Abstract
To determine whether one or more opioid receptor types might be preferentially involved in gliogenesis, primary mixed glial cultures derived from mouse cerebra were continuously treated with varying concentrations of opioid agonists selective for mu (μ) i.e., DAGO ([D-Ala2,MePhe4, Gly(ol)5]enkephalin), delta (δ) i.e., DPDPE ([D-Pen2,D-Pen5]enkephalin), or kappa (κ) i.e., U69,593 opioid receptor types. In addition, a group of cultures was treated with [Met5]enkephalin, an agonist for δ opioid receptors as well as putative zeta (ζ) opioid receptors. Opioid-dependent changes in growth were assessed by examining alterations in (1) the number of cells in mixed glial cultures at 3, 6, and 8 days in vitro (DIV), (2) [3H]thymidine incorporation by glial fibrillary acidic protein (GFAP) immunoreactive, flat (type 1) astrocytes at 6 DIV, and (3) the area and form factor of GFAP-immunoreactive, flat (type 1) astrocytes. DPDPE at 10−8 or 10−10 M, as well as [Met5]enkephalin at 10−6, 10−8 or 10−10 M, significantly reduced the total number of glial cells in culture; but this effect was not observed with DAGO or U69,593 (both at 10−6, 10−8 or 10−10 M). Equimolar concentrations (i.e., 10−6 M) of [Met5]enkephalin or U69,593, but not DPDPE or DAGO, suppressed the rate of [3H]thymidine incorporation by GFAP-immunoreactive, flat (type 1) astrocytes. DAGO had no effect on growth, although in previous studies morphine was found to inhibit glial numbers and astrocyte DNA synthesis. [Met5]enkephalin (10−6 M) was the only agonist to significantly influence astrocyte area. Collectively, these results indicate that δ (and perhaps μ) opioid receptor agonists reduce the total number of cells in mixed-glial cultures; while [Met5]enkephalin (and perhaps κ) responsive opioid receptors mediate DNA synthesis in astrocytes. This implies that δ opioid receptors, as well as [Met5]enkephalin-sensitive, non-δ opioid receptors mediate opioid-dependent regulation of astrocyte and astrocyte progenitor growth. These data support the concept that opioid-dependent changes in central nervous system growth are the result of endogenous opioid peptides acting through multiple opioid receptor types.
Keywords: Endogenous opioid systems, Astrocyte, Glial development, Cell proliferation, Opioid receptor types, Drug abuse
INTRODUCTION
There is considerable evidence that endogenous opioid systems (i.e., endogenous opioid peptides and opioid receptors) are involved in the regulation of neural growth in vivo (Vertes et al., 1982; Zagon and McLaughlin, 1983; Hauser et al., 1987, 1989; Schmahl et al., 1989) and in vitro (Ruffin et al., 1969; Vernadakis et al., 1982; Kornblum et al., 1987; Davila-Garcia and Azmitia, 1989; Zagon and McLaughlin, 1989; Stiene-Martin and Hauser, 1989, 1990). By manipulating the interaction between endogenous opioids and their receptors, the number and rate of differentiation of neural cells can be modified in vivo with opioid peptides acting as negative trophic regulators of growth (Zagon and McLaughlin, 1983; Hauser et al., 1987, 1989). More recently, a direct role for opioid systems as regulators of growth is revealed by in vitro studies demonstrating that endogenous opioids (Stiene-Martin and Hauser, 1990) and opiate drugs (Stiene-Martin, et al, 1991) can directly inhibit DNA synthesis by developing astrocytes.
Opioid action is mediated through multiple, functionally distinct opioid receptor types (see Martin et al., 1976; Gilbert and Martin, 1976; Goldstein, 1987). An unresolved question is which opioid receptor type(s) mediate(s) the neurodevelopmental actions of opioids. Mu (μ)-, delta (δ)-, and kappa (κ)- opioid binding sites are present early in rodent brain development (Spain et al., 1985; Kornblum et al., 1987; Ruis et al., 1991). Opiate-specific binding sites have also been found in chick embryonic brain both in culture (Gibson and Vernadakis, 1983) as well as in vivo (Geladopoulos et al., 1987). Reaggregate cultures from guinea pig cerebellum express multiple types of opioid sites at 0, 7, and 14 days in vitro suggesting that opioid receptors are expressed early and that expression of these sites can be sustained for at least two weeks in chemically defined media in vitro (Barg and Simantov, 1989). Zagon and co-workers initially reported the presence of an opioid binding site in tumor cell lines (Zagon et al., 1989) that preferentially binds [Met5]enkephalin (i.e., zeta [ζ] receptor). Moreover, an identical opioid site present in the developing rat and human cerebellum (Zagon et al., 1990a), is not detectable in the adult. The ζ opioid receptor is reported to have a pharmacological selectivity and specificity profile that is unique compared to μ, δ, and κ opioid receptors (Zagon et al., 1990a).
μ-Selective ligands have been reported to directly inhibit neuronal (pyramidal cell) growth in the rat cerebral cortex in vivo (Hammer et al., 1989; Ricalde and Hammer, 1990), to depress [3H]thymidine incorporation in rat brain in a naloxone reversible manner (Kornblum et al., 1987); increase ornithine decarboxylase in glial cell cultures (Vernadakis et al., 1982); and to suppress [3H]thymidine incorporation by cerebellar neural cells in reaggregate cultures (Barg et al., 1990). Conversely, other studies report direct opioid-dependent modulation of growth by prototypic ligands that are selective for different opioid receptor types. Based on correlative evidence that ζ opioid receptors are present in some developing tissues, and direct evidence that [Met5]enkephalin can inhibit the growth of S20Y murine neuroblastoma cells in culture, it has been inferred that ζ opioid receptors are preferentially involved in opioid-dependent growth (Zagon et al., 1990b). In addition to μ and ζ-selective opioid agents, substances selective for other opioid receptor types also have been reported to modify growth (Haynes, 1984).
In general, studies of opioid action on nervous system development have been contradictory (Ghadirian, 1969; Willson et al., 1976; Ilyinsky et al., 1987). Numerous side effects (e.g., opiates modify nutrition, respiration, and circulating hormone levels) make it difficult to assess the in vivo action of opiate drugs per se (Sparber and Lichtblau, 1983), and perhaps contribute to disparate results. In addition, brain region-specific critical periods of opioid responsiveness or vulnerability (Sakellaridis and Vernadakis, 1986; Hammer et al., 1989), as well as species (Pert et al., 1974; Mauer, 1982) and strain (Licata et al., 1990) differences in opiate responsiveness that are, as yet, poorly defined, may additionally contribute to conflicting results in vivo and in vitro. Because few reports have addressed the specific effects of selective opioid ligands on the growth of identified neurons or glia in primary cultures, we decided to assess the action of equimolar amounts of agonists prototypic for μ, δ and κ opioid receptors using a murine astrocyte culture model known to be developmentally sensitive to opioids (Stiene-Martin and Hauser, 1989; 1990). The experiments presented herein are part of a series of investigations which have simultaneously assessed the action of multiple opioid agonists, as well as an opiate drug with abuse liability (i.e., morphine) using glial cell growth itself as an end-point (Stiene-Martin et al., 1991). The studies presented herein test the hypothesis that opioid effects on glial cells are mediated by specific opioid receptors by using prototypic agonists selective for currently well established (i.e., μ, δ and κ) opioid receptor types. Because [Met5]enkephalin has a demonstrated growth inhibiting effect on astrocytes (Stiene-Martin and Hauser, 1990), it was included in several groups for direct comparison with other agonists with the awareness that [Met5]enkephalin may be acting on more than one opioid receptor type including putative ζ opioid receptor sites. The results provide evidence that opioid-dependent alterations in the developing nervous system are mediated by complex interrelationships between multiple opioid peptides and opioid receptor types.
MATERIALS AND METHODS
Cell Culture
Primary cultures of glial cells were obtained from 1-day-old ICR mice (Harlen Sprague-Dawley, Indianapolis, IN) as previously described (Stiene-Martin and Hauser, 1990) using modifications of initial glial isolation steps reported by McCarthy and de Vellis (1978). Briefly, using aseptic technique, mouse pups were decapitated, and the olfactory bulbs, cerebellum and meninges removed from each brain. The cerebral hemispheres were minced, and dissociated in 2.5% trypsin containing DNase (1 μg/ml) at 37°C by repeated agitation. Cell suspensions were centrifuged at 40 × g for 5 min and the pellets resuspended in 3–4 ml growth media containing Dulbecco's modified Eagle's medium (DMEM) with 0.5% glucose, 0.06% Na2CO3, and 10% fetal calf serum (FCS) (Hazelton, Lenexa, KS). The cell suspension was triturated to break up cell aggregates and filtered through Nitex 130 (Tetko Co., Elmsford, NY) to remove any remaining large clumps. The cell suspension was then centrifuged at 40 × g for 3 min and the pellet resuspended in 1 ml of growth media with 10% FCS. The cells were counted using a hemacytometer and were diluted to a density of approximately 5 × 105 cells/ml with growth media and FCS. For labeling index studies 16 mm glass coverslips coated with poly-L-lysine (40.75 kDa; Sigma, St Louis, MO) were placed into 22 mm wells and seeded with 1 ml of cell suspension. For absolute counts, 0.5 ml of cell suspension was delivered to 16 mm diameter culture wells (Primaria, Falcon, Oxnard, CA). Cultures were incubated at 34–34.5 °C in 5% CO2/95% air and high humidity.
The following opioid agonists were used in subsequent culture treatments: DAGO ([D-Ala2, MePhe4, Gly(ol)5] enkephalin) (Handa et al., 1981) and DPDPE ([D-Pen2, D-Pen5]enkephalin) (Mosberg et al, 1983) obtained from Sigma (St. Louis, MO); U69,593 (5a,7a,8b)-(−)-N-methyl-N-(7-(1-pyrrolidinyl)-l-oxaspiro (4.5)dec-8-yl)benzeneacetamide) (Lahti et al., 1985) a gift from the Upjohn Co., Kalamazoo, MI; [Met5]enkephalin (Tyr-Gly-Gly-Phe-Met) acquired from Peninsula Laboratories (Belmont, CA); and naloxone from E.I. Dupont, Wilmington, DE.
Two independent measures were used to assess glial growth in this culture system. First, total cell numbers were examined in mixed glial cultures. Total cell numbers are dependent on both the rate of cell proliferation and the rate of cell death. Second, to specifically assess the effects of selective opioid agonists on astrocyte DNA synthesis, [3H]thymidine incorporation was examined in glial fibrillary acidic protein (GFAP) immunoreactive astrocytes at 6 days in vitro. In these studies, [3H]thymidine was administered for a 16 h period from days 5 to 6 and labeling index assessed at 6 days in vitro, because previous studies indicated that (at least in the case of [Met5]enkephalin and morphine) astrocyte DNA synthesis is most reliably suppressed by opioids from 4 to 6 days in vitro (Stiene-Martin and Hauser, 1989, 1990; Stiene-Martin et al., 1991; Stiene-Martin and Hauser, unpublished).
Cell Counts
Methods for determining absolute cell counts have been previously reported (Stiene-Martin and Hauser, 1990). Briefly, 24 h after plating (day 1), growth media and unattached cells were removed from each culture well and replaced with either growth media plus 10% FCS serum alone (controls), or growth media containing 10% FCS plus either 10−6 M, 10−8 M or 10−10 M of the individual agonist drugs tested. Fresh medium was added to each culture well on alternate days. On days 3, 6, and 8, cells were released from groups of culture wells representing each treatment by trituration in the presence of 0.25% trypsin and 0.05% EDTA after 10 min incubation at 34.5°C. Counts were performed using a hemacytometer; microscopic examination verified that over 99% of cells were removed by this treatment.
Combined [3H]Thymidine Autoradiography and GFAP Immunocytochemistry
Twenty four hours after plating (day 1), growth media and unattached cells were removed from each well (12 wells per group) and replaced with growth media containing 10−6 M of agonists selective for μ (DAGO), δ (DPDPE), κ (U69,593), or δ and ζ ([Met5]enkephalin) opioid receptor types. Another set of culture groups was treated with each of the preceding opioid agonists plus 3 × 10−6 M naloxone. In addition, a control group was established and treated with growth media alone, and one group received 3 × 10−6 M naloxone alone.
Combined [3H]thymidine autoradiography and GFAP immunocytochemistry were performed as previously described (Stiene-Martin and Hauser, 1990). Briefly, 0.24 μCi/ml (6.7 Ci/mM) of [3H]thymidine (ICN Radiochemicals, Irvine, CA) was added to each culture well on the evening of the 5th day in culture for a total of 16 h. On in vitro day 6, radioactive media were removed and the cultures were washed twice with cold DMEM, fixed in Zamboni's fixative containing 3% paraformaldehyde for 1 h followed by 5 rinses in cold PBS. To identify astrocytes, coverslips were stained immunocytochemically for the astrocyte marker, GFAP, using a primary anti-GFAP polyclonal antibody (Bio-Genex Laboratories, Dublin, CA) and a VectastainR-ABC kit (Vector Laboratories, Burlingame, CA). Diaminobenzidine 4-HCl (Sigma, St Louis, MO) was used to visualize the reaction. The coverslips were then dipped in NTB-2 emulsion (Kodak) and exposed for 4 weeks at 4 °C. After development in D-19 (Kodak) for 5 min at 12 °C, the coverslips were counterstained with Ehrlich's hematoxylin.
Labeling Index
At least 500 GFAP-positive astrocytes were counted per culture well (N = 12 wells/group) as previously described (Stiene-Martin and Hauser, 1990). Based on morphology, GFAP-immunoreactive astrocytes were categorized as being either (i) flat and polygonal (i.e., morphologically similar to A2B5-/GFAP+ type 1 astrocytes, or (ii) process-bearing (i.e., morphologically similar to A2B5+/GFAP+ type 2 astrocytes (see Raff et al., 1983; Miller et al., 1985). Independent analyses were performed for each astrocyte type with an "unbiased" observer unaware of which group was being sampled. Nuclei with a density of autoradiographic grains greater than 5× background levels (typically 10 or more) were considered to be positively labeled with [3H]thymidine. Using these criteria and assuming a Poisson distribution for autoradiographic grains/cell, the probability of falsely counting a cell with a high background signal is less than 0.001 (Arnold, 1981). The labeling index was calculated for both flat-polygonal, and process-bearing astrocytes. The labeling index was defined as the number of [3H]thymidine labeled cells divided by the total number of labeled plus unlabeled cells for a given morphologic type. All cell counts were performed using a Leitz microscope (40×, 0.65 NA objective).
Morphometry
The cytoplasmic areas of randomly selected single, flat, GFAP-positive cells for each treatment group (12–15 cells/4–6 culture wells/group) were outlined using a cursor-guided digitizing tablet attached to a computerized video imaging system (MicroComp Software; Southern Micro Instruments, Atlanta, GA) connected to a Leitz microscope. The system was calibrated and the cells outlined using a 40× objective. For each cell, perimeter, area (μm2), and "form factor" were determined. Form factor is an index of the number of processes of a cell and is defined as (4[π])(area)/perimeter2) (MicroComp Software).
Statistics
Data were reported as the mean ± the standard error of the mean. Overall differences due to experimental treatments were tested using analysis of variance (ANOVA) and subsequent (post hoc) comparisons were made using Newman-Keuls test (Statistica; StatSoft, Tulsa, OK). Differences were considered significant if P < 0.05. For clarity, data for individual agonists were presented separately. All experiments were independently replicated and representative examples reported.
RESULTS
Cell Numbers
Mu (μ) opioid receptors
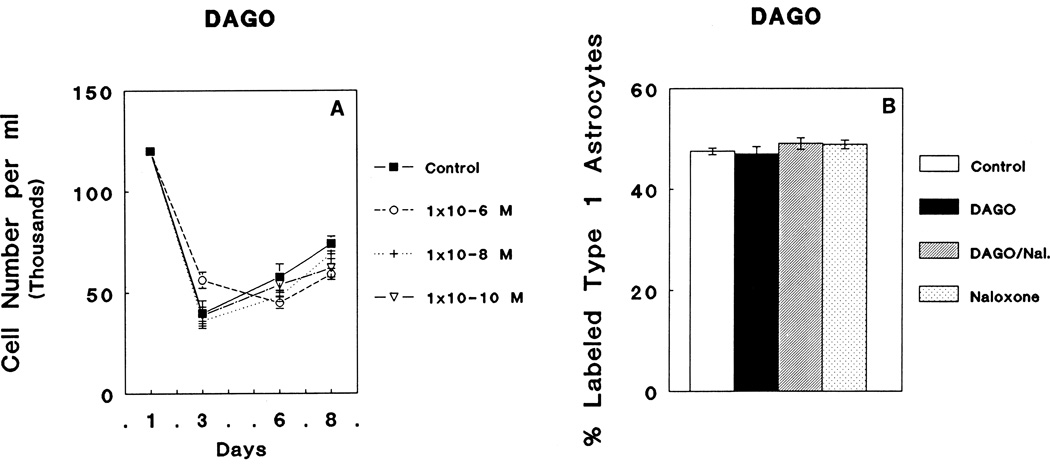
DAGO treatment 10−6 M, 10−8 M, or 10−10 M concentrations had no effect on the total numbers of cells in mixed-glial cultures at 3, 6, or 8 days in vitro (DIV) compared to controls (Fig. 1A). Moreover, treatment with 10−6 M DAGO, 10−6 M DAGO combined with 3 × 10−6 M naloxone, or 3 × 10−6 M naloxone did not influence the rate of [3H]thymidine incorporation by GFAP-immunoreactive flat, polygonal (type 1) astrocytes compared to control levels (Fig. 1B).
Fig. 1.
A: Continuous exposure to varying concentrations (10−6 M, 10−8 M, or 10−10 M) of the μ opioid receptor agonist, DAGO, did not affect the total number of cells in mixed-glial cultures at 3, 6 and 8 days in vitro (DIV). The mean baseline cell number for all cultures prior to the onset of treatment is indicated at day 1. The decrease in cell numbers between 1 and 3 DIV reflects, in part, the death of neurons as well as the loss of some cells that do not incompletely attach to the surface of the culture well. B: DAGO (10−6 M) did not influence the rate of incorporation of [3H]thymidine by glial fibrillary acidic protein- (GFAP) immunoreactive flat (type 1) astrocytes; 3 × 10−6 M Naloxone (Nal.).
Delta (δ) opioid receptors
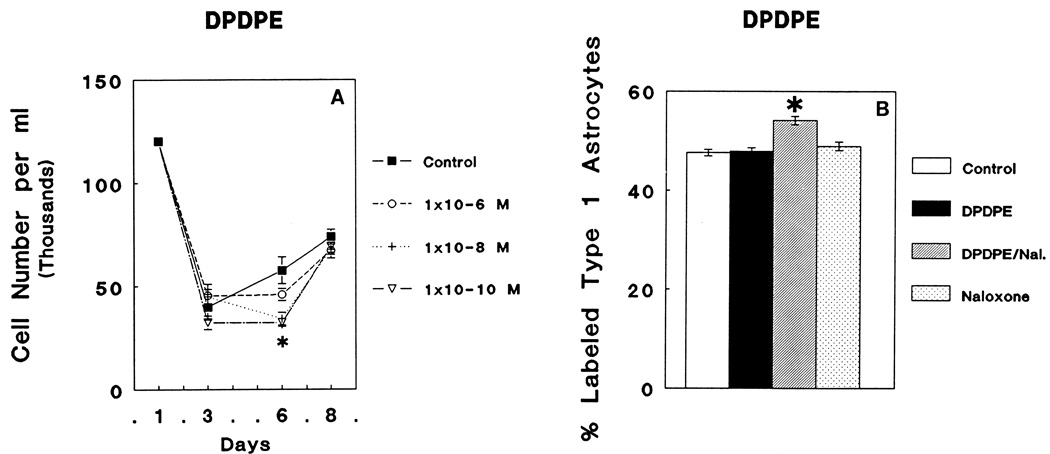
There was a significant overall decline in the total number of cells present in mixed-glial cultures caused by treatment with 10−6 M, 10−8 M, or 10−10 M of DPDPE (ANOVA; F3,81 = 5.28; P < 0.003). At 6 DIV, significant decreases in total cell numbers were elicited by 10−8 or 10−10, but not 10−6 M concentrations of DPDPE compared to control values (Newman-Keuls; P < 0.05) (Fig. 2A). There were significant overall treatment effects on the rate of [3H]thymidine incorporation by GFAP-immunoreactive flat, polygonal (type 1) astrocytes (ANOVA: F3,42 = 16.11; P < 0.01). Subsequent comparisons showed that either 10−6 M DPDPE treatment or 3 × 10−6 M naloxone treatments alone did not influence [3H]Thymidine incorporation by astrocytes compared to controls. However, a significant 13% increase in [3H]thymidine incorporation compared to controls (Newman-Keuls; P < 0.05) was observed at day 6 in GFAP-immunoreactive flat, polygonal (type 1) astrocytes treated with 10−6 M DPDPE plus 3 × 10−6 M naloxone (Fig. 2B).
Fig. 2.
A: Continuous exposure to varying concentrations (10−6 M, 10−8 M, or 10−10 M) of the δ opioid receptor agonist, DPDPE, caused marked differences in the total number of cells in mixed-glial cultures (ANOVA; F3,81 = 5.28; P < 0.003). Post hoc comparisons using Newman-Keuls test revealed that the number of cells in mixed glial cultures were significantly decreased by 10−8 M or 10−10 M DPDPE at day 6 compared to control values (*P < 0.05) (2A). At 8 days in vitro (DIV), DPDPE treated groups were similar to control values; net increases in cell numbers in mixed glial cultures become smaller as these cells become confluent. B: Neither DPDPE (10−6 M) nor naloxone (Nal.; 3 × 10−6 M) treatments alone suppressed [3H]thymidine incorporation in glial fibrillary acidic protein- (GFAP) immunoreactive, type 1 astrocytes at 6 DIV. However, there was an increase in the proportion of astrocytes incorporating [3H]thymidine compared to controls (Newman-Keuls; *P < 0.05) when treated with both 10−6 M DPDPE and 3 × 10−6 M naloxone.
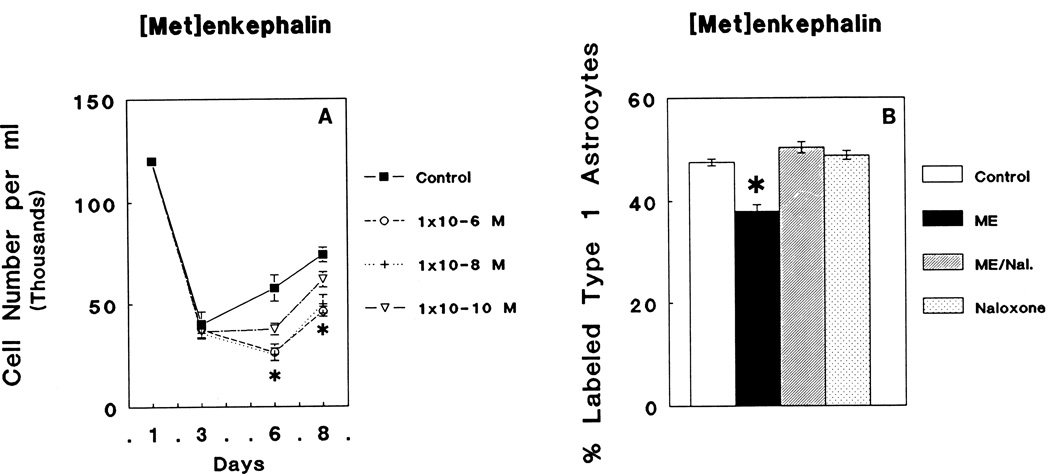
[Met5]enkephalin treatment caused an overall reduction in mixed-glial cell numbers (ANOVA; F3,80 = 16.06; P < 0.05.) (Fig. 3A). [Met5]enkephalin-dependent suppression of cell numbers was dose-dependent with 10−6 M (45% of controls at day 6 and 62% of controls at day 8) or 10−8 M (44% of control on day 6 and 60% of control on day 8) yielding the greatest amount of growth inhibition, while 10−10 M [Met5]enkephalin treatment resulted in intermediate values (65% of controls at day 6 and 70% of controls at day 8) (Newman-Keuls significance in all of the above cases; P < 0.05). At 6 days in vitro, there were significant overall treatment effects on [3H]thymidine incorporation by GFAP-immunoreactive flat, polygonal (type 1) astrocytes (ANOVA: F3,44 = 26.3; P < 0.05). When individual groups were compared, 10−6 M [Met5]enkephalin-treated GFAP-immunoreactive flat, polygonal (type 1) astrocytes displayed a marked 19% decline in the rate [3H]thymidine incorporation compared to control levels (Fig 3B) (Newman-Keuls; P < 0.05). [Met5]enkephalin-dependent changes were prevented by concomitant treatment with 3 × 10−6 M naloxone, whereas naloxone (3 × 10−6 M) treatment alone had no effect on [3H]thymidine incorporation by astrocytes compared with control values.
Fig. 3.
A: Continuous exposure to varying concentrations (10−6 M, 10−8 M, or 10−10 M) of δ and ζ opioid receptor agonist, [Met5]enkephalin, altered the total number of cells in mixed-glial cultures (ANOVA; F3,80 = 5.49; P < 0.002). Significant decreases in cell numbers occurred at 6 and 8 DIV as a result of treatment with all three [Met5]enkephalin concentrations (Newman-Keuls; *P < 0.05). B: [Met5]enkephalin (ME) treatment caused a significant suppression in the rate [3H]thymidine incorporation by glial fibrillary acidic protein- (GFAP) immunoreactive, flat (type 1) astrocytes compared to controls (Newman-Keuls; *P < 0.05) that was prevented by treatment with 3 × 10−6 M naloxone (Nal.).
Kappa (κ) opioid receptors
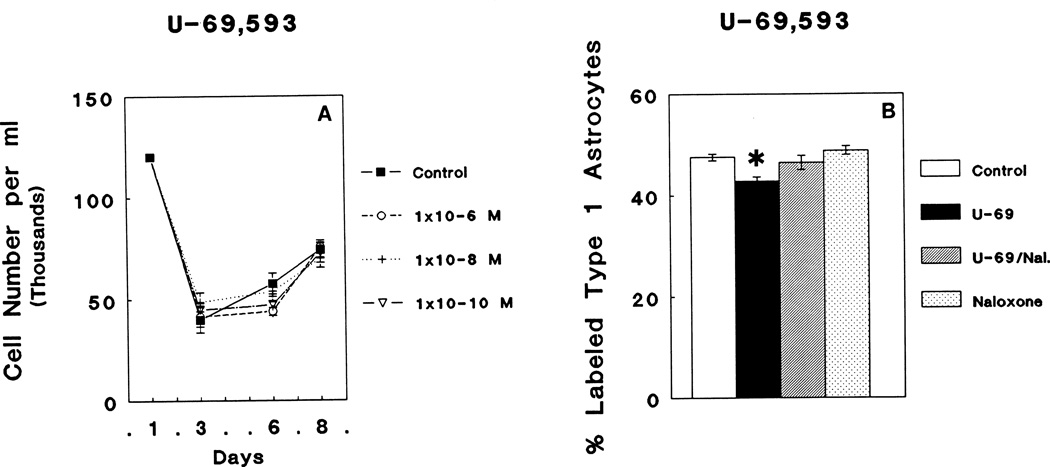
Treatment with 10−6 M, 10−8 M, or 10−10 M U69,593 did not alter the total number of cells in mixed-glial cultures at 3, 6, or 8 days in culture (Fig. 4A). However, examination of GFAP-immunoreactive flat, polygonal (type 1) astrocytes continuously exposed to 10−6 M U69,593, 10−6 M U69,593 plus 3 × 10−6 M naloxone, or 3 × 10−6 M naloxone alone demonstrated a marginally significant alteration in [3H]thymidine incorporation at 6 DIV (ANOVA; F3,44 = 6.95; P < 0.05). Post hoc comparisons revealed a significant reduction in [3H]thymidine incorporation in astrocytes (Newman Keuls; P < 0.05) in response to 10−6 M U69,593 treatment compared to control values. The reduction in [3H]thymidine incorporation in astrocytes by 10−6 M U69,593 treatment was prevented by the simultaneous addition of 3 × 10−6 M naloxone; 3 × 10−6 M naloxone alone had no effect (Fig. 4B).
Fig. 4.
A: Continuous exposure to varying concentrations (10−6 M, 10−8 M, or 10−10 M) of the κ opioid receptor agonist, U69,593 (U-69), did not affect the total number of cells in mixed glial cultures compared to control values. B: However, 10−6 M U69,593 caused a significant decrease in [3H]thymidine incorporation by glial fibrillary acidic protein- (GFAP) immunoreactive, flat (type 1) astrocytes when compared to controls (Newman-Keuls; *P < 0.05) that was prevented by 3 × 10−6 M naloxone (Nal.).
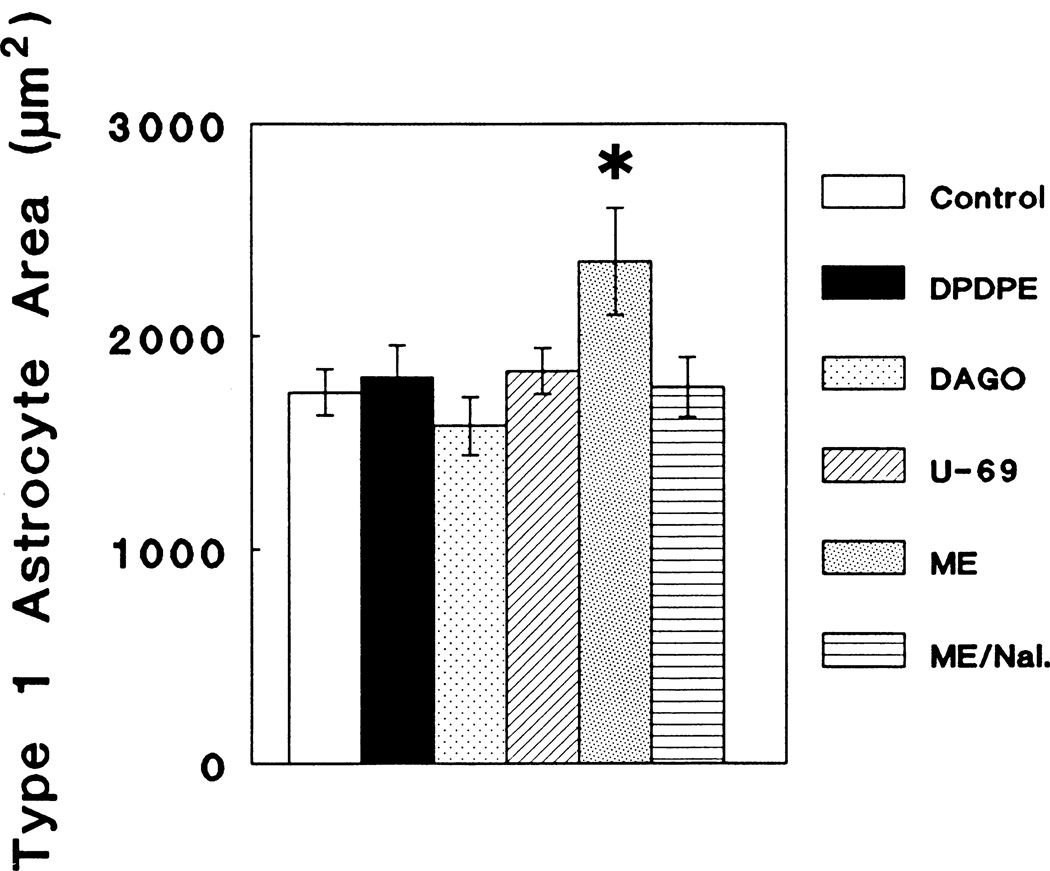
Astrocyte Area
An assessment of the area of individual astrocytes following continuous exposure to opioid agonists selective for individual opioid receptor types resulted in a significant overall change in the area of GFAP-immunoreactive, flat (type 1) astrocytes at 6 DIV compared to controls (ANOVA; F5,46 = 3.29; P < 0.05), that was attributable to 10−6 M [Met5]enkephalin treatment (Newman-Keuls; P < 0.05) (Fig. 5). Neither 10−6 M DPDPE, DAGO nor U69,593 treatment had any effect on astrocyte area. No differences were noted in the form factor parameter as a result of treatment with 10−6 M concentrations of either DPDPE, DAGO, U69,593 or [Met5]enkephalin.
Fig. 5.
Continuous exposure to 10−6 M [Met5]enkephalin (ME) caused a significant increase in the area of glial fibrillary acidic protein- (GFAP) immunoreactive, flat (type 1) astrocytes when compared to controls at 6 days in vitro (Newman-Keuls; *P < 0.05) that was prevented by the simultaneous treatment with 3 × 10−6 naloxone (Nal.). Identical concentrations (10−6 M) of the selective agonists DAGO, DPDPE, and U69,593 (U-69) did not alter astrocyte area.
DISCUSSION
The results indicate that opioid-dependent alterations in glial cell growth are mediated by multiple, yet selective opioid receptor-effector coupling mechanisms. Our discussion will address each of the opioid receptor ligands examined in this study (DAGO, DPDPE, U69,593 and [Met5]enkephalin) with respect to their effects on glial growth.
Our findings that the μ selective opioid agonist DAGO had no effect on glial growth were somewhat unexpected since previous findings indicated that morphine can inhibit astrocyte growth in culture (Stiene-Martin et al., 1991), and both substances are reported to preferentially bind μ opioid receptors. One explanation might be that a μ opioid receptor subtype (i.e., μ-1 or μ-2) is involved in growth and that DAGO and morphine bind different μ subtypes. For example, there is evidence that β-funaltrexamine-sensitive μ opioid receptors (believed to represent the μ-2 subtype) are not involved in growth in vivo (Zagon and McLaughlin, 1986). Reports from other laboratories, however, argue against this explanation. Morphine, which affects growth in vivo (Loughlin et al., 1985; Kornblum et al., 1987; Hammer et al., 1989) and in vitro (Ruffin et al., 1969; Sakellaridis and Vernadakis, 1986; Stiene-Martin et al., 1991), binds both μ-1 and μ-2 opioid receptor subtypes (Spiegel et al., 1982). DAGO also acts as an agonist at both μ-1 and μ-2 opioid sites (Reviewed by Wollemann, 1990); therefore, it would appear unlikely that μ opioid receptor subtypes explain the differences between DAGO and morphine. A second explanation is that morphine is not acting via μ opioid receptors. Studies on the selectivity of DPDPE for the δ opioid site have shown DAGO, morphine, and normorphine to have high affinity for a portion of the δ opioid receptor (Cotton, et al., 1985). To add to the complexity, in some systems δ and μ opioid receptor types do not function independently; these two receptor types are reported to interact to generate novel effects (Rothman and Westfall, 1983; Schoffelmeer et al., 1989; Heyman et al., 1989; Rothman et al., 1991) and may, in fact, be products of the same gene (Cabon et al., 1988). Thus, because DAGO had no noticeable effect on glial growth, morphine's ability to inhibit glial growth is likely to be mediated by a non-μ opioid receptor type.
δ Opioid agonists modify some aspects of glial growth. DPDPE, at concentrations of 10−8 M or 10−10 M significantly reduced the number of cells present in mixed glial cultures. This suggests that δ opioid receptors influence the net production of immature glia which consist primarily of astrocytes and astrocyte progenitors. However, 10−6 M concentrations of the δ-selective agonist DPDPE (Goldstein, 1989; Iyengar and Wood, 1990), had no effect on the rate of [3H]thymidine incorporation or area of astrocytes. Conversely, equimolar concentrations of [Met5]enkephalin, a less selective and lower affinity δ opioid agonist than DPDPE, significantly alters both these parameters. One explanation is that 10−6 M is an inappropriate concentration of DPDPE, since 10−8 and 10−10 M concentrations resulted in more pronounced reductions in glial cell numbers. Such "U-shaped" dose-response curves have been previously described for DPDPE action on learning and memory (Schulteis et al., 1988), and further assessment of astrocyte growth in response to 10−8 M and 10−10 M DPDPE is warranted. A second explanation might be that DPDPE and [Met5]enkephalin are acting through a non-traditional opioid binding site such as the reported μ-δ receptor complex mentioned above. It is of interest that a recent report indicated that DPDPE has a high affinity interaction with a receptor complex such as the μ-δ complex in vitro (Rothman et al., 1991). Further evidence that δ opioid receptors per se are not involved in the opioid-dependent growth effects found in this study is the fact that δ opioid receptors are not reported to be present during early postnatal development in rodents (however, see Zagon et al., 1990a). In rats, δ opioid receptors were first detected 10 – 14 days after birth (Spain et al., 1985; McDowell and Kitchen, 1986; Kornblum et al., 1987). In mice, δ opioid receptors were undetectable at postnatal day 3, and constituted only 25% of the total opioid binding by postnatal day 15 (Tavani et a., 1985). Because our cultures were derived from 1-day-old ICR mice and maintained in vitro for 6 days, it is improbable that δ opioid receptors were present unless opioid receptor expression is altered as an artifact of our culture conditions.
The effects of DPDPE on [3H]thymidine incorporation by astrocytes underscore the fact that the current understanding of opioid receptor types and subtypes is incomplete. When DPDPE was combined with naloxone, there was a significant (Newman-Keuls; p < 0.05) and reproducible increase in [3H]thymidine incorporation by astrocytes, yet neither DPDPE nor naloxone alone had any measurable influence on this parameter. Perhaps continuous exposure to opioid agonists and/or antagonists (as occurred in this study) elicits complex patterns of up- or down- regulation of selective opioid receptor types and/or confers some agonist properties to the antagonist, naloxone (Barg et al., 1989).
[Met5]enkephalin sensitive, non-δ opioid receptors mediate aspects of glial growth. Some enkephalin analogues (e.g., D-Ala5-D-Leu5-enkephalin [DADLE]) have affinity for both μ-2 and δ opioid receptors (Iyengar and Wood, 1990). However, the lack of response to DAGO treatment, as well as the observed reduction in glial cell numbers in the presence of low concentrations (e.g., 10−10 M) of [Met5]enkephalin which is well below its Kd for μ-opioid receptors, suggests that μ opioid receptor subtypes are not involved. Could [Met5]enkephalin action be mediated by ζ opioid receptors? As mentioned, a novel ζ opioid receptor type has been reported in the developing nervous system that has high affinity for [Met5]enkephalin but that does not appreciably bind DPDPE (Zagon et al., 1989; 1990b). Because both DPDPE and [Met5]enkephalin yielded similar growth effects at low concentrations, there is no compelling reason to assume that ζ receptors influence glial numbers based on the cell count data alone. However, findings that [Met5]enkephalin suppressed [3H]thymidine incorporation by astrocytes; whereas DPDPE did not alter this parameter, are compatible with the concept of a ζ opioid receptor.
[Met5]enkephalin was the only opioid agonist in this study to cause increases in the cytoplasmic area of astrocytes. Moreover, increased area was not associated with a concomitant change in form factor as previously observed in astrocytes treated with 10−6 M morphine. One explanation is that the increased cell area may reflect astrocyte swelling (Kimelberg and Ransom, 1986) in response to opioids. For example, binding of opioid receptor(s) may result in altered ion fluxes and consequent osmotic swelling. An alternate explanation is that astrocytes tend to be more spherical when proliferating compared to non-replicating astrocytes. Because opioids are presumed to inhibit cellular proliferation (Stiene-Martin and Hauser, 1990), fewer astrocytes are likely to be round.
The κ agonist, U69,593 caused a marginally significant suppression of [3H]thymidine incorporation by astrocytes despite findings that U69,593 had no effect on cell numbers. One explanation might be that κ receptors mediate growth effects in a small population of astrocytes that was not detectable by total cell counts of mixed glial-cell cultures. It is also of interest to note that κ agonists have been reported to act as antagonists to both the μ and δ receptors in a variety of tissues (Iyengar and Wood, 1990).
In summary, based on current knowledge of opioid receptor selectivity, no single opioid type can sufficiently account for all of our results. Collectively, the present results suggest that agonists selective for δ and ζ and perhaps κ opioid receptors can all modify some aspects of glial growth. This is in agreement with recent observations that δ and κ, but not μ opioid receptor agonists cause a dose-dependent reduction in forskolin stimulated cAMP formation by rat astrocytes in vitro (Eriksson et al., 1990). Thus, the present findings provide new insights concerning the role of endogenous opioid systems in the regulation of growth because they suggest for the first time that multiple opioid receptor types mediate the transduction of opioid-dependent signals affecting glial growth. Thus, critical periods of opioid sensitivity in the developing nervous system are likely to be defined by the differential expression of multiple opioid receptor types. Understanding the details of these interactions will be of great importance toward understanding the role of endogenous opioid systems in nervous system maturation.
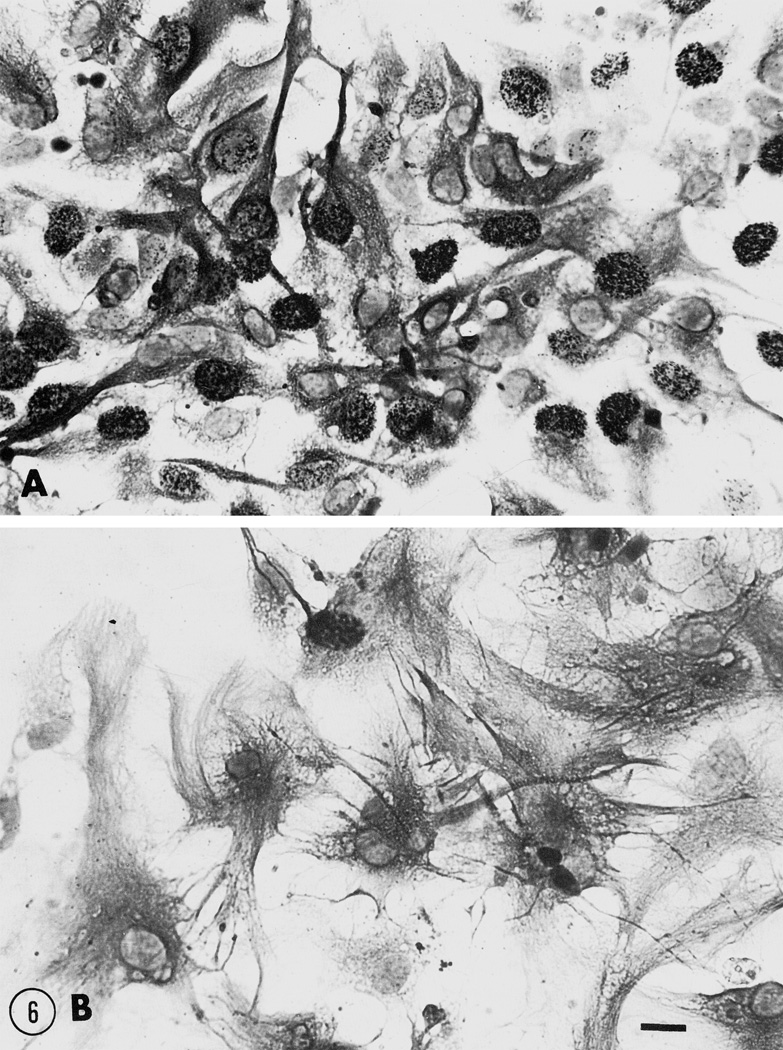
Fig. 6.
Brightfield photomicrographs of cultured glial fibrillary acidic protein- (GFAP) immunoreactive, flat (type 1) astrocytes at 6 DIV following continuous exposure to (A) growth media alone or (B) to growth media supplemented with 10−6 M [Met5]enkephalin. [Met5]enkephalin caused a marked increase in astrocyte area when compared to control cultures (see Fig. 5 for a quantitative assessment of opioid-dependent changes in astrocyte area). Scale bar = 20 μm.
Acknowledgments
The authors wish to thank Drs. William Bartlett, Narayan Bhat, William R. Martin, Adrienne Salm, and Harold Traurig for expert advice and comments; Dr. John Porter for the use of the image analysis system; and Dr. M.B. Nikitovitch-Winer for continued support and encouragement. Supported by a grant from the National Institute on Drug Abuse (DA 06204).
REFERENCES
- Arnold AP. Quantitative analysis of steroid autoradiograms. J Histochem Cytochem. 1981;29:207–211. [PubMed] [Google Scholar]
- Barg J, Levy R, Simantov R. Paradoxical and subtype-specific effects of opiate antagonists on the expression of opioid receptors in rat brain cultures. J Neurosci Res. 1989;22:322–330. doi: 10.1002/jnr.490220312. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva M, Coscia CJ. Regulation of 3H-thymidine incorporation by opioids in rat brain aggregating cultures. Soc Neurosci Abstr. 1990;16:367. [Google Scholar]
- Cabon F, Cupo A, Baumann N, Zalc B. Mu and delta opioid receptors are the products of the same gene. Int Narcotics Res Conf Abstr. 1988:15. [Google Scholar]
- Cotton R, Kosterlitz HW, Paterson SJ, Rance MJ, Traynor JR. The use of [3H]-[D-Pen2,D-Pen5]enkephalin as a highly selective ligand for the δ-binding site. Br J Pharmacol. 1985;84:927–932. doi: 10.1111/j.1476-5381.1985.tb17387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila-Garcia MI, Azmitia EC. Effects of acute and chronic administration of Leu-enkephalin on cultured serotonergic neurons: evidence for opioids as inhibitory neuronal growth factors. Dev Brain Res. 1989;49:97–103. doi: 10.1016/0165-3806(89)90062-x. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Ronnback L. δ and κ Opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res. 1990;15:1123–1126. doi: 10.1007/BF01101714. [DOI] [PubMed] [Google Scholar]
- Geladopoulos T, Sakellaridis N, Vernadakis A. Differential maturation of μ and δ opioid receptors in the chick embryonic brain. Neurochem Res. 1987;12:279–288. doi: 10.1007/BF00972138. [DOI] [PubMed] [Google Scholar]
- Ghadirian A. A tissue culture study of morphine dependence on the mammalian CNS. Can Psychiatr Ass J. 1969;14:607–615. doi: 10.1177/070674376901400609. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Vernadakis A. Effects of N-LAAM on [3H]etorphine binding in neuronal-enriched cell cultures. Neurochem Res. 1983;8:1197–1202. doi: 10.1007/BF00964933. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Martin WR. The effects of morphine- and nalorphine-like drugs in the nondependent, morphine-dependent and cyclazocine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;198:66–82. [PubMed] [Google Scholar]
- Goldstein A. Binding selectivity profiles for ligands of multiple receptor types: focus on opioid receptors. Trends Pharmacol Sci. 1987;8:456–459. [Google Scholar]
- Hammer RP, Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology. 1989;10:475–484. [PubMed] [Google Scholar]
- Handa B, Lane AC, Lord JAH, Morgan BA, Rance MJ, Smith CFC. Analogues of β-LPH61–64 possessing selective agonist activity at μ-opiate receptors. Eur J Pharmacol. 1981;70:531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987;416:157–161. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J Comp Neurol. 1989;281:13–22. doi: 10.1002/cne.902810103. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LW. Opioid systems and the developing brain. Trends Pharmacol Sci. 1984;5:453–454. [Google Scholar]
- Heyman JD, Vaught JL, Mosberg HI, Haaseth RC, Porreca F. Modulation of μ-mediated antinociception by δ agonists in the mouse: selective potentiation of morphine and normorphine by [D-Pen2,D-Pen5]enkephalin. Eur J Pharmacol. 1989;165:1–10. doi: 10.1016/0014-2999(89)90764-4. [DOI] [PubMed] [Google Scholar]
- Ilyinsky OB, Kozlova MV, Kondrikova ES, Kalentchuk VU, Titov MI, Bespalova ZdD. Effects of opioid peptides and naloxone on nervous tissue in culture. Neurosci. 1987;22:719–735. doi: 10.1016/0306-4522(87)90368-x. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Wood PL. Multiple opiate receptors: functional definitions. In: Szekely JI, Ramabadran K, editors. Opioid Peptides. IV. Boston: CRC Press; 1990. pp. 115–132. [Google Scholar]
- Kimelberg HK, Ransom BR. Physiological and pathological aspects of astrocytic swelling. In: Fedoroff S, Vernadakis A, editors. Astrocytes, Cell Biology and Pathology of Astrocytes. Vol. 3. New York: Academic Press; 1986. pp. 129–166. [Google Scholar]
- Kornblum HI, Loughlin SE, Leslie FM. Effects of morphine on DNA synthesis in neonatal rat brain. Dev Brain Res. 1987a;31:45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Hurlbut DE, Leslie FM. Postnatal development of multiple opioid receptors in rat brain. Dev Brain Res. 1987b;37:21–41. doi: 10.1016/0165-3806(87)90226-4. [DOI] [PubMed] [Google Scholar]
- Lahti RA, Mickelson MM, McCall JM, von Voigtlander PF. [3H]U-69,593 a highly selective ligand for the opioid κ receptor. Eur J Pharmacol. 1985;109:281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Licata SP, Nalwal JW, Hough LB. Actions of morphine on histamine dynamics in the mouse brain: a strain comparison. Biochem Pharmacol. 1990;39:978–981. doi: 10.1016/0006-2952(90)90219-b. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Massamiri TR, Kornblum HI, Leslie FM. Postnatal development of opioid systems in rat brain. Neuropeptides. 1985;5:469–472. doi: 10.1016/0143-4179(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effect of morphine and nalorphine-like drugs in the non-dependent and morphine-chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Mauer R. Multiplicity of opiate receptors in different species. Neurosci Lett. 1982;30:303–307. doi: 10.1016/0304-3940(82)90417-7. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. α-Adrenergic receptor modulation of beta-adrenergic, adenosine, and prostaglandin E1 increased adenosine 3';5'-cyclic monophosphate levels in primary cultures of astrocytes. J Cyc Nucl Res. 1978;4:15–36. [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Ontogenesis of δ opioid receptors in rat brain using [3H][D-Pen2-D-Pen5] enkephalin as a binding ligand. Eur J Pharmacol. 1986;128:287–293. doi: 10.1016/0014-2999(86)90780-6. [DOI] [PubMed] [Google Scholar]
- Miller RH, David S, Patel R, Abney ER, Raff MC. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev Biol. 1985;111:35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HJ, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci (USA) 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Aposhian D, Snyder SH. Phylogenetic distribution of opiate receptor binding. Brain Res. 1974;75:356–361. doi: 10.1016/0006-8993(74)90761-6. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in culture of developing rat white matter: differences in morphology, surface gangliosides and growth characteristics. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricalde AA, Hammer PR., Jr Perinatal opiate treatment delays growth of cortical dendrites. Neurosci Lett. 1990;15:137–143. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Westfall TC. Further evidence for an opioid receptor complex. J Neurobiol. 1983;14:341–345. doi: 10.1002/neu.480140502. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Mahboubi A, Bykov V, Kim CH, Jacobson AE, Rice KC. Probing the opioid receptor complex with (+)-trans-superfit. I. Evidence that [D-Pen2,D-Pen5]enkephalin interacts with high affinity at the δcx binding site. Peptides. 1991;12:359–364. doi: 10.1016/0196-9781(91)90026-l. [DOI] [PubMed] [Google Scholar]
- Ruffin NE, Reed BL, Finnin BC. Effects of morphine withdrawal on cells in continuous culture. Life Sci. 1969;8:671–675. doi: 10.1016/0024-3205(69)90001-0. [DOI] [PubMed] [Google Scholar]
- Ruis RA, Barg J, Bem WT, Coscia CJ, Loh YP. The prenatal development profile of opioid peptides and receptors of the mouse brain. Dev Brain Res. 1991;58:237–241. doi: 10.1016/0165-3806(91)90010-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellaridis N, Vernadakis A. An unconventional response of adenylate cyclase to morphine and naloxone in the chicken during early development. Proc Natl Acad Sci (USA) 1986;83:2738–2742. doi: 10.1073/pnas.83.8.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl W, Funk R, Miaskowski U, Plendl J. Long-lasting effects of naltrexone, an opioid receptor antagonist, on cell proliferation in developing rat forebrain. Brain Res. 1989;486:297–300. doi: 10.1016/0006-8993(89)90515-5. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, Hansen HA, Stoof JC, Mulder AH. Blockade of D2 dopamine receptors strongly enhances the potency of enkephalins to inhibit dopamine-sensitive adenylate cyclase in rat neostriatum: involvement of delta- and mu-opioid receptors. J Neurosci. 1986;6:2235–2239. doi: 10.1523/JNEUROSCI.06-08-02235.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Martinez JL, Hruby VJ. Stimulation and antagonism of opioid delta receptors produce opposite effects on active avoidance conditioning in mice. Behav Neurosci. 1988;102:678–688. doi: 10.1037//0735-7044.102.5.678. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta and kappa) J Neurosci. 1985;5:585–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber SB, Lichtblau L. Neonatal undernutrition alters responsiveness to morphine in mature rats: a possible source of epiphenomena in developmental drug studies. J Pharmacol Exp Ther. 1983;225:1–7. [PubMed] [Google Scholar]
- Spiegel K, Kourides IA, Pasternak GW. Different receptors mediate morphine-induced prolactin and growth hormone release. Life Sci. 1982;31:2177–2180. doi: 10.1016/0024-3205(82)90112-6. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Opioids directly modulate the growth of mixed-glial cultures: suppression of astrocyte proliferation by Met-enkephalin. Soc Neurosci Abstr. 1989;15:278. [Google Scholar]
- Stiene-Martin A, Hauser KF. Opioid-dependent growth of glial cultures: suppression of astrocyte DNA synthesis by Met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: evidence for direct effect of opiates on neural maturation. Dev Brain Res. 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavani A, Robson LE, Kosterlitz HW. Differential postnatal development of mu, delta and kappa-opioid binding sites in mouse brain. Dev Brain Res. 1985;23:306–309. doi: 10.1016/0165-3806(85)90056-2. [DOI] [PubMed] [Google Scholar]
- Vernadakis A, Estin C, Gibson DA, Amott S. Effects of methadone on ornithine decarboxylase and cyclic nucleotide phosphohydrolase in neuronal and glial cell cultures. J Neurosci Res. 1982;7:111–117. doi: 10.1002/jnr.490070203. [DOI] [PubMed] [Google Scholar]
- Vertes Z, Melegh G, Vertes M, Kovacs S. Effect of naloxone and D-Met2-Pro5-enkephalinamide treatment on the DNA synthesis in the developing rat brain. Life Sci. 1982;31:119–126. doi: 10.1016/0024-3205(82)90423-4. [DOI] [PubMed] [Google Scholar]
- Willson NJ, Schneider JF, Roizin L, Fleiss JF, Rivers W, Demartini JE. Effects of methadone HCl on the growth of organotypic cerebellar cultures prepared from methadone tolerant and control rats. J Pharmacol Exp Ther. 1976;199:368–374. [PubMed] [Google Scholar]
- Wollemann M. Recent developments in the research of opioid receptor subtype molecular characterization. J Neurochem. 1990;54:1095–1101. doi: 10.1111/j.1471-4159.1990.tb01934.x. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Increased brain size and cellular content in infant rats treated with an opiate antagonist. Science. 1983;221:1179–1180. doi: 10.1126/science.6612331. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. β-funaltrexamine (β-FNA) and the regulation of body and brain development in rats. Brain Res Bull. 1986;17:5–9. doi: 10.1016/0361-9230(86)90155-3. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Endogenous opioid systems regulate growth of neural tumor cells in culture. Brain Res. 1989;490:14–25. doi: 10.1016/0006-8993(89)90425-3. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Goodman SR, McLaughlin PJ. Characterization of zeta (ζ): a new opioid receptor involved in growth. Brain Res. 1989;482:297–305. doi: 10.1016/0006-8993(89)91192-x. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Goodman SR, McLaughlin PJ. Demonstration and characterization of zeta (ζ), a growth-related opioid receptor, in a neuroblastoma cell line. Brain Res. 1990a;511:181–186. doi: 10.1016/0006-8993(90)90159-9. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Gibo DM, McLaughlin PJ. Adult and developing human cerebella exhibit different profiles of opioid binding sites. Brain Res. 1990b;523:62–68. doi: 10.1016/0006-8993(90)91635-t. [DOI] [PubMed] [Google Scholar]