Abstract
Bevacizumab is a monoclonal antibody targeting the vascular endothelial growth factor receptor and a key drug for advanced non‐small cell lung cancer. There are few reports describing bevacizumab‐induced chronic interstitial pneumonia.
A 62‐year‐old man with advanced non‐small cell lung cancer was admitted to our hospital with dyspnea. He previously received four courses of carboplatin plus paclitaxel with bevacizumab combination therapy and thereafter received four courses of maintenance bevacizumab monotherapy. A chest‐computed tomography scan on admission revealed diffuse ground glass opacity. He had not received any other drugs and did not have pneumonia. Thus, he was diagnosed with bevacizumab‐induced chronic interstitial pneumonia and was treated with a high dose of corticosteroids. After steroid treatment, his dyspnea and radiological findings improved.
This case report is the first description of bevacizumab‐induced chronic interstitial pneumonia during maintenance therapy in a patient with non‐small cell lung cancer.
Keywords: bevacizumab, chemotherapy, drug‐induced chronic interstitial pneumonia, molecular target drug, non‐small cell lung cancer
Introduction
Bevacizumab (BEV) is a humanized monoclonal antibody targeting vascular endothelial growth factor receptor and is one of new molecular targeting drugs for colon, cerebral, ovarian, and advanced non‐small cell lung cancer (NSCLC). Recently, carboplatin (CBDCA) plus paclitaxel (PTX) combined with BEV therapy (CP‐BEV) has become a first‐line regimen for advanced‐NSCLC patients 1. In this study, progression‐free survival in patients treated with CP‐BEV was longer than that in patients treated with CBDCA plus PTX alone. Therefore, although we can administer BEV to a limited number of patients with advanced non‐squamous NSCLC, it is very important to determine whether these patients can receive additional BEV treatment combined with CBDCA and PTX. After effective treatment with this combination therapy, patients can receive BEV as maintenance monotherapy. It was reported that patients treated with the epidermal growth factor receptor tyrosine kinase inhibitors developed lung injury. However, to our knowledge, there are no reported cases of BEV‐induced lung injury or development of chronic interstitial pneumonia (CIP) during maintenance therapy. Here, we report a rare case of BEV‐induced CIP during maintenance therapy after four courses of CP‐BEV in an advanced‐NSCLC patient.
Case Report
A 62‐year‐old Asian Japanese man was admitted to our hospital with development of dyspnea. Seven months before this admission, he was diagnosed with advanced NSCLC. He had received four courses of CP‐BEV followed by four courses of BEV monotherapy as maintenance therapy. He received the final BEV treatment 20 days before this admission. His initial vital signs on admission were body temperature 35.6°C, respiratory rate 22 breaths/min, oxygen saturation on room air lying quietly 92%, and oxygen saturation on room air after 6 min of waking 78%. A physical examination revealed fine crackles in both middle and lower lung fields. The laboratory test values were white blood cells 8900/μL, neutrophils 6050/μL, serum lactate dehydrogenase 316 IU/L (normal, 119–229 IU/L), serum sialylated carbohydrate antigen KL‐6 (KL‐6) 2670 U/mL (normal <500 U/mL), serum pulmonary surfactant protein D (SP‐D) 408 ng/mL (normal <110 ng/mL), serum C‐reactive protein 0.6 mg/dL (normal <0.3 mg/dL), serum procalcitonin 0.06 ng/mL (normal <0.1 ng/mL), and serum β‐d‐glucan, 7.1 pg/mL (normal <20 pg/mL). The arterial blood gas values on room air were pH 7.40, partial pressure of arterial oxygen 62.3 Torr, partial pressure of arterial carbon dioxide 41 Torr, and bicarbonate 25.2 mg/dL. A sputum Gram stain revealed only normal bacterial flora. A rapid flu test was performed and yielded negative results. Mycoplasma antigen was negative. A chest X‐ray film (Figure 1B) showed reticular shadows in both lung fields. A chest high‐resolution computed tomography (HRCT) scan showed diffuse ground glass opacity and irregular areas of reticular shadow without traction bronchiectasis (Figure 2B). No interstitial shadows were observed by chest X‐ray (Figure 1A) or HRCT (Figure 2A) at the beginning of maintenance BEV treatment. Pulmonary function tests revealed abnormal findings of vital capacity 2.32 L and diffusion capacity to carbon monoxide 13.4% mL/min/mmHg. On the second day after admission, we performed a bronchial alveolar lavage (BAL) from the left B4 bronchus. The total cell count and lymphocyte percentage in the BAL fluid were elevated to 2.2 × 105/mL (normal range, 0.7‐2.0 × 105/mL) and 28%, respectively. There was negative result of a polymerase chain reaction (PCR) test of BAL fluid for Pneumocystis jirovecii. The patient had not received any other drugs, including anti‐cancer medicines. There was no history of interstitial pneumonia or interstitial shadow on chest HRCT before this admission. Additionally, drug lymphocyte stimulation testing with BEV was strongly positive (stimulation index 489%). Therefore, the patient was diagnosed with development of CIP induced by BEV and was treated with methylprednisolone (1 g/day for 3 days). After initiation of steroid pulse therapy, his respiratory condition, chest X‐ray findings (Figure 1C), and chest computed tomography scan (Figure 2C) findings improved.
Figure 1.
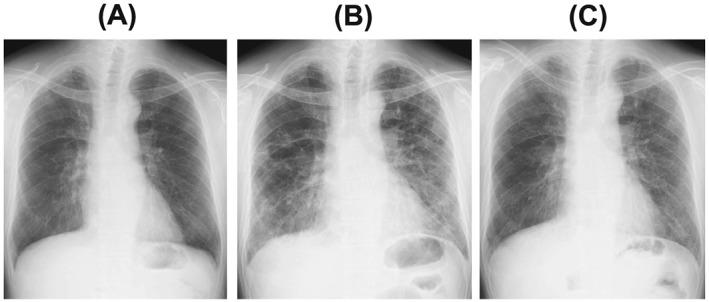
Chest X‐ray. (A) No abdominal findings at the beginning of bevacizumab maintenance monotherapy. (B) Ground glass opacity and reticular shadows in both lower lung fields at admission. (C) Improvement of ground glass opacity and reticular shadow after treatment with methylprednisolone.
Figure 2.
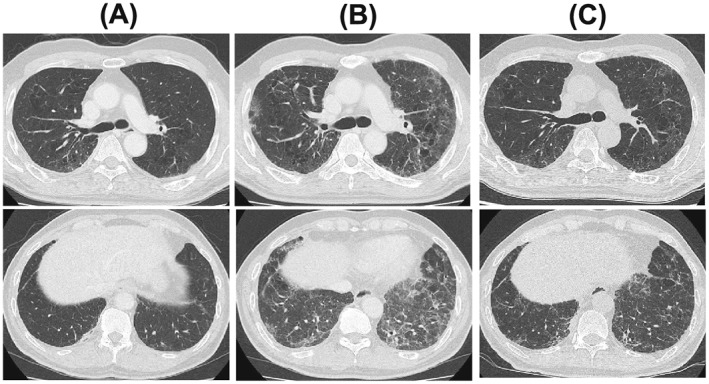
Chest high‐resolution computed tomography. (A) No abdominal findings at the beginning of bevacizumab maintenance monotherapy. (B) Diffuse panlobular ground glass opacity and an irregular area of reticular shadow without traction bronchiectasis at admission. (C) Improvement of ground glass opacity and reticular shadow after treatment with methylprednisolone.
Discussion
To the best of our knowledge, this is the first case report describing development of CIP during BEV maintenance therapy after CP‐BEV in a patient with advanced NSCLC. It had been reported that the incidence of CIP or lung injury was 0.19% in patients with advanced NSCLC in a post‐marketing investigation. There are two studies examining the safety and efficacy of CP‐BEV with additional BEV monotherapy in patients with interstitial lung disease (ILD). In one study, no patients developed CIP 2. In the second study, one patient developed CIP, out of 10 patients with the combination of advanced NSCLC and ILD 3. However, the patient number was too small to evaluate efficacy or toxicity of chemotherapy. In our case, drug‐induced CIP developed in a patient without interstitial shadow on chest HRCT and no past history of ILD.
It has been reported that the time to develop drug‐induced lung injury may be rapid in the case of bronchospasm or longer in the case of drug‐induced CIP 4. However, there are no reports about which drugs or regimens are associated with the development of interstitial pneumonia either shortly after or at a later time after initiation of suspect drugs. Although there are many cases of drug‐induced pneumonia during the early period after initiating suspect drug treatment, it has been reported that several patients developed interstitial pneumonia a long time after initiation of any suspect drugs, as in our case. Recently, advanced NSCLC patients received maintenance therapy after standard first‐line chemotherapy, especially combination therapy with cisplatin or carboplatin (CBDCA) plus pemetrexed (PEM), with or without BEV, as well as CP‐BEV. Patients treated with platinum agents and PEM with or without BEV received PEM with or without BEV as maintenance therapy, while patients treated with CP‐BEV received BEV monotherapy as maintenance therapy. For maintenance therapy with PEM alone, there is only one case report describing CIP induced by PEM maintenance therapy after six courses of CBDCA plus PEM in a patient with advanced NSCLC 5. It is known that steroid dosage is different between platinum doublet therapy and maintenance therapy, whereas the steroid dose in CP‐BEV is 18 mg of dexamethasone and no steroids are administered with BEV maintenance therapy in our hospital. Lack of steroid treatment may be important in developing CIP during BEV maintenance therapy.
We usually confirm KL‐6 and SP‐D levels to distinguish between different types of drug‐induced lung toxicity. KL‐6 levels in patients diagnosed with diffuse alveolar damage or CIP are significantly higher than those in patients with other types of drug‐induced lung toxicity, including hypersensitivity pneumonia, eosinophilic pneumonia, and organized pneumonia 6. Elevation of both KL‐6 and SP‐D is considered to indicate fibrosis progression or lung injury as our patient. The β‐d‐glucan level in this patient was very low; therefore, we excluded fungal infection. We also excluded pneumocystis pneumonia based on the negative results of a PCR test of BAL fluid for P. jirovecii with negative result of β‐d‐glucan. Before diagnosis with BEV‐induced CIP, this patient did not have infectious symptoms, such as high fever. We excluded any infections, including influenza by flu test and post‐infective bronchiolitis obliterans organizing pneumonia from the possible causes of the patient's symptoms and findings by our results. We intended to perform a transbronchial lung biopsy; however, we instead performed a BAL because his respiratory condition worsened. We tested the BAL fluid and confirmed negative results on bacterial examination, P. jirovecii PCR testing, and eosinophil elevation, which enabled us to exclude bacterial infection, Pneumocystis pneumonia, and eosinophilic pneumonia. We assessed non‐specific interstitial pneumonia development by chest HRCT findings (ground glass opacity and peribronchovascular reticulation) and fibrosing markers. This patient was diagnosed with BEV‐induced CIP by observing the clinical course, chest computed tomography findings, and result of drug lymphocyte stimulation testing, while excluding infection, heart failure, and other agent inducing CIP. Therefore, we initiated treatment with steroid pulse therapy.
In conclusion, this is the first case of CIP development during BEV maintenance therapy. It is known that BEV is low risk for development of CIP. However, it might be necessary to increase awareness of CIP after changing from a standard regimen with BEV to maintenance BEV monotherapy.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Sekimoto, Y. , Kato, M. , Shukuya, T. , Koyama, R. , Nagaoka, T. , and Takahashi, K. (2016) Bevacizumab‐induced chronic interstitial pneumonia during maintenance therapy in non‐small cell lung cancer. Respirology Case Reports, 4: n/a. doi: 10.1002/rcr2.151.
References
- 1. Sandler A, Gray R, Perry MC, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N. Engl. J. Med. 2006; 14: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki H, Hirashima T, Kobayashi M, et al. Carboplatin plus paclitaxel in combination with bevacizumab for the treatment of adenocarcinoma with interstitial lung diseases. Mol. Clin. Oncol. 2013; 1: 480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimizu R, Fujimoto D, Kato R, et al. The safety and efficacy of paclitaxel and carboplatin with or without bevacizumab for treating patients with advanced nonsquamous non‐small cell lung cancer with interstitial lung disease. Cancer Chemother. Pharmacol. 2014; 74: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 4. Matsuno O. Drug‐induced interstitial lung disease: mechanisms and best diagnostic approaches. Respiratory Research 2012, 13:39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhakal B, Singh V, Shrestha A, et al. Pemetrexed induced pneumonitis. Clinics and Practice. 2011; 1: 232–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costabel U, Uzaslan E, Guzman J. Bronchoalveolar lavage in drug‐induced lung disease. Clin. Chest Med. 2004, 25:25–35. [DOI] [PubMed] [Google Scholar]


