability of the composite response index to discriminate among therapies using results from a previously published randomized controlled trial (RCT). The following paragraphs describe each step in greater detail.
Figure 1.
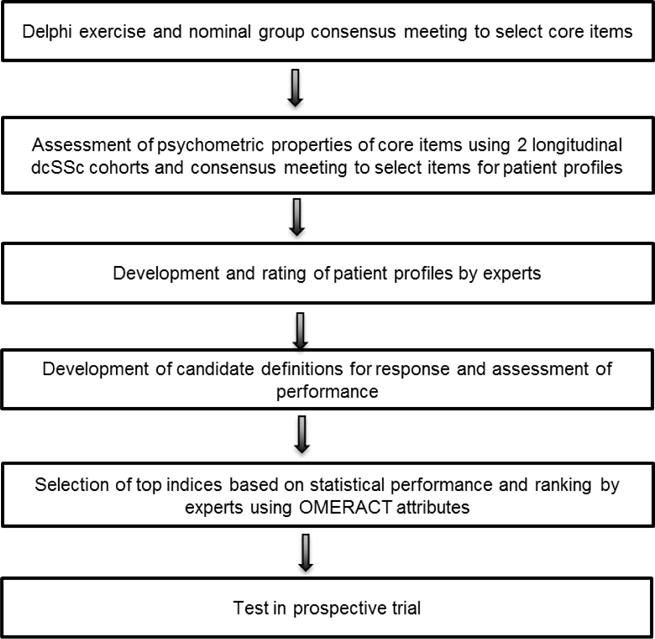
Expert consensus and data-driven approaches used to develop CRISS
(i). Structured consensus exercise to develop domains and core items
We conducted a structured, 3-round Delphi exercise to reach consensus on core items for clinical trials of SSc the details of which have been published elsewhere (5). Briefly, an initial list of potential domains and items was composed by a steering committee and then the members of the Scleroderma Clinical Trials Consortium (SCTC). Round 1 asked the SCTC members to list items in 11 pre-defined domains and Round 2 asked respondents to rate the importance of the chosen items on a 1–9 ordinal scale. This was followed by a face-to-face meeting where, under expert facilitators, consensus was reached using the Nominal Group Technique (13) about the domains and core items to test in a database (5). During this exercise, the Steering Committee discussed the feasibility, reliability, redundancy, and validity of the items.
(ii). Data collection and evaluation of psychometric properties in a longitudinal observational cohort
Due to a lack of positive trials in dcSSc and as a consequence of the fact that previous trials did not include some of the core items chosen in the consensus exercise (15), we launched a longitudinal observational cohort (the CRISS Cohort) of patients with early dcSSc (< 5 years from 1st non-Raynaud’s phenomenon sign or symptom) at 4 US Scleroderma Centers (16). The observational cohort, recruited over 1 year, included 200 patients with dcSSc, defined as skin thickening proximal, as well as distal, to the elbows or knees, with or without involvement of the face and neck. Patients were followed for 12 months and outcomes were collected at baseline and 12 months. Exclusion criteria included life expectancy of less than 1 year and non-proficiency in English. All core items that emerged from the consensus meeting were included to enable an assessment of their psychometric properties (e.g., feasibility, reliability, and face, content, and construct validity [including sensitivity to change]) (17). Feasibility was defined as completion of the core set item by > 50% of subjects at two time points, redundancy was defined as either a Spearman or Pearson correlation coefficient of at least 0.80 at baseline or during follow up. Sensitivity to change was calculated over the 1-year period using appropriate patient and physician anchor and transition questions. For example, a modified Likert scale (transition health question) was employed by physicians and patients at the 1-year follow-up visit to determine the change in overall condition during the prior year on a scale from 1 (“much better”) to 5 (“much worse”). Responses of 1 or 2 were considered an improvement in health, ratings of 4 or 5 were considered a decline in health, and a rating of 3 was considered to mean that there was no appreciable change in overall health. For this analysis, those who answered “1” or “2” were categorized as “improved” on both transition questions and those who scored “3”, “4” or “5” were categorized as “not improved”. Effect size (ES) was calculated using the transition questions as anchors and Cohen’s “rule-of-thumb” for interpreting ES: values of 0.20–0.49 represent a small change, values between 0.50–0.79 a medium change, and ≥0.80 a large change (18). Core items that were significant at predefined p< 0.20 (for dichotomous measures) or had an effect size ≥ 0.20 in the “Improved” group (with respect to either patient or physician assessments) were included in the next stage.
Eight Steering Committee members (see Acknowledgement section) reviewed the data and scored each core item on an ordinal scale (1–4) for feasibility, reliability, and face, content, and construct validity [including sensitivity to change] using the modified content validity index matrix (19): a score of 4 (highest score) was assigned when the item referred to a value or an attribute well-established in the literature or through systematically obtained information; a score of 3 indicated a value or an attribute somewhat known and accepted, but that may need minor alteration or modification; a score of 2 indicated that the rater was unable to assess the attribute without additional information or research; and a score of 1 (lowest score) meant that the attribute should definitely not be used as a core item. Experts could also assign “not applicable” if they were unfamiliar with an item or different aspects of feasibility, reliability, and validity for the item. Items scored as 3 or 4 were considered supportive of an individual item.
Based on results from psychometrics analysis and expert input, a modified Nominal Group Technique exercise was conducted via webinar by E. Giannini where consensus was defined a priori as ≥75% agreement on each item of the matrix and overall inclusion/exclusion of the item as a core item. During the NGT webinar, summary statistics were provided for each core set item and the moderator encouraged to discuss each item by each committee member and then as a group. This process ensured all participants had an opportunity to contribute. Subsequently, each item was rescored (if the committee member felt that it should be changed) and summary statistics were generated. Items that were found to lack feasibility, reliability, and validity (<75% raters assigning score of 3 or better) were excluded from the next step.
(iii). Development and ratings of representative patient profiles
In this step, we developed 150 paper patient profiles using actual data from the CRISS Cohort. To have sufficient data for the representative patients, we also obtained data from early dcSSc (defined as the disease duration < 5 years) in the Canadian Scleroderma Research Group (CSRG) database (20), a large observational Canadian scleroderma cohort. Since patient interviews were not performed as part of the consensus meeting (Step i), the medical literature was searched to assess the most prevalent/bothersome issues faced by patients with SSc (21–23). Based on this, pain and fatigue (assessed by the SF-36 vitality scale), were included as part of the patient profiles.
Fifty-four international scleroderma experts in clinical care and trial design were subsequently invited to participate in a web-based evaluation of 20 patient profiles each. The profiles were randomly assigned to experts based on their location (North America [N=29] vs. Europe [N=21] vs. Australia [N=4]) and years of experience with management of SSc (>10 years [N=38] vs. ≤ 10 years of scleroderma experience [N=16]) to prevent systematic bias in rating due to practice patterns. For each patient profile, the rater was asked three questions:
Do you think the patient has improved, stabilized, or worsened (or unable to tell) over 1 year?
If the patient was rated as improved or worsened, by how much did the patient’s condition change?: considerably, somewhat, or a little.
How would you rank the three most important core items that influenced your decision regarding change or stability?
Consensus was met if at least 75% among those who rated the same patient profile agreed that the patient had improved, stabilized, or worsened. When there was lack of consensus, the Steering Committee members were asked to rate the profiles that were not assigned to them before, followed by a web-based Nominal Group Technique exercise to discuss each profile in detail. These patient profile ratings were then added to the previous voting and percentage consensus was recalculated. If the proportion of agreement on a patient profile was ≥ 75%, the case was deemed as having reached consensus. This process produced a final list of 16 core items. Finally, we sought consensus among SSc experts on the level of change in internal organ involvement that would classify a patient as not improved.
(iv). Development of response definitions
Using only profiles where consensus was reached, we fit logistic regression models to the binary outcome, i.e., whether a patient had been rated by experts as being improved (=1) vs. not improved (=0), Not improved included scenarios rated as either no change or worsened. We examined various models, increasing at each step the number of predictors (core set items) included in the logistic regression model. For each model, we calculated sensitivity, specificity, and area under the curve (AUC). Additionally, using the estimates of the logistic regression beta coefficients, we derived, for each patient profile, the predicted log-odds, and thus, the predicted probability, that the patient would be rated as improved. We then compared the predicted probability to the raters’ consensus opinion on the patient. Accuracy of the predictions was evaluated in several ways. Using the predicted probabilities in their continuous form, accuracy in the predictions was quantified by the Brier score (24); the model with the lowest Brier Score is interpreted to have the best predictive performance.
We also tested whether the predicted probabilities had a different distribution for the patient profiles which were rated improved by the experts and for those that were rated not improved. We assessed the difference in the two distributions via the non-parametric Mann-Whitney test. We examined whether the predicted probabilities could be transformed into binary classifications by choosing a threshold and defining “improved” for all patients for which the predicted probability is above the chosen threshold and “not improved” for all patients for which the predicted probability is below the threshold. To identify which threshold (i.e., cut point) to use, we considered different possible cut points from 0.1 to 1.0. For each of the thresholds considered, we derived the corresponding sensitivity and specificity of the predicted binary classification of patients into improved (=1) or not improved (=0). We made a plot of the sensitivity and specificity as a function of each threshold and determined which threshold had the highest sensitivity and specificity. The data-driven definitions were discussed with the Steering Committee regarding content and face validity.
To determine whether there was a clear distinction among the 16 core items in their helpfulness to guide raters in determining whether a patient was improved or not, we conducted a cluster analysis. To evaluate the contribution of each core component to the final CRISS, we computed the generalized coefficient of determination or pseudo R2 for logistic regression (25).
(v). Preliminary evaluation in an independent cohort
The composite index was tested in a randomized controlled trial of methotrexate vs. placebo in early dcSSc (26). This trial was chosen as individual patient data were recorded and all final core items were available in this database. We applied the CRISS to the subjects with complete data and, for each subject, derived the predicted probability that a subject was improved using the predicted probability equation (see Results section). We transformed the continuous predicted probabilities ranging from 0 to 1 into a binary classification, by defining each subject “improved” or “not improved” depending on whether the predicted probability was above the threshold with the highest sensitivity and specificity (identified in Step # iv). We then tested whether the probability of being improved was independent of being on methotrexate (e.g., whether the probability of being improved was the same in the two groups of subjects – placebo and methotrexate) by performing a chi-square test. We also assessed whether the distributions of the predicted probabilities for the subjects on methotrexate and subjects on placebo were different using the Mann-Whitney test.
Results
(i). Structured Consensus Exercise to develop domains and core items
A total of 50 SCTC investigators participated in Round 1, providing 212 unique items for the 11 domains, and rated 177 items in Round 2. The ratings of 177 items were reviewed by the Steering Committee, and 11 domains and 31 items were identified as the core items that met the Outcome Measures in Rheumatology (OMERACT) filters of truth, feasibility, and discrimination. The 11 domains included: skin, musculoskeletal, cardiac, pulmonary, gastrointestinal, renal, Raynaud’s phenomenon, digital ulcers, health-related quality of life and function, global health, and biomarkers. Attendees of OMERACT conference in 2008 provided input during the consensus exercise (4, 27).
(ii). Data collection and evaluation of psychometric properties in a longitudinal observational cohort
CRISS Cohort
Two hundred patients with early dcSSc were recruited at baseline and 150 had both baseline and 1-year data. In these 150 patients, mean (SD) age was 50.4 (11.7), years, 74.7% were female, 78% were Caucasian and 10.7 % were Hispanic with mean disease duration (dated from 1st non-Raynaud’s sign or symptom) of 2.3 (1.5) years, mean modified Rodnan skin score (MRSS) of 21.4 (10.1) units, mean FVC% predicted of 82.3% (18.5), and mean HAQ-DI of 1.0 (0.8; Table 1).
Table 1.
Baseline demographics of patients who participated in the CRISS Cohort with baseline and 1 year data
| Baseline N | ||
|---|---|---|
|
| ||
| Age, mean (SD) | 150 | 50.4 (11.7) |
|
| ||
| Female, N (%) | 112 (75%) | |
|
| ||
| Race, N (%) | 150 | |
| Caucasian | 117 (78%) | |
| African American | 13 (9%) | |
| Asian | 11 (7%) | |
| Other or not provided | 9 (6%) | |
|
| ||
| Ethnicity, N (%) | 150 | |
| Hispanic | 16 (11%) | |
| Non-Hispanic | 134 (89%) | |
|
| ||
| Disease duration from first non-Raynaud symptom (yrs), mean (SD) | 144 | 1.59 (1.34) |
|
| ||
| Years since first Raynaud symptom, mean (SD) | 128 | 2.87 (2.49) |
|
| ||
| Years since first non-Raynaud symptom, mean (SD) | 129 | 2.32 (1.5) |
|
| ||
| Body mass index, mean (SD) | 96 | 26.02 (7.1) |
|
| ||
| Modified Rodnan skin score, mean (SD) | 150 | 21.4 (10.1) |
|
| ||
| Durometer, mean (SD) | 113 | 272.4 (64.5) |
|
| ||
| Forced vital capacity % predicted, mean (SD) | 140 | 82.32 (18.5) |
|
| ||
| Total lung capacity % predicted, mean (SD) | 109 | 87.83 (20.4) |
|
| ||
| Diffusion capacity of carbon monoxide % predicted, mean (SD) | 140 | 65.05 (20.9) |
|
| ||
| High-resolution computer tomography consistent with interstitial lung disease, N (%) | 99 | 79 (80) |
|
| ||
| 6-minute walking distance, mean (SD) | 50 | 421.6 (139.2) |
|
| ||
| Borg dyspnea (0–10 scale), mean (SD) | 46 | 1.92 (1.51) |
|
| ||
| Tendon friction rubs, N (%) | 140 | 40 (29) |
|
| ||
| Small joint contractures, N (%) | 133 | 78 (59) |
|
| ||
| Large joint contractures, N (%) | 133 | 39 (29) |
|
| ||
| Digital ulcers, N (%) | 150 | 15 (10) |
|
| ||
| Health assessment questionnaire-disability index, mean (SD) | 150 | 1.0 (0.8) |
|
| ||
| Digital ulcers VAS (0–150), mean (SD) | 134 | 20.9 (40.9) |
|
| ||
| Raynaud’s VAS (0–150), mean (SD) | 135 | 32.7 (40.8) |
|
| ||
| Breathing VAS (0–150), mean (SD) | 138 | 23.1 (36.7) |
|
| ||
| GI VAS (0–150), mean (SD) | 136 | 22.6 (34.4) |
|
| ||
| Disease severity VAS (0–150), mean (SD) | 138 | 56.4 (42.9) |
|
| ||
| Pain VAS (0–10), mean (SD) | 140 | 4.0 (2.8) |
|
| ||
| SF-36 PCS, mean (SD) | 138 | 37.6 (12.9) |
|
| ||
| SF-36 MCS, mean (SD) | 138 | 44.2 (6.0) |
|
| ||
| Physician global assessment VAS (0–10 cm), mean (SD) | 143 | 4.4 (2.2) |
|
| ||
| Patient global assessment VAS (0–10 cm), mean (SD) | 140 | 4.1 (4.0) |
|
| ||
| Antinuclear antibody, N (%) | 116 | 94 (81) |
|
| ||
| Anti-SCL-70 antibody, N (%) | 115 | 34 (30) |
|
| ||
| Serum creatine phosphokinase (IU/L), mean (SD) | 127 | 143.9 (184.5) |
|
| ||
| Serum platelets (k/uL), mean (SD) | 143 | 315.2 (102.5) |
|
| ||
| Serum brain natriuretic peptide (pg/ml), mean (SD) | 105 | 161.3 (824.0) |
|
| ||
| Serum erythrocyte sedimentation rate (mm/hr), mean (SD) | 121 | 23.4 (22.6) |
|
| ||
| Serum C-reactive protein (mg/dL), mean (SD) | 116 | 2.1 (4.9) |
VAS=visual analog scale; PCS=Physical component scale; MCS=Mental component scale
Core items that lacked feasibility due to low completion rate (< 50%) at 1 year included durometer (a device to measure the skin hardness (28)), right heart catheterization, Borg dyspnea index, 6-minute walk test, and Raynaud’s Condition Score (29) (required daily patient diary records).
Using the patient global assessment as the metric to classify patients as improved vs. not, 57% of patients were rated as “improved” and 43% were rated as “not improved”. Using physician global assessment, 58% of patients were rated as “improved” and 42% were rated as “not improved”. The Spearman correlation among the definitions was 0.46, supporting use of 2 global transition questions. Using these transition questions, 5 items were found to be not responsive to change or occurred in less than 10% of the cohort: tender joint count, presence of renal crisis, estimated GFR, body mass index, presence of digital ulcers, and erythrocyte sedimentation rate. A modified Nominal Group review was performed wherein consensus was achieved on 16 core items that should be used for the development of paper patients. It was decided to keep renal crisis and presence/absence of digital ulcers as core items due to their impact on prognosis in early dcSSc. No redundancy was noted in the core items at baseline and change scores as assessed by the correlation coefficients (Appendix Tables 1–2).
(iii). Development and ratings of representative patient profiles
A total of 150 patient profiles were rated by 40 of 54 invited experts (74% completion) (20 profiles rated by each expert; examples shown in the Appendix Tables 3–5). The median number of experts that rated a profile was 6, and the range was 4–13. In response to the instruction, “Please rank the most important core items that influenced your decision regarding change or stability”, experts ranked MRSS as the “most important” 44% of the time, followed by FVC% predicted (14.5%), patient global assessment (11.0%), physician global assessment (9.1%), and HAQ-DI (8.0%; Table 2). All other core items were ranked as most influential in the decision making less than 2% of the time.
Table 2.
Final CRISS model consisting of 5 core items with highest face validity
| Core items (calculated as changed from baseline to 1 year) | Area under the curve (AUC) | Sensitivity (95% CI) | Specificity (95% CI) | Unadjusted Beta coefficients | Standard errors |
|---|---|---|---|---|---|
|
| |||||
| MRSS | −0.81 | 0.21 | |||
| FVC predicted | 0.21 | 0.08 | |||
| HAQ-DI | 0.9861 | 0.9821 | 0.9310 | −0.40 | 0.24 |
| Patient global assessment | (0.9816, 0.9827) | (0.9300, 0.9321) | −0.44 | 0.26 | |
| Physician global assessment | −3.41 | 1.75 | |||
MRSS= modified Rodnan skin score, FVC= Forced vital capacity, HAQ-DI= health assessment questionnaire-disability index, MRSS= modified Rodnan skin score
Initially, consensus was achieved for 107 (71.3%) of the patient profiles. The Steering Committee then rescored the remaining 43 profiles as improved, worsened, or stable, and final consensus was achieved in 118 (78.7%) profiles. These profiles were then used for developing the response definitions.
(iv). Development of response definitions
Logistic regression models
There were 118 profiles for which consensus was reached; these profiles were used in the statistical models that examined response definitions regarding improvement based on change in the 16 core items. In 1-core item models (models where only one covariate was included), AUC ranged from 0.47 (for the model including as single covariate the change in presence/absence of new digital ulcers) to 0.92 (for the model including as single covariate the change in MRSS; Appendix Table 6). In a 2-core item model, change in MRSS and change in FVC% predicted yielded the highest AUC (0.96; Appendix Table 7) but was deemed not to have content validity as it did not include either the patient or physician perspective. Different definitions of response and their corresponding AUC, sensitivity, and specificity were discussed by the Steering Committee (data available from the corresponding author). The 5-core item model including change in MRSS, FVC% predicted, physician global assessment, patient global assessment, and HAQ-DI was voted as having the greatest face validity (Table 2). The clustering algorithm supported 5-core item model with the first cluster contained the following 5 items—MRSS, FVC% predicted, patient global assessment, physician global assessment, and HAQ-DI and the second cluster included all the remaining core items (Table 3). This model had a sensitivity of 0.9821 (95% CI: [0.9816, 0.9827]), specificity of 0.9310 (95% CI: [0.9300, 0.9321]), and AUC of 0.9861. The Brier score was 0.038 (lower score indicates a better predictive performance). As the data were not normally distributed, non-parametric tests were used to assess whether the distributions of the predicted probability of improving were different for the subjects who improved and those who did not (p-value < 0.0001; Figure 2a). Using depiction of sensitivity vs. specificity for improved vs. not improved group, a threshold of 0.6 had the best combination of specificity and sensitivity values (Figure 2b). The 5-core item logistic regression model can be used not only to derive predicted probabilities of improving on a 0–1 scale, but also to derive the log-odds of improving for each subject. The latter can take any value: a log-odds of 0 means that a subject has equal odds to improve as to not improve (i.e. predicted probability of 0.5 or 50%) while a positive (negative) log-odds means that a subject has greater (lower) odds of improving.
Table 3.
The table describes ranking of the 16 core items by scleroderma experts and results of the cluster analysis
| Core item | Rank 1 (%) | Rank 2 (%) | Rank 3 (%) | Cluster |
|---|---|---|---|---|
| MRSS | 374 (44.1%) | 131 (15.5%) | 75 (8.9%) | 1 |
| FVC% predicted | 123 (14.5%) | 148 (17.5%) | 72 (8.5%) | 1 |
| Physician global assessment | 77 (9.1%) | 116 (13.7%) | 88 (10.4%) | 1 |
| Patient global assessment | 93 (11%) | 69 (8.2%) | 115 (13.6%) | 1 |
| HAQ-DI | 68 (8%) | 112 (13.2%) | 99 (11.7%) | 1 |
| Vitality SF-36 | 12 (1.4%) | 37 (4.4%) | 101 (11.9%) | 2 |
| GI VAS | 25 (2.9%) | 44 (5.2%) | 43 (5.1%) | 2 |
| Pain | 11 (1.3%) | 38 (4.5%) | 82 (9.7%) | 2 |
| Tendon friction rubs | 11 (1.3%) | 33 (3.9%) | 23 (2.7%) | 2 |
| Breathing VAS | 13 (1.5%) | 25 (3%) | 32 (3.8%) | 2 |
| Digital ulcers VAS | 7 (0.8%) | 38 (4.5%) | 17 (2%) | 2 |
| Raynaud’s VAS | 11 (1.3%) | 18 (2.1%) | 43 (5.1%) | 2 |
| Patient skin interference last month | 2 (0.2%) | 21 (2.5%) | 22 (2.6%) | 2 |
| Number of digital ulcers | 9 (1.1%) | 11 (1.3%) | 17 (2%) | 2 |
| Presence of renal crisis | 11 (1.3%) | 3 (0.4%) | 2 (0.2%) | 2 |
| Body mass index | 1 (0.1%) | 3 (0.4%) | 15 (1.8%) | 2 |
MRSS= modified Rodnan skin score, FVC= Forced vital capacity, HAQ-DI= health assessment questionnaire-disability index, GI= gastrointestinal, VAS= visual analog scale, MRSS= modified Rodnan skin score
Figure 2.
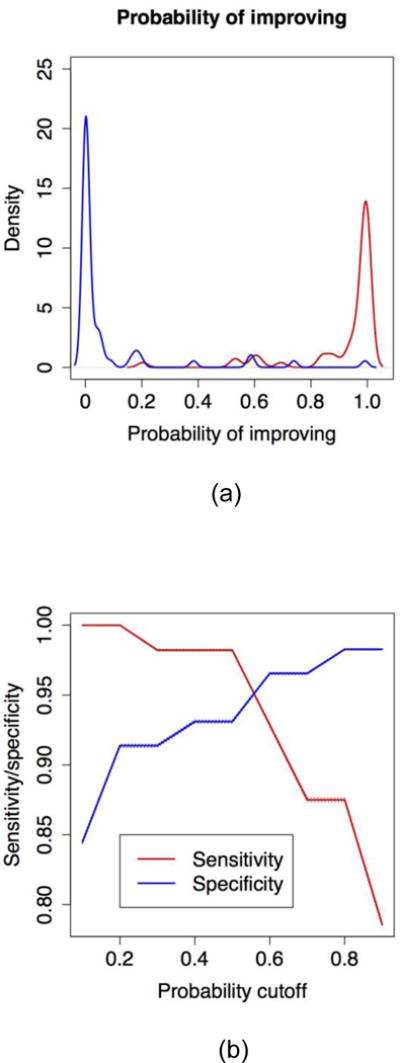
(a) Distribution of the predicted probability of improving for patients rated improved by the experts (red curve) and patients rated not improved by experts (blue curve). (b) Sensitivity (red line) and specificity (blue line) of the predicted classification of patients into “improved” and “not improved” as a function of the predicted probability cutoff. The cutoffs considered are 0.1, 0.2, 0.3, … 0.9 and the predicted classifications are derived as follow: if the predicted probability for a subject is greater than the probability cutoff, the subject is rated as “improved”, otherwise subject is not.
Contribution of 5 core components to the CRISS
We computed the pseudo R2 for the logistic regression models that included all the 5 core items of the CRISS as well as the pseudo R2 for logistic regression models including each single predictor. Combined, the 5 core items explained 89.3% of the variability in the data. Individually, when used in a single-core item logistic regression model, MRSS explained 66.3% of the variation, FVC% predicted explained 36.1% of the variation, physician global assessment explained 24.5% of the variation, patient global assessment explained 23.7% of the variation, and HAQ-DI explained 28.5% of the variation.
To assess how changes in the core items are related to the predicted probabilities of improving on each patient profile, Appendix Figure 1(a)–(e) presents a scatterplot of the change in MRSS, change in FVC% predicted, change in the patient global, change in physician global, and change in HAQ-DI versus the predicted probabilities for the 118 patient profiles, all calculated from baseline to 12 months. A change in MRSS, FVC% predicted and HAQ-DI are strong indicators of whether a patient is likely to be improved or not. In each scenario, a decrease of MRSS or HAQ-DI from baseline to follow-up and an increase in FVC% predicted corresponds to very high probabilities of improving. For patient global and physician global, the association between probability of improving and change in these two core components is less evident.
Defining a patient who is not improved irrespective of improvement in other core items
The Steering Committee considered circumstances in which a patient may improve in a particular outcome measure (such as MRSS or FVC% predicted) but have clinically significant worsening or end organ damage to another organ (e.g., development of renal crisis or pulmonary arterial hypertension). There was consensus that such patients should be defined as not improved in a clinical trial. The Steering Committee voted and determined that the following items met this definition: new onset of renal crisis, new-onset or worsening lung fibrosis, new onset of pulmonary arterial hypertension, or new onset of left ventricular failure (Table 4). The international experts subsequently endorsed these definitions as well.
Table 4.
Application of CRISS in a clinical trial
| CRISS is a 2-step process. | ||
| Step 1: Subjects who develop new or worsening of cardiopulmonary and/or renal involvement due to systemic sclerosis are considered as not improved (irrespective of improvement in other core items) and assigned a probability of improving equal to 0.0. Specifically if a subject develops any of the following | ||
| – | New scleroderma renal crisis (43) | |
| – | Decline in forced vital capacity (FVC)% predicted ≥15% (relative), confirmed by another FVC% within a month, high resolution computer tomography (HRCT) to confirm interstitial lung disease (ILD; if previous high resolution computer tomography of chest did not show ILD) and FVC% predicted below 80% predicted* | |
| – | New onset of left ventricular failure (defined as left ventricular ejection fraction ≤45%) requiring treatment* | |
| – | New onset of pulmonary arterial hypertension (PAH) on right heart catheterization (44) requiring treatment*. PAH is defined as mean pulmonary artery pressure ≥ 25 mm Hg at rest and an end-expiratory pulmonary artery wedge pressure ≤ 15 mm Hg and a pulmonary vascular resistance >3 Wood units | |
| • | ||
| *= | Attributable to systemic sclerosis | |
|
Step 2: For the remaining subjects, Step 2 involves computing the predicted probability of improving for each subject using the following equation (equation to derive predicted probabilities from a logistic regression model):
| ||
Definition of scleroderma renal crisis [adapted from (43)]
| ||
(v). Preliminary evaluation in a randomized controlled clinical trial
We used the individual patient data from a clinical trial comparing treatment of dcSSc with methotrexate vs. placebo to assess our definition of response (26). Data for change in MRSS, FVC% predicted, patient global assessment, physician global assessment, and HAQ-DI was available for 35 of 71 patients at 1 year. Using the CRISS we derived the predicted probability of improving for each of the 35 patients with complete baseline and 1-year data and classified them into improved and not improved using a probability cutoff of 0.6 (decided analytically in Step #iv). With this criterion, 11 of 19 subjects who received methotrexate were rated as improved whereas 3 of 16 subjects in the placebo group were rated as improved (p=0.04; Appendix Figure 2). When the data were assessed as a continuous measure, the distribution of the predicted probability for improvement was statistically different between the placebo and the methotrexate groups (p= 0.02).
Application in a clinical trial
The CRISS was developed with a goal to summarize the changes in the clinical and patient-reported outcomes in a single composite score that conveys the likelihood (or probability) that the patient has improved. If there is an effective agent for treatment of dcSSc, the assumption is that the patient treated with the agent will have a higher probability of improvement as summarized by CRISS vs. placebo or an ineffective agent. CRISS is a 2-step process for use in a clinical trial and is described in Table 4. In Step 1, subjects who develop new onset of renal crisis, new-onset or worsening lung fibrosis, new onset of pulmonary arterial hypertension, or new onset of left ventricular failure during the trial are considered as not improved and assigned a probability of improving equal to 0.0. For the remaining subjects with complete data, Step 2 involves computing the predicted probability of improving for each subject using the equation in Table 4. Subjects for whom the predicted probability is greater or equal to 0.60 are considered improved, while subjects for whom the predicted probability is below 0.60 are considered not improved. The 2 groups (drug vs. placebo or an active comparator) can then be compared in a 2–2 table using appropriate significance tests. The predicted probabilities obtained using the CRISS can also be assessed as a continuous variable and the distributions of the probability of improving for patients on drug vs. placebo can be compared using non-parametric tests. For trials that incorporate components of CRISS at multiple time points, the CRISS was developed using data at 12 month. Therefore, there is lack of data to support its performance at earlier time periods. We recommend using 12-month data as primary/secondary outcome measure and using others such as baseline to 3, 6, and/or 9 months as exploratory outcomes. We recommend capturing the data at each patient visit using specific case report forms for organ involvement. We also encourage developing an adjudication committee that can help with validating that cardio-pulmonary-renal involvement occurred. If case report forms are not developed and included in the trial, then these should be captured as part of adverse events [all of them should be classified as serious adverse events]. Specifically, non-availability of this data [if no specific case report forms are developed upfront] should not be taken as missing data as these should be captured as adverse events/serious adverse events. If there is missing data for the components of Step 2, we recommend considering the reason for missingness and using appropriate statistical methods. Missing data for the 5 components in Step 2 should be imputed till Month 12 before calculating the score.
Discussion
We have developed a composite response index for trials (CRISS) in early dcSSc using well-established consensus and data-driven approaches. The CRISS includes core items that assess change in two common and prominent manifestations of early dcSSc (skin and interstitial lung disease), functional disability (as assessed by the HAQ-DI), and patient and physician global assessments. In addition, the CRISS captures clinically meaningful declines in internal organ involvement requiring treatment that classify the patient as having not improved (regardless of changes in other parameters) during the clinical trial. We subsequently tested CRISS using data from a clinical trial and showed that the CRISS identified different probabilities of improvement for early dcSSc subjects in the placebo and methotrexate groups, suggesting that methotrexate has the potential to improve the overall health condition in the dcSSc subjects after 1 year.
Traditionally, trials in early dcSSc have focused on skin or lung involvement (30, 31). MRSS has been used as the primary outcome measure for the trials of skin fibrosis (6). MRSS meets the OMERACT criteria as a fully validated measure of outcome (32), but is also a surrogate of internal organ involvement and mortality in early dcSSc (33, 34). However, clinical trials in dcSSc to date have largely been “negative” and MRSS has been questioned as a primary outcome measure where post-hoc analysis of negative trials has shown stability/improvement in MRSS over time (35, 36). The CRISS incorporates multisystem involvement in dcSSc and includes the patient perspective and the impact of the disease on functional disability. CRISS was developed with a goal to summarize the changes in the clinical and patient-reported outcomes in a single composite score that conveys the likelihood (or probability) that the patient has improved. For an effective treatment for dcSSc, the assumption is that patients treated with the agent will have a higher probability of improvement as summarized by CRISS vs. placebo or an ineffective agent.
The CRISS is calculated as a 2-step process (Table 4). The first step evaluates clinically significant decline in renal or cardiopulmonary involvement that requires treatment; if present, the patient is classified as not improved. The definitions chosen for internal organ involvement were based on published data and expert opinion that was felt to be clinically significant and would trigger pharmacologic management. The second step assesses remaining patients and calculates the predicted probability of improvement. Here, the Steering Committee discussed different response definitions and decided on using a data-driven definition as suggested by the ACR Criteria subcommittee (37). In addition, data-driven definitions of disease activity have been successfully used for regulatory approval in other rheumatic diseases (38, 39).
The goal of CRISS is to assess if new pharmacologic agents have an impact on overall disease activity/severity. Our hope is that the use of CRISS in clinical trials on dcSSc will greatly facilitate the interpretation of results and form the basis for drug approvals. Rather than using numerous outcomes that vary from trial to trial, the core set of items used in CRISS will produce a single efficacy measure. This process will lessen the ambiguity associated with the presentation of multiple test statistics, some of which may be significant and others not, and facilitate meta-analyses. It will likely also allow a decrease in the number of patients necessary for appropriately powered clinical trials, as has been the case for other composite indices in rheumatoid arthritis. It should also be noted that the use of CRISS does not preclude the addition of other items in a trial; it simply provides one standardized outcome that can be easily compared and understood across trials. The individual components of CRISS would each likely be important secondary outcomes to assess in any trial. If the goal of a trial is to focus on a particular organ (e.g., use of vasodilators for underlying digital ulcers), then the CRISS can be used as a secondary measure.
The initial panel of domains (N=11) and items (N=31) offered a comprehensive view of the marked heterogeneity of SSc and at first was modeled on the comprehensive structure of the BILAG and SLEDAI measures used in trials of systemic lupus erythematous (40). However, many items were discarded based on lack of sensitivity to change in our actual data gathering exercise and others were shown to lack feasibility. As an example, the CRISS does not include items for worsening gastrointestinal disease or digital ulcers but it is anticipated that patient and physician global assessments will capture these. The data-driven approach used in the development of the CRISS strongly supports the relatively simple and accessible panel of items.
There are other indices that have been developed in SSc. The European Scleroderma Study Group (41) has proposed a composite index to assess SSc-related disease activity in routine clinical care but it has not been validated as an outcome measure in clinical trials. A severity index (42), a measure that encompasses disease activity and damage has been proposed and can be used in trials to complement CRISS.
This study has several strengths. It is the first concerted effort by the scleroderma research community to address the lack of a robust composite index for this multisystem disease. We used well-accepted expert consensus and data-driven methodologies and successfully derived the index in early dcSSc. The index addresses several domains of illness by capturing single-organ involvement in early dcSSc, patient assessment of overall disease, functional disability, and physician global assessment. We were only able to test the index in a single, small RCT that had loss to follow-up; CRISS therefore requires further validation in a prospective RCT of adequate size.
Our study is not without limitations. The CRISS was developed for early dcSSc and may not be valid for late dcSSc or limited cutaneous SSc (lcSSc). A similar exercise in late lcSSc might focus on vascular complications such as digital ulcers, calcinosis, or pulmonary arterial hypertension but might not include MRSS. The majority of past and ongoing therapeutic clinical trials are focused on early dcSSc due to dynamic changes in skin and internal organ involvement that may be responsive to pharmacologic intervention. We did not obtain patient input during the development of the index. We acknowledge this limitation and searched the literature for patient input regarding scleroderma (21, 22); this led to inclusion of fatigue and pain during the development of patient profiles but neither measure remained in the final core set of items following the Nominal Group exercises. Nonetheless, two of the constituent core items of the CRISS include patient global assessment and patient-reported functional assessment. We also note that CRISS should be considered as a preliminary index. Although the index was tested in a RCT, missing data in the trial (>50%) precludes definitive conclusion and the CRISS may need to be revised as more data becomes available from future trials. We had 118 paper patient profiles where there was expert consensus and these profiles were used to develop different response definitions. Although this is standard methodology, this may be suboptimal for testing 16 core set items. This may also explain high AUC of 0.968 for the index.
Lastly, as our goal was to develop a response index for change, baseline scores are not included in the algorithm. Other indices such as ACR 20 for rheumatoid arthritis or ACR 30 for juvenile arthritis also employ only changes in core items and not baseline values. Although the baseline scores can influence the changed scores, randomization should provide a balanced cohort.
In conclusion, we have developed a novel composite index for use in clinical trials in early dcSSc. The index should be considered provisional and needs to be validated in RCTs of dcSSc.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number UO1 AR055057. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Drs. Khanna and Berrocal were also supported by NIH/NIAMS K24 AR063120. Dr. Johnson is supported by the Canadian Institutes of Health Research Clinician Scientist Award.
The Steering committee included: Murray Baron, MD, Philip J. Clements, MD, MPH, Daniel E. Furst, MD, Dinesh Khanna, MD, MS, Maureen D. Mayes, MD, MPH, Peter A. Merkel, MD, MPH, James R. Seibold, MD, and Virginia Steen, MD
We thank following colleagues for participating in rating of patient profiles
Avouac, Jerome; Carreira, Patricia; Chung, Lorinda; Csuka, Mary Ellen; Czirjak, Laszlo; Frech, Tracy; Herrick, Ariane; Hinchcliff, Monique; Hsu, Vivian; Inanc, Murat; Jimenez, Sergio; Kahaleh, Bashar; Kowal-Bielecka, Otylia; Medsger Jr., Thomas A; Müller-Ladner, Ulf; Nikpour, Mandana; Shah, Ami; Stevens, Wendy; Valentini, Gabriele ; van Laar, Jacob M; Varga, John; Vonk, Madelon; Walker, Ulrich A
Appendix Figure 1.
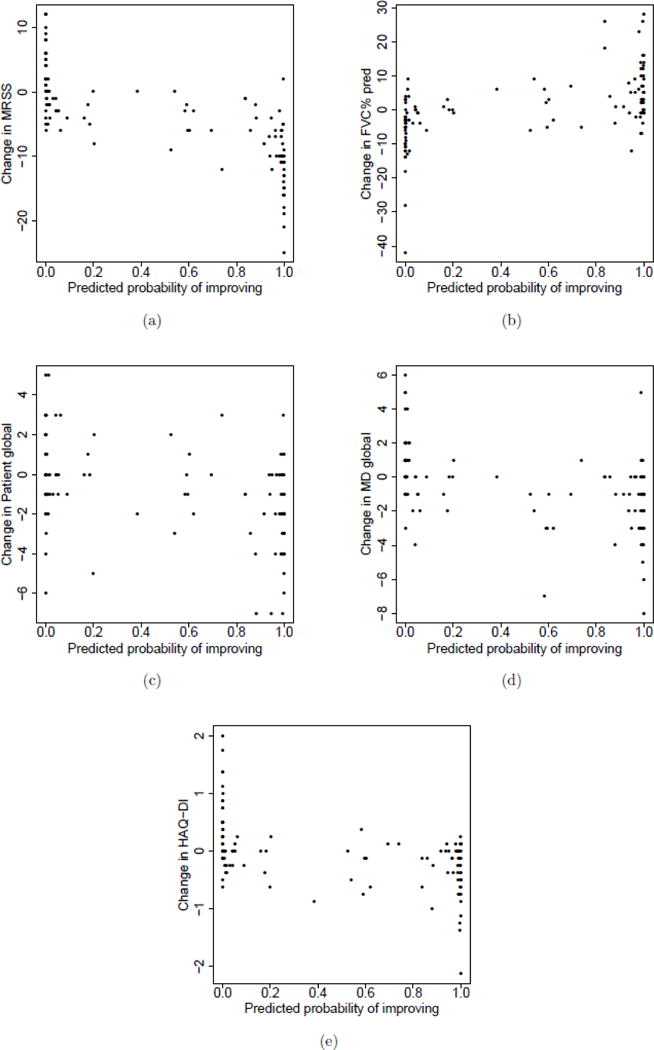
(a) Change in MRSS, (b) Change in FVC% predicted, (c) Change in patient global assessment, (d) Change in physician global assessment, and (e) Change in HAQ-DI versus the predicted probability of improving yielded by CRISS.
Appendix Figure 2.
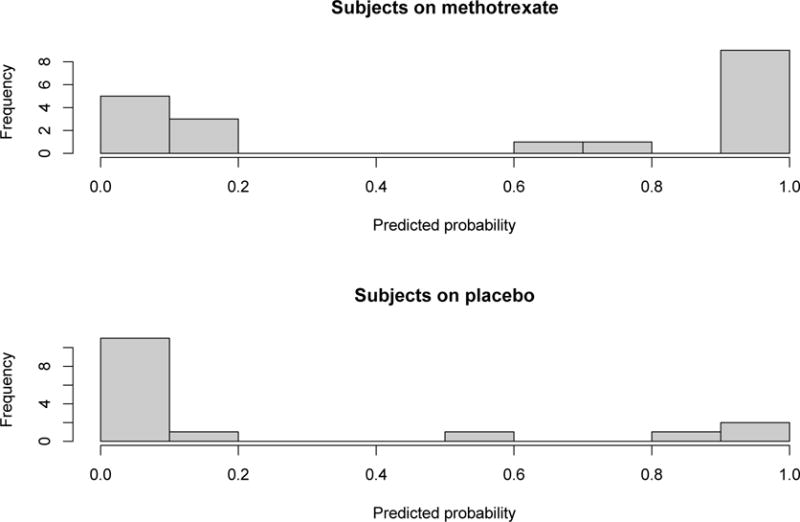
Histogram of the predicted probabilities of improving in subjects in the RCT study of methotrexate vs. placebo.
Appendix Table 1.
Correlation between the continuous core items among the 14 core items at baseline.*
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | V10 | V11 | V12 | V13 | V14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 1.0 | −0.26 | 0.43 | 0.60 | 0.33 | 0.49 | 0.31 | 0.04 | 0.16 | 0.09 | 0.09 | 0.04 | 0.03 | 0.17 |
| V2 | 1.0 | −0.22 | −0.33 | −0.23 | −0.20 | −0.18 | 0.02 | −0.03 | −0.17 | −0.003 | −0.11 | −0.27 | −0.16 | |
| V3 | 1.0 | 0.46 | 0.57 | 0.66 | 0.56 | 0.23 | 0.26 | 0.17 | 0.02 | −0.06 | 0.28 | 0.25 | ||
| V4 | 1.0 | 0.45 | 0.54 | 0.33 | 0.17 | 0.18 | 0.11 | 0.04 | 0.08 | 0.13 | 0.10 | |||
| V5 | 1.0 | 0.55 | 0.57 | 0.35 | 0.35 | 0.19 | −0.02 | 0.01 | 0.41 | 0.30 | ||||
| V6 | 1.0 | 0.60 | 0.19 | 0.44 | 0.26 | 0.11 | 0.06 | 0.30 | 0.22 | |||||
| V7 | 1.0 | 0.17 | 0.47 | 0.41 | 0.11 | 0.09 | 0.34 | 0.33 | ||||||
| V8 | 1.0 | 0.15 | 0.06 | −0.05 | 0.06 | 0.26 | 0.07 | |||||||
| V9 | 1.0 | 0.35 | 0.20 | 0.15 | 0.39 | 0.45 | ||||||||
| V10 | 1.0 | 0.16 | 0.11 | 0.20 | 0.23 | |||||||||
| V11 | 1.0 | −0.04 | −0.02 | 0.02 | ||||||||||
| V12 | 1.0 | 0.19 | 0.07 | |||||||||||
| V13 | 1.0 | 0.36 | ||||||||||||
| V14 | 1.0 |
V1=MRSS, V2=FVC% predicted, V3=HAQ-DI, V4=Physician global, V5=Patient global, V6=Patient skin interference, V7=Pain, V8=Vitality, V9=Raynaud VAS, V10=Digital Ulcers VAS, V11=Number of digital ulcers, V12=BMI, V13=Breathing VAS, V14=GI VAS
renal crisis and tendon friction rubs not included
Appendix Table 2.
Correlation between the change scores in the 14 core continuous core items.*
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | V10 | V11 | V12 | V13 | V14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 1.0 | −0.30 | 0.22 | 0.26 | 0.16 | 0.32 | 0.21 | 0.12 | 0.17 | 0.17 | −0.10 | 0.07 | 0.08 | 0.17 |
| V2 | 1.0 | −0.39 | −0.31 | −0.27 | −0.29 | −0.33 | 0.03 | −0.06 | −0.17 | 0.10 | 0.002 | −0.30 | −0.10 | |
| V3 | 1.0 | 0.17 | 0.27 | 0.31 | 0.23 | −0.005 | 0.08 | −0.05 | −0.009 | −0.18 | 0.30 | 0.05 | ||
| V4 | 1.0 | 0.25 | 0.46 | 0.19 | −0.09 | 0.18 | 0.03 | −0.08 | 0.04 | 0.33 | 0.26 | |||
| V5 | 1.0 | 0.13 | 0.25 | −0.007 | 0.002 | 0.05 | −0.14 | −0.10 | 0.16 | 0.25 | ||||
| V6 | 1.0 | 0.28 | −0.08 | 0.15 | −0.07 | −0.02 | 0.22 | 0.30 | 0.02 | |||||
| V7 | 1.0 | 0.07 | 0.27 | 0.10 | 0.22 | 0.11 | 0.33 | 0.23 | ||||||
| V8 | 1.0 | 0.001 | −0.12 | −0.03 | 0.01 | −0.12 | −0.14 | |||||||
| V9 | 1.0 | 0.20 | 0.35 | 0.20 | 0.23 | 0.47 | ||||||||
| V10 | 1.0 | −0.13 | 0.11 | 0.05 | 0.36 | |||||||||
| V11 | 1.0 | 0.008 | 0.06 | 0.05 | ||||||||||
| V12 | 1.0 | 0.16 | −0.07 | |||||||||||
| V13 | 1.0 | 0.28 | ||||||||||||
| V14 | 1.0 |
V1=MRSS, V2=FVC% predicted, V3=HAQ-DI, V4=Physician global, V5=Patient global, V6=Patient skin interference, V7=Pain, V8=Vitality, V9=Raynaud VAS, V10=Finger Ulcers VAS, V11=Number of digital ulcers, V12=BMI, V13=Breathing VAS, V14=GI VAS
renal crisis and tendon friction rubs not included
Appendix Table 3.
Example of a patient rated “improved” by the experts. Predicted probability of improving is 0.99 according to CRISS.
| Baseline | Follow-up | Absolute change | |
|---|---|---|---|
| Age | 51.6 years | ||
| Disease duration (months) | 12.98 | ||
| Global assessments | |||
| Patient global assessment (0–10)* | 3 | 1 | −2 |
| Physician global assessment (0–10)* | 3 | 3 | 0 |
| Musculoskeletal | |||
| HAQ-DI (0–3)* | 0.625 | 0 | −0.625 |
| Tendon friction rubs* | No | No | No change |
| Skin | |||
| MRSS (0–51)* | 13 | 3 | −10 |
| Patient skin interference last month | 2 | 0 | −2 |
| Lung | |||
| FVC% predicted* | 62 | 75 | 13 |
| Breathing VAS (0–10) | 2 | 0 | −2 |
| Renal | |||
| Renal crisis** | No | No | No change |
| Gastrointestinal | |||
| GI VAS (0–10) | 3 | 3 | 0 |
| Body Mass Index (BMI) | 25.40 | 26.58 | 1.18 |
| Raynaud’s | |||
| Raynaud’s VAS (0–10) | 2 | 1 | −1 |
| Digital ulcers | |||
| Digital ulcers VAS (0–10) | 0 | 0 | 0 |
| Number of digital ulcers | 0 | 0 | 0 |
| HRQOL | |||
| Pain VAS (0–10) | 3 | 1 | −2 |
| Fatigue (SF-36 Vitality scale) (0–100) | 42.31 | 35.12 | −7.19 |
included in Step 2;
included in Step 1
HAQ-DI= health assessment questionnaire-disability index, MRSS= modified Rodnan skin score, FVC= Forced vital capacity, GI= gastrointestinal, VAS= visual analog scale
Appendix Table 4.
Example of a patient rated “improved” by the experts. Predicted probability of improving is 0.60 according to CRISS.
| Baseline | Follow-up | Absolute change | |
|---|---|---|---|
| Age | 64.65 years | ||
| Disease duration (months) | 30.74 | ||
| Global assessments | |||
| Patient global assessment (0–10)* | 1 | 0 | −1 |
| Physician global assessment (0–10)* | 7 | 4 | −3 |
| Musculoskeletal | |||
| HAQ-DI (0–3)* | 0.375 | 0.250 | −0.125 |
| Tendon friction rubs* | No | No | No change |
| Skin | |||
| MRSS (0–51)* | 21 | 15 | −6 |
| Patient skin interference last month | 8 | 5 | −3 |
| Lung | |||
| FVC% predicted* | 86 | 81 | −5 |
| Breathing VAS (0–10) | 0 | 0 | 0 |
| Renal | |||
| Renal crisis** | Yes | Yes | No change |
| Gastrointestinal | |||
| GI VAS (0–10) | 0 | 0 | 0 |
| Body Mass Index (BMI) | 25.12 | 24.82 | −0.3 |
| Raynaud’s | |||
| Raynaud’s VAS (0–10) | 3 | 4 | 1 |
| Digital ulcers | |||
| Digital ulcers VAS (0–10) | 0 | 8 | 8 |
| Number of digital ulcers | 0 | 0 | 0 |
| HRQOL | |||
| Pain VAS (0–10) | 0 | 2 | 2 |
| Fatigue (SF-36 Vitality scale) (0–100) | 35.12 | 35.12 | 0.0 |
included in Step 2;
included in Step 1
HAQ-DI= health assessment questionnaire-disability index, MRSS= modified Rodnan skin score, FVC= Forced vital capacity, GI= gastrointestinal, VAS= visual analog scale
Appendix Table 5.
Example of a patient rated “worsened” by the experts. Predicted probability of improving is 0.002 according to the CRISS.
| Baseline | Follow-up | Absolute Change | |
|---|---|---|---|
| Age | 53.6 years | ||
| Disease duration (months) | 43.3 | ||
| Global assessments | |||
| Patient global assessment (0–10)* | 1 | 2 | 1 |
| Physician global assessment (0–10)* | 1 | 2 | 1 |
| Musculoskeletal | |||
| HAQ-DI (0–3)* | 0 | 0 | 0 |
| Tendon friction rubs* | No | Yes | Change to worsen |
| Skin | |||
| MRSS (0–51)* | 7 | 5 | −2 |
| Patient skin interference last month | 3 | 2 | −1 |
| Lung | |||
| FVC% predicted* | 87 | 80 | −7 |
| Breathing VAS (0–10) | 0 | 1 | 1 |
| Renal | |||
| Renal crisis** | No | No | No change |
| Gastrointestinal | |||
| GI VAS (0–10) | 0 | 1 | 1 |
| Body Mass Index (BMI) | 24.68 | 24.68 | 0 |
| Raynaud’s | |||
| Raynaud’s VAS (0–10) | 0 | 3 | 3 |
| Digital ulcers | |||
| Digital ulcers VAS (0–10) | 0 | 0 | 0 |
| Number of digital ulcers | 0 | 0 | 0 |
| HRQOL | |||
| Pain VAS (0–10) | 1 | 1 | 0 |
| Fatigue (SF-36 Vitality scale) (0–100) | 37.52 | 35.10 | −2.42 |
included in Step 2;
included in Step 1
HAQ-DI= health assessment questionnaire-disability index, MRSS= modified Rodnan skin score, FVC= Forced vital capacity, GI= gastrointestinal, VAS= visual analog scale
Appendix Table 6.
One core item logistic model using expert consensus definition of improved vs. not
| Core item | Area under the curve (AUC) | Sensitivity | Specificity | Brier Score |
|---|---|---|---|---|
| MRSS | 0.9231 | 0.8392 | 0.8793 | 0.108 |
| FVC% predicted | 0.7906 | 0.6429 | 0.7586 | 0.184 |
| Physician global | 0.7743 | 0.7143 | 0.7241 | 0.197 |
| Patient global | 0.7448 | 0.7143 | 0.6207 | 0.204 |
| HAQ-DI | 0.7107 | 0.6429 | 0.6897 | 0.200 |
| Pain | 0.6857 | 0.6071 | 0.7586 | 0.218 |
| Vitality | 0.6856 | 0.4643 | 0.7414 | 0.225 |
| VAS Breathing | 0.6670 | 0.375 | 0.8103 | 0.219 |
| GI VAS | 0.6667 | 0.7857 | 0.4483 | 0.220 |
| Patient skin interference last month | 0.6601 | 0.5179 | 0.7586 | 0.226 |
| Raynaud’s VAS | 0.6190 | 0.4286 | 0.7241 | 0.238 |
| Tendon friction rubs | 0.5640 | 0.2321 | 0.8966 | 0.245 |
| Digital ulcers VAS | 0.5503 | 0.2857 | 0.7931 | 0.247 |
| Body mass index | 0.4946 | 0.1786 | 0.8276 | 0.250 |
| Number of digital ulcers | 0.4764 | 0.0179 | 0.931 | 0.249 |
HAQ-DI= health assessment questionnaire-disability index, MRSS= modified Rodnan skin score, FVC= Forced vital capacity, GI= gastrointestinal, VAS= visual analog scale
Appendix Table 7.
Two core item logistic model using expert consensus definition of improved vs. not
| Core item | Area under the curve (AUC) | Sensitivity | Specificity | Brier Score |
|---|---|---|---|---|
| MRSS, FVC% predicted | 0.9632 | 0.8929 | 0.9138 | 0.068 |
| MRSS, HAQ-DI | 0.9615 | 0.9107 | 0.8793 | 0.076 |
| MRSS, Patient global | 0.9560 | 0.875 | 0.8966 | 0.081 |
| MRSS, physician global | 0.9450 | 0.875 | 0.9310 | 0.094 |
| FVC% predicted, HAQ-DI | 0.8519 | 0.7679 | 0.8448 | 0.158 |
| FVC% predicted, Patient global | 0.8548 | 0.7679 | 0.8448 | 0.152 |
| FVC% predicted, physician global | 0.8544 | 0.750 | 0.8103 | 0.158 |
| HAQ-DI, patient global | 0.7982 | 0.7143 | 0.7241 | 0.184 |
| HAQ-DI, physician global | 0.8094 | 0.6607 | 0.7931 | 0.181 |
| Patient global, physician global | 0.8265 | 0.7321 | 0.7759 | 0.170 |
HAQ-DI= health assessment questionnaire-disability index, MRSS= modified Rodnan skin score, FVC= Forced vital capacity
Footnotes
CONFLICTS
Dr. Khanna has/had consultancy relationship with and/or has received research funding from Bayer, Biogen Idec, Bristol-Myers Squibb, Celgene, Cytori, EMD Serono, Forward, Genentech/Roche, Gilead, Glaxo SmithKline, Lycera, Medac, Sanofi-Aventis/Genzyme, and Seattle Genetics.
Drs. Assassi, Baron, Berrocal, Clements, Giannini, Mayes, Schiopu, Phillips, Pope, Wong and Wells have no conflicts related to this project.
Dr. Seibold has consultancies relevant to the present work with Bayer, Boehringer-Ingelheim, EMD Serono, FibroGen, Novartis, Sanofi-Aventis, Celgene, DART, InterMune and Sigma Tau.
Dr. Merkel has/had consultancy relationships with Actelion, ChemoCentryx, Glaxo-Smith-Kline, and Sanofi and has received research funding from Actelion, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, and Genentech/Roche.
Dr. Steen has/had consultancy relationship with and/or has received research funding from Actelion, Bayer, Bristol-Myers Squibb, Celgene, CSL Behring, Cytori, Genentech/Roche, Gilead, InterMune, Sanofi-Aventis/Genzyme, and United Therapeutics.
Dr. Simms is on the Gilead Speakers bureau, has/had consultancies with Actelion, Cytori. Has/had grant support from Actelion, Gilead, Medimmune, and InterMune.
Dr. Allanore has/had consultancy relationship and/or has received research funding with Actelion, Bayer, Behring, Biogen Idec, Genentech/Roche, Inventiva, Pfizer, Sanofi/Genzyme, Servier, and UCB.
Dr. Denton has/had consultancy relationship and/or has received research funding from Actelion, Genentech/Roche, Pfizer, GlaxoSmithKline, BMS, CSL Behring, Novartis, Sanofi-Aventis, Inventiva, and Biogen-Idec.
Dr. Distler has/had consultancy relationship and/or has received research funding in the area of SSc and related conditions from Actelion, Pfizer, Ergonex, Bristol-Myers Squibb, Sanofi-Aventis, United BioSource Corporation, Genentech/Roche, Medac, Biovitrium, Boehringer Ingelheim Pharma, Novartis, 4 D Science, Active Biotec, Bayer-Schering, Sinoxa/Serodapharm, EpiPharm, Biogen Idec, Inventiva, GSK and Pharmacyclics.
Dr. Johnson is supported by the Canadian Institutes of Health Research Clinician Scientist Award.
Dr. Matucci Cerinic has/had consultancy relationship and/or has received research funding with Actelion, Bayer, Behring, Bristol-Myers Squibb, MSD Pfizer, and UCB.
Dr. Proudman has research grants and consultancies for Actelion, Bayer and Glaxo Smith Kline.
Dr. Siegel is an employee of Genentech.
Dr. Furst has/had research grants with AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Gilead, GSK, NIH, Novartis, Pfizer, Genentech/Roche, UCB and has/had consultancies with AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Genentech/Roche, UCB and does CME programs with AbbVie, Actelion, and UCB.
References
- 1.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, Medsger TA, Jr, Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118(1):2–10. doi: 10.1016/j.amjmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2012;51(6):1017–26. doi: 10.1093/rheumatology/ker269. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Kowal-Bielecka O, Khanna PP, Lapinska A, Asch SM, Wenger N, et al. Quality indicator set for systemic sclerosis. Clin Exp Rheumatol. 2011;29(2 Suppl 65):33–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna D, Distler O, Avouac J, Behrens F, Clements PJ, Denton C, et al. Measures of response in clinical trials of systemic sclerosis: the combined response index for systemic sclerosis (CRISS) and Outcome Measures in Pulmonary Arterial Hypertension related to Systemic Sclerosis (EPOSS) J Rheumatol. 2009;36(10):2356–61. doi: 10.3899/jrheum.090372. [DOI] [PubMed] [Google Scholar]
- 5.Khanna D, Lovell DJ, Giannini E, Clements PJ, Merkel PA, Seibold JR, et al. Development of a provisional core set of response measures for clinical trials of systemic sclerosis. Ann Rheum Dis. 2008;67(5):703–9. doi: 10.1136/ard.2007.078923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna D, Merkel PA. Outcome measures in systemic sclerosis: an update on instruments and current research. Curr Rheumatol Rep. 2007;9(2):151–7. doi: 10.1007/s11926-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 7.Chung L, Denton CP, Distler O, Furst DE, Khanna D, Merkel PA. Clinical trial design in scleroderma: where are we and where do we go next? Clin Exp Rheumatol. 2012;30(2 Suppl 71):97–102. [PubMed] [Google Scholar]
- 8.Abraham DJ, Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26(11):587–95. doi: 10.1016/j.it.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Nagaraja V, Denton CP, Khanna D. Old medications and new targeted therapies in systemic sclerosis. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Heijde DM, t Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49(11):916–20. doi: 10.1136/ard.49.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulus HE, Egger MJ, Ward JR, Williams HJ. Analysis of improvement in individual rheumatoid arthritis patients treated with disease-modifying antirheumatic drugs, based on the findings in patients treated with placebo. The Cooperative Systematic Studies of Rheumatic Diseases Group. Arthritis Rheum. 1990;33(4):477–84. doi: 10.1002/art.1780330403. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 13.Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum. 2011;41(2):95–105. doi: 10.1016/j.semarthrit.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh JA, Solomon DH, Dougados M, Felson D, Hawker G, Katz P, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum. 2006;55(3):348–52. doi: 10.1002/art.22003. [DOI] [PubMed] [Google Scholar]
- 15.Merkel PA, Silliman NP, Clements P, Denton CP, Furst DE, Mayes M, et al. Patterns and Predictors of Change in Outcome Measures in Clinical Trials in Scleroderma. Arthritis Rheum. 2005;52:282–3. doi: 10.1002/art.34427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiese AB, Berrocal VJ, Furst DE, Seibold JR, Merkel PA, Mayes MD, et al. Correlates and responsiveness to change of measures of skin and musculoskeletal disease in early diffuse systemic sclerosis. Arthritis Care Res (Hoboken) 2014:1731–9. doi: 10.1002/acr.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays RD, Hadorn D. Responsiveness to change: an aspect of validity, not a separate dimension. Qual Life Res. 1992;1(1):73–5. doi: 10.1007/BF00435438. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. pp. 273–406. [Google Scholar]
- 19.Davies EH, Surtees R, DeVile C, Schoon I, Vellodi A. A severity scoring tool to assess the neurological features of neuronopathic Gaucher disease. J Inherit Metab Dis. 2007;30(5):768–82. doi: 10.1007/s10545-007-0595-x. [DOI] [PubMed] [Google Scholar]
- 20.Fan X, Pope J, Baron M. What is the relationship between disease activity, severity and damage in a large Canadian systemic sclerosis cohort? Results from the Canadian Scleroderma Research Group (CSRG) Rheumatol Int. 2009:1205–10. doi: 10.1007/s00296-009-1129-7. [DOI] [PubMed] [Google Scholar]
- 21.Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology (Oxford) 2011;50(4):762–67. doi: 10.1093/rheumatology/keq310. [DOI] [PubMed] [Google Scholar]
- 22.Suarez-Almazor ME, Kallen MA, Roundtree AK, Mayes M. Disease and Symptom Burden in Systemic Sclerosis: A Patient Perspective. J Rheumatol. 2007;34(8):1718–26. [PubMed] [Google Scholar]
- 23.Stamm TA, Mattsson M, Mihai C, Stocker J, Binder A, Bauernfeind B, et al. Concepts of functioning and health important to people with systemic sclerosis: a qualitative study in four European countries. Ann Rheum Dis. 2011;70(6):1074–9. doi: 10.1136/ard.2010.148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gneiting T, Raftery A. Strictly proper scoring rules. J Am Stat Assoc. 2007;102:359–78. [Google Scholar]
- 25.Nagelkerke NGD. A note on a general definition of the coefficient of Determination. Biometrika. 1991;78(3):691–2. [Google Scholar]
- 26.Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44(6):1351–8. doi: 10.1002/1529-0131(200106)44:6<1351::AID-ART227>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Furst D, Khanna D, Matucci-Cerinic M, Clements P, Steen V, Pope J, et al. Systemic sclerosis – continuing progress in developing clinical measures of response. J Rheumatol. 2007;34(5):1194–1200. [PubMed] [Google Scholar]
- 28.Merkel PA, Silliman NP, Denton CP, Furst DE, Khanna D, Emery P, et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 2008;59(5):699–705. doi: 10.1002/art.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002;46(9):2410–20. doi: 10.1002/art.10486. [DOI] [PubMed] [Google Scholar]
- 30.Khanna D, Clements PJ, Furst DE, Korn JH, Ellman M, Rothfield N, et al. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(4):1102–11. doi: 10.1002/art.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 32.Merkel PA, Clements PJ, Reveille JD, Suarez-Almazor ME, Valentini G, Furst DE. Current status of outcome measure development for clinical trials in systemic sclerosis. Report from OMERACT 6. J Rheumatol. 2003;30(7):1630–47. [PubMed] [Google Scholar]
- 33.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;43(11):2445–54. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 34.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43(11):2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Merkel PA, Silliman NP, Clements PJ, Denton CP, Furst DE, Mayes MD, et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma: an individual patient meta-analysis of 629 subjects with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2012;64(10):3420–9. doi: 10.1002/art.34427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amjadi S, Maranian P, Furst DE, Clements PJ, Wong WK, Postlethwaite AE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: Analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60(8):2490–8. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh JA, Solomon DH, Dougados M, Felson D, Hawker G, Katz P, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum. 2006;55(3):348–52. doi: 10.1002/art.22003. [DOI] [PubMed] [Google Scholar]
- 38.van der Heijde DM, van’t Hof MA, van Riel PL, van Leeuwen MA, van Rijswijk MH, van de Putte LB. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis. 1992;51(2):177–81. doi: 10.1136/ard.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luijten KM, Tekstra J, Bijlsma JW, Bijl M. The Systemic Lupus Erythematosus Responder Index (SRI); a new SLE disease activity assessment. Autoimmun Rev. 2012;11(5):326–9. doi: 10.1016/j.autrev.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Yee CS, Isenberg DA, Prabu A, Sokoll K, Teh LS, Rahman A, et al. BILAG-2004 index captures systemic lupus erythematosus disease activity better than SLEDAI-2000. Ann Rheum Dis. 2008;67(6):873–6. doi: 10.1136/ard.2007.070847. [DOI] [PubMed] [Google Scholar]
- 41.Valentini G, D’Angelo S, Della RA, Bencivelli W, Bombardieri S. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. IV. Assessment of skin thickening by modified Rodnan skin score. Ann Rheum Dis. 2003;62(9):904–5. doi: 10.1136/ard.62.9.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26(10):2159–67. [PubMed] [Google Scholar]
- 43.Steen VD, Mayes MD, Merkel PA. Assessment of kidney involvement. Clin Exp Rheumatol. 2003;21(3 Suppl 29):29–31. [PubMed] [Google Scholar]
- 44.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


