Abstract
Key points
Despite the clinical importance of pre‐emptive analgesia, the mechanisms by which it attenuates pain associated with central sensitization are poorly understood.
We find that fentanyl and the α2‐adrenoceptor agonist dexmedetomidine (Dex) differ significantly in their modulatory actions on noxious mechanical and noxious heat‐evoked nociception in vivo.
Unlike fentanyl, Dex modified descending control of nociception by decreasing the threshold for descending inhibition and/or increasing the threshold for descending facilitation.
Dex exhibited after‐actions on activities of thalamus in prolongation of noxious heat‐evoked paw withdrawal latency that persisted for at least 7 days.
This study provides insight into the organization of thalamic modulation in pre‐emptive analgesia.
Abstract
We investigated and compared the antinociceptive effects of intraperitoneal administration of fentanyl (2–60 μg kg−1) and dexmedetomidine (Dex, 1–10 μg kg−1; a highly selective α2‐adrenoceptor agonist) in the regulation of nociception assessed by measuring noxious paw withdrawal reflexes in rats. Fentanyl elevated noxious mechanical paw withdrawal threshold and prolonged paw withdrawal heat latency within 1–1.5 h (P < 0.05). Dex failed to affect the mechanical paw withdrawal threshold, yet significantly prolonged the paw withdrawal heat latency in a bi‐phasic manner; a short transient 1–1.5 h period followed by a second, slowly developing increase in latency that persisted for at least 7 days (P < 0.05). Lesion of the dorsolateral funiculus (DLF) did not influence fentanyl‐induced antinociceptive effects, indicating peripheral and spinal antinociceptive mechanisms. By contrast, the Dex‐induced second, but not the first, phase of the prolonged paw withdrawal heat latency was significantly blocked by the lesion of either DLF or thalamic ventromedial (VM) nuclei, and was attenuated by intracerebral administration of either atipamezole (α2‐adrenoceptor antagonist) or WAY‐100635 (5‐HT1A receptor antagonist) into the VM nuclei (P < 0.05). Upon intramuscular 5.8% saline‐induced muscle nociception, pre‐emptive injection of fentanyl enhanced mechanical hyperalgesia and blocked heat hypoalgesia, whereas Dex significantly prevented the occurrence of mechanical hyperalgesia and enhanced heat hypoalgesia. It is suggested that Dex, but not fentanyl, significantly enhances descending inhibition and/or decreases descending facilitation to modulate pain and nociception. The present study provides novel insight into thalamus‐mediated mechanisms in pre‐emptive analgesia.
Key points
Despite the clinical importance of pre‐emptive analgesia, the mechanisms by which it attenuates pain associated with central sensitization are poorly understood.
We find that fentanyl and the α2‐adrenoceptor agonist dexmedetomidine (Dex) differ significantly in their modulatory actions on noxious mechanical and noxious heat‐evoked nociception in vivo.
Unlike fentanyl, Dex modified descending control of nociception by decreasing the threshold for descending inhibition and/or increasing the threshold for descending facilitation.
Dex exhibited after‐actions on activities of thalamus in prolongation of noxious heat‐evoked paw withdrawal latency that persisted for at least 7 days.
This study provides insight into the organization of thalamic modulation in pre‐emptive analgesia.
Abbreviations
- Dex
dexmedetomidine
- GS
gastrocnemius
- i.c.
intracerebal
- DLF
dorsolateral funiculus
- MD
mediodorsal
- VM
ventromedial
Introduction
At the beginning of the 20th century, Crile (1913) introduced the concept of pre‐emptive analgesia, i.e. that pharmacological interventions performed prior to surgery may attenuate and weaken the operation‐induced pain. Recent developments in pre‐emptive analgesia have heightened the need and importance of peri‐operative clinical manipulation in the prevention and inhibition of central sensitization induced by surgical interventions (Woolf & Chong, 1993). Despite clinical validation, however, it appears that complete blockade of peripheral noxious inputs from the surgical wound during and after the operation, in particular deep tissue surgery, to prevent sensitization of the nociceptive system might not be realistic. Thus, regulation of endogenous controls of pain, i.e. enhancement of descending inhibition, by pharmacological and/or non‐pharmacological interventions is considered to be an alternative means to prevent the induction and maintenance of surgery‐induced hypersensitivity.
It is widely accepted that spinal nociceptive processing is controlled by descending modulation, facilitation and/or inhibition, from supraspinal structures (Fields et al. 1976; Belcher et al. 1978; Haber et al. 1980; Cervero & Wolstencroft, 1984; for reviews, see Basbaum & Fields, 1978; Besson & Chaouch, 1987; Fields, 1992; Millan, 2002). With respect to endogenous descending modulation, we recently proposed the novel concept of thalamic ‘nociceptive discriminators’, which discriminate and modulate peripheral noxious stimuli‐induced nociception (You et al. 2010, 2013, 2014; Lei & You, 2013, Lei et al. 2011). We suggest that endogenous descending modulation, facilitation and inhibition, derived from thalamic ‘nociceptive discriminators’ is inactive under physiological pain‐free conditions. Once triggered and initiated by sufficient innocuous and noxious C‐afferents associated with temporal and spatial summation, thalamic ‘nociceptive discriminators’ (thalamic mediodorsal (MD) and ventromedial (VM) nuclei) perform distinct actions; thalamic MD nuclei have a facilitatory role in noxious mechanically evoked activities while thalamic VM nuclei have an inhibitory action in heat‐mediated responses (You et al. 2010, 2013, 2014). The descending modulation mediated by thalamic ‘nociceptive discriminators’ was further demonstrated to have different triggering thresholds: a lower threshold for descending facilitation and a higher threshold for descending inhibition (You et al. 2010, 2013, 2014; Lei et al. 2011). This lower triggering threshold of descending facilitation may limit effective relief of pain by initiation of descending inhibition alone without activation of descending facilitation. To date, it is accepted that pathological pain associated with central sensitization is subject to abnormal, imbalanced descending modulation: enhanced descending facilitation and weakened descending inhibition (Porreca et al. 2002; Ren & Dubner, 2009; Heinricher et al. 2009; Henderson et al. 2013; Ossipov et al. 2014). With this in mind, appropriate pre‐emptive pharmacological intervention (i.e. pre‐emptive anaesthesia) might be able to enhance descending inhibition and weaken descending facilitation. As such, it may provide an effective way to control surgery‐induced pain and pathological pain associated with central sensitization.
Despite extensive clinical use, the efficacy of treatment with fentanyl in the prevention of surgery‐induced pain has been debated (Celerier et al. 2000). In the current study, we systematically investigated and compared the effects of fentanyl, an opioid μ‐receptor potent agonist, and dexmedetomidine (Dex), a highly selective α2‐adrenoceptor agonist, on noxious mechanically and heat‐evoked paw withdrawal reflexes. Potential peripheral and central mechanisms underlying pre‐emptive analgesic effects of fentanyl and Dex on i.m. 5.8% saline‐induced acute muscle pain were further explored.
Methods
Ethical approval and animals
Male Sprague‐Dawley rats weighing 250–300 g (about 10 weeks of age) were used in the present study. The animals were provided by the Animal Center of the College of Medicine, Xi'an Jiaotong University, and housed pairwise in plastic boxes under a 12:12 h light dark cycle (lights on at 08.00 h) at 22–26°C with food and water available ad libitum. All experiments were approved by the Xi'an Jiaotong University Animal Care Committee in accordance with the Principles of Laboratory Animal Care (NIH publication no. 85‐23, revised 1985), and comply with the policies and regulations of The Journal of Physiology (Grundy, 2015). The animals were acclimatized to the laboratory and habituated to the test boxes for at least 1 h each day 5 days prior to the behavioural testing. The rats were used only once and killed at the end of the experiment by i.p. injection of an overdose of sodium pentobarbital (200 mg kg−1). All efforts were made to minimize the number of animals used.
Behavioural test
Experimental study groups were randomized and blinded. According to the different experimental purposes, rats recruited in the current study were randomly divided into several individual groups; ten rats randomly assigned in each group were included for the investigation.
Measurement of mechanical sensitivity
According to mapping of the withdrawal field of the gastrocnemius (GS) muscle (You et al. 2003, 2005, 2010, 2014), mechanical and heat stimulation were applied onto the heel part of the hind paw. Both ipsilateral and contralateral paw withdrawal thresholds 30 min prior to and 30 min, 1–8 h and 1–7 days after the i.p. administration of different drugs at different doses were measured.
For measurement of the noxious mechanically evoked paw withdrawal responses, rats were placed in individual Plexiglas chambers with mesh floors and transparent covers (20 × 20 × 25 cm). An electronic von Frey device (2290 Electrovonfrey, IITC, Woodland Hills, CA, USA) was used to detect the mechanical paw withdrawal threshold. The filament that elicited a withdrawal response in 50% of trials was taken to be the mechanical threshold (g). A reduced or increased threshold for the paw withdrawal response compared with the threshold before the pharmacological treatment was defined as hyperalgesia or hypoalgesia, respectively.
Measurement of heat sensitivity
Noxious heat‐evoked paw withdrawal responses were determined using a 390 G plantar stimulator Analgesia Meter (IITC). The rats were tested individually in a Plexiglas cubicle placed onto a constant temperature‐controlled transparent glass plate used to avoid temperature sink from the tested hind paws. The heat stimulus was a high‐intensity beam (setting = 30–40% intensity of full power) aimed at the heel part of the hind paw. Withdrawal latency was defined as the time from the onset of noxious heat stimulation to withdrawal of the tested hind paw. The intensity of the beam was adjusted so that the latency of the paw withdrawal reflex was around 10–11 s in untreated animals. A painful, but tolerable, sensation could be elicited using this 10–11 s heat stimulation on the operator's hand. To avoid excessive tissue injury, manual cut‐off of the heat stimulus was performed if no paw withdrawal reflex could be evoked during 20 s of heat stimulation.
Intraperitoneal administration of different drugs
For investigation of the role of opioid receptor ligands and α2‐adrenoceptor agonists on noxious mechanically and heat‐evoked paw withdrawal reflexes, the effects of the i.p. administration of μ‐opioid receptor agonist, fentanyl (2‐60 μg kg−1, Yichang Humanwell Pharmaceutical Co., Ltd, Yichang, China), and the highly selective α2‐adrenoceptor agonist, dexmedetomidine hydrochloride (Dex, 1–10 μg kg−1, Jiangsu Hengrui Medicine Co., Ltd, Jiangsu, China) was investigated for 7 days. All drugs were freshly dissolved in 0.9% saline, which was used as control for the different drugs.
Surgical lesion of the dorsolateral funiculus
To exclude potential effects from supraspinal structures (Basbaum & Fields, 1979), surgery for lesion of the dorsolateral funiculus (DLF) was performed in some experiments.
Rats were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.), and then moved to a stereotaxic frame (MP8003, RWD Life Science Co., Nan Shan District, Shenzhen, China), followed by a laminectomy performed at T3–4 for lesion of DLF. In brief, the surgical site was aseptically treated, a sagittal incision was made after local lidocaine (2%, Sanjing Pharmaceutical Co., Ltd, Nangang, Harbin, Heilongjiang, China) anaesthesia, and blunt dissection revealed the dorsal surface of T3–4 vertebrae. After that, the dura mater and arachnoid membrane of the spinal cord were carefully removed under a dissection microscope (SMZ645, Nikon Co., Tokyo, Japan). As described elsewhere (Saadé et al. 1990), lesion of unilateral DLF (lateral 1.5–2.7 mm from midline, dorsoventral 0–1.2 mm from the pia mater) was made using a sharp needle blade controlled precisely by a manual hydraulic micro drive (MO‐81, Narishige Co., Tokyo, Japan). During the lesion procedure, special attention was paid to avoid any damage to blood vessels. After lesion of the DLF, the lesions of the spinal cord were filled by gelfoam on the exposed spinal cord. The wound was washed with sterile 0.9% saline, treated with antibiotic (benzylpenicillin sodium, 80 mg kg−1, Harbin Pharmaceutical Group Holding Co. Ltd, Nangang, Harbin, Heilongjiang China), and the muscles and skin were sutured by layers. The whole operation was performed under strictly sterile conditions. In sham lesion rats, similar surgical procedures were followed except for lesion of the spinal cord. After the surgery, the animals were returned to their plastic boxes for 7 days of recovery.
Electrolytic lesion of thalamic nuclei
The anaesthetized (sodium pentobarbital, 50 mg kg−1, i.p.) rats were mounted in a stereotaxic frame with fixation of the head by ear bars and tooth plate (MP8003, RWD Life Science). After local lidocaine anaesthesia, the scalp was cut and the cranium was drilled. The target thalamic VM nucleus was electrolytically lesioned. For electrolytic lesion of the VM nucleus, an insulated stainless steel electrode (shank diameter 200 μm, tip diameter 50 μm, exposed tip 50 μm) was advanced stereotactically into the targeting areas at the following coordinates: VM: anteroposterior –(2.3–3.3) mm, lateral 1.6 mm from midline, dorsoventral 7–7.4 mm from the cranium (Paxinos & Watson, 1998). An electrolytic lesion was made by means of an electrical stimulator generating a 150–200 μA anodal direct current for 30 s through the electrode tip. The lesion current was monitored continuously by using an oscilloscope to measure the voltage drop across a 100 Ω resistor in series with the electrode. After the electrolytic lesion, the microelectrode was slowly withdrawn, and the skull was closed with dental cement. A recovery period of 7 days was allowed, during which the animals were treated with benzylpenicillin sodium (80 mg kg−1), and behaviour and motor function were strictly monitored. Animals showing permanent neurological deficits, i.e. deficits in reflexes and postural adjustment, stepping patterns and interlimb coordination abnormalities, or motor dysfunction assessed by means of the Rota‐Rod treadmill were excluded from the remaining experiments.
Intracerebral microinjection with different antagonists
A craniotomy was made with a dental drill in order to perform the bilateral intracerebral (i.c.) catheterization. According to the coordinates described above, one guide cannula (OD: 0.35 mm; ID: 0.25 mm; RWD Life Science) was advanced stereotactically into the bilateral thalamic VM nuclei areas. After catheterization, the wounds were washed with sterile 0.9% saline, treated with benzylpenicillin sodium (80 mg kg−1), and the skull was closed with dental cement. The animals were then put back in the box for 7 days of recovery during which the animals’ behaviour and motor function were strictly monitored. Animals showing permanent neurological deficits or motor dysfunction were excluded.
The effects of the α2‐adrenoceptor antagonist atipamezole (2–10 μg, Sigma‐Aldrich Chemie Gmbh, Munich, Germany) and the 5‐HT1A receptor antagonist WAY‐100635 (0.5–2 μg, Sigma‐Aldrich Chemie) on Dex‐induced effects were investigated. In the present study, all antagonists were freshly prepared and dissolved in 0.9% saline. One day after the i.p. administration of Dex, a bolus of 0.5 μl solution containing the each of the drugs listed above was injected bilaterally through the intrathalamic catheter using a 1 μl microsyringe while the rat was gently restrained by hand. All drugs were slowly infused at a constant speed over 30 s. Effects of each of the drugs were tested within 4 h. After the experiment, the drug injection sites were marked by microinjection with Pontamine Sky Blue dye (0.5 μl; 2% in 0.5 m sodium acetate).
Muscle nociception elicited by i.m. injection of 5.8% saline
As described elsewhere (You et al. 2010; Lei et al. 2011), a volume of 0.2 ml hypertonic (5.8%) saline was intramuscularly injected into the GS muscle of the hind limb to elicit persistent muscle nociception. The injection site was in the middle part of the GS muscle, and the depth of the injection was about 0.5 cm. The injection procedure was performed manually and lasted around 45 s.
Assessment of motor function
Briefly, animals were placed on a Rota‐Rod treadmill (Model 755, IITC) rotating at a gradually increasing speed from 5 to 30 r.p.m. for 30 s and maintained for another 120 s at 30 r.p.m. Rats with motor dysfunction after the DLF lesion, chronic electrolytic lesion of thalamic VM nucleus and i.c. catheterization were strictly excluded from the remaining experiments.
Histology for identification of the DLF lesion, electrolytic thalamic lesion and i.c. microinjection
At the end of the period of behavioural testing (about 7 days), the animals receiving the DLF lesion, electrolytic lesion of thalamic nucleus and i.c. microinjection were deeply anaesthetized by sodium pentobarbital (75 mg kg−1, i.p.) and transcardially perfused with 10% formalin. The T3–4 segments of the spinal cord and the brain were then isolated and stored in 30% sucrose for 2 days. Freezing serial sections (15 μm thickness for the spinal cord; 45 μm thickness for the brain) were cut and stained with Nissl stain, and were screened under a microscope (Leica 2000, Wetzlar, Germany). Schematic reconstructions of the lesions and microinjection sites were drawn according to the stereotaxic atlas of rats (Paxinos & Watson, 1998). Reported results are based on observations made on rats with accurate location of the spinal cord and the thalamic VM nucleus. Histological determination of the lesion and location of the cannula tip in the brain, and lesion of DLF area in the spinal cord was performed without knowledge of the behavioural results.
Statistical analysis
All results are expressed as means ± SEM. The data were analysed using SigmaStat (Systat Software Inc., San Jose, CA, USA) and compared by means of one‐way/two‐way repeated measures ANOVA with post hoc Bonferroni t‐tests for analysis of the differences in the observation time among different groups. P < 0.05 was considered statistically significant.
Results
Effects of fentanyl and Dex on noxious mechanically and heat‐evoked paw withdrawal reflexes
Bilateral paw withdrawal reflexes to noxious mechanical and heat stimuli were evaluated before and 30 min, 1–8 h and 1–7 days after the i.p. administration of either fentanyl or Dex at different doses (Figs 1 and 2). Following the i.p. injection of fentanyl at doses of 2–60 μg kg−1, the noxious mechanically evoked paw withdrawal reflexes were significantly depressed bilaterally (mechanical hypoalgesia; P < 0.05 and P < 0.001, one‐way ANOVA, Fig. 1 A, B). Depression of mechanically evoked responses was apparent within 30 min of drug injection and progressively returned to the baseline level within 2–3 h. Noxious heat‐evoked paw withdrawal reflexes were likewise significantly and bilaterally depressed over a similar time course (heat hypoalgesia; P < 0.05, one‐way ANOVA, Fig. 1 C, D). Two hours after the i.p. fentanyl injection, no significant differences were found between 0.9% saline treatment and fentanyl treatment (P > 0.05, one‐way ANOVA, Fig. 1). The dose‐dependent manner of fentanyl‐induced antinociceptive effects was found on both noxious mechanically and heat‐evoked paw withdrawal responses. The duration of antinociceptive effects of fentanyl at a dose of 2 μg kg−1 on paw withdrawal mechanical threshold and withdrawal thermal latency was noticeably shorter than that seen with higher doses.
Figure 1. Fentanyl on bilateral mechanically and heat‐evoked paw withdrawal reflexes .
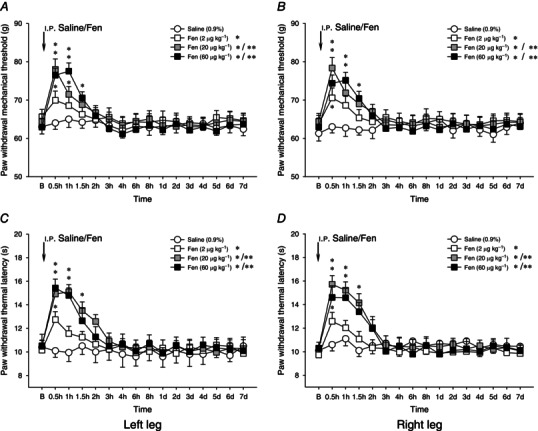
Effects of i.p. administration of fentanyl (Fen, 2–60 μg kg−1) on bilateral mechanically (A and B) and heat (C and D) evoked paw withdrawal reflexes. Fentanyl induced mechanical and heat hypoalgesia, which were observed within 2–3 h following the i.p. administration. *P < 0.05 and **P < 0.001 compared with the i.p. injection of 0.9% saline. B, baseline responses before the i.p. injection of 0.9% saline or fentanyl (n = 10 for each group).
Figure 2. Dexmedetomidine on bilateral mechanically and heat‐evoked paw withdrawal reflexes .
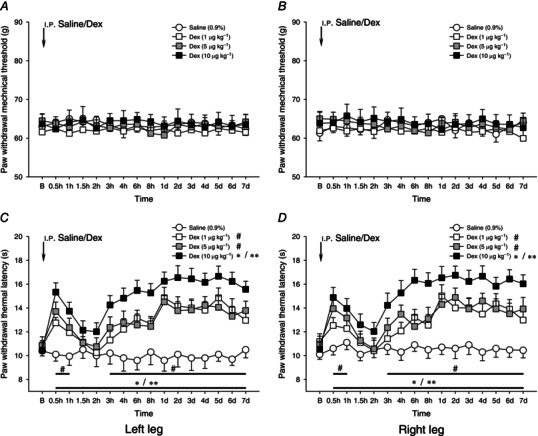
Effects of i.p. administration of dexmedetomidine (Dex, 1–10 μg kg−1) on bilateral mechanically (A and B) and heat (C and D) evoked paw withdrawal reflexes. Dex at a dose of 1–10 μg kg−1 failed to affect the mechanical paw withdrawal threshold. In contrast, Dex significantly prolonged the paw withdrawal heat latency in a bi‐phasic manner; there was a short‐term (around 1.5–2 h) prolongation of paw withdrawal heat latency, followed by a long‐term increase in heat latency lasting over 7 days (C and D). *P < 0.05, **P < 0.001 and # P < 0.05 compared with 0.9% saline injection (n = 10 for each group). B, baseline responses before the i.p. injection of 0.9% saline or Dex.
Intraperitoneal injection of Dex at 1–10 μg kg−1 failed to influence bilateral noxious mechanically evoked paw withdrawal responses (P > 0.05, one‐way ANOVA, Fig. 2 A, B). In some experiments, we further tested the effects of Dex at a dose of 20 μg kg−1 on mechanically evoked responses. Dex at 20 μg kg−1 produced profound sedative effects rather than antinociceptive effects, as a sustained reduction in locomotion of animals receiving the Dex injection was found after the Rota‐Rod treadmill test (data not shown).
By contrast, a significant prolongation of heat‐evoked paw withdrawal latency was observed within 30 min of the administration of 1–10 μg kg−1 Dex (P < 0.05 and P < 0.001, Fig. 2 C, D). This prolongation of paw withdrawal latency reverted to the baseline level within 2 h (Fig. 2 C, D). Following this, however, a second phase of drug effects emerged. Three hours after the i.p. Dex, a second phase of prolongation of heat‐evoked paw withdrawal latency occurred. This progressed to a peak level at 1 day after the drug injection, and persisted throughout the 7 day observation period (Fig. 2 C, D). The Dex‐induced long‐lasting heat hypoalgesia returned to the baseline level within about 2 weeks.
Involvement of descending modulation in the actions of fentanyl and Dex
To identify peripheral or central antinociceptive effects of fentanyl and Dex on noxious mechanically and heat‐evoked nociception, the DLF was transected unilaterally. Seven days after the transection, the effects of i.p. injection of fentanyl at 20 μg kg−1 and Dex at 5 μg kg−1 were tested. DLF lesion failed to affect noxious mechanically and heat‐evoked paw withdrawal responses (Fig. 3). Also, as before (Fig. 2 A, B) Dex failed to affect paw withdrawal responses to noxious mechanical stimulation whereas fentanyl was effective (Fig. 3 A, B). This antinociceptive effect of fentanyl was unaffected by the DLF lesion. Likewise, the DLF lesion failed to affect the prolongation of heat‐evoked paw withdrawal latency induced by the i.p. injection of fentanyl (Fig. 3 C, D). However, the occurrence of Dex‐induced prolongation of heat‐evoked paw withdrawal latency in the second, but not the first, phase ipsilateral to the DLF lesion was completely blocked (P < 0.05, Fig. 3 C). In contrast, the contralateral prolonged paw withdrawal latency induced by Dex was not affected after the ipsilateral DLF lesion (P > 0.05, Fig. 3 D).
Figure 3. Ipsilateral lesion of dorsolateral funiculus on fentanyl‐ and dexmedetomidine‐induced antinociceptive effects .
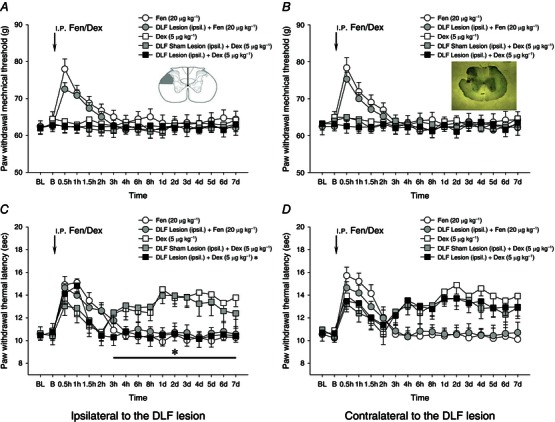
Effects of ipsilateral (ipsil.) lesion of dorsolateral funiculus (DLF) on fentanyl‐ (Fen, 20 μg kg−1) and dexmedetomidine‐ (Dex, 5 μg kg−1) induced antinociceptive effects. A and B, mechanical responses; C and D, heat responses. The insets in A and B are schematic diagram and representative image showing lesion of the DLF. After ipsilateral lesion of the DLF, the ipsilateral second, but not the first, phase of heat hypoalgesia induced by i.p. administration of Dex was completely blocked (C). In contrast, no significant effects of DLF lesion on fentanyl‐induced mechanical and heat hypoalgesia were found. *P < 0.05 compared with the DLF shame lesion (n = 10 for each group). BL, baseline responses before the DLF lesion; B, baseline responses before the Fen/Dex treatment.
We further investigated the effects of an electrolytic lesion of the thalamic VM nucleus on the i.p. Dex‐induced antinociceptive effects. Figure 4 A shows the schematic reconstructions of the electrolytic lesion sites (n = 30) of the thalamic VM nuclei. One week after either unilateral (ipsilateral/contralateral) or bilateral electrolytic lesion of the target thalamic VM nuclei, variations of paw withdrawal reflexes exposed to the i.p. administration of Dex were examined. Neither unilateral nor bilateral lesion of the thalamic VM nucleus affected the noxious mechanically evoked paw withdrawal reflex (Fig. 5 A, B). Bilateral, but not unilateral (ipsilateral/contralateral), lesion of the thalamic VM nuclei significantly blocked the Dex‐elicited occurrence of heat hypoalgesia in the second phase: from 2 h to 2 days(P < 0.05), but not in the first phase (0.5–1.5 h) (P > 0.05, Fig. 5 C, D).
Figure 4. Locations of electrolytic lesions of thalamic ventromedial nuclei and of microinjection sites within the bilateral ventromedial nuclei .
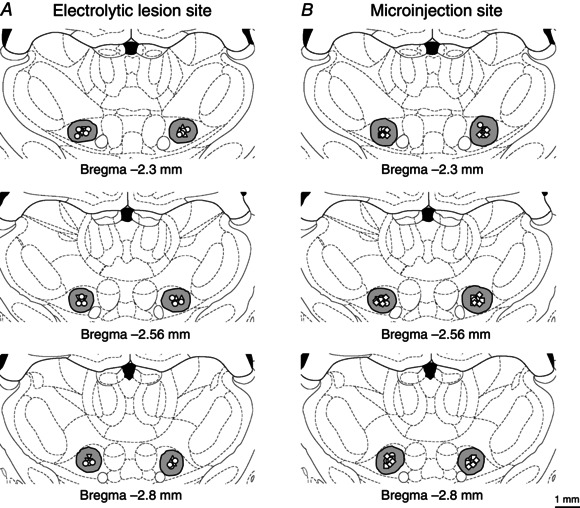
Reconstructions of the locations of 30 electrolytic lesions of thalamic ventromedial (VM) nuclei (A), and of 50 microinjection sites within the bilateral VM nuclei (B). The spread regions of lesion and microinjection are marked by grey areas. Effective sites for ipsilateral, contralateral and bilateral electrolytic lesions are indicated by up‐triangles, down‐triangles and circles in A, respectively. Microinjection sites of 0.9% saline, atipamezole and WAY‐100635 are indicated by circles, squares and diamonds in B, respectively. Note that some microinjection sites overlap with others in this reconstruction.
Figure 5. Effects of ipsilateral, contralateral or bilateral lesion of thalamic ventromedial nucleus on dexmedetomidine‐induced influence on noxious mechanically and heat‐evoked paw withdrawal reflexes .
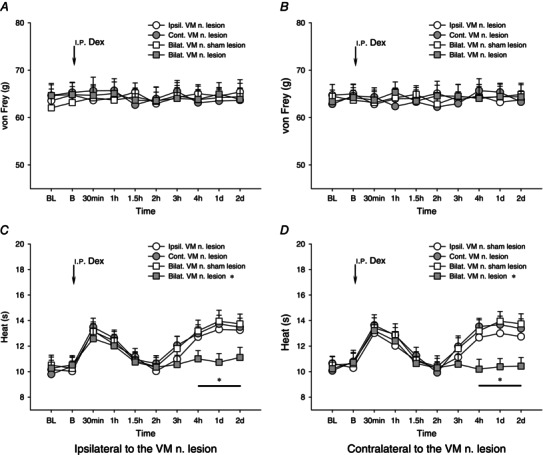
Effects of ipsilateral (Ipsil.), contralateral (Cont.) or bilateral (Bilat.) lesion of thalamic ventromedial (VM) nucleus (n.) on dexmedetomidine‐ (Dex, 5 μg kg−1) induced influence on noxious mechanically (A and B) and heat (C and D) evoked paw withdrawal reflexes. After the bilateral, but not ipsilateral or contralateral, electrolytic lesion of the thalamic VM nucleus, the Dex‐induced second phase of heat hypoalgesia was significantly blocked. *P < 0.05 compared with the sham lesion of the bilateral VM nuclei (n = 10 for each group). BL, baseline responses before the VM nucleus lesion; B, baseline responses before the i.p. administration of Dex.
Mechanism of effect of Dex on descending control of nociception
We investigated potential mechanisms underlying the effects of Dex on the descending inhibition. Different doses of either α2‐adrenoceptor antagonist atipamezole (2–10 μg/0.5 μl) or 5‐HT1A receptor antagonist WAY‐100635 (0.5–2 μg/0.5 μl) were intracerebrally administrated into the bilateral thalamic VM nuclei 1 day after the i.p. administration of 5 μg kg−1 Dex. Figure 4 B shows the schematic reconstructions of the microinjection sites of different antagonists in the bilateral thalamic VM nuclei.
Compared with vehicle (0.9% saline) treatment, none of the above tested drugs showed significant effects on noxious mechanically evoked paw withdrawal reflex (P > 0.05, Fig. 6 A, B). By contrast, the prolongation of noxious heat‐evoked paw withdrawal latency (heat hypoalgesia) in the i.p. Dex‐induced second phase was dose‐dependently depressed by atipamezole (P < 0.05, Fig. 6 C, D). Likewise, i.c. administration of WAY‐100635 at 2 μg/0.5 μl, but not 0.5 μg/0.5 μl, significantly attenuated the development of Dex‐induced heat hypoalgesia (Fig. 6 C, D). It is suggested that endogenous noradrenaline (NA) and serotonin (5‐HT) are both involved in the second phase of Dex‐mediated antinociceptive effects on noxious heat‐evoked activities.
Figure 6. Different doses of antagonists into the bilateral thalamic VM nuclei on dexmedetomidine‐induced effects on mechanically and heat‐evoked paw withdrawal reflexes .
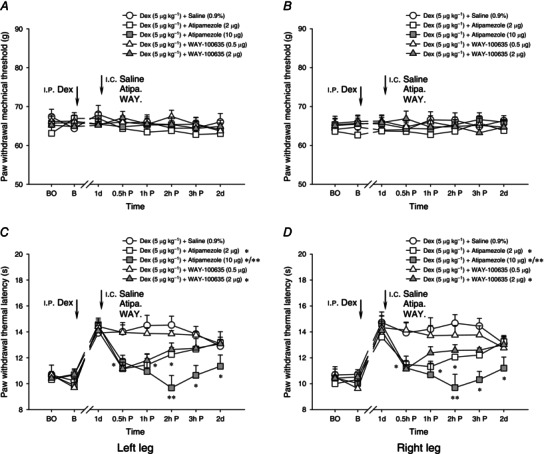
Effects of intracerebral (i.c.) administration of different doses of antagonists, atipamezole (Atipa.: 2–10 μg) and WAY‐100635 (WAY.: 0.5–2 μg) into the bilateral thalamic VM nuclei on i.p. dexmedetomidine‐ (Dex, 5 μg kg−1) induced effects on mechanically (A and B) and heat (C and D) evoked paw withdrawal reflexes. All antagonists including vehicle (0.9% saline) were administrated 1 day after the i.p. injection of Dex. Atipamezole and WAY‐100635 dose‐dependently reduced Dex‐induced heat hypoalgesia, suggesting that the second phase of heat hypoalgesia following the treatment with Dex is controlled by endogenous descending noradrenergic and serotonergic pathways. In contrast to the blocking effects on heat hypoalgesia, no effects of these antagonists on mechanically evoked paw withdrawal reflexes were observed. *P < 0.05 and **P < 0.001 compared with the vehicle (i.c. 0.9% saline) treatment (n = 10 for each group). BO, baseline responses before the I.C. Catheterization; B, baseline responses before the i.p. administration of Dex.
Effects of pre‐emptive fentanyl or Dex on muscle nociception induced by 5.8% saline
As shown in figures 1 and 2, the antinociceptive effects of fentanyl at a dose of 20 μg kg−1 on mechanically and heat‐evoked nociception returned to the baseline level within 3 h, whereas the second phase of heat hypoalgesia induced by Dex at 5 μg kg−1 occurred 2 h after the i.p. injection. To explore drug actions relevant to pre‐emptive analgesia, fentanyl at a dose of 20 μg kg−1 or Dex at 5 μg kg−1 were injected i.p. 3.5 and 1.5 h prior to the i.m. injection of 0.2 ml 5.8% saline, respectively. In line with previous reports (You et al. 2010, Lei et al. 2011), unilateral i.m. injection of 0.2 ml 5.8% saline into the GS muscle produced bilateral mechanical hyperalgesia within 30 min in control rats (Fig. 7 A, B), and a more slowly developing, bilateral, secondary heat hypoalgesia which appeared 1 day later (Fig. 7 C, D).
Figure 7. Pre‐emptive injection of fentanyl on unilateral 5.8% saline‐induced bilateral mechanical hyperalgesia and heat hypoalgesia .
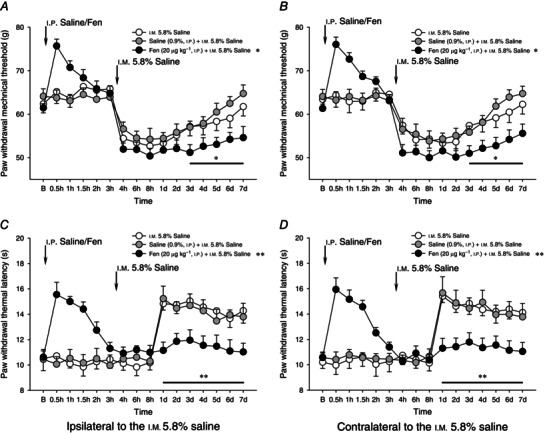
Effects of pre‐emptive injection of fentanyl (Fen, 20 μg kg−1, i.p.) on unilateral i.m. 5.8% saline‐induced bilateral mechanical hyperalgesia (A and B) and heat hypoalgesia (C and D). Three and a half hours after the i.p. administration of fentanyl, 0.2 ml of 5.8% saline was intramuscularly injected into the GS muscle. Pre‐emptive injection of fentanyl significantly enhanced mechanical hyperalgesia (3–7 days after the fentanyl treatment), and blocked heat hypoalgesia during the 5.8% saline intramuscularly induced muscle nociception. *P < 0.05 and **P < 0.001 compared with the vehicle (i.p. 0.9% saline) treatment (n = 10 for each group). B, baseline responses before the i.p. administration of either 0.9% saline or fentanyl.
The mechanical hyperalgesia produced by i.m. 5.8% saline injection was bilaterally increased by pre‐emptive injection of fentanyl (20 μg kg−1). Significant effects (P < 0.05, two‐way ANOVA) emerged 3 days after i.m. 5.8% saline injection (Fig. 7 A, B). Pre‐emptive injection of fentanyl also prevented the secondary heat hypoalgesia that followed the i.m. injection of 5.8% saline throughout the 7 day observation period (Fig. 7 C, D).
Pre‐emptive i.p. injection of Dex (5 μg kg−1) completely prevented the mechanical hyperalgesia induced by i.m. 5.8% saline injection (P < 0.05, two‐way ANOVA, Fig. 8 A, B). By contrast, it did not attenuate or block 5.8% saline intramuscularly induced secondary heat hypoalgesia (Fig. 8 C, D). Dex significantly and bilaterally increased the speed of onset of heat hypoalgesia (P < 0.05, two‐way ANOVA, Fig. 8 C, D). This Dex‐induced augmentation of hypoalgesia persisted throughout the 7 day experimental period.
Figure 8. Pre‐emptive injection of dexmedetomidine on unilateral 5.8% saline‐induced bilateral mechanical hyperalgesia and heat hypoalgesia .
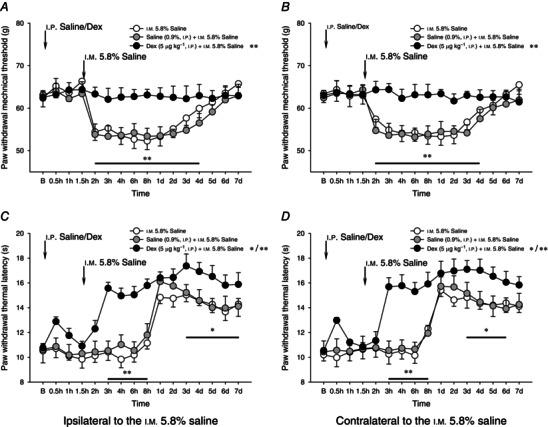
Effects of pre‐emptive injection of dexmedetomidine (Dex, 5 μg kg−1, i.p.) on unilateral i.m. injection of 5.8% saline‐induced bilateral mechanical hyperalgesia (A and B) and heat hypoalgesia (C and D). One and a half hours after the i.p. administration of Dex, a bolus of 0.2 ml 5.8% saline was intramuscularly injected into the GS muscle to elicit acute muscle nociception. Pre‐emptive injection of Dex significantly enhanced heat hypoalgesia during the 5.8% saline‐induced muscle nociception, whereas mechanical hyperalgesia was significantly prevented throughout the 7 day observation period. *P < 0.05 and **P < 0.001 compared with the vehicle (0.9% saline) treatment (n = 10 for each group). B, baseline responses before the i.p. administration of either 0.9% saline or Dex.
Discussion
We report here different effects of fentanyl and Dex on modulation of noxious mechanically and heat‐evoked nociception. Whereas fentanyl produced rapidly developing antinociceptive effects in both situations, Dex failed to affect mechanically evoked nociception. Dex did, however, produce both rapid and slowly developing attenuation of noxious heat‐induced pain. This slowly developing effect was quite novel as it took hours to develop and persisted for at least 7 days following a single i.p. injection. These findings as well as the effects of thalamic and DLF lesions on drug effects suggest that Dex downregulates the triggering threshold of descending inhibition, and/or upregulates the triggering threshold of descending facilitation. Descending inhibition is therefore increased and/or descending facilitation is attenuated. Pre‐emptive injection of Dex may engage this mechanism to provide long‐term relief of pain produced by i.m. injection of 5.8% saline. By contrast, pre‐emptive injection of fentanyl in this situation may actually worsen rather than attenuate pain.
Effects of fentanyl and Dex on modulation of nociception: peripheral or central mechanisms
To date, opioids and their analogues have been widely regarded as the first‐line analgesic in treatment and relief of surgical pain and other pathological pain, i.e. cancer pain (Laux‐Biehlmann et al. 2013). Despite their analgesic effects, the long‐term effectiveness of opioids has been much debated with respect to their ability to produce tolerance and dependence, which can lead to misuse and over‐prescription (Colpaert, 1996). There is also evidence that long‐term administration of opioid analgesics can produce hyperalgesia (Celerier et al. 2000). This paradoxical phenomenon of ‘analgesia–hyperalgesia’ raises interest in exploring the targeting site of opioids, in particular opioid μ‐receptor agonists, on neural activities.
From anatomical perspectives, it has been accepted that opioid receptors localize at both the peripheral and the central nervous systems (Mansour et al. 1987; Coggeshall et al. 1997). The antinociceptive effects of i.p. fentanyl on noxious paw withdrawal reflexes presented in Fig. 1 were initially assumed to be dependent on both peripheral and central effects. The findings shown in Fig. 3 seem to only support fentanyl's peripheral and spinal actions rather than supraspinal antinociceptive actions as the lesion of DLF failed to influence the fentanyl‐induced analgesia. However, the peripheral and spinal actions do not explain the effects of pre‐emptive fentanyl injection on 5.8% saline intramuscularly induced muscle nociception: a slowing developing increase in mechanical hyperalgesia and an attenuation of heat hypoalgesia (Fig. 7). Our previous study demonstrated that opioid μ‐receptors are involved in thalamic MD nucleus‐mediated endogenous descending facilitation (You et al. 2014). We suggest here that the fentanyl‐induced peripheral and spinal antinociceptive actions mask and counteract its supraspinal facilitatory control of nociception. The significant increase in mechanical hyperalgesia during 5.8% saline‐induced muscle nociception might be attributable to descending facilitatory action of fentanyl metabolities (You et al. 2014). Due to this ‘analgesia–hyperalgesia’ effect, it is further suggested that single bolus, but not continuous, administration of opioids, in particular a μ‐opioid receptor agonist, is not a valid way to meet the criteria for clinical pre‐emptive analgesia.
It is well known that Dex administrated in the perioperative period has sedative, analgesic, sympatholytic and anxiolytic effects, and may reduce the requirements for volatile anaesthetics, sedatives and analgesics without causing significant respiratory depression (Buerkle & Yaksh, 1998; Paris & Tonner, 2005; Tan & Ho, 2010). With respect to its effects on the regulation of pain, the action site of Dex at the level of either the spinal cord or the supraspinal regions has been debated (Stone et al. 1997; Malmberg et al. 2001; Sanders et al. 2005). Furthermore, it is not yet known if anaesthesic and sedative effects induced by Dex persist over time during and after the administration (Pertovaara et al. 1994). We here report that 1–10 μg kg−1 Dex failed to affect the mechanical paw withdrawal threshold, but significantly prolonged the noxious heat‐evoked paw withdrawal latency in a bi‐phasic manner (Fig. 2). Of particular importance, the prolonged increase in noxious heat‐evoked paw withdrawal latencies in the second phase 2–3 h following the i.p. Dex was significantly different from that in the first phase. Lesion of either the DLF or the bilateral thalamic VM nuclei completely blocked the occurrence of Dex‐induced second phase analgesia, indicating an important involvement of descending modulation from the supraspinal region. It has been documented that the effects of Dex may not persist for more than 2–3 h (Ebert et al. 2000; Riker et al. 2009). From the present study, i.c. administration of either atimezole or WAY‐100635 into the bilateral thalamic VM nuclei dose‐dependently reversed the Dex‐induced prolonged heat‐evoked paw withdrawal latencies in the second phase (Fig. 6). These results confirm previous findings showing that both adrenergic and serotonergic pathways are involved in descending modulation of nociception (Tjølsen et al. 1991; Heinricher et al. 2009; You et al. 2010, Lei et al. 2011; Pertovaara, 2013). Combining these, the late occurrence of antinociceptive effects of Dex found in the current study is believed to be not dependent on Dex per se, but relies on its central effects on activities of the thalamic VM nuclei, which exhibited descending inhibition (Lei & You, 2013; You et al. 2013, 2014).
Pre‐emptive analgesia and its action on endogenous descending modulation of pain: enhancing inhibition and weakening facilitation
It is accepted that perioperative pain control by various pharmacological manipulations that are referred to as pre‐emptive analgesia may prevent the establishment of central sensitization, and therefore decrease the perception of pain during and after the operation (Woolf & Chong, 1993). Despite procedures to limit central sensitization by blocking nociceptive afferents via local anaesthetic, post‐operative pain from deep tissue, in particular visceral organs, during and after the surgery cannot to be effectively and simply treated in this way. There is therefore an urgent need to develop therapies aimed at central structures, in particular supraspinal structures, so as to depress surgically induced central sensitization.
With regard to descending modulation, the more active descending inhibitory activity, the more pronounced antinociceptive effects would be expected. However, in the unstimulated, pain‐free condition descending facilitatory and inhibitory controls are physiologically ‘silent’ or inactive, and can be triggered by sufficient activity in C‐fibre afferents associated with temporal and spatial summation (You et al. 2010). Most importantly, the triggering threshold for descending facilitation is significantly lower than that for inhibition (You et al., 2010, 2013, 2014; Lei et al. 2011; Lei & You, 2013). It therefore remains to be determined whether descending inhibition can be selectively activated without excitation of descending facilitation (Fig. 9 A).
Figure 9. Variations of triggering thresholds of endogenous descending controls of nociception .
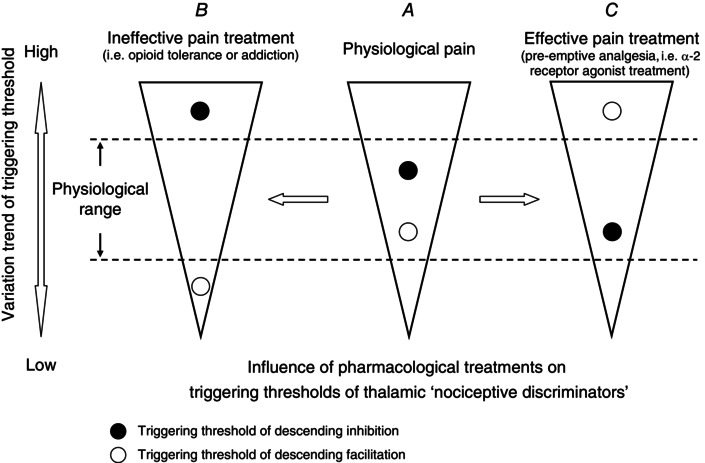
Conceptual diagram of variations of triggering thresholds of endogenous descending controls of nociception governed by thalamic ‘nociceptive discriminators’ during the conditions of physiological pain (A), ineffective pain treatment (B) and pain receiving efficacy control, i.e. α2 receptor agonist induced pre‐emptive analgesia (C). The triggering thresholds of the thalamic ‘nociceptive discriminators’, in control of descending facilitation and inhibition, are not solid, but exhibit plastic characteristics when exposed to pain and its pharmacological interference. Fentanyl decreases the triggering threshold of descending facilitation, and elevates the triggering threshold of descending inhibition (B). By contrast, Dex reverses the triggering thresholds of descending modulation as a result of a lower threshold of descending inhibition associated with higher threshold of descending facilitation (C). For a detailed interpretation see the Discussion.
After exposure to a single bolus injection of fentanyl, enhanced mechanical hyperalgesia with an absence of secondary heat hypoalgesia was found during the i.m. 5.8% saline‐induced muscle nociception (Fig. 7). Combined with the previous finding (You et al. 2014), we hypothesize that fentanyl's central action may have opposite effects on descending modulation: downregulation of the triggering threshold of descending facilitation and upregulation of the triggering threshold of descending inhibition (Fig. 9 B). From this point of view, it is not difficult to interpret why after the fentanyl treatment the descending inhibition of noxious heat‐evoked nociception was absent during the 5.8% saline intramuscularly induced muscle nociception as the i.m. noxious afferents irritated by 5.8% saline are probably insufficient to trigger the elevated triggering threshold of descending inhibition induced by fentanyl.
The other striking finding from the present study is that the post‐effects of i.p. Dex on mechanically and heat‐evoked paw withdrawal reflexes during the 5.8% saline‐induced muscle nociception were significantly different from that of in fentanyl treatment (Figs. 7 and 8). With regard to the pharmacokinetic profile, the terminal half‐life of Dex plasma concentration is about 1 h in rats (Bol et al. 1997). The effect of Dex may not persist for more than 2–3 h in clinical trials (Ebert et al. 2000; Riker et al. 2009). However, the noxious heat‐evoked prolongation of paw withdrawal latency appeared 1–2 day after the administration of Dex (Fig. 2 C, D). This finding, while preliminary, further suggests that the triggering thresholds of descending facilitation and inhibition are not invariable but exhibit plastic characteristics during exposure to pharmacological interference. Treatment with Dex may significantly reverse the triggering thresholds of descending facilitation and inhibition as a result of a lower threshold of descending inhibition associated with a much higher threshold of descending facilitation (Fig. 9 C). As such, it is not surprising that no mechanical hyperalgesia was found during persistent muscle nociception given that after the pre‐emptive Dex treatment i.m. insults by 5.8% saline might not be sufficient to reach the elevated triggering threshold of ‘silent’ or inactive descending facilitation. However, the detailed electrophysiological characteristics of thalamic ‘nociceptive discriminators’, i.e. MD and VM nuclei, in responses to noxious stimulation and pharmacological interventions should be explored in the future.
In conclusion, compared with fentanyl's potent peripheral and spinal antinociceptive effects followed by supraspinal pro‐nociceptive effects, we here report Dex‐induced specific actions on endogenous, discriminative modulation of nociception. Dex enhanced and weakened descending inhibition and facilitation by means of upregulation of the triggering threshold of descending facilitation and downregulation of the triggering threshold of descending inhibition. From pharmacological perspectives, with exploration of the action of analgesics, except for studies performed principally on their immediate effects, more attention should been paid to post‐effects, which may exert complex modulatory actions on activities of descending controls of pain. On this basis, we hypothesize that medical prescription involving a single bolus injection of fentanyl might not be appropriate in producing pre‐emptive analgesia.
Additional information
Conflict of Interest
The authors have declared that no conflict of interest exists.
Author contributions
H.‐J.Y. contributed to the conception and design of the experiments, conducted the experiments and performed data analysis and interpretation, and drafted the paper. J.L. conducted the experiments and performed the data analyses, data discussion and interpretation, and assisted in the writing. Y.X., G.Y., Z.‐H. S., N.N. and L.Y. contributed to the conduction of the experiments and data analysis. All authors approved the final version of the manuscript.
Funding
The present work was supported by grants from the National Natural Science Foundation of China (81271228, 81473752) to H.‐J.Y.
References
- Basbaum AI & Fields HL (1978). Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 4, 451–462. [DOI] [PubMed] [Google Scholar]
- Basbaum AI & Fields HL (1979). The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 187, 513–531. [DOI] [PubMed] [Google Scholar]
- Belcher G, Ryall RW & Schaffner R (1978). The differential effects of 5‐hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non‐nociceptive dorsal horn interneurones in the cat. Brain Res 151, 307–321. [DOI] [PubMed] [Google Scholar]
- Besson JM & Chaouch A (1987). Peripheral and spinal mechanisms of nociception. Physiol Rev 67, 67–186. [DOI] [PubMed] [Google Scholar]
- Bol CJJG, Danhof M, Stanski DR & Mandema JW (1997). Pharmacokinetic–pharmacodynamic characterization of the cardiovascular, hypnotic, EEG and ventilatory responses to dexmedetomidine in the rat. J Pharmacol Exp Ther 283, 1051–1058. [PubMed] [Google Scholar]
- Buerkle H & Yaksh TL (1998). Pharmacological evidence for different alpha2‐adrenergic receptor sites mediating analgesia and sedation in the rat. Br J Anaesth 81, 208–215. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P & Simonnet G (2000). Long‐lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology 92, 465–472. [DOI] [PubMed] [Google Scholar]
- Cervero F & Wolstencroft JH (1984). A positive feedback loop between spinal cord nociceptive pathways and antinociceptive areas of the cat's brain stem. Pain 20, 125–138. [DOI] [PubMed] [Google Scholar]
- Crile GW (1913). The kinetic theory of shock and its prevention through anoci‐association (shockless operation). Lancet 185, 7–16. [Google Scholar]
- Coggeshall RE, Zhou S & Carlton SM (1997). Opioid receptors on peripheral sensory axons. Brain Res 764, 126–132. [DOI] [PubMed] [Google Scholar]
- Colpaert FC (1996). System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev 48, 355–402. [PubMed] [Google Scholar]
- Ebert TJ, Hall JE, Barney JA, Uhrich TD & Colinco MD (2000). The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 93, 382–394. [DOI] [PubMed] [Google Scholar]
- Fields HL, Anderson SD, Clanton CH & Basbaum AI (1976). Nucleus raphe magnus: a common mediator of opiate‐ and stimulus‐produced analgesia. Trans Am Neurol Assoc 101, 208–210. [PubMed] [Google Scholar]
- Fields HL (1992). Is there a facilitating component to central pain modulation? Am Pain Soc J 1, 71–78. [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber LH, Martin RF, Chung JM & Willis WD (1980). Inhibition and excitation of primate spinothalamic tract neurons by stimulation in region of nucleus reticularis gigantocellularis. J Neurophysiol 43, 1578–1593. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL & Lumb BM (2009). Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 60, 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Peck CC, Petersen ET, Rae CD, Youssef AM, Reeves JM, Wilcox SL, Akhter R, Murray GM & Gustin SM (2013). Chronic pain: lost inhibition? J Neurosci 33, 7574–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux‐Biehlmann A, Mouheiche J, Vérièpe J & Goumon Y (2013). Endogenous morphine and its metabolites in mammals: history, synthesis, localization and perspectives. Neuroscience 233, 95–117. [DOI] [PubMed] [Google Scholar]
- Lei J & You HJ (2013). Endogenous descending facilitation and inhibition differ in control of formalin intramuscularly induced persistent muscle nociception. Exp Neurol 248, 100–111. [DOI] [PubMed] [Google Scholar]
- Lei J, Jin L, Zhao Y, Sui MY, Huang L, Tan YX, Chen YK & You HJ (2011). Sex‐related differences in descending norepinephrine and serotonin controls of spinal withdrawal reflex during intramuscular saline induced muscle nociception in rats. Exp Neurol 228, 206–214. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Hedley LR, Jasper JR, Hunter JC & Basbaum AI (2001). Contribution of α2 receptor subtypes to nerve injury‐induced pain and its regulation by dexmedetomidine. Br J Pharmacol 132, 1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour et al., 1987 Mansour A, Khachaturian H, Lewis ME, Akil H & Watson SJ (1987). Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7, 2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Millan MJ (2002). Descending control of pain. Prog Neurobiol 66, 355–474. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K & Porreca F (2014). Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 8, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris A & Tonner PH (2005). Dexmedetomidine in anaesthesia. Current Opin Anaesthesiol 18, 412–418. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1998). The Rat Brain in Stereotaxic Coordinates, 4th edn Elsevier Academic Press, San Diego. [Google Scholar]
- Pertovaara A, Hämäläinen MM, Kauppila T, Mecke E & Carlson S (1994). Dissociation of the α2‐adrenergic antinociception from sedation following microinjection of medetomidine into the locus coeruleus in rats. Pain 57, 207–215. [DOI] [PubMed] [Google Scholar]
- Pertovaara A (2013). The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol 716, 2–7. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH & Gebhart GF (2002). Chronic pain and medullary descending facilitation. Trends Neurosci 25, 319–325. [DOI] [PubMed] [Google Scholar]
- Ren K & Dubner R (2009). Descending control mechanisms In Science of Pain, ed. Basbaum AI, Bushnell MC, pp. 723–762. Elsevier, San Diego. [Google Scholar]
- Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW & Rocha MG (2009). Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 301, 489–499. [DOI] [PubMed] [Google Scholar]
- Saadé NE, Atweh SF, Jabbur SJ & Wall PD (1990). Effects of lesion in the anterolateral columns and dorsolateral funiculi on self‐mutilation behaviour in rats. Brain Res 42, 313–323. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Giombini M, Ma D, Ohashi Y, Hossain M, Fujinaga M & Maze M (2005). Dexmedetomidine exerts dose‐dependent age‐independent antinociception but age‐dependent hypnosis in Fischer rats. Anesth Analg 100, 1295–1302. [DOI] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE & Wilcox GL (1997). The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic‐opioid synergy. J Neurosci 17, 7157–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JA & Ho KM (2010). Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta‐analysis. Intensive Care Med 36, 926–939. [DOI] [PubMed] [Google Scholar]
- Tjølsen A, Berge OG & Hole K (1991). Lesions of bulbo‐spinal serotonergic or noradrenergic pathways reduce nociception as measured by the formalin test. Acta Physiol Scand 142, 229–236. [DOI] [PubMed] [Google Scholar]
- Woolf CJ & Chong MS (1993). Preemptive analgesia – treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77, 363–379. [DOI] [PubMed] [Google Scholar]
- You HJ, Mørch CD, Chen J & Arendt‐Nielsen L (2003). Differential antinociceptive effects induced by a selective cyclooxygenase‐2 inhibitor (SC‐236) on dorsal horn neurons and spinal withdrawal reflexes in anesthetized spinal rats. Neuroscience 121, 459–472. [DOI] [PubMed] [Google Scholar]
- You HJ, Colpaert FC & Arendt‐Nielsen L (2005). Nociceptive spinal withdrawal reflexes but not spinal dorsal horn wide‐dynamic range neuron activities are specifically inhibited by halothane anaesthesia in spinalized rats. Eur J Neurosci 22, 354–360. [DOI] [PubMed] [Google Scholar]
- You HJ, Lei J, Sui MY, Huang L, Tan YX, Tjølsen A & Arendt‐Nielsen L (2010). Endogenous descending modulation: spatiotemporal effect of dynamic imbalance between descending facilitation and inhibition of nociception. J Physiol 588, 4177–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, Lei J, Niu N, Yang L, Fan XL, Tjølsen A & Li Q (2013). Specific thalamic nuclei function as novel ‘nociceptive discriminators’ in the endogenous control of nociception in rats. Neuroscience 232, 53–63. [DOI] [PubMed] [Google Scholar]
- You HJ, Lei J, Ye G, Fan XL & Li Q (2014). Influence of intramuscular heat stimulation on endogenous modulation of nociception: complex role of different subtypes of central opioid receptors in descending facilitation and inhibition. J Physiol 592, 4365–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]


