Abstract
Reactive oxygen species (ROS) and oxidative stress have long been linked to aging and diseases prominent in the elderly such as hypertension, atherosclerosis, diabetes and atrial fibrillation (AF). NADPH oxidases (Nox) are a major source of ROS in the vasculature and are key players in mediating redox signalling under physiological and pathophysiological conditions. In this review, we focus on the Nox-mediated ROS signalling pathways involved in the regulation of ‘longevity genes’ and recapitulate their role in age-associated vascular changes and in the development of age-related cardiovascular diseases (CVDs). This review is predicated on burgeoning knowledge that Nox-derived ROS propagate tightly regulated yet varied signalling pathways, which, at the cellular level, may lead to diminished repair, the aging process and predisposition to CVDs. In addition, we briefly describe emerging Nox therapies and their potential in improving the health of the elderly population.
Keywords: aging, cardiovascular diseases, reduced nicotinamide–adenine dinucleotide phosphate (NADPH) oxidase, reduced nicotinamide–adenine dinucleotide phosphate oxidase therapeutics (Nox therapeutics)
INTRODUCTION
Aging is the predominant risk factor in cardiovascular diseases (CVDs) [1] and the latest statistics show that of the 83.6 million American adults with some form of CVD, 42.2 million are estimated to be ≥60 years of age [2]. Currently, CVD is the leading cause of death in the U.S.A.; approximately 66 % of CVDs occurring in people 75 years of age or more [2]. It is projected that by 2030 nearly 40.5 % of the U.S.A. population will have more than one type of CVD [3]. With increasing age, clinical manifestations and prognosis of vascular diseases worsen as a result of molecular changes associated with the aging process, often similar to specific pathophysiological changes observed in disease. Moreover, aging is a cumulative multifactorial process and not linked to a single cause. To date, multiple theories of biological aging are discussed within the literature, e.g. the ‘wear and tear’ theory, auto-immunity theory, programmed cellular theory, somatic mutation theory or homoeostatic theory have been discussed previously [4,5]. However, many of these viewpoints share ‘oxidative stress’ as the common denominator in aging. The free radical theory of aging suggests that accumulated damage caused by free radicals and reactive oxygen species (ROS) accelerates the aging process [6]. Although there is still considerable debate that a single theory can explain the aetiology of aging, the free radical theory of aging still draws considerable attention and intrigue. It is now generally accepted that superoxide anion and other ROS can exert beneficial/protective effects under normal conditions, via the promotion of antioxidant enzyme expression and through adaptive cellular signalling responses. Harmful effects, on the other hand, are the result of aberrant redox signalling and direct damage to biologically sensitive targets. Typically, this oxidative damage results in a pro-senescent phenotype, which is one mechanism explaining biological aging [7,8]. Although senescence can be triggered by telomere attrition, mitogen-mediated extracellular signalling or decreased replicative capacity, oxidative stress remains a prominent instigator of this phenotype [9–11]. While associated, biological aging, senescence and longevity are distinct processes. Therefore, an improved understanding of the biology governing age-related decline of cellular function and homoeostasis is imperative.
Furthermore, although differences in cardiovascular function between young and old have been extensively described in the literature, the majority of the in vivo studies related to CVD have been performed in young animals. Hence, it is important to understand how ROS contributes to aging and is, in turn, a critical contributor to age-related diseases. Perspectives on the role of NADPH oxidase (Nox)-derived ROS in aging and the potential signalling mechanisms that underlie these age-related changes as well as how they relate to CVD in older population are the focus of the present review. The potential for currently available Nox therapeutic strategies to intervene in the cellular aging process and thereby slow the onset of age-related CVD are also discussed.
OXIDATIVE STRESS THEORY, AGING AND NADPH OXIDASES
The free radical theory of aging, when first proposed by Harman [6], suggested that longevity is governed by the production of endogenous oxygen radicals in cells, resulting in a pattern of cumulative damage in a random and indiscriminate manner. This theory, therefore, implied that an overall reduction in oxidative stress, either by reducing the pro-oxidant load or by increasing antioxidant defences or both, protects against aging and increases lifespan.
Although cellular defences [including catalase, Cu/Zn superoxide dismutase (SOD1) and glutathione peroxidases (Gpxs)] combating oxidative damage are normally plentiful, they are not entirely effective, as oxidatively damaged macromolecules still accumulate with biological aging [12]. There is supporting evidence from studies in Caenorhabditis elegans and Drosophila, two well-established models to study aging, which revealed that decreased ROS production may improve resistance to oxidative stress and therefore increase lifespan [13,14]. In mammals, however, studies are largely correlative, based on findings of increased oxidatively damage DNA and/or proteins in aged individuals [15]. Moreover, studies performed in vertebrate animals using transgenic and knockout mouse models [e.g. SOD, Gpx, thioredoxin 2 (Trx2) and catalase] provide conflicting evidence regarding effects on lifespan or longevity [16–24]. It is worth noting that antioxidant enzyme systems are not the only factors involved in cellular homoeostasis. Other enzymes, such as Noxs and myeloperoxidase (MPO), historically assumed to generate ROS for destructive purposes (i.e. in host defence), are now known to serve as important signal-transducing enzymes. Their activation is elicited in response to a variety of growth factors, cytokines and G-protein-coupled receptors [25–30]. ROS are also produced via a variety of other enzyme systems including xanthine oxidase (XO), cytochrome P450 and by uncoupling of nitric oxide (NO) synthase (NOS), yet Noxs appear to predominate in cellular ROS production. ROS have also emerged as locally produced in multiple cellular compartments including at the plasma membrane and within the mitochondrion, peroxisomes and the cytoplasm. Furthermore, basal ROS mediate important biological functions by regulating the activity of diverse intracellular signalling pathways involved in growth, apoptosis, survival, metabolism and migration [26–28,31–34]. It stands to reason therefore that specific signal transduction by any one Nox in a particular cell is a consequence of its co-localization with upstream and downstream effectors of each pathway. A protective role for ROS nothwith-standing, a wide array of studies suggests that ROS participate in biological aging and age-related CVDs such as atherosclerosis, hypertension, stroke and atrial fibrillation (AF) [35–51].
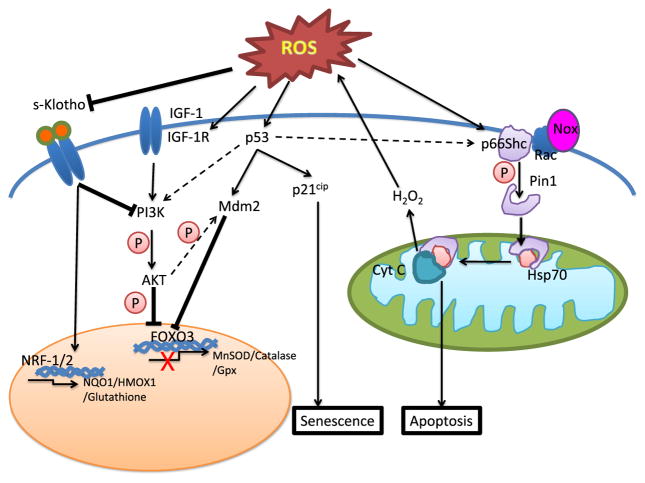
Despite evidence suggesting a causal link between aging and disease, little is known regarding the molecular mechanisms that dictate age-related processes and their association with diseases. A number of studies, at both the cellular and the organismal levels, have reported increased lifespan with increased resistance to oxidative stress. However, whether this may be attributed to a common signal transduction pathway that regulates aging and response to oxidative stress or a variety of pathways is yet to be determined. Logic informs us that multiple pathways are most likely involved. Figure 1 illustrates a few of the important signalling pathways implicated in the process of aging. Indeed, one mechanistic link between aging and oxidants comes from studies showing that mice lacking p66Shc have a ~30 % increase in lifespan, which is correlated to reduced intracellular ROS. These studies also showed that oxidative regulation of p53 and FKHRL1 [forkhead-box protein O3 (FOXO3)] is mediated by p66Shc [52–55]. Other proteins and pathways linked to aging are SIRT1 (sirtuin 1; mammalian homologue of Sir2 protein found in lower organisms) [56–59], Klotho [60–62], AMP-activated protein kinase (AMPK) [63], protein skinhead 1 (SKN-1) [homologue of nuclear respiratory factor 1] [64], Clock 1 (clk-1) [65,66], insulin/insulin-like growth factor (IGF)-1 signalling [67] and mammalian target of rapamycin (mTOR) signalling [68,69].
Figure 1. Prominent redox-sensitive signalling pathways involved in aging.
Schematic representation of known redox-sensitive signalling pathways which regulate cellular aging and age-related CVDs. Arrows denote positive activation of downstream targets. Blunt-ended arrow denotes inhibitory effects. Dashed arrow denotes cross-talk between pathways. Secreted Klotho prolongs lifespan by increasing the expression of antioxidant genes via Nrf-1/2 or by inhibiting IGF-1 signalling. Binding of growth hormones, such as IGF-1 to their respective receptors or oxidative stress leads to autophosphorylation of β-subunits on the IGF-1R, leading to subsequent activation of PI3K/Akt pathway. Akt-mediated phosphorylation of FOXO3 results in nuclear exclusion and inhibition of FOXO3-mediated transcription of target antioxidants, such as MnSOD and Gpx. Oxidative stress induces phosphorylation and translocation of p66Shc via PKCβ and Pin1 to the mitochondrial inner membrane leading to cytochrome c-mediated apoptosis. ROS also causes post-translational modification of p53 resulting in its activation triggering p21cip-mediated cell senescence. Akt activation or p53-induced transcriptional activation of Mdm2 leads to ubiquitination-dependent degradation of FOXO3. Abbreviations: PI3K, phosphoinositide 3-kinase; s-Klotho, secreted Klotho.
Mammalian NADPH oxidase
The NADPH oxidase family of enzymes are considered the ‘professional’ enzymatic source of ROS in vascular cells and are therefore known to be a major contributor to vascular dysfunction and disease [43,44,47,48,70–76]. In this regard, Nox isozymes plausibly play a prominent role in age-related susceptibility to vascular occlusive diseases such as coronary artery disease (CAD) and peripheral artery disease (PAD), hypertension and diabetes, through redox signalling pathway activation [29,30,45,46,49,50,77–86]. The structure and function of Nox isozymes are complex and more thoroughly reviewed elsewhere [43,44,47,48,74,87]. It is the aim of the current review to outline their role in ROS production and their ability to contribute to the aging process. In brief, the Nox family consists of seven isoforms [Nox1–5 and Duox1–2 (dual oxidase 1–2)], of which Nox1, Nox2, Nox4 and Nox5 are known to be expressed in human vascular tissue [88]. Moreover, Nox enzymes are varied in their cellular expression and are identified by their catalytic core, and extensive evidence demonstrates their contribution to vascular dysfunction [77]. Importantly, Nox1, Nox2 and Nox4 isoforms, but not Nox5, require the smaller membrane-bound p22phox component for membrane stabilization and activity [89]. Nox1 and Nox2 are regulated by sometimes distinct, otherwise interchangeable, cytosolic components loosely termed as activators or organizers, which are generally recruited to the membrane components following cellular stimuli to assemble an active Nox [90–92]. To date, Nox2 remains the most extensively studied family member in a variety of physiological and pathophysiological signalling pathways. The active Nox2 enzyme complex comprises the catalytic Nox2 subunit in association with p22phox to form the cytochrome B558 complex, p47phox (organizer), p67phox (activator), p40phox and the small Rho-family GTP-binding protein Rac1/2 [30]. Arguably, Nox2 is the most widely distributed of the isoforms, present in phagocytes, heart, lung and the vasculature, among multiple other tissues, and is suggested to be increased in aging vascular cells [93]. Similarly, the active Nox1 system is composed of the Nox1 catalytic subunit, p22phox and in its canonical subunits NoxO1 (p47phox homologue), NoxA1 (p67phox homologue) and Rac1/2 [94]. Nox1 is highly expressed in the colon, but is currently also known to be present in the heart, lungs and blood vessels. In contrast, the Nox4 system is composed of the Nox4 catalytic subunit and p22phox, with the only other known associated protein described for its activity being Poldip2 [95]. It is widely held that Nox4’s activity is regulated transcriptionally, and, interestingly, it is up-regulated in aging VSMCs (vascular smooth muscle cells) [96]. Incidentally, Nox4 is expressed in all three main vascular cell types: the endothelial cell, smooth muscle cell and fibroblast. Another distinct feature of Nox4 compared with other Nox is that it is constitutively active and appears to preferentially produce H2O2 over superoxide anion. It has been suggested that Nox4 may act as an oxygen sensor [97]. Finally, Nox5, which does not require p22phox for membrane stabilization, is regulated by calcium binding through EF-hand motifs [98]. Although Nox enzymes have been implicated to play a crucial role in several signalling pathways, a lack of crystal structure for Nox isoenzymes as well as structural similarities among Nox family members have confounded progress towards the generation of specific antibodies and small-molecule inhibitors. Therefore, when implicating Nox isoenzymes in any study, it is critical that key positive controls are used to account for antibody limitations, as is discussed by Baniulis et al. [99]; namely neutrophil homogenates for Nox2, colon homogenates for Nox1, kidney homogenates for Nox4 or heterologous overexpression cell systems. In addition, mRNA analyses [78,100,101] are highly recommended to corroborate changes detected at the protein level.
ROS production by activated NADPH oxidase is mediated by a wide variety of factors including mechanical forces, environmental factors (e.g. hypoxia) and cytokines and hormones such as angiotensin II (AngII), aldosterone, endothelin-1 (ET-1), platelet-derived growth factor (PDGF), TGFβ (transforming growth factor β) and TNFα (tumour necrosis factor α) [71,75,82,102–106]. In the light of this broad and often deleterious role for Nox, the quest for efficacious and isoform-selective Nox inhibitors is expected to come into even greater focus in Nox-related disease research, i.e. advances in biochemical characterization and ROS detection agents are anticipated to facilitate viable therapy development [107–110]. As Nox-derived ROS are an important culprit in the free radical theory of aging, selective inhibition is anticipated to be a viable therapeutic strategy in age-related disease, and the roles of Nox in the aging process are expected to be explored in further depth in the coming years as a consequence of an increasingly aging population in the developed world.
ROLE OF ROS SIGNALLING IN PROTEINS INVOLVED IN AGING
p66Shc adaptor protein
p66Shc protein is one of the three isoforms encoded by the mammalian Shc locus. Shc proteins (p66Shc, p52Shc and p46Shc) are cytoplasmic signal transducers involved in the transmission of mitogenic signals from activated receptors to Ras proteins. Although the three splice variants carry similar structural domains, p66Shc contains an additional collagen-homology region in the N-terminus, allowing p66Shc to function in intracellular pathway(s) that regulate ROS metabolism and apoptosis [52,111]. Whereas activation with growth factors (such as epidermal growth factor receptor, EGF) induce tyrosine phosphorylation and Ras activation, environmental stress factors, such as H2O2 or UV irradiation, induce serine phosphorylation on p66Shc through protein kinase Cβ (PKCβ) [112] and JNK [113], causing increased apoptosis. Deletion of p66Shc protein was shown to reduce oxidative stress and high-fat-induced atherogenesis and increase cellular resistance to apoptosis as well as life expectancy by 30 % [52,53]. Since that study, several others have reported a link between the redox regulation of p66Shc protein and downstream signalling via p53/p21cip and FOXO3 to mechanisms that modulate longevity [54,55]. Increasing evidence supports that oxidative stress-induced serine phosphorylation of p66Shc leads to mitochondrial accumulation of p66Shc, where it binds to cytochrome c via the CH2–PTB domain. This, in turn, leads to redox activation and the transfer of electrons to O2, leading to generation of mitochondrial H2O2. Increased mitochondrial H2O2 results in opening of permeability transition pore (PTP) leading to increased swelling and apoptosis [112,114]. Pinton et al. [112] showed that oxidative-stress-mediated activation of PKCβ leads to phosphorylation of p66Shc, which effects translocation of p66Shc to the mitochondrial membrane. This process is mediated by prolyl isomerase 1 (Pin1). Another study describing a mechanistic pathway underlying the apoptogenic effect of p66Shc suggested that UV-irradiation-induced dissociation of p66Shc and mitochondrial heat-shock protein (Hsp) 70 results in decreased membrane potential and increased trans-mitochondrial permeability, leading to apoptosis and decreased lifespan [115]. Many other studies strengthen the link between p66Shc, mitochondrial oxidative damage and aging by providing evidence of phosphorylation of protein kinase B/Akt, up-regulation of p53-dependent apoptosis, inactivation of FOXO3a and suppression of MnSOD [54,55,116,117]. Most of those studies have linked mitochondrial ROS as a causal source of p66shc-mediated oxidative damage. Only one previous study showed that NADPH oxidase-dependent superoxide production in macrophages of p66Shc-knockout mice was attenuated by 40 % [118]. In that study, Tomilov et al. [118] demonstrated that the deficit in NADPH oxidase-dependent ROS production was independent of expression levels of Nox2 subunits (i.e. Rac1/2, p40phox, p47phox, p67phox, p22phox and gp91phox), but rather governed by reduced phosphorylation and membrane translocation of p47phox (organizing subunit of Nox2 complex) in macrophages isolated from p66Shc-knockout mice, as well as in p66Shc siRNA-transfected RAW264.7 macrophages. The authors also observed decreased activation of PKCδ, Akt and extracellular-signal-regulated kinase (ERK) in the p66Shc mutants, suggesting that a Shc-dependent defect in signalling via PKCδ, Akt and ERK activation contributed to the defect in p47phox phosphorylation and Nox2-dependent superoxide production. With that said, these studies, taken together, suggest the potential for p66Shc/Nox2 signalling pathways in oxidative-stress-mediated effects on inflammation, atherosclerosis and longevity.
p53/p21 pathway
The redox-sensitive transcription factor p53 functions as a longevity-associated gene due to its strong tumour-suppressor activity [119]. In unstressed mammalian cells, p53 has a short half-life and is maintained at low levels by ubiquitination catalysed by Mdm2 [120,121], COP1 (constitutively photomorphogenic 1) [122] and Pirh2 (p53-induced protein with a RING-H2 domain) [123]. The stability and activity of p53 are subject to diverse covalent post-translational modifications such as ubiquitination [124,125], phosphorylation [126], acetylation [127], NEDDylation [128], SUMOylation [129] and methylation [130]. Among these post-translational modifications, ROS have been implicated in the phosphorylation of p53 mediated through p38α MAPK (mitogen-activated protein kinase) [131,132] and Polo-like kinase 3 (Plk3) [133]. Moreover, p53 itself is redox-active due to the presence of cysteines that contain redox-sensitive thiol groups [134]. In human p53, two clusters of cysteines in the DNA-binding domain exist and these are essential for the specific binding of p53 to its consensus sequence for transcription of p53-dependent genes [135,136]. Importantly, the role of p53 in premature aging is evident from several mouse studies where persistent low-level activation and increased expression, by ROS signalling, telomere erosion or DNA damage, promotes senescence or irreversible cell cycle arrest [137–139]. In turn, vascular cell senescence is one proposed mechanism surrounding unstable atherosclerotic plaque progression [140]. The ability for p53 to control senescence is consistent with its function to restrain vascularized tumour development, but the precise role for maintenance of longevity requires further investigation [141]. Whereas ROS are demonstrated to promote p53 activation, p53 has also been shown to enhance ROS signalling, presumably via up-regulation of the p67phox subunit as identified previously [142]. However, p53 is also known to participate in the induction of vascular cell apoptosis, which could enhance disease progression [34,143]. But, as p53 also promotes the expression of a number of antioxidant genes, it remains to be clearly demonstrated whether this could account for p53’s ability to control oxidative stress and therefore dictate whether a cell becomes apoptotic or senescent [144,145]. Induction of senescence by p53 is associated with the regulation of p53-dependent genes which participate in cell cycle arrest. Of these, p21cip was identified as the key factor governing the switch to a senescent phenotype. p21cip or cyclin-dependent kinase inhibitor 1, functions to inhibit cell cycle progression by placing a block on the G1- to S-phase transition of the cell cycle [146,147]. Therefore, activation of p53 by ROS and downstream up-regulation of p21cip appears to play a key role in the adaptive response of the vasculature in disease. This is evident in a nutrient deprivation model in vitro where serum-starved human endothelial cells underwent p53/p21cip-dependent cell cycle arrest via Nox2-derived superoxide anion [148]. Furthermore, Nox1- and Nox4-derived ROS were demonstrated to play a role in lung fibroblast senescence via p53-dependent p21cip up-regulation following matricellular CCN1 exposure [149]. Moreover, Kodoma et al. [150] support these findings wherein Nox1- and Nox4-derived ROS play central roles in p53 activation, leading to premature fibroblast senescence. In that study, siRNA against Nox1 and/or Nox4 inhibited Ras-induced premature senescence. This was the first piece of evidence that suppression of selective Nox isoforms could be instrumental in the maintenance of vascular longevity. Finally, redox activation of p53/p21cip signalling leading to senescence is evident in murine models of hypertension [151,152], diabetes [153,154], atherosclerosis [155,156] and pulmonary hypertension [121,157].
Klotho
The Klotho gene was first identified by Kuro-o et al. [60] in 1997, as a putative anti-aging gene, since Klotho-null mice displayed phenotypes resembling premature human aging, including vascular calcification, pulmonary emphysema, skin atrophy, muscle atrophy, osteoporosis, arteriosclerosis and cognitive impairment, among others [60,158]. Several studies have linked Klotho’s influences to intracellular signalling pathways involved in oxidative stress responses and aging via inhibition of IGF-1 [61,62,159] and p53/p21cip [160] and activation of FOXO3 [62]. Klotho is a transmembrane protein that functions as a FGF23 co-receptor with FGFR and is critical in maintaining proper calcium, phosphate and vitamin haemostasis [60]. Although the Klotho gene is mainly expressed in kidneys and the choroid plexus of the brain, the secreted soluble form may cause paracrine effects in distant organs. Recent studies show that Klotho exerts a protective effect against oxidative damage associated with age-related and retinal and neurodegenerative disorders, such as age-related macular degeneration (AMD) and Alzheimer’s disease [161,162]. These studies suggest that binding of the secreted Klotho protein to the membrane results in phosphorylation and translocation of FOXO3a to the nucleus, leading to transcriptional activation of MnSOD and peroxiredoxin-2 (Prx2), and thereby increasing resistance to oxidative stress [62]. Klotho is also shown to be protective of arterial calcification by preventing differentiation of VSMCs to an osteoblast-like phenotype and by restoring FGF23 signalling [163,164]. Another study by Saito et al. [165] showed the involvement of ROS and iron metabolism in AngII-mediated down-regulation of renal Klotho expression. Although Klotho is not known to be expressed in blood vessels but is a circulating hormone, it reportedly protects against endothelial dysfunction in age-associated disorders through endothelium-derived NO production [166–169]. The role of Klotho in age-related CVD was initially based on the development of atherosclerosis in Klotho-deficient mice [60]. Since then it has been suggested that Klotho may have a protective role in the cardiovascular system through endothelium-derived NO production and regulation of angiogenesis [166–168]. Subsequent population-based studies in humans have identified a functional variant of Klotho, i.e. KL-VS, which is associated with the early-onset occult CAD [170] and cardiovascular risk factors such as high-density lipoprotein cholesterol, high blood pressure and stroke [171]. Although a number of studies show that Klotho protein is protective under oxidative stress, the precise mechanisms underlying these phenomena are not completely understood [172]. A study recently showed that the protective effect of Klotho against oxidative damage is a consequence of increased endogenous antioxidant capacity (increased SOD2 and thioredoxin) via increased nuclear factor erythroid-derived 2-related factors 1 and 2 (Nrf1/2) transcriptional activity [173,174]. In a study, Wang et al. [175] showed that direct delivery of Klotho suppresses AngII-induced Nox2-derived superoxide anion production and oxidative damage and attenuates apoptosis via the cAMP/PKA pathway in rat aortic smooth muscle cells (RASMCs). This study suggests the potential cross-talk between Klotho and Nox2 signalling pathways in oxidative-stress-mediated effects on endothelial dysfunction, hypertension, atherosclerosis and lifespan.
IGF-1 pathway
The C. elegans homologue of IGF-1, daf-2, was one the early genes to be identified as a longevity-related gene [67]. IGF-1 is synthesized by almost all tissues and is involved in cell growth, differentiation and transformation processes. It is also well known that IGF-1 exerts pleiotropic effects by virtue of its binding to both IGF-1 receptor (IGF-1R) and insulin–IGF-1 hybrid receptors [176]. Several studies have shown that oxidative stress such as that caused by H2O2 and AngII stimulates IGF-1 and IGF-1R, which is mediated by a tyrosine kinase-dependent redox-sensitive mechanism [177,178]. A number of studies in C. elegans, since the discovery of IGF-1 as a longevity-related gene, have shown that attenuation of IGF-1 signalling increases lifespan. This involves cross-talk with several other genes/pathway(s) implicated in aging, such as FOXO transcription factor (daf-16) [179], heat-shock transcription factor (HSF)-1 [180], SKN-1, an Nrf-like xenobiotic factor [64] and mTOR pathway. These transcription factors, in turn, modulate a diverse set of genes that act cumulatively to produce large beneficial effects on the lifespan of C. elegans. Indeed, mutations known to impair IGF-1R in several centenarian human cohorts strengthen the case that insulin/IGF-1 signalling is negatively associated with lifespan [181,182]. On the other hand, studies in rodents and humans have raised reservations regarding the role IGF-1 plays in aging. Studies show that mice deficient in IGF-1 die shortly after birth, whereas some that live to adulthood have retarded growth [183]. In humans, an age-related decline in endocrine IGF-1 levels is reported, and a large body of evidence suggests that IGF-1 improves cardiovascular function and is atheroprotective [184–187]. IGF-1 overexpression in the heart prevented myocardial cell death after infarction and reduced ventricular dilation, hypertrophy and diabetic cardiomyopathy via attenuation of p53 activation and AngII production, thereby leading to reduced ROS and cellular damage [188–191]. Another study showed that anti-atherogenic effects of IGF-1 infusion in the pro-atherogenic ApoE-knockout mouse model occur via suppression of oxidative stress and up-regulation of vascular eNOS expression leading to increased NO bioavailability [187]. Csiszar et al. [192] reported that in Ames dwarf long-lived mice, low IGF-1 serum concentrations were associated with high oxidative stress in the vasculature and reduced eNOS expression, leading to endothelial dysfunction. More importantly, exogenous IGF-1 enhanced expression of antioxidant enzymes such as MnSOD, Cu/ZnSOD, Gpx1 in explants of mouse aortas and human coronary arterial endothelial cells [192]. In a study, Bailey-Downs et al. [193] showed that IGF-1-deficient mice were also lacking in Nrf-2 and Nrf-2-driven antioxidant genes, NAD(P)H:quinone oxidoreductase1 (NQO1) and heme oxygenase-1 (HMOX-1), thereby impairing the ability of vascular cells to respond to oxidative stress caused by high glucose, oxidized LDL (low-density lipoprotein) and/or H2O2. Endocrine IGF-1 deficiency in the vasculature promotes adverse vascular phenotype under disease states and results in accelerated vascular impairments in aging [193]. Altogether, these studies suggest that IGF-1 has antioxidant-like effects.
Thus, it appears important to note that opposing effects of IGF-1 signalling are at work in different phases of the human life cycle. This disparity in IGF-1-mediated effects is probably a result of the difference between autocrine and paracrine effects of IGF-1. Interestingly, Li et al. [194] used the LID (liver IGF-1-deficiency) mouse model to examine the mechanism involved in IGF-1-deficiency-induced effect on cardiac aging. They showed that severe IGF-1 deficiency reduced aging-associated cardiac contractile dysfunction, prolonged relaxation and ablated intracellular Ca2+ dysfunction. Moreover, the IGF-1-deficiency-elicited effects on cardiac aging were correlated with antagonism against aging-induced reduction in Akt, AMPK phosphorylation, Klotho and up-regulated p53 [194]. A similar study involving the response to a challenge with pro-oxidant paraquat in LID mice demonstrated that IGF-1 deficiency effectively enhanced resistance to oxidative-stress-induced cardiac dysfunction, attenuated ROS and carbonyl production, ultimately resulting in improved survival rate against paraquat-induced mortality [195]. In experimental aortic abdominal constriction (AAC) studies in mice, liver-specific IGF-1 deficiency mitigated AAC-induced cardiac hypertrophy and contractile changes via attenuating AAC-induced Akt phosphorylation and glucose transporter 4 (GLUT4) up-regulation and rescuing down-regulated miR-1 and miR-133a levels [196]. Therefore, whether reduced IGF-1 levels are negatively or positively correlated to age-associated decline in cardiovascular physiological functions is yet to be fully elucidated.
NOX IN AGING
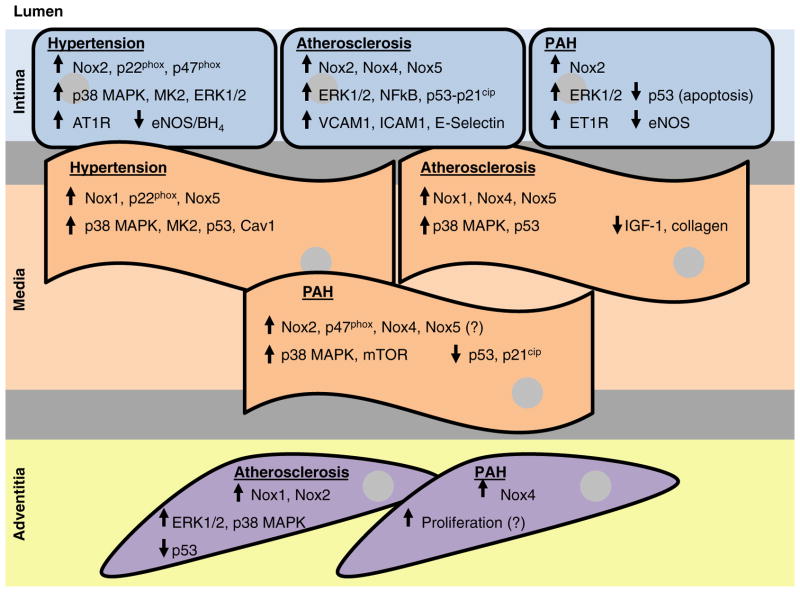
Progressive anatomical, mechanical and biochemical changes occur in the heart as an adaptive response with increasing age. Aging is accompanied by an increase in arterial systolic pressure, reduced or maintained diastolic pressure, increased pulse pressure, aortic dilatation and wall thickening [197]. However, one of the major caveats in the study of aging and its effect on CVDs is that multifactorial changes that occur during the aging process are indistinguishable from vascular phenotypes such as endothelial dysfunction, increased collagen deposition, fibrosis and vessel remodelling presented in CVDs. In one of the early studies carried out in individuals who died from causes other than CVDs, it was reported that aging human hearts are characterized by an increase in the volume fraction of myocytes, myocyte loss, cellular hypertrophy and reduction in the ventricular mass. These cellular processes represent underlying causes for the onset of myocardial dysfunction and failure in the aging population [198]. Wang et al. [199] examined the involvement of Nox in age-associated cardiac remodelling in a rodent model of aging and found that age-dependent increases in blood pressure, cardiomyocyte hypertrophy, coronary artery remodelling and cardiac fibrosis were associated with increased myocardial Nox2 activity. The increase in Nox2-dependent ROS were accompanied by increased renin–angiotensin–aldosterone system (RAAS) activation in the myocardium, increased expression of connective tissue growth factor (CTGF) and TGFβ1 and a significant activation of matrix metalloproteinase (MMP)-2 and membrane type-1 (MT1)-MMP, leading to age-associated cardiac remodelling [199]. It is widely acknowledged that Nox4 is localized to the mitochondria of many different cell types and that mitochondrial oxidative stress promotes aging. In this regard, Ago et al. [200] reviewed the role of mitochondrial Nox4 in the aging process of the heart. It was speculated that oxidant stress in mitochondria builds when antioxidants are unable to counteract the ROS produced by Nox4, triggering the aging process of the heart. It is tempting, therefore, to speculate that Nox4 may be the initiator of mitochondrial ‘stress’ and oxidant production or vice versa. However, Nox4 has been identified to be beneficial against disease-induced cardiac dysfunction and hypertrophy and is therefore assumed to be ‘protective’ [44]. Whatever the relationship, it appears that these two very important sources of ROS could be linear in their role. Taken together, these studies suggest that NADPH oxidase-derived ROS play a pivotal role in the process of aging and age-related cardiovascular changes, whether these are in conjunction with or independent of the mitochondrion. However, despite their importance in vascular function and disease, very few studies provide mechanistic insight into the role of Nox in the aging process. Figure 2 depicts the proposed effects of Nox-derived ROS on redox-sensitive longevity signalling in the vessel wall.
Figure 2. Effects of Nox-derived ROS on redox-sensitive longevity signalling in the vessel wall.
Summary of the effects of hypertension, atherosclerosis and PAH on age-related redox-sensitive signalling pathways linked to the different vascular cells comprising the vessel wall (intima: endothelial cells; media: VSMCs; adventitia: fibroblasts/myofibroblasts) as a consequence of Nox-derived ROS. Arrows depict either increased or decreased protein expression or signalling pathway activation. A question mark (?) depicts a potential effect wherein more conclusive evidence is required to fully support the observations in the literature.
NOX IN AGE-ASSOCIATED CVD
Atherosclerosis
Age is an important independent risk factor for the development of atherosclerosis, which persists even when other risk factors such as hypertension, diabetes, smoking and LDL cholesterol levels are controlled. This may be attributable to characteristic features of aging vessels, including reduced medial VSMC number, increased collagen deposition and breakdown of the elastic lamellae, which collectively can lead to medial thickening and vessel remodelling. These changes are likely to further promote increased extracellular matrix and vascular stiffness, subsequently leading to systolic hypertension. Adhesion and recruitment of pro-inflammatory molecules, also a result of luminal and lamellae damage, logically result in lipid accumulation and foam cell formation following monocyte infiltration [201–204]. As a consequence, the effects of atherosclerosis are superimposed on normal aging of the underlying vessel and therefore it is of utmost importance to unravel the molecular mechanisms involved. Towards that end, it is now generally agreed that elevated vascular ROS triggers an atherosclerotic process by oxidatively modifying lipoproteins causing activation of pro-inflammatory signalling and endothelial dysfunction resulting in further elevated oxidative stress. Pro-oxidant stimuli further result in reduced cell proliferation, irreversible growth arrest and apoptosis, elevated DNA damage, epigenetic modifications and telomere shortening, all of which are distinguishing features of cellular senescence as well as accelerated vascular aging associated with atherosclerosis [205]. Many of these cellular events are modulated by ROS-mediated signal transduction catalysed by Nox [74,206]. Because all cells comprising the lining and vessel wall harbour one or the other isoform of Nox, singularly or collectively they provide a mechanism of localized or paracrine release of ROS, which can influence redox-sensitive signalling pathways with fundamental effects on atherogenesis. One of the first points of evidence for NADPH oxidase-derived superoxide anion as a clinical risk factor for atherosclerosis was reported by Guzik et al. [71]. This study, based on clinical samples, showed that NADPH oxidase is the main source of superoxide production in human saphenous veins, which is associated with impaired NO-mediated endothelial function and increased atherosclerotic risk factors. Two other studies identified the cellular sources of intracellular superoxide anion production within atherosclerotic and non-atherosclerotic human coronary arteries and found increased expression of p22phox, Nox2 (gp91phox) and Nox4 in the shoulder regions of the plaque, whereas expression of Nox1 was reduced [72,78,207]. Interestingly, Sorescu et al. [78] noted a strong association of Nox2 to plaque macrophage content and Nox4 to SMCs and advanced fibrocellular plaques, but not in more advanced atherosclerotic lesions [78]. More importantly, intense ROS in the plaque regions corresponded to increased expression of p22phox and Nox2, implicating NADPH oxidase-derived ROS in plaque rupture [78]. Further, Barry-Lane et al. [79] demonstrated the role of p47phox subunit in the development of atherosclerosis and showed that p47phox−/−/ApoE−/− mice have significantly lower aortic superoxide production, decreased proliferative response to growth factors and lesion formation compared with ApoE−/− mice. A later study by the same group showed that the atheroprotective effect of p47phox deficiency in ApoE-null mice was a result of a decrease in Nox2-derived ROS from monocytes/macrophages that reduced levels of oxidized LDL as well as from that of vessel wall cells, which attenuated the expression of cellular adhesion molecules [208]. Several studies further strengthen the involvement of Nox2 in atherogenesis. Judkins et al. [209] demonstrated that Nox2 expression was up-regulated in the aortic endothelium of ApoE−/− mice, before the appearance of lesions. They also showed that the absence of Nox2 in Nox2−/y/ApoE−/− mice was manifested by a reduced lesion area in the descending aorta by 50 % and was associated with decreased aortic ROS and increased NO bioavailability [209]. These studies provide a strong rationale for Nox-dependent ROS as potential players contributing to premature aging of the vasculature via propagation of atherosclerosis.
Previously, it was reported that an endothelium-specific increase in Nox2-derived superoxide anion production is sufficient to increase macrophage recruitment and endothelial cell activation, leading to an increased atherosclerotic plaque initiation. However, this early event does not alter the progression of atherosclerosis [210]. Sheehan et al. [80] investigated the role of Nox1-smooth muscle cell component in atherogenesis and demonstrated that deletion of Nox1 reduced diet-induced lesion formation by 28 %, decreased macrophage infiltration and ROS levels compared with ApoE−/− mice. They showed that loss of Nox1 attenuated cell proliferation and increased collagen content within the neointima following carotid ligation. A greater reduction in lesion formation in ApoE/Nox2 double null mice observed in previous studies compared with Nox1−/y/ApoE−/− was explained by the absence of Nox1 in circulating cells [80]. These studies linked the increased expression of NADPH oxidase and intracellular superoxide anion production to oxidative modification of LDL and plaque instability via superoxide anion-induced expression of matrix-degrading proteases, such as MMP-2 and MMP-9 as well as associated SMCs and endothelial dysfunction. Additional evidence comes from a study showing the involvement of Nox1-derived ROS in diabetes mellitus-induced atherosclerosis. That study demonstrated that deletion of Nox1 reduced the development of aortic atherosclerosis in the diabetic ApoE−/− mice. This decrease in atherosclerosis was associated with reduced ROS formation, attenuation of chemokine expression, vascular adhesion of leucocytes, macrophage infiltration and reduced expression of pro-inflammatory and profibrotic markers [211].
Not much is known regarding the role of Nox5 in atherosclerosis, primarily because of the limitation posed by Nox5’s absence in rats and mice. However, Guzik et al. [81] showed that expression of Nox5 is dramatically increased in atherosclerotic human coronary artery vessel wall which was associated with a 7-fold increase in calcium-dependent NADPH oxidase activity. Interestingly, Nox5 was localized to the endothelial cells in the early lesions and in the smooth muscle layer in advanced lesions [81]. Supporting evidence comes from a study showing that endothelial Nox5 is activated by AngII and ET-1 through Ca2+/calmodulin-dependent and Rac-1-independent mechanisms, leading to increased ROS generation and ERK1/2-regulated growth and inflammatory signal transduction associated with endothelial dysfunction and vascular pathologies [212]. Nevertheless, the affected signal transduction pathways intertwining vascular aging and atherogenesis are poorly understood and are largely derived from studies on cellular senescence. ROS-mediated oxidative DNA damage is known to promote cellular senescence, which occurs both in the mitochondrial DNA and in the nuclear DNA, as well in both telomeric and non-telomeric regions of VSMC and macrophages in plaques [73,213]. Other than directly causing DNA damage, ROS-induced activation of p53 has been shown to promote DDR (DNA damage recognition and repair) and reduce the expression of the IGF-1R in VSMCs as well as the subsequent Akt survival signals [214]. Since both p53 and IGF-1 have been associated with longevity, signal transduction by these molecules might be responsible for NADPH oxidase-mediated damage in atherosclerosis.
Hypertension
Hypertension is one of the most common diseases in the elderly. The prevalence of hypertension increases markedly with advancing age, primarily a result of elevations in blood pressure attributed to alterations in the structure, function and rigidity of vessel wall [215], as well as from a shift in control by the autonomic nervous system [216]. Several studies have shown that aging and hypertension are associated with the impairment of endothelium-dependent vascular relaxation and endothelial dysfunction. Both are known to increase the risk of cardiovascular and cerebrovascular diseases [71,75,217,218]. Oxidative stress is well established as a key regulator of age-induced endothelial dysfunction and RAAS. These influence the sympathetic and parasympathetic arms of the autonomic nervous system and promote hypertension. Moreover, it is well known that RAAS activation may itself potentiate oxidative stress [29,70]. Several clinical and animal model studies of hypertension have shown that increased NADPH oxidase-derived ROS is associated with endothelial dysfunction [76,102,103,105]. Zalba et al. [102] showed that endothelium-dependent relaxation in response to acetylcholine was significantly attenuated in 30-week-old compared with 16-week-old hypertensive rats. This impaired NO-dependent relaxation in AngII-mediated hypertension was linked to increased NADPH oxidase activity and expression of p22phox mRNA [102]. Another study of aging in a spontaneously hypertensive rat (SHR) model showed that RAAS attenuation in vivo by temocaprilat, hydralazine and olmesartan reversed age-related advanced cardiac hypertrophy due to suppression of cardiac oxidative stress by attenuating the expression of Nox2 oxidase components, p22phox, p47phox and Nox2 [219]. Another study demonstrated the role of MAPK-activated protein kinase 2 (MK2) in AngII-induced hypertension. Ebrahimian et al. [82] showed that MK2, which is a direct downstream target of p38 MAPK, mediates an AngII-induced increase in Nox2-dependent superoxide production and activation of pro-inflammatory molecules through up-regulation of the p47phox subunit of NADPH oxidase and suppressed antioxidant enzyme catalase. The AngII-mediated up-regulation of MK2 resulted in a vicious cycle that potentiated inflammatory responses to AngII and led to increased VSMC proliferation [82]. Other than Nox2, several studies have demonstrated a role for Nox1 in systemic hypertension. Using transgenic mice overexpressing Nox1 in the smooth muscle cells, Dikalova et al. [220] provided a causal link between Nox1-derived superoxide production and increased systolic blood pressure and aortic hypertrophy in response to AngII. Matsuno et al. [83] generated Nox1−/y mice and showed that Nox1 in AngII-mediated hypertension underlies the pressor response. Similar studies showed that lack of Nox1 attenuated AngII-induced ROS generation and Ca2+ signalling in VSMCs and Nox1-derived ROS regulated cell-surface expression of AT1R (AngII type 1 receptor) through mechanisms involving caveolin phosphorylation [221]. Although all the above studies suggest that hypertension, NADPH oxidase-derived ROS and oxidative stress are linked, none of them specifically examine the so-named longevity genes in hypertension. Wang and Sun [222] studied the role of Klotho in the pathogenesis of hypertension. In this study, they showed that Klotho gene delivery decreased aortic Nox2 expression and NADPH oxidase activity in SHRs, whereas renal expression of NOS, Nox1 and Nox4 remained unaltered. Further mechanistic studies delving into signalling that confer specificity of Nox inhibition by Klotho in systemic hypertension are warranted. Similarly, the role of Nox in redox signalling with age-related hypertension-linked vessel remodelling remains to be investigated.
One of the common forms of hypertension that is increasingly being diagnosed in elderly population is cor pulmonale or pulmonary arterial hypertension (PAH) [223–225]. PAH is a haemodynamic and pathophysiological condition defined by an increase in mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest. It is the consequence of an increasing pulmonary vascular resistance (PVR) that leads to right ventricular (RV) overload, hypertrophy, reduction in cardiac output and eventually RV failure and death [226]. It is characterized by an intense and characteristic remodelling of small pulmonary arteries, due to structural and functional changes in endothelial, smooth muscle and adventitial fibroblast layers of the vessel wall [227]. One of the key environmental factors that cause extensive irreversible medial and adventitial thickening of vessel wall and muscularization of previously non-muscularized distal vessels is chronic hypoxia (CH). Several studies have implicated ROS in acute hypoxic vasoconstriction and vascular remodelling associated with CH and a pivotal role of NADPH oxidase-derived ROS in this process is now well established. In one of the initial studies, Liu et al. [84] showed that CH-induced increase in superoxide anion production was attenuated in Nox2-null mice. This was associated with a decrease in pathological changes seen in CH-induced vascular remodelling such as mean RV pressure, medial wall thickening of small pulmonary arteries and right heart hypertrophy. They also linked increased ROS production to increased p47phox expression and activity, rather than increased mRNA expression levels of NADPH oxidase subunits, Nox2, p22phox, p40phox, Rac1 and Rac2 [84]. Later studies demonstrated that in the lung vasculature, Nox4-derived ROS play a crucial role in mediating PASMC and fibroblast proliferation-associated vascular remodelling in PAH. This was linked to increased Nox4 mRNA and protein expression [85,228–230]. It was suggested that the difference in localization of Nox2 and Nox4, to the endothelial and smooth muscle cell layers respectively, is responsible for the differential role of Nox2 and Nox4 in hypoxia-induced pulmonary hypertension. Although it is known that RV function is the critical determinant of lifespan in patients with PAH, not much is known about the role Nox isoforms play in causing RV failure independent of an elevated pulmonary artery pressure. Using a pulmonary artery banding (PAB) model in mice, which is a surgical model of chronic progressive RV pressure overload not associated with structural alterations of the lung circulation, we recently showed PAB results in an increase in Nox4-derived ROS production, which is responsible for cardiac dysfunction and RV failure. We also showed that expression of antioxidant enzymes, SOD, catalase and Gpx did not change [45]. All of these studies elegantly link Nox-derived ROS to structural changes associated with PAH and subsequent RV failure, comparative studies in young and old animals are necessary to understand how these processes are modulated in aging heart.
Atrial fibrillation
AF is the most common type of cardiac arrhythmia. AF refers to abnormal rhythm of the heartbeat caused by perturbed electrophysiology of the myocardium. The incidence of AF increases quite dramatically with age (1.5 % at 50–59 years to 23.5 % at 80–89 years) [231] and even more so with prior history of cardiac surgery and cardiopulmonary diseases [232–235]. A number of studies have shown that oxidative stress plays a vital role in the pathogenesis of AF [236–239]. It is also noteworthy that oxidative stress is also involved in the pathogenesis of several predisposing factors and conditions associated with AF. These include hypertension, CHD (chronic heart disease), obesity and diabetes, among many others. There is a large body of evidence showing the role that the NADPH oxidase family of enzymes plays in the development of AF. Both myocardial Nox2 and myocardial Nox4 have been shown to participate in ROS-mediated effects in AF [38,39,86,240,241]. Initial studies in animal models suggested a Rac1-dependent effect of Nox-derived ROS leading to AF [86,242], which were supported by a number of clinical studies showing a correlation among Nox2 up-regulation, oxidative stress and AF [38,39,243]. Kim et al. [38] suggested that NADPH oxidase-derived ROS play an important role in the atrial oxidative injury associated with AF. In this study, they reported up-regulation of NADPH oxidase subunits p22phox, p47phox p67phox and Nox2, but not of Nox1 or Nox4, in tissue homogenates and isolated atrial myocytes from the right atrial appendage of patients undergoing cardiac surgery [38]. Importantly, Reilly et al. [241] suggested that an initial insult caused by Nox activation probably accounts for early development of AF, whereas ROS derived from mitochondria and uncoupled eNOS are involved in long-term AF. The change in the sources of ROS with atrial remodelling therefore could explain why statins are effective in the primary prevention of AF, but not in its management [241]. In vitro studies using HL-1 atrial myocytes have implicated Nox2 and Nox4 in TGFβ1-mediated myofibril degradation via calpain activation leading to calcium overload. Collectively, these were shown to elicit electrical and structural remodelling of cardiac tissue in AF [240,244]. Although these studies suggest a causal link between oxidative stress, the role of Nox and AF, the downstream signalling cascade by which atrial structural remodelling contributes to the pathogenesis of AF has not yet been described. A study by Adam et al. [245] demonstrated a marked up-regulation of CTGF, which is associated with increased fibrosis and tissue AngII concentration. With regard to an underlying mechanism, this study suggested that AngII up-regulated CTGF via activation of Rac1 and NADPH oxidase, leading to up-regulation of N-cadherin and connexin43 (Cx43) and therefore contributing to structural remodelling associated with AF [245].
Nox therapeutics in treatment of CVDs
From the aforementioned studies, it is apparent that NADPH oxidase-derived ROS play a pivotal role in age-associated cardiovascular changes and CVDs. Therefore, Nox could be important drug targets for the treatment of atherosclerosis, hypertension, stroke, AF, diabetes and many other diseases of the elderly. The role of Nox as drug targets in CVDs has been extensively reviewed [246,247]. Despite a large armamentarium of drugs currently approved for specific therapy such as AngII receptor antagonists (azilsartan and benicar), renin inhibitor (aliskiren), calcium channel blockers (amlodipine), synthetic prostacyclin and its analogues (epoprostenol, treprostinil, beraprost, iloprost and selexipag), ET-1 receptor antagonists (bosentan, ambrisentan and macinentan) and type 5 phosphodiesterase (PDE5) inhibitors (sildenafil, tadalafil, vardenafil and riociguat) for PAH, blood thinners (aspirin, warfarin, dabigatran, rivaroxaban and apixaban) and dofetilide and dronedarone for AF, CVDs still contribute to a high level of mortality in the elderly. The beneficial effects of these drugs may, in part, come from their ability to reduce oxidative stress, either directly via attenuating the NADPH oxidase-derived ROS or indirectly by improving NO bioavailability [248,249]. Although antioxidants such as vitamins A, E and C, polyphenols and flavonoids have been used as therapeutic agents with some success to reduce oxidant stress and blood pressure [250], most of the large clinical trials have failed to demonstrate a beneficial cardiovascular effect [251,252]. Compared with the antioxidants, which degrade free radical intermediates and prevent further oxidation of molecules, inhibitors of Nox isoforms decrease the rate of enzymatic ROS formation. These inhibitors either modify Nox expression or target assembly and activation of Nox complexes, thereby reducing the immediate oxidant environment and suppressing ROS-induced signalling cascades.
Although some classical Nox inhibitors (AEBSF, apocynin, PR-39 and S17834) and two commonly used drugs, HMG-CoA reductase inhibitors (statins) and angiotensin receptor antagonists [ARBs (angiotensin II receptor blockers) and ACE (angiotensin-converting enzyme) inhibitors], are regularly being used in animal models and treatment therapy respectively, most of the currently available Nox inhibitors including the above-mentioned are not isoform-specific and may potentially affect both physiological and pathophysiological signalling pathways. Therefore, the development of selective and isoform-specific Nox inhibitors that preferably target only pathological Nox signalling has become a foremost objective for both academia and pharmaceutical companies. A number of different small-molecule inhibitors have become available that could become useful in their own right or by way of their derivatives [253–263]. Nox1/4 inhibitor GKT 136901 was reported to inhibit NADPH oxidase-dependent aortic ROS formation, attenuate CD44- and HA-dependent inflammatory cell recruitment and aortic lesion area in atherosclerotic lesions of ApoE−/− mice [264]. Similarly, GKT137831 inhibited ROS production and attenuated diabetes-induced macrophage infiltration, inflammation and fibrosis associated with atherosclerotic plaque formation in ApoE−/− mice as well as attenuated hypoxia-induced H2O2 release and improved indices of ventricular remodelling and pulmonary artery wall thickening in a mouse hypoxia model of PAH [211,265]. Like the first-generation GKT inhibitor, issues of Nox specificity are still a concern with regard to untoward and off-target effects of these drugs. Novel approaches and rational drug design strategies have yielded isoform selectivity for Nox2 and been discussed in greater detail [107,108,247,258,266]. Assuredly with these innovative approaches, new isoform-specific Nox therapeutics are expected to gain greater traction. That said, peptidic inhibitors still appear to offer the greatest level of Nox selectivity [267–270] and have exhibited high levels of potency in cell-free and whole-cell assays. For this reason, they have been widely employed in animal studies. Admittedly, an inherent concern in the pharmaceutical industry regarding oral bioavailability of these potential therapeutics has stunted development, in spite of the potential for alternative modes of delivery and stabilization to increase clinical application [107,271].
CONCLUSIONS AND PERSPECTIVES
A higher level of care, treatment options and better socioeconomic conditions have resulted in a dramatic increase in the quality and lifespan of the elderly population with cardiovascular complications over the last half-century. Since the free radical theory of aging was first proposed, tremendous progress has been made to identify and understand the molecular mechanisms involved in aging and age-related CVDs. A seminal role of Nox in CVD is quite evident. Despite these advances, manifestation of combined aetiologies (co-morbidities) together with age-related changes further complicates our understanding of the pathophysiological and pathobiological factors contributing to decline in the older patient. Indeed, much more work needs to be done to determine whether there is a unified effect of ROS on longevity and CVD. That is, whether specific Nox isoforms are responsible for modulating CVD and the biology of the aging process and whether these are both modulated by a common gene or set of longevity genes are yet to be fully appreciated. Furthermore, with Nox-targeted therapies showing promising results in animal models and pre-clinical trials, it is possible that, in the near future, we may be able to adapt these interventions to further improving cardiovascular health and/or slow the aging process.
Acknowledgments
We thank Ms. Laura Pliske for editorial support during the preparation of the manuscript.
FUNDING
This work was supported by the National Institutes of Health [grant numbers RO1HL079207 and PO1HL103455-02 (to P.J.P.)]; the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Abbreviations
- AAC
aortic abdominal constriction
- AEBSF
4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride
- AF
atrial fibrillation
- AKT
synonym for Protein kinase B
- AngII
angiotensin II
- ApoE
apolipoprotein E
- CAD
coronary artery disease
- CCN1 or CYR61
cysteine-rich angiogenic inducer 61
- FGF
fibroblast growth factor
- CD44
cluster of differentiation 44
- CH
chronic hypoxia
- CTGF
connective tissue growth factor
- CVD
cardiovascular disease
- DUOX
dual oxidase
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular-signal-regulated kinase
- ET-1
endothelin-1
- FGF-R
fibroblast growth factor receptor
- FOXO3
forkhead-box protein O3
- GKT
GenKyotex
- Gpx
glutathione peroxidase
- HA
hyaluronic acid
- HMG-coA
3-hydroxy-3-methyl-glutaryl-coenzyme A
- IGF-1
insulin-like growth factor
- IGF-1R
IGF-1 receptor
- JNK
c-Jun N-terminal kinases
- KL-VS
variant of Klotho
- LDL
low-density lipoprotein
- LID
liver IGF-1-deficiency
- MAPK
mitogen-activated protein kinase
- Mdm2
mouse double minute 2 homolog
- MK2
mitogen-activated protein kinase-activated protein kinase 2
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- NOS
nitric oxide synthase
- Nox
NADPH oxidase(s)
- Nrf
nuclear factor erythroid-derived 2-related factor
- PAB
pulmonary artery banding
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cell
- Pin1
prolyl isomerase 1
- PKA
protein kinase A
- PKC
protein kinase C
- PKCδ
Protein Kinase C delta
- Poldip2
polymerase (DNA-directed), delta interacting protein 2
- Shc
src homology 2 domain containing
- PR-39
proline-arginine (PR)-rich antibacterial peptide
- PTB
phospho tyrosine binding
- RAAS
renin–angiotensin–aldosterone system
- Ras
Rat sarcoma
- RING
Really Interesting New Gene
- ROS
reactive oxygen species
- RV
right ventricular
- S17834
1,4-dimethyl-2,3,5,6-tetraiodobenzene
- SHR
spontaneously hypertensive rats
- Sir2
silent information regulator 2
- SKN-1
protein skinhead 1
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- TGFβ
transforming growth factor β
- Trx2
Thioredoxin2
- VSMC
vascular smooth muscle cell
References
- 1.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, et al. Forecasting the future of cardiovascular disease in the united states: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Jin K. Modern biological theories of aging. Aging Dis. 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Viña J, Borras C, Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 10.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 11.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Lithgow GJ, Kirkwood TB. Mechanisms and evolution of aging. Science. 1996;273:80. doi: 10.1126/science.273.5271.80. [DOI] [PubMed] [Google Scholar]
- 13.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 14.Taub J, Lau JF, Ma C, Hahn JH, Hoque R, Rothblatt J, Chalfie M. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-c and Clk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. Cu/ZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 18.Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc Natl Acad Sci USA. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sentman ML, Granstrom M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, Hertzog P, Kola I. Fibroblasts derived from gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richardson A. Knockout mice heterozygous for sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–H1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 25.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of h2o2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 27.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 29.Cifuentes ME, Rey FE, Carretero OA, Pagano PJ. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin ii-infused mice. Am J Physiol Heart Circ Physiol. 2000;279:H2234–H2240. doi: 10.1152/ajpheart.2000.279.5.H2234. [DOI] [PubMed] [Google Scholar]
- 30.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin ii. Proc Natl Acad Sci USA. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 33.Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H. G alpha(i) and g alpha(o) are target proteins of reactive oxygen species. Nature. 2000;408:492–495. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- 34.Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part i: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 36.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN, Cai H. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 39.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 40.Zalba G, Beaumont J, San Jose G, Fortuno A, Fortuno MA, Diez J. Vascular oxidant stress: Molecular mechanisms and pathophysiological implications. J Physiol Biochem. 2000;56:57–64. doi: 10.1007/BF03179777. [DOI] [PubMed] [Google Scholar]
- 41.Griendling KK, Alexander RW. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- 42.Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation. 2001;104:2638–2640. [PubMed] [Google Scholar]
- 43.Csanyi G, Taylor WR, Pagano PJ. Nox and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol. 2014;306:H197–H205. doi: 10.1152/ajpheart.00977.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Ghouleh I, Frazziano G, Rodriguez AI, Csanyi G, Maniar S, St Croix CM, Kelley EE, Egana LA, Song GJ, Bisello A, et al. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res. 2013;97:134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frazziano G, Champion HC, Pagano PJ. NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. Am J Physiol Heart Circ Physiol. 2012;302:H2166–H2177. doi: 10.1152/ajpheart.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, et al. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin ii-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 51.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin ii. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 52.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 53.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 55.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, et al. A p53-p66shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 56.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and Sir2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 57.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 61.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor skn-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong A, Boutis P, Hekimi S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1995;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lakowski B, Hekimi S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science. 1996;272:1010–1013. doi: 10.1126/science.272.5264.1010. [DOI] [PubMed] [Google Scholar]
- 67.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 68.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in drosophila by modulation of genes in the tor signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaeberlein M, Powers RW, III, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 70.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 71.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 72.Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, Awano K, Kobayashi K, Maeda K, Hata K, et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: Important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2002;22:1838–1844. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 73.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 74.Bedard K, Krause KH. The Nox family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 75.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 76.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 77.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 79.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. P47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ., Jr Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–326. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, et al. Calcium-dependent nox5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ebrahimian T, Li MW, Lemarie CA, Simeone SM, Pagano PJ, Gaestel M, Paradis P, Wassmann S, Schiffrin EL. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin ii-induced inflammation and hypertension: regulation of oxidative stress. Hypertension. 2011;57:245–254. doi: 10.1161/HYPERTENSIONAHA.110.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin ii-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 84.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 85.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit Nox4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 86.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 87.Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhofer S, Radermacher KA, Janssen B, Gorlach A, Schmidt HH. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J Mol Med. 2012;90:1391–1406. doi: 10.1007/s00109-012-0963-3. [DOI] [PubMed] [Google Scholar]
- 88.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which Nox to target in vascular disease? Trends Endocrinol Metab. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 89.von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 Phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes–prototype of the Nox electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pick E. Role of the rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task. Small GTPases. 2014;5:e27952. doi: 10.4161/sgtp.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meijles DN, Fan LM, Howlin BJ, Li JM. Molecular insights of p47phox phosphorylation dynamics in the regulation of NADPH oxidase activation and superoxide production. J Biol Chem. 2014;289:22759–22770. doi: 10.1074/jbc.M114.561159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turgeon J, Haddad P, Dussault S, Groleau J, Maingrette F, Perez G, Rivard A. Protection against vascular aging in Nox2-deficient mice: Impact on endothelial progenitor cells and reparative neovascularization. Atherosclerosis. 2012;223:122–129. doi: 10.1016/j.atherosclerosis.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Helmcke I, Heumuller S, Tikkanen R, Schroder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 95.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Touyz RM, Montezano AC. Vascular nox4: a multifarious NADPH oxidase. Circ Res. 2012;110:1159–1161. doi: 10.1161/CIRCRESAHA.112.269068. [DOI] [PubMed] [Google Scholar]
- 97.Nisimoto Y, Diebold BA, Constentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bedard K, Jaquet V, Krause KH. Nox5: from basic biology to signaling and disease. Free Radic Biol Med. 2012;52:725–734. doi: 10.1016/j.freeradbiomed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 99.Baniulis D, Nakano Y, Nauseef WM, Banfi B, Cheng G, Lambeth DJ, Burritt JB, Taylor RM, Jesaitis AJ. Evaluation of two anti-gp91phox antibodies as immunoprobes for Nox family proteins: Mab 54.1 recognizes recombinant full-length Nox2, Nox3 and the C-terminal domains of Nox1-4 and cross-reacts with Grp 58. Biochim Biophys Acta. 2005;1752:186–196. doi: 10.1016/j.bbapap.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 100.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin ii-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and Nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol. 2004;24:1614–1620. doi: 10.1161/01.ATV.0000139011.94634.9d. [DOI] [PubMed] [Google Scholar]
- 102.Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, Diez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]
- 103.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 104.Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA, Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin ii hypertension. Hypertension. 2010;55:116–123. doi: 10.1161/HYPERTENSIONAHA.109.135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin ii-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oskarsson HJ, Heistad DD. Oxidative stress produced by angiotensin too. Implications for hypertension and vascular injury. Circulation. 1997;95:557–559. doi: 10.1161/01.cir.95.3.557. [DOI] [PubMed] [Google Scholar]
- 107.Cifuentes-Pagano E, Meijles DN, Pagano PJ. The quest for selective nox inhibitors and therapeutics: challenges, triumphs and pitfalls. Antioxid Redox Signal. 2014;20:2741–2754. doi: 10.1089/ars.2013.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The Nox toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, II, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG. A novel transforming protein (Shc) with an Sh2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 112.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, et al. Protein kinase c beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 113.Le S, Connors TJ, Maroney AC. C-jun n-terminal kinase specifically phosphorylates p66shca at serine 36 in response to ultraviolet irradiation. J Biol Chem. 2001;276:48332–48336. doi: 10.1074/jbc.M106612200. [DOI] [PubMed] [Google Scholar]
- 114.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, et al. Electron transfer between cytochrome c and p66shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, et al. The life span determinant p66shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates transmembrane potential. J Biol Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 116.Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H. Age-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol. 2008;294:C145–C152. doi: 10.1152/ajpcell.00350.2007. [DOI] [PubMed] [Google Scholar]
- 117.Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M. P66shc-generated oxidative signal promotes fat accumulation. J Biol Chem. 2008;283:34283–34293. doi: 10.1074/jbc.M804362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomilov AA, Bicocca V, Schoenfeld RA, Giorgio M, Migliaccio E, Ramsey JJ, Hagopian K, Pelicci PG, Cortopassi GA. Decreased superoxide production in macrophages of long-lived p66shc knock-out mice. J Biol Chem. 2010;285:1153–1165. doi: 10.1074/jbc.M109.017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 120.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 121.Mouraret N, Marcos E, Abid S, Gary-Bobo G, Saker M, Houssaini A, Dubois-Rande JL, Boyer L, Boczkowski J, Derumeaux G, et al. Activation of lung p53 by nutlin-3a prevents and reverses experimental pulmonary hypertension. Circulation. 2013;127:1664–1676. doi: 10.1161/CIRCULATIONAHA.113.002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O’Rourke K, Koeppen H, Dixit VM. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 123.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 124.Lee JT, Gu W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 2010;17:86–92. doi: 10.1038/cdd.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brooks CL, Gu W. P53 regulation by ubiquitin. FEBS Lett. 2011;585:2803–2809. doi: 10.1016/j.febslet.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 127.Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2:456–462. doi: 10.1007/s13238-011-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abida WM, Nikolaev A, Zhao W, Zhang W, Gu W. Fbxo11 promotes the neddylation of p53 and inhibits its transcriptional activity. J Biol Chem. 2007;282:1797–1804. doi: 10.1074/jbc.M609001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santiago A, Li D, Zhao LY, Godsey A, Liao D. P53 sumoylation promotes its nuclear export by facilitating its release from the nuclear export receptor crm1. Mol Biol Cell. 2013;24:2739–2752. doi: 10.1091/mbc.E12-10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leonova KI, Brodsky L, Lipchick B, Pal M, Novototskaya L, Chenchik AA, Sen GC, Komarova EA, Gudkov AV. P53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs. Proc Natl Acad Sci USA. 2013;110:E89–E98. doi: 10.1073/pnas.1216922110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bragado P, Armesilla A, Silva A, Porras A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007;12:1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 132.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem. 2000;275:35778–35785. doi: 10.1074/jbc.M004267200. [DOI] [PubMed] [Google Scholar]
- 133.Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M, Dai W. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276:36194–36199. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 134.Hainaut P, Mann K. Zinc binding and redox control of p53 structure and function. Antioxid Redox Signal. 2001;3:611–623. doi: 10.1089/15230860152542961. [DOI] [PubMed] [Google Scholar]
- 135.Rainwater R, Parks D, Anderson ME, Tegtmeyer P, Mann K. Role of cysteine residues in regulation of p53 function. Mol Cell Biol. 1995;15:3892–3903. doi: 10.1128/mcb.15.7.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Buzek J, Latonen L, Kurki S, Peltonen K, Laiho M. Redox state of tumor suppressor p53 regulates its sequence-specific DNA binding in DNA-damaged cells by cysteine 277. Nucleic Acids Res. 2002;30:2340–2348. doi: 10.1093/nar/30.11.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, et al. P53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 138.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vigneron A, Vousden KH. P53, ROS and senescence in the control of aging. Aging. 2010;2:471–474. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu S, Chamseddine AH, Carrell S, Miller FJ., Jr Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014;2:642–650. doi: 10.1016/j.redox.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 142.Italiano D, Lena AM, Melino G, Candi E. Identification of Ncf2/p67phox as a novel p53 target gene. Cell Cycle. 2012;11:4589–4596. doi: 10.4161/cc.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 144.Olovnikov IA, Kravchenko JE, Chumakov PM. Homeostatic functions of the p53 tumor suppressor: regulation of energy metabolism and antioxidant defense. Semin Cancer Biol. 2009;19:32–41. doi: 10.1016/j.semcancer.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zuckerman V, Wolyniec K, Sionov RV, Haupt S, Haupt Y. Tumour suppression by p53: The importance of apoptosis and cellular senescence. J Pathol. 2009;219:3–15. doi: 10.1002/path.2584. [DOI] [PubMed] [Google Scholar]
- 146.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R, Khokhar AR, Kuang J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene. 2005;24:2929–2943. doi: 10.1038/sj.onc.1208474. [DOI] [PubMed] [Google Scholar]
- 148.Li JM, Fan LM, George VT, Brooks G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med. 2007;43:976–986. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kodama R, Kato M, Furuta S, Ueno S, Zhang Y, Matsuno K, Yabe-Nishimura C, Tanaka E, Kamata T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 151.Morgan RG, Ives SJ, Walker AE, Cawthon RM, Andtbacka RH, Noyes D, Lesniewski LA, Richardson RS, Donato AJ. Role of arterial telomere dysfunction in hypertension: Relative contributions of telomere shortening and telomere uncapping. J Hypertens. 2014;32:1293–1299. doi: 10.1097/HJH.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, et al. Angiotensin ii induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 153.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171:523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tavana O, Puebla-Osorio N, Sang M, Zhu C. Absence of p53-dependent apoptosis combined with nonhomologous end-joining deficiency leads to a severe diabetic phenotype in mice. Diabetes. 2010;59:135–142. doi: 10.2337/db09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S, White A, Biswas S, Khamis R, Chong CK, et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler Thromb Vasc Biol. 2014;34:985–995. doi: 10.1161/ATVBAHA.114.303415. [DOI] [PubMed] [Google Scholar]
- 156.Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, et al. Lincrna-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abid S, Houssaini A, Mouraret N, Marcos E, Amsellem V, Wan F, Dubois-Rande JL, Derumeaux G, Boczkowski J, Motterlini R, Adnot S. P21-dependent protective effects of a carbon monoxide-releasing molecule-3 in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014;34:304–312. doi: 10.1161/ATVBAHA.113.302302. [DOI] [PubMed] [Google Scholar]
- 158.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 160.de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 161.Kokkinaki M, Abu-Asab M, Gunawardena N, Ahern G, Javidnia M, Young J, Golestaneh N. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci. 2013;33:16346–16359. doi: 10.1523/JNEUROSCI.0402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR. The neuroprotective effect of klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289:24700–24715. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 165.Saito K, Ishizaka N, Mitani H, Ohno M, Nagai R. Iron chelation and a free radical scavenger suppress angiotensin ii-induced downregulation of klotho, an anti-aging gene, in rat. FEBS Lett. 2003;551:58–62. doi: 10.1016/s0014-5793(03)00894-9. [DOI] [PubMed] [Google Scholar]
- 166.Fukino K, Suzuki T, Saito Y, Shindo T, Amaki T, Kurabayashi M, Nagai R. Regulation of angiogenesis by the aging suppressor gene Klotho. Biochem Biophys Res Commun. 2002;293:332–337. doi: 10.1016/S0006-291X(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 167.Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M. Endothelial dysfunction in the Klotho mouse and downregulation of Klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738–746. doi: 10.1007/s000180050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 169.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 170.Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. Klotho allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the klotho gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 172.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 173.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW. Alpha-klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L566–L575. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem. 2001;276:18399–18406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 175.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 177.Du J, Peng T, Scheidegger KJ, Delafontaine P. Angiotensin ii activation of insulin-like growth factor 1 receptor transcription is mediated by a tyrosine kinase-dependent redox-sensitive mechanism. Arterioscler Thromb Vasc Biol. 1999;19:2119–2126. doi: 10.1161/01.atv.19.9.2119. [DOI] [PubMed] [Google Scholar]
- 178.Delafontaine P, Ku L. Reactive oxygen species stimulate insulin-like growth factor i synthesis in vascular smooth muscle cells. Cardiovasc Res. 1997;33:216–222. doi: 10.1016/s0008-6363(96)00179-4. [DOI] [PubMed] [Google Scholar]
- 179.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of daf-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 180.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by daf-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 181.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor i receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, et al. Association of common genetic variation in the insulin/igf1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor i (IGF-1) and type 1 IGF receptor (IGF1R) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 184.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 185.Kaplan RC, McGinn AP, Pollak MN, Kuller L, Strickler HD, Rohan TE, Cappola AR, Xue X, Psaty BM. High insulin-like growth factor binding protein 1 level predicts incident congestive heart failure in the elderly. Am Heart J. 2008;155:1006–1012. doi: 10.1016/j.ahj.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 187.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 188.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. Igf-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin ii-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 190.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 191.Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. IGF-i alleviates diabetes-induced rhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–R802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 192.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, et al. Liver-specific knockdown of igf-1 decreases vascular oxidative stress resistance by impairing the nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–733. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li Q, Yang X, Sreejayan N, Ren J. Insulin-like growth factor i deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: role of oxidative stress. Rejuvenation Res. 2007;10:501–512. doi: 10.1089/rej.2007.0552. [DOI] [PubMed] [Google Scholar]
- 196.Hua Y, Zhang Y, Ren J. Igf-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: role of microRNA-1 and microRNA-133a. J Cell Mol Med. 2012;16:83–95. doi: 10.1111/j.1582-4934.2011.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 198.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 199.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging. 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 202.Franklin SS, Gustin W, 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham heart study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 203.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 204.Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo SY, Liu TH, Ou DY, O’Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 205.Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- 206.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 208.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 209.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 210.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(−)/(−) mice. Cardiovasc Res. 2012;94:20–29. doi: 10.1093/cvr/cvs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 212.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin ii and endothelin-1 is mediated via calcium/calmodulin-dependent, Rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin a acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 214.Kavurma MM, Figg N, Bennett MR, Mercer J, Khachigian LM, Littlewood TD. Oxidative stress regulates IGF1R expression in vascular smooth-muscle cells via p53 and HDAC recruitment. Biochem J. 2007;407:79–87. doi: 10.1042/BJ20070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Higashi Y, Oshima T, Ozono R, Matsuura H, Kajiyama G. Aging and severity of hypertension attenuate endothelium-dependent renal vascular relaxation in humans. Hypertension. 1997;30:252–258. doi: 10.1161/01.hyp.30.2.252. [DOI] [PubMed] [Google Scholar]
- 216.Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10(Suppl 1):S127–S136. doi: 10.1111/j.1447-0594.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 217.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 218.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 219.Ito N, Ohishi M, Yamamoto K, Tatara Y, Shiota A, Hayashi N, Komai N, Yanagitani Y, Rakugi H, Ogihara T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am J Hypertens. 2007;20:792–799. doi: 10.1016/j.amjhyper.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 220.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, et al. Nox1 overexpression potentiates angiotensin ii-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 221.Basset O, Deffert C, Foti M, Bedard K, Jaquet V, Ogier-Denis E, Krause KH. NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin ii-receptor localization in vascular smooth muscle. Antioxid Redox Signal. 2009;11:2371–2384. doi: 10.1089/ars.2009.2584. [DOI] [PubMed] [Google Scholar]
- 222.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, et al. The changing picture of patients with pulmonary arterial hypertension in the united states: how reveal differs from historic and non-us contemporary registries. Chest. 2011;139:128–137. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 224.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 225.Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the compera registry. Int J Cardiol. 2013;168:871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 226.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 227.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 228.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. Nox4 regulates ros levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 229.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, et al. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol. 2014;34:1704–1715. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 232.Kalus JS, White CM, Caron MF, Coleman CI, Takata H, Kluger J. Indicators of atrial fibrillation risk in cardiac surgery patients on prophylactic amiodarone. Ann Thorac Surg. 2004;77:1288–1292. doi: 10.1016/j.athoracsur.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 233.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 234.Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol. 1998;32:197–204. doi: 10.1016/s0735-1097(98)00221-6. [DOI] [PubMed] [Google Scholar]
- 235.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, et al. Development of a risk score for atrial fibrillation (Framingham heart study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil. 2008;15:735–741. doi: 10.1097/HJR.0b013e328317f38a. [DOI] [PubMed] [Google Scholar]
- 237.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 238.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC., Jr Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 240.Zhang J, Youn JY, Kim AY, Ramirez RJ, Gao L, Ngo D, Chen P, Scovotti J, Mahajan A, Cai H. Nox4-dependent hydrogen peroxide overproduction in human atrial fibrillation and hl-1 atrial cells: relationship to hypertension. Front Physiol. 2012;3:140. doi: 10.3389/fphys.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U, Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 242.Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 243.Chang JP, Chen MC, Liu WH, Yang CH, Chen CJ, Chen YL, Pan KL, Tsai TH, Chang HW. Atrial myocardial nox2 containing NADPH oxidase activity contribution to oxidative stress in mitral regurgitation: potential mechanism for atrial remodeling. Cardiovasc Pathol. 2011;20:99–106. doi: 10.1016/j.carpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 244.Yeh YH, Kuo CT, Chan TH, Chang GJ, Qi XY, Tsai F, Nattel S, Chen WJ. Transforming growth factor-beta and oxidative stress mediate tachycardia-induced cellular remodelling in cultured atrial-derived myocytes. Cardiovasc Res. 2011;91:62–70. doi: 10.1093/cvr/cvr041. [DOI] [PubMed] [Google Scholar]
- 245.Adam O, Lavall D, Theobald K, Hohl M, Grube M, Ameling S, Sussman MA, Rosenkranz S, Kroemer HK, Schafers HJ, et al. Rac1-induced connective tissue growth factor regulates connexin 43 and n-cadherin expression in atrial fibrillation. J Am Coll Cardiol. 2010;55:469–480. doi: 10.1016/j.jacc.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 246.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 247.Streeter J, Thiel W, Brieger K, Miller FJ., Jr Opportunity Nox: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther. 2013;31:125–137. doi: 10.1111/j.1755-5922.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 248.Fan YF, Zhang R, Jiang X, Wen L, Wu DC, Liu D, Yuan P, Wang YL, Jing ZC. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res. 2013;99:395–403. doi: 10.1093/cvr/cvt109. [DOI] [PubMed] [Google Scholar]
- 249.Muzaffar S, Shukla N, Srivastava A, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate (NCX 911) are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary artery endothelial cells. Br J Pharmacol. 2005;146:109–117. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Juraschek SP, Guallar E, Appel LJ, Miller ER., III Effects of vitamin c supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079–1088. doi: 10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, Manson JE, Glynn RJ, Buring JE, Gaziano JM. Multivitamins in the prevention of cardiovascular disease in men: The physicians’ health study ii randomized controlled trial. JAMA. 2012;308:1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, Young IS. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (dapit): a randomised placebo-controlled trial. Lancet. 2010;376:259–266. doi: 10.1016/S0140-6736(10)60630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor Vas2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 254.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 256.Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol. 2012;19:752–763. doi: 10.1016/j.chembiol.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang Q, Zhou H, Gao H, Chen SH, Chu CH, Wilson B, Hong JS. Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J Neuroinflammation. 2012;9:32. doi: 10.1186/1742-2094-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cifuentes-Pagano E, Saha J, Csanyi G, Ghouleh IA, Sahoo S, Rodriguez A, Wipf P, Pagano PJ, Skoda EM. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox2) inhibitors. Medchemcomm. 2013;4:1085–1092. doi: 10.1039/C3MD00061C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 261.Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, et al. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual nox4/nox1 inhibitors. Bioorg Med Chem. 2011;19:6989–6999. doi: 10.1016/j.bmc.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 262.Shin YS, Song SJ, Kang SU, Hwang HS, Choi JW, Lee BH, Jung YS, Kim CH. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1h-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: in vitro and in vivo study. Neuroscience. 2013;232:1–12. doi: 10.1016/j.neuroscience.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 263.Kofler PA, Pircher H, von Grafenstein S, Diener T, Holl M, Liedl KR, Siems K, Jansen-Durr P. Characterisation of nox4 inhibitors from edible plants. Planta Med. 2013;79:244–252. doi: 10.1055/s-0032-1328129. [DOI] [PubMed] [Google Scholar]
- 264.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS. NADPH oxidases regulate cd44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J Biol Chem. 2010;285:26545–26557. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol. 2012;47:718–726. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2014;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 268.Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G, Pagano PJ. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem. 2013;288:36437–36450. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 b-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Csanyi G, Pagano PJ. Strategies aimed at Nox4 oxidase inhibition employing peptides from Nox4 b-loop and C-terminus and p22 (Phox) N-terminus: an elusive target. Int J Hypertens. 2013;2013:842827. doi: 10.1155/2013/842827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Agrawal U, Sharma R, Gupta M, Vyas SP. Is nanotechnology a boon for oral drug delivery? Drug Discov Today. 2014;19:1530–1546. doi: 10.1016/j.drudis.2014.04.011. [DOI] [PubMed] [Google Scholar]