Abstract
Key points
Aerobic exercise, such as running, enhances adult hippocampal neurogenesis (AHN) in rodents.
Little is known about the effects of high‐intensity interval training (HIT) or of purely anaerobic resistance training on AHN.
Here, compared with a sedentary lifestyle, we report a very modest effect of HIT and no effect of resistance training on AHN in adult male rats.
We found the most AHN in rats that were selectively bred for an innately high response to aerobic exercise that also run voluntarily and increase maximal running capacity.
Our results confirm that sustained aerobic exercise is key in improving AHN.
Abstract
Aerobic exercise, such as running, has positive effects on brain structure and function, such as adult hippocampal neurogenesis (AHN) and learning. Whether high‐intensity interval training (HIT), referring to alternating short bouts of very intense anaerobic exercise with recovery periods, or anaerobic resistance training (RT) has similar effects on AHN is unclear. In addition, individual genetic variation in the overall response to physical exercise is likely to play a part in the effects of exercise on AHN but is less well studied. Recently, we developed polygenic rat models that gain differentially for running capacity in response to aerobic treadmill training. Here, we subjected these low‐response trainer (LRT) and high‐response trainer (HRT) adult male rats to various forms of physical exercise for 6–8 weeks and examined the effects on AHN. Compared with sedentary animals, the highest number of doublecortin‐positive hippocampal cells was observed in HRT rats that ran voluntarily on a running wheel, whereas HIT on the treadmill had a smaller, statistically non‐significant effect on AHN. Adult hippocampal neurogenesis was elevated in both LRT and HRT rats that underwent endurance training on a treadmill compared with those that performed RT by climbing a vertical ladder with weights, despite their significant gain in strength. Furthermore, RT had no effect on proliferation (Ki67), maturation (doublecortin) or survival (bromodeoxyuridine) of new adult‐born hippocampal neurons in adult male Sprague–Dawley rats. Our results suggest that physical exercise promotes AHN most effectively if the exercise is aerobic and sustained, especially when accompanied by a heightened genetic predisposition for response to physical exercise.
Key points
Aerobic exercise, such as running, enhances adult hippocampal neurogenesis (AHN) in rodents.
Little is known about the effects of high‐intensity interval training (HIT) or of purely anaerobic resistance training on AHN.
Here, compared with a sedentary lifestyle, we report a very modest effect of HIT and no effect of resistance training on AHN in adult male rats.
We found the most AHN in rats that were selectively bred for an innately high response to aerobic exercise that also run voluntarily and increase maximal running capacity.
Our results confirm that sustained aerobic exercise is key in improving AHN.
Abbreviations
- AHN
adult hippocampal neurogenesis
- BDNF
brain‐derived neurotrophic factor
- BrdU
bromodeoxyuridine
- HIT
high‐intensity interval training
- HRT
high‐response trainer
- LRT
low‐response trainer
- RW
running wheel
- Sed
sedentary
- TBS
Tris‐buffered saline
maximal oxygen uptake
Introduction
Adult hippocampal neurogenesis (AHN) is a continuous process through which cells proliferate in the subgranular zone of the dentate gyrus, mature into granule cells and, ultimately, become incorporated into hippocampal neuronal networks (for review, see Aimone et al. 2014). In rodents, adult‐born hippocampal neurons seem crucial for a variety of adaptive behaviours, such as learning (Shors et al. 2001), pattern separation (Clelland et al. 2009) and responses to stress (Snyder et al. 2011). Aerobic exercise, e.g. running, increases AHN and improves cognitive performance in both male and female adult rodents (van Praag et al. 1999, 2005; Creer et al. 2010; Marlatt et al. 2012; Fardell et al. 2012). The increase in AHN in response to running is reported to be due in part to an increase in the number of surviving neuronal precursor cells (type 2) rather than to the shortening of the cell cycle (Fischer et al. 2014). There are also studies indicating that running increases the survival and incorporation of newly divided hippocampal cells, generated days before commencing training, to increase net neurogenesis (Lee et al. 2013; Castilla‐Ortega et al. 2014). The increase in AHN is considered to be mediated by an upregulation of factors including brain‐derived neurotrophic factor (BDNF; Farmer et al. 2004; Li et al. 2008; Marlatt et al. 2012) and insulin‐like growth factor 1 (Carro et al. 2000; Trejo et al. 2001). In addition to the hippocampus, exercise also increases adult neurogenesis in the subventricular zone (Bednarczyk et al. 2009; Chae et al. 2014) and in the hypothalamus (Niwa et al. 2015), suggesting that the neurogenic effect of exercise might occur throughout the brain.
Examining the effects of exercise on AHN in animal models has been, for the most part, limited to studying the effects of running. Among humans, different forms of anaerobic exercise, such as resistance training, modelled in rats by using a progressive training regimen on a vertical ladder with weights attached to the base of the tail (Hornberger & Farrar, 2004), as well as combinations of aerobic and anaerobic exercise, such as high‐intensity interval training (HIT; for review, see Gibala et al. 2012), are gaining in popularity. High‐intensity interval training in rodents refers to a regimen of alternating brief bouts of very intense anaerobic exercise, such as running on a treadmill at ∼85–90% of maximal speed, with short recovery periods and repeating this cycle to accomplish a training session of up to an hour in duration (see, for example, Haram et al. 2009). High‐intensity interval training can increase maximal oxygen uptake (), a commonly used measure of aerobic fitness, in rodents to a similar extent or even more than moderate sustained aerobic exercise (Haram et al. 2009). A recent study conducted on healthy adult male rats reported a larger increase in inducers of neuroprotective factors (H2O2 and tumour necrosis factor α), as well as in BDNF and glial cell line‐derived neurotrophic factor in whole‐brain samples in response to HIT compared with continuous training (Afzalpour et al. 2015). To our knowledge, the direct effects of HIT on AHN have not been studied, and few reports exist on the effects of purely anaerobic exercise on AHN. In adult male rats, progressive resistance training on a vertical ladder increased insulin‐like growth factor 1 but not BDNF expession in the hippocampus (Cassilhas et al. 2012 a). Resistance training is also reported to promote hippocampal cell proliferation (Novaes Gomes et al. 2014) and to improve spatial learning (Cassilhas et al. 2012 a,b). It should be noted that forced exercise usually involves the use of a reward or punishment to motivate animals to perform. For example, mild electrical shocks are routinely used to encourage rats to keep running on a treadmill. These shocks are likely to cause negative stress to the animals. It is well documented that especially prolonged and unpredictable stress inhibits adult neurogenesis (for a recent review, see Lucassen et al. 2015), whereas mild stress can enhance AHN (Parihar et al. 2011).
In addition to the type of physical exercise, the effects of exercise on AHN are likely to be influenced, in part, by individual variation in responsiveness to exercise training. Although aerobic exercise is, on average, beneficial for health, its effects vary between individuals, presumably as a result of considerable genetic variance. For some, aerobic training provides substantial gain in maximal aerobic capacity () and metabolic health, whereas for others the same amount of training results in little or even negative change (Bouchard & Rankinen, 2001; see also Timmons, 2011; Bouchard et al. 2012). Several clinically supervised exercise training studies report that up to 20% of participants fail to increase and can be considered non‐responders. Studies examining predictors of non‐response show that groups that exercise at greater volumes (longer durations at same relative intensity) are associated with a higher probability of responding (Sisson et al. 2009).
Recently, we developed a contrasting rat model system for low (LRT) and high (HRT) response to aerobic exercise training (Koch et al. 2013). Starting with a founder population of genetically heterogeneous rats (N/NIH stock), we applied two‐way artificial selection based on the magnitude of change in running capacity after completing 8 weeks of standardized aerobic treadmill training. After 15 generations of selection, rats bred as HRT increased maximal treadmill running distance from 646 to 869 m (change, 223 ± 20 m), whereas rats bred as LRT decreased from 620 to 555 m (change, −65 ± 15 m) after completing the same absolute amount of training (Koch et al. 2013). Note that only rats that complete the exercise regimen are used for breeding to ensure that no underlying difference in motivation to exercise develops between the two rat lines. As a result of selection, the vast majority of HRT rats show a clear increase in a number of markers of cardiorespiratory fitness, such as running capacity, and cardiac muscle cell function (Wisløff et al. 2015). In contrast, these same indices of cardiorespiratory fitness do not change or even decrease in LRT rats, despite successful completion of the training regimen. Importantly, no baseline difference in cardiorespiratory fitness exists between the LRT and HRT rats, i.e. in non‐trained conditions, both LRT and HRT rats have comparable exercise capacities (Wisløff et al. 2015). Compared with HRT rats, LRT rats demonstrate impaired skeletal muscle angiogenesis, have altered signal transduction for JNK and p38 mitogen‐activated protein kinase (Lessard et al. 2013) and have lower levels of mitochondrial biogenesis‐regulating factors (peroxisome proliferator‐activated receptor‐gamma coactivator 1 alpha, nuclear respiratory factor 1 and mitochondrial transcription factor A; Marton et al. 2015), suggesting diminished exercise‐induced plasticity in the muscle. A microarray of RNA from skeletal muscle indicates large differences between LRT and HRT rats in transcriptional responses to the same exercise bout (Lessard et al. 2013). Indeed, the reason for development of the HRT and LRT rats was to provide contrasting polygenic models in order to explore in more mechanistic detail the so‐called energy transfer hypothesis (Koch & Britton, 2008). According to this hypothesis, the capacity for energy transfer, as measured typically through maximal aerobic exercise capacity, could be the central mechanistic determinant underlying the divide between complex metabolic disease and health.
Here, using this unique contrasting rat model system of HRT and LRT rats, we aimed to test whether HIT (experiment 1) and resistance training (experiments 2 and 3) are comparable to aerobic exercise (wheel running and endurance treadmill training) in terms of promoting AHN in male rats. Based on the literature reviewed above, we expected HIT to promote AHN. In terms of resistance training, we anticipated to see an increase in the number of proliferating cells in the hippocampus (Novaes Gomes et al. 2014). We hypothesized that there would be no effect of genetic predisposition on baseline AHN, but that compared with sedentary control animals, exercise activity, in general, would promote AHN more in HRT rats relative to LRT rats.
Methods
Ethical approval
All the experimental procedures were implemented in accordance with the directive 2010/63/EU of the European Parliament and approved by the National Animal Experiment Board, Finland.
Animals and groups
All animals were housed on the premises of the animal research unit at the University of Jyväskylä. Food and water were freely available, and room temperature and humidity were controlled at 21 ± 2°C and 50 ± 10%, respectively. All rats had aspen chips (Tapvei, Kaavi, Finland) at the bottom of the cage as bedding material. Rats in cages without running wheels were provided with wooden toys. Rats were maintained on a 12 h–12 h light–dark cycle, with lights on at 08.00 h. All procedures were conducted during the light portion of the cycle.
Experiment 1
The outline of the experiment is depicted in Fig. 1 A. The animals were 88 adult male LRT and HRT rats, representing the 17th generation of these rat lines developed by selective breeding and maintained at the University of Michigan (Ann Arbor, MI, USA; Koch et al. 2013). All rats were singly housed and were ∼8 months old at the beginning of the experiment. The mean ± SEM body weight was 388 ± 6 g in HRT rats and 403 ± 6 g in LRT rats. The animals were not phenotyped for their training response in order to keep them naive to exercise training. Animals from both LRT and HRT lines were divided into the following four treatment groups. (i) Sedentary (Sed) rats were not subjected to any physical exercise and spent the entire time in their home cage (Tecniplast 1354, Italy; size: 595 mm × 380 mm × 200 mm). (ii) Control (C) rats were tested for aerobic capacity at the beginning and end of the 7 week experiment. Half of the animals in the C group were housed in standard cages and half in cages (Tecniplast 2154; 480 mm × 265 mm × 210 mm) fitted with a disabled running wheel. (iii) Running (RW) rats were housed in cages fitted with active running wheels for the duration of the exercise intervention (7 weeks). Animals in the RW group were also tested for aerobic capacity before and after exposure to running wheels. (iv) High‐intensity interval training (HIT) rats were housed in regular cages and trained on a treadmill three times a week. Rats in the HIT group were tested for aerobic capacity once a week to keep the training parameters optimal (please see the details in the section on ‘Tests of exercise capacity and training regimens’ below). As a result, the following eight groups were formed: LRT‐Sed (n = 8), LRT‐C (n = 12), LRT‐RW (n = 12), LRT‐HIT (n = 12), HRT‐Sed (n = 8), HRT‐C (n = 12), HRT‐RW (n = 12) and HRT‐HIT (n = 12).
Figure 1. Experiment 1 indicated that the number of doublecortin‐positive new neurons was highest in rats genetically predisposed to a high response to training and in which aerobic fitness consequently increased significantly in response to voluntary wheel running .
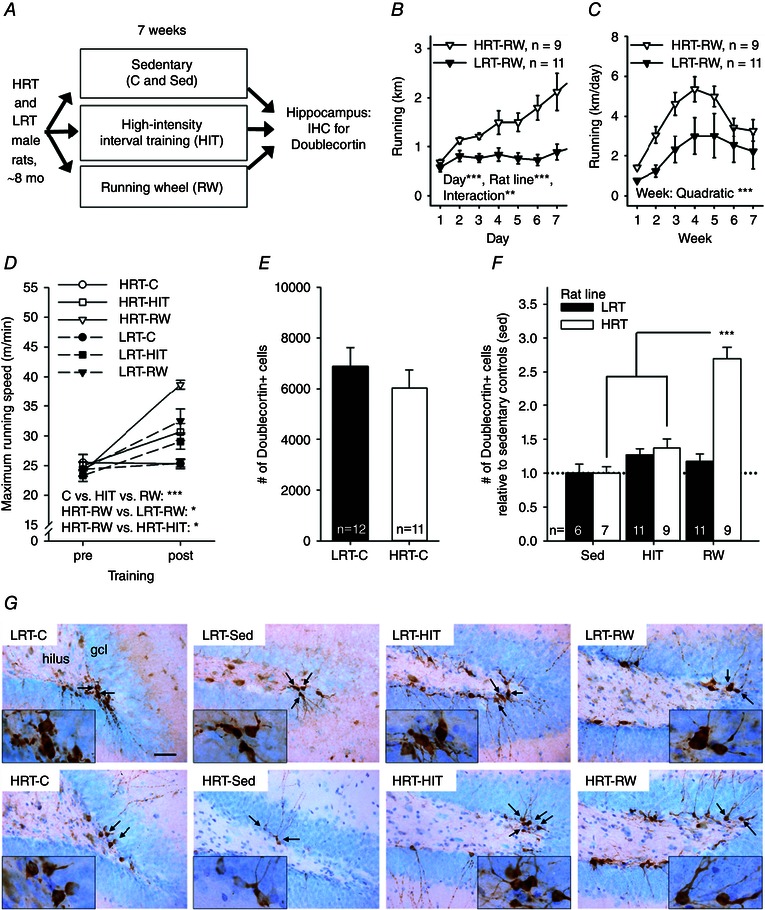
Abbreviations: C, control; gcl, granule cell layer; HIT, high‐intensity interval training; HRT, high‐response trainer; IHC, immunohistochemistry; LRT, low‐response trainer; mo, months old; RW, running wheel; and Sed, sedentary. A, outline of the experiment. B, running in rats allowed access to running wheels throughout the first 7 days. The HRT rats increased running more compared with LRT rats. C, throughout the whole 7 week training period, the running distance was comparable in both rat lines and changed in a quadratic manner. D, running speed increased in both LRT and HRT rats in response to either HIT or RW. The biggest improvement was seen in HRT rats voluntarily training on a running wheel. E, there was no baseline difference in adult hippocampal neurogenesis in the two rat lines. F, however, when allowed to run freely on a wheel in the home cage for 7 weeks, the HRT rats showed a higher number of new neurons in the hippocampus compared with rats of the same rat line either not trained at all (Sed) or subjected to HIT three times a week. G, representative examples of doublecortin‐positive cells (brown) in the dentate gyrus in each group of rats are illustrated. The background stain (Cresyl Violet) shows cell bodies of mature neurons in light blue. The hilus and the granule cell layer are indicated in the top left panel. The scale bar length is 50 μm, and the scale is the same in all panels (original magnification, ×400). Insets depict cell groups positive for doublecortin at ×1000 magnification, and arrows point to these cells in the ×400 photos. In B–F, vertical lines depict the SEM. Asterisks refer to statistically significant effects/differences, as follows: *P < 0.05, **P < 0.01 and ***P < 0.001.
Experiment 2
The outline of the experiment is depicted in Fig. 2 A. The animals were 28 adult male LRT and HRT rats representing the 18th generation of these rat lines. Upon arrival at the University of Jyväskylä, the rats were allowed to acclimate for 4–5 weeks. After this, when the rats were ∼6 months old, they were tested for their response to aerobic exercise. All rats were subjected to an 8 week exercise regimen, during which they were trained on a treadmill three times a week (see section ‘Tests of exercise capacity and training regimens’ for details). The rats were then divided into four training groups matched for body weight and capacity for aerobic training response, and training was either continued as aerobic treadmill endurance or changed to resistance ladder climb training, as follows: (i) LRT rats subjected to resistance training [LRT‐Res, n = 6; 417 ± 17 g, adaptive response to 8 weeks of aerobic training (see section “Tests of exercise capacity and training regimes” for details): 9 ± 5%]; (ii) LRT rats subjected to aerobic endurance training (LRT‐End, n = 8; 421 ± 14 g; 10 ± 6%); (iii) HRT rats subjected to resistance training (HRT‐Res, n = 6; 386 ± 15 g, 24 ± 16%); and (iv) HRT rats subjected to aerobic endurance training (HRT‐End, n = 8; 359 ± 9 g, 24 ± 16%).
Figure 2. Experiment 2 indicated more doublecortin‐positive new hippocampal neurons in rats subjected to endurance training on a treadmill compared with rats subjected to resistance training on a vertical ladder .
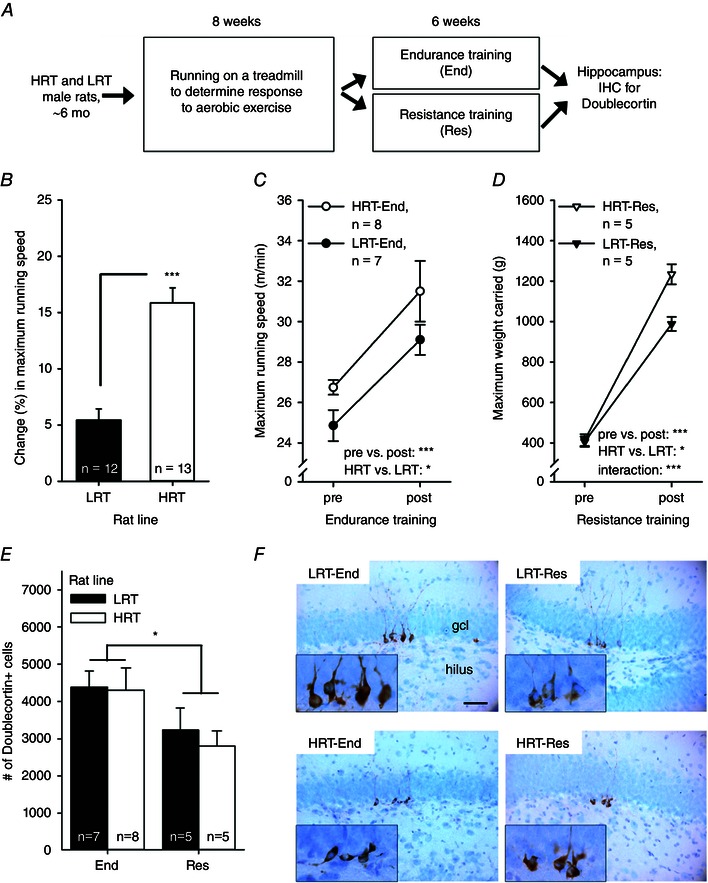
Abbreviations: End, endurance training; Res, resistance training; other abbreviations are as in the legend to Fig. 1. A, outline of the experiment. B, HRT rats showed a greater adaptive response to aerobic training, i.e. their percentage change in maximal running speed increased more than the LRT rats. C, subsequent endurance training on a treadmill resulted in a comparable increase in maximal running speed in both rat lines, but HRT rats outperformed LRT rats overall. D, resistance training led to a bigger improvement in strength in HRT compared with LRT rats. E, rats subjected to endurance training (regardless of rat line) had a greater number of new neurons in the hippocampus. F, representative examples of doublecortin‐positive cells (brown) in the tip of the dentate gyrus in each group of rats. The background stain (Cresyl Violet) shows cell bodies of mature neurons in light blue. The hilus and the granule cell layer are indicated in the top left panel. The scale bar length is 50 μm, and the scale is the same in all panels (original magnification, ×400). Insets illustrate the doublecortin‐positive cells at ×1000 magnifcation. In B–E, vertical lines depict the SEM. Asterisks refer to statistically significant differences, as follows: *P < 0.05 and ***P < 0.001.
Experiment 3
The outline of the experiment is depicted in Fig. 3 A. The animals were 20 outbred Sprague–Dawley male rats obtained from Harlan (The Netherlands). The rats were first allowed to acclimate for 2 weeks, after which experiments were started. The rats were 9 weeks old at the onset of experiments and weighed on average 276 ± 2 g. The rats were housed in pairs; one animal in each cage was subjected to resistance training (Res, n = 10) and the other was kept sedentary (Sed, n = 10).
Figure 3. Experiment 3 indicated no effect of 8 weeks of resistance training on a vertical ladder on adult hippocampal neurogenesis in male Sprague–Dawley rats .
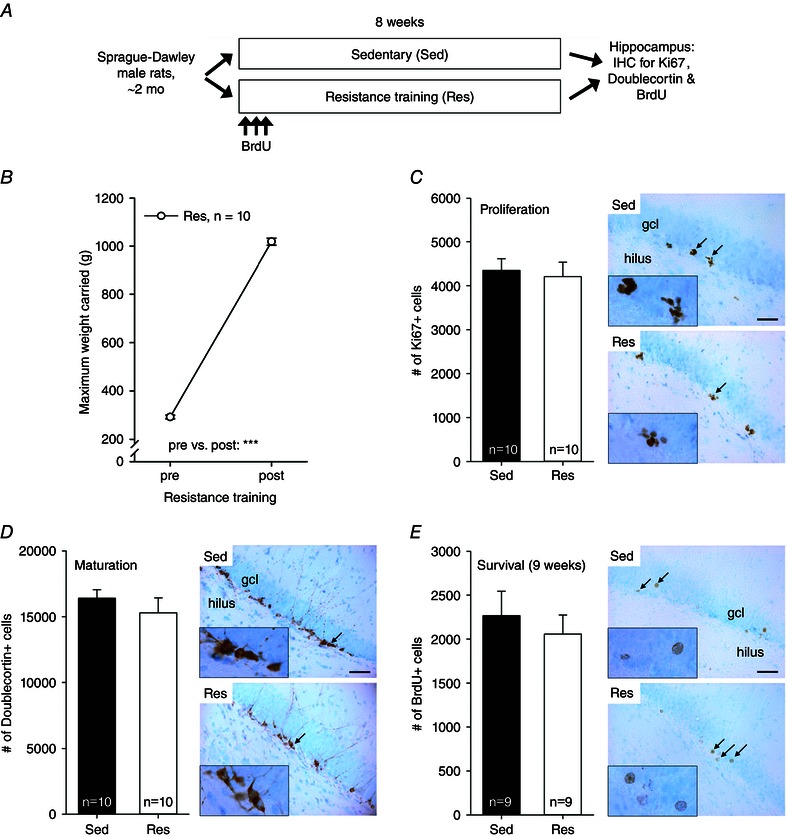
Abbreviations: BrdU, bromodeoxyuridine; End, endurance training; Res, resistance training; other abbreviations are as in the legend to Fig. 1. A, outline of the experiment. B, strength increased in all rats subjected to resistance training. Asterisks refer to a significant difference (***P < 0.001). Vertical lines depict the SEM. C, the proliferation of cells in the hippocampus was similar in the resistance training and sedentary groups. Black arrows mark the locations of clusters of Ki67‐positive cells (×400 magnification) illustrated in the insets (×1000 magnification). The background stain (Cresyl Violet) shows cell bodies of mature neurons in light blue. The hilus and the granule cell layer are indicated in the top panel. The scale bar length is 50 μm, and it applies to all the ×400 photomicrographs. D, animals subjected to resistance training and those kept sedentary had a similar number of immature adult‐born new neurons in the hippocampus. Doublecortin‐positive cells in brown; mature neurons in light blue. E, the survival of adult‐born neurons in the hippocampus was also not affected by resistance training. Bromodeoxyuridine‐positive cells in brown; mature neurons in light blue.
Tests of exercise capacity and training regimens
All protocols for testing maximal running capacity and for endurance training, conducted on a treadmill, were adapted from the protocol developed for selective breeding purposes (Koch et al. 2013). Across 15 generations (n = 3114), we find that more than 90% of phenotyped rats are successful in completing this type of training programme. Further details are presented below.
Experiment 1
Exercise test for maximal running capacity
The exercise test for maximal running speed was conducted before and after the training period. For all experiments, a custom‐made treadmill was used for testing maximal running capacity. The running space for each rat was 9 cm × 70 cm, and a 9 cm × 9 cm electrified grid was located at the end of the lane. The electrified grid at the end of the lane delivered a mild electrical shock (adjustable between 0.2 and 2 mA; usually set to 1 mA) and was used to help educate rats to run on the treadmill and to evaluate maximal running capacity. If a rat slid off the treadmill belt and stayed on the electrified grid for more than 5 s rather than continued running, the running test was stopped and the rat removed from the treadmill. Unfortunately, the treadmill did not include a counter for frequency of shock delivery. At the completion of each session, time spent on the treadmill running was recorded. The test protocol was as follows. Rats were first allowed to warm up for 5 min at a speed of 8–9 m min−1. The test itself began at a speed of 10 m min−1, after which the speed was increased by 2.4 m min−1 every other minute. The test was conducted three times, on consecutive days, and the best result (speed measured in metres per minute) obtained for each rat was considered the best estimate of maximal running capacity, as previously described (Koch & Britton, 2001).
Voluntary training on a running wheel
Rats had free access to a RW in the home cage for 7 weeks. The distance run was recorded continuously by a computerized system using custom‐made software. Data collection started when the RW started moving, and data were stored in 2 min bouts. No motivational punishments or rewards were used in conjunction with voluntary running on a running wheel.
High‐intensity interval training
Rats were subjected to HIT three times a week, with 1–2 days of recovery between each session, for 7 weeks. Each HIT session started with 5 min on the treadmill at a speed corresponding to 50–60% of the rat's individual maximal speed in the maximal capacity test. After that, each rat ran for 3 min at a speed corresponding to 85–90% of its maximum and then again for 2 min at a speed equal to 50% of its maximum. This 5 min HIT trial was conducted a total of three times per session, meaning that the duration of the session was 20 min overall. The inclination of the treadmill was 10 deg uphill during the entire training period. To keep the parameters for the HIT training regimen up to date, maximal running capacity was tested once a week (one test session, substituted for HIT training). During HIT, the electrified grid at the end of the treadmill lane was used to encourage rats to keep running (see also ‘Exercise test for maximal running capacity’ above); however, to minimize the use of shocks the rats were gently pushed by hand to help avoid stepping off the treadmill belt and onto the grid. If a rat repeatedly slid off the treadmill belt and stayed on the electrified grid for more than 5 s rather than continued running, the training was stopped and the rat removed from the treadmill.
Sedentary
Rats in the sedentary (C and Sed) groups did not exercise. Rats in the C group were tested for maximal running capacity (see ‘Exercise test for maximal running capacity’ above) before and after the 7 week intervention. Rats in the Sed group were not tested for maximal running capacity or given any type of physical training during the study.
Experiment 2
Test for adaptive response to aerobic training
The protocol included the following four phases: (i) familiarization; (ii) baseline maximal running capacity test before training; (iii) 8 weeks of aerobic training on a motorized treadmill; and (iv) maximal running capacity test after training, as previously described (Koch et al. 2013). In short, all the rats were familiarized with running on a treadmill for at least 3 days, 10–20 min at a time. Treadmill speed was mildly increased in each session so that each rat was able to run at a speed of 10 m min−1 at the end of familiarization. After that, the rats had at least 1 day of rest before they performed the pretraining maximal running capacity test. Speed‐ramped treadmill running tests were conducted three times to acquire a reliable measure of the baseline performance level. Each test was conducted with a 15 deg inclination, starting with a speed of 10 m min−1 without a warm‐up. The running speed was increased by 1 m min−1 every other minute until the rat was exhausted. Note that this test result reflects the baseline maximal running capacity of the rats, and no difference was expected (or observed; please see Results) between the HRT and the LRT rat lines.
Two to four days after the last baseline test, aerobic training was started. On the first day, the rats ran at a speed of 10 m min−1 for 20 min. The speed was gradually increased (by 1 m min−1) every other session up to a maximal speed of 20 m min−1, if the rat was able to maintain the speed during the training session. Also, the duration of training was increased by 0.5 min every session up to 30 min per session. Training was carried out three times a week (Monday, Wednesday and Friday) for 8 weeks. The treadmill inclination was kept at a constant 15 deg. Similar to HIT, pushing by hand and mild electric shocks were used to motivate animals to keep running on the treadmill. After training, the maximal running capacity was again tested with a procedure identical to that used during the baseline tests and maximal running speed recorded. Note that at this point, the HRT rats were expected (and observed; please see Results) to have increased their maximal running capacity more in comparison to the LRT rats, in which little or no increase was expected (and observed; please see Results and Fig. 2 B).
Endurance training
Training on a motorized treadmill was conducted three times per week for 6 weeks, with at least 1 day of rest between each of the training sessions. The inclination of the belt was kept constant at 15 deg uphill. The first training session lasted 25 min, and the duration of the session increased every week by 1 min. The starting speed for training was 60% relative to the average maximal running speed in each rat line and increased by 1 m min−1 every week. During each individual training session, the treadmill speed was kept constant. Maximal running capacity was tested once every 2 weeks and at the the end of the intervention.
Resistance training
The resistance training protocol was a modification from one used by Hornberger & Farrar (2004), and the training lasted for 6 weeks. The HRT and LRT rats were familiarized with a custom‐made vertical ladder (height × width: 90 cm × 15 cm, 2 cm separation between steps, 85 deg incline) on three occasions during the first week. On the first day, the rats climbed without an extra load. On the next 2 days, a load pouch containing lead weights corresponding to <50% of the rat's body weight was fixed to the proximal part of the tail with double‐sided tape and a Velcro strap. Next, the rats began a progressive resistance training period. The training sessions took place three times a week (Monday, Wednesday and Friday). The first load was 75% of the body weight of a rat. When the rat successfully accomplished the climb with this load (one trial), the load was increased by 30 g and the test repeated. The load was increased in 30 g increments until the rat could no longer reach the top of the ladder. The highest load the rat successfully carried to the top of the ladder was considered as the maximal carrying capacity for that session. Subsequent training sessions consisted of nine trials. During the first three climbs, 50, 75 and 90% of the previous maximal load was used. Then the load was increased by 30 g until a new maximal load was reached. Three trials were then attempted with this new maximal load. Between the climbing trials, the rats were allowed to rest for 90 s in an open chamber (length × width × height: 30 cm × 15 cm × 11 cm) located at the top of the ladder. Note that the rats were not punished or rewarded to motivate them to climb.
Experiment 3
Resistance training of young male Sprague–Dawley rats was carried out as explained in the previous subsection, but the duration of the regimen was increased to 8 weeks. Sedentary cage mates were placed in a custom‐made plywood box (20 cm × 30 cm × 25 cm) with a Plexiglas wall and ceiling, and placed in the training room for the duration of the training session.
Immunohistochemistry
Adult neurogenesis was studied using doublecortin (all experiments), bromodeoxyuridine (BrdU; experiment 3) and Ki67 (experiment 3) as markers.
Bromodeoxyuridine injections
Bromodeoxyuridine (Sigma, catlogue no. B5002) was injected i.p. at a dose of 200 mg kg−1 (15 mg ml−1, diluted in physiological saline) to mark dividing cells. Bromodeoxyuridine injections were performed during the first week of training, 2–6 h prior to the resistance training session. Thus, a total of three injections per rat were administered, once every other day. The dose is comparable to that used in several previous studies to mark dividing cells in the hippocampus, in order to study AHN (see, for example, Gould et al. 1999; Nokia et al. 2012), and no adverse effects of the injections were observed. The dose of 200 mg kg−1 is high enough to label the majority of dividing cells, yet low enough not to be toxic (Cameron & McKay, 2001). Based on a number of previous reports, ∼80% of the hippocampal dividing cells labelled with BrdU mature into neurons, indicated by double labelling for BrdU and neuronal markers such as doublecortin (Dalla et al. 2009), NeuN (Leuner et al. 2010) or TuJ1 (Cameron & McKay, 2001; Leuner et al. 2010).
Tissue preparation
Forty‐eight hours after the last training session/exposure to running wheels, animals were killed by exposure to a rising concentration of CO2, and death was verified by cardiac puncture. The brain was immediately extracted and postfixed in 4% paraformaldehyde in PBS solution (pH 7.4) at 4°C for 48 h, after which the paraformaldehyde solution was replaced with 0.1 m PBS (pH 7.4). Next, coronal sections (40 μm thick) were cut through the entire dentate gyrus of the left or right (randomized) hippocampus with a vibratome (Leica VT 1000 S). Every 12th section was collected into a tube filled with cryoprotectant solution (30% sucrose + 30% ethylene glycol in 0.1 m phosphate buffer, pH 7.6). The samples were then stored at −20°C until staining.
Staining
Immunohistochemical staining was performed using free‐floating samples. It was ensured that samples from each group to be compared later were stained at the same time. First, the cryoprotectant solution was washed out with 0.1 m PBS (pH 7.6; three 15 min washes). Then, necessary steps for blocking and DNA denaturation were performed. For doublecortin, samples were first subjected to citric acid solution (pH 6) at 80°C for 30 min, then blocked in H2O2. For Ki67, samples were also boiled in citric acid, then blocked with both H2O2 and normal goat serum. For BrdU staining, samples were first soaked in 2 m HCl for 15 min at 37°C, then blocked with H2O2 and normal goat serum. After these pretreatments, samples were washed (three 5 min washes) in Tris‐buffered saline (TBS) supplemented with 0.3% Triton X‐100 (TBS‐T, pH 7.6). Primary anti‐BrdU antibody was obtained from Becton‐Dickinson (BD Pharmingen, catalogue no. 347580, made in the mouse), as was the primary antibody for Ki67 (catalogue no. 556003, mouse). A 1:100 dilution was used for BrdU and 1:500 for Ki67. Doublecortin staining was performed using an anti‐doublecortin antibody from Santa Cruz (sc‐8066, goat; 1:250 dilution). Antibodies were diluted into TBS‐T (doublecortin) or PBS‐T (Ki67 and BrdU) and supplemented with 2% normal goat serum when using a secondary antibody made in goats (BrdU and Ki67). Free‐floating sections were incubated with primary antibodies overnight at room temperature. The next day, samples were first washed with the buffer solution. Then, corresponding biotinylated secondary antibodies (BrdU and Ki67: Abcam, ab98691, goat anti‐mouse, 1:500 dilution; and doublecortin: Vector Labs, BA‐5000, rabbit anti‐goat, 1:500 dilution) were diluted into the buffer solution, and samples were incubated at room temperature for 2 h. Next, samples were again washed with the buffer solution and then incubated in HRP‐labelled streptavidin (GE Healthcare, RPN1231, 1:1000 dilution) diluted in the buffer solution for 2 h at room temperature. Again, samples were washed with the buffer solution. To visualize the immunostaining, samples were immersed in diaminobenzidine solution (DAB; Sigma‐Aldrich, D5905; 0.25 mg ml−1 in Tris buffer, pH 7.6) supplemented with 0.075% H2O2. The reaction was stopped after a maximum of 30 min with 0.1 m phosphate buffer (pH 7.6), and sections were then mounted on slides using a gelatin solution and dried at 37°C overnight. Finally, samples mounted on slides were counterstained with 0.1% Cresyl Violet (for details on Cresyl Violet staining, see Nokia et al. 2012), cleared in xylene and coverslipped (Depex).
Microscopic analysis
From the stained slices, estimates of total numbers of BrdU‐, Ki67‐ or doublecortin‐labelled cells were obtained with a modified unbiased stereology protocol. The experimenters were unaware of the experimental conditions when counting the cells. In essence, the number of labelled cells in the granule cell layer and the hilus were counted using a light microscope from every 12th unilateral section throughout the dentate gyrus (one slide per rat, a total of nine slices, 6.3–1.8 mm posterior to bregma; Paxinos, 1998). The number of cells was multiplied by 24 to obtain an estimate of the total number of labelled cells in the hippocampus. As can be seen from Fig. 3 C–E, the new cells were loosely packed and few, so counting them at ×400 magnification was deemed justified. As for the fractionator method, even though it might lead to some clusters of cells being overlooked, this should not be systematic in any way, and should thus not affect the results of group comparisons between cell counts. In addition, we were interested in the relative difference between the experimental groups, not so much in the absolute number of cells.
Statistical analyses
All statistical analyses were conducted using IBM SPSS Statistics version 22. Repeated‐measures ANOVA was used for analysing changes across sessions/time. One‐way and univariate ANOVA was used for simple group comparisons. Bonferroni corrected P values were used for post hoc comparisons. Pearson's correlation coefficient (r) was used in analysing connections between variables. When the sample size was small (fewer than five), correlations were probed using a non‐parametric measure of dependence, the Spearman's rank correlation coefficient (r s).
Results
Experiment 1: adult hippocampal neurogenesis was most abundant in HRT rats in response to voluntary wheel running
Only animals with successful sectioning and staining for doublecortin were included in the analyses. In addition, one animal in the LRT‐RW group was dropped because it was observed to rotate the RW only with its forepaws, thus producing unreliable data on running distance. Final group sizes are reported below and in each figure. There was no difference between the HRT and LRT rat lines for baseline maximal running speed (one‐way ANOVA: F 1,61 = 0.57, P = 0.454). Accordingly, rats of both lines assigned to the RW groups (HRT‐RW, n = 9; LRT‐RW, n = 11) ran an equal distance on the first day (one‐way ANOVA: F 1,18 = 0.763, P = 0.394). During the first week of training (7 days; see Fig. 1 B), running distance increased more in HRT‐RW than LRT‐RW rats (repeated‐measures ANOVA: main effect of day, F 6,108 = 9.27, P < 0.001; main effect of rat line, F 1,18 = 17.13, P = 0.001; interaction, F 6,108 = 5.05, P = 0.009). This was to be expected based on the inherently better response to aerobic exercise training in HRT rats. Mean daily running distances throughout the whole training period for animals in the RW groups are presented in Fig. 1 C. There was no statistically significant difference for wheel running between the LRT and HRT rat lines (repeated‐measures ANOVA: main effect of week, F 6,108 = 13.4, P < 0.001; interaction of rat line and week, F 6,108 = 1.38, P = 0.264; main effect of rat line, F 1,18 = 3.96, P = 0.062). Running distance increased in both rat lines throughout the first few weeks and then levelled off, in a quadratic manner (P < 0.001). The average total running distance (sum of 7 weeks) was 183 ± 18 km for HRT rats and 106 ± 32 km for LRT rats.
On average, HRT rats subjected to HIT completed 89 ± 4% and LRT rats completed 95 ± 1% of training. There was no difference between rat lines (one‐way ANOVA: F 1,18 = 2.18, P = 0.157). Consistently, the total running distances throughout the 7 weeks of HIT training in HRT and LRT rats were comparable (F 1,18 = 0.04, P = 0.844) at 6733 ± 454 and 6824 ± 241 m, respectively (note the difference in scale compared with running distances in RW groups). To summarize, LRT and HRT rats exercised to a comparable degree when subjected to HIT.
Consistent with the above findings, both HIT and voluntary running on a wheel increased running capacity in both rat lines (Fig. 1 D), whereas little change occurred in the control group (HRT‐C, n = 11; LRT‐C, n = 12; univariate ANOVA: main effect of rat line, F 1,57 = 2.19, P = 0.145; main effect of training type, F 2,57 = 14.24, P < 0.001; interaction of rat line and training type, F 2,57 = 2.56, P = 0.086). Maximal running speed increased the most in response to voluntary wheel running (Bonferroni‐corrected post hoc tests: RW vs. HIT, P = 0.048; RW vs. C, P < 0.001) but there was also a significant improvement in the HIT groups compared with the control groups (P = 0.037). The maximal running speed increased by 3 ± 9% in the HRT‐C group and by 7 ± 6% in the LRT‐C group. The corresponding percentages for the HRT‐HIT and LRT‐HIT groups were 29 ± 11 and 26 ± 8%, respectively. For the HRT‐RW and the LRT‐RW groups, the corresponding changes in maximal running speed were 67 ± 12 and 34 ± 8%, respectively. Planned comparisons between rat lines within each exercise group using one‐way ANOVA indicated a significant difference in training response between the HRT‐RW and LRT‐RW groups (F 1,18 = 5.74, P = 0.028) but not between the HRT‐HIT and LRT‐HIT groups (F 1,18 = 0.34, P = 0.857). Furthermore, planned comparisons between the two exercise groups within each rat line indicated a higher training response in the HRT‐RW compared with the HRT‐HIT group (F 1,16 = 5.59, P = 0.031). A similar difference was not evident in the LRT rats (F 1,20 = 0.57, P = 0.459). In conclusion, voluntary running was more effective for demonstrating a differential response to training between LRT and HRT rat lines and for increasing running capacity in HRT rats (see Fig. 1 D).
Next, we studied possible differences between groups in AHN measured as the number of doublecortin‐positive cells in the hippocampus. To avoid confounding effects related to the time of staining, comparisons were either limited to groups stained at the same time (HRT‐C vs. LRT‐C) or the number of neurons was standardized relative to the sedentary control group (Sed vs. HIT vs. RW). Figure 1 E shows that the number of doublecortin‐positive cells in the hippocampi of HRT‐C and LRT‐C rats was comparable (one‐way ANOVA: F 1,21 = 0.01, P = 0.933). That is, LRT and HRT selected lines did not show a difference in AHN in the baseline (i.e. non‐trained) conditions. Figure 1 F, however, shows that HIT and voluntary running affected neurogenesis in differing ways in the HRT and LRT rats (univariate ANOVA: main effect of rat line, F 1,47 = 27.68, P < 0.001; main effect of exercise type, F 2,47 = 28.34, P < 0.001; interaction, F 2,47 = 24.81, P < 0.001). Specifically, there were more doublecortin‐positive cells in the hippocampi of animals subjected to voluntary running compared with those subjected to HIT (Bonferroni‐corrected post hoc test: P < 0.001) or those kept sedentary (Sed, P < 0.001; HIT vs. Sed, P = 0.057). More importantly, compared with HIT or sedentary, there were more doublecortin‐positive cells in the hippocampi of HRT rats subjected to voluntary running (one‐way ANOVA: F 2,22 = 41.04, P < 0.001; Bonferroni‐corrected post hoc test: RW vs. Sed, P < 0.001; RW vs. HIT, P < 0.001; HIT vs. Sed, P = 0.242). This effect was not present in the LRT rats (one‐way ANOVA: F 2,25 = 1.32, P = 0.285). That is, voluntary wheel running was most effective in producing a divide between LRT and HRT rats for running capacity (Fig. 1 D) and also for increasing AHN (Fig. 1 F).
The relationship between running capacity and AHN was also evaluated. First, we calculated correlations without separating HRT and LRT rat lines. Then, each rat line was analysed separately. The standardized measure of AHN was used to avoid any confounding effects related to differences in staining date between rat lines. There was no correlation between post‐training maximal running capacity and AHN in rats subjected to HIT (rat lines combined: r = 0.188, P = 0.427, n = 20). A separate analysis for each rat line confirmed this result (HRT‐HIT, r = 0.443, P = 0.232, n = 9; LRT‐HIT, r = −0.146, P = 0.668, n = 11). There was also no correlation between AHN and the change (expressed as a percentage) in maximal running capacity in rats subjected to HIT (combined, r = −0.309, P = 0.185, n = 20; HRT‐HIT, r = −0.147, P = 0.706, n = 9; LRT‐HIT, r = −0.579, P = 0.062, n = 11). However, a significant positive correlation was found between the total distance run during HIT and AHN (r = 0.538, P = 0.014, n = 20; see Fig. 4 A). Furthermore, this correlation was statistically significant only in the HRT rats (r = 0.797, P = 0.010, n = 9) and not in the LRT rats (r = 0.100, P = 0.771, n = 11), when rat lines were analysed separately (see Fig. 4 A). That is, the amount of forced aerobic exercise predicted the outcome on AHN only in HRT rats but not in the LRT rats.
Figure 4. Post‐training maximal running capacity and total running distance are correlated with adult hippocampal neurogenesis in adult male rats .
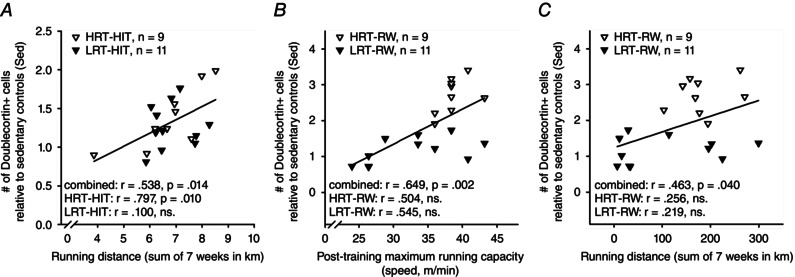
Abbreviations are as in the legend to Fig. 1. All data in this figure are from experiment 1. A, the greater the distance an animal ran during HIT training on the treadmill across 7 weeks of training, the more doublecortin‐positive new neurons were present in the hippocampus at the end of the experiment. B, the higher the maximal running capacity of a given rat subjected to voluntary running on a running wheel was at the end of the training period, the more new neurons were found in the hippocampus. C, the greater the distance an animal ran voluntarily in the running wheel throughout the 7 weeks of training, the more doublecortin‐positive new neurons were present in the hippocampus at the end of the experiment.
The same analyses applied to rats subjected to voluntary wheel running indicated a significant correlation between post‐training maximal running capacity and AHN (rat lines combined, r = 0.649, P = 0.002, n = 20; see Fig. 4 B) but not between the change (expressed as a percentage) in maximal running capacity and AHN (r = 0.378, P = 0.100, n = 20). Further examination of each rat line individually revealed no statistically significant correlations between post‐training maximal running capacity and AHN (HRT‐RW, r = 0.504, P = 0.167, n = 9; LRT‐RW, r = 0.545, P = 0.083, n = 11; see Fig. 4 B) or between the change (expressed as a percentage) in maximal running capacity and AHN (HRT‐RW, r = −0.481, P = 0.190, n = 9; LRT‐RW, r = 0.371, P = 0.261, n = 11). We also calculated the correlation between the total running distance across 7 weeks and AHN. There was a significant correlation overall (rat lines combined, r = 0.463, P = 0.040, n = 20; see Fig. 4 C) but not when each rat line was examined separately (HRT‐RW, r = 0.256, P = 0.506, n = 9; LRT‐RW, r = 0.219, P = 0.518, n = 11; see Fig. 4 C). Thus, the significant correlation between maximal running capacity and AHN as well that between running distance and AHN reflected the divide in training response between the two rat lines, HRT and LRT.
Experiment 2: more AHN was observed in rats subjected to endurance training compared with those subjected to resistance training
Only animals with successful sectioning and staining for doublecortin were included in the following analyses (final group sizes reported below). All animals in this experiment were first subjected to 8 weeks of a standardized absolute amount of exercise training on a treadmill to measure the adaptive response to aerobic training (Koch et al. 2013). Before any training, HRT and LRT animals were able to run at comparable speeds (one‐way ANOVA: F 1,23 = 2.02, P = 0.168). In response to 8 weeks of training on a treadmill, the maximal running speed increased more in HRT compared with LRT rats (see Fig. 2 B; change: 15.85 ± 1.35 vs. 5.43 ± 1.01%, respectively; one‐way ANOVA: F 1,23 = 37.47, P < 0.001).
Half of the LRT and HRT rats were then subjected to sustained endurance training on a treadmill for an additional 6 weeks performed at 60% relative to maximal running speed. The HRT rats started with a speed of 17 m min−1 and LRT rats with a speed of 14 m min−1 (63.5 ± 1.6 and 58.3 ± 1.4% of the maximal running speed, respectively). The velocity was increased by 1 m min−1 every week so that at the end of the training the HRT rats ran at a speed of 22 m min−1 and LRT rats ran at a speed of 19 m min−1. Thus, the HRT rats ran a total running distance of 8016 ± 276 m (n = 8), whereas the LRT rats ran 6972 ± 258 m (n = 7). That is, HRT rats trained over longer distances than LRT rats (one‐way ANOVA: F1,13) = 8.82, P = 0.011). However, with this relative adjustment, both rat lines were able to complete the training sessions to an equivalent degree (HRT, 99 ± 1 vs. LRT, 95 ± 3%; F 1,13 = 3.13, P = 0.100). Endurance training resulted in similar improvements in the maximal running capacity of both HRT and LRT rats (see Fig. 2 C; repeated‐measures ANOVA: main effect of exercise, F 1,13 = 22.75, P < 0.001; interaction of rat line and exercise, F 1,13 = 0.07, P = 0.792). On average, HRT rats improved their running capacity by 18.04 ± 6.33%. The corresponding increase in running capacity for the LRT rats was 17.48 ± 3.43%. Overall, maximal running capacity during endurance training was higher in HRT compared with LRT rats (main effect of rat line, F 1,13 = 4.67, P = 0.050). This overall difference was the result of a difference in the relative running speed at the start of the training period (one‐way ANOVA: F 1,13 = 5.37, P = 0.037). Both HRT and LRT rats ran at comparable speeds during the post‐training test (F 1,13 = 1.87, P = 0.195).
The other half of the rats, which received resistance training on a vertical ladder for 6 weeks, demonstrated an increase in strength [measured as the maximal load (in grams) that the rat was able to carry to the top of the ladder] in both LRT and HRT rat lines (see Fig. 2 D; repeated‐measures ANOVA: main effect of exercise, pre‐ vs. post‐training: F 1,8 = 797.73, P < 0.001). However, the increase in strength was more prominent in the HRT (n = 5) compared with the LRT rats (n = 5; interaction of rat line and exercise, F 1,8 = 22.99, P = 0.001; main effect of rat line, F 1,8 = 8.19, P = 0.021). The increase in strength was on average 201 ± 13% in the HRT rats, whereas in the LRT rats the respective change was on average 147 ± 17%.
We also tested whether the individual variation in the adaptive response to training during the initial 8 week absolute training period predicted the outcome during subsequent relative endurance or resistance training. We found no correlation between the increase in maximal running speed (expressed as a percentage) during the initial absolute training with the increase in running capacity during continued relative endurance training (r = −0.044, P = 0.875, n = 15) when both rat lines were included in the analysis. However, further analysis carried out for each rat line separately indicated a significant correlation between the change (expressed as a percentage) in maximal running speed during initial training and the increase (expressed as a percentage) in running capacity during subsequent endurance training in the LRT rats (r = 0.942, P = 0.002, n = 7) but not in the HRT rats (r = −0.459, P = 0.252, n = 8). This suggests that genetic factors selected for in the high response to aerobic exercise are different from the genetic factors selected for in the low‐response line.
For resistance training, we found a significant positive correlation between the response to training during the initial 8 week absolute training period and the increase in strength (expressed as a percentage) during resistance training (rat lines combined, r = 0.740, P = 0.014, n = 10). However, there was no correlation between initial training and resistance training when each rat line was analysed separately (HRT, r s = 0.359, P = 0.553, n = 5; LRT, r s = 0.400, P = 0.505, n = 5). This suggests that although the rat's performance during aerobic training predicts performance during strength training, the genetic factors selected for within low and high response to aerobic training are not uniquely associated with a response to resistance training.
Finally, we examined the effects of endurance vs. resistance training on AHN (see Fig. 2 E and F). Animals subjected to relative endurance training had significantly more doublecortin‐positive hippocampal cells compared with animals subjected to resistance training, regardless of rat line (univariate ANOVA: main effect of exercise type, F 1,21 = 5.72, P = 0.026; interaction of exercise type and rat line, F 1,21 = 0.11, P = 0.743; main effect of rat line, F 1,21 = 0.23, P = 0.636). Using correlation analysis, we found no relationship between maximal running speed after endurance training and the number of new neurons (rat lines combined, r = 0.156, P = 0.579, n = 15; HRT, r = 0.212, P = 0.614, n = 8; LRT, r = 0.082, P = 0.861, n = 7) or between the change (expressed as a percentage) in maximal running speed owing to endurance training and the number of new neurons (rat lines combined, r = 0.057, P = 0.840, n = 15; HRT, r = 0.172, P = 0.684, n = 8; LRT, r = −0.334, P = 0.464, n = 7). Although the relative training was different for the LRT and HRT rats, there was also no correlation between the total distance run during endurance training and the number of new hippocampal neurons (rat lines combined, r = −0.029, P = 0.917, n = 15; HRT, r = −0.123, P = 0.771, n = 8; LRT, r = 0.205, P = 0.659, n = 7). In addition, there was no correlation between the absolute strength after resistance training and the number of new neurons (r = 0.000, P = 1, n = 10) or the change (expressed as a percentage) in strength and the number of new neurons (r = 0.304, P = 0.393, n = 10). Separate analyses for each rat line yielded similar results (absolute strength × neurogenesis: HRT/LRT, r s = 0.600/0.791, P = 0.285/0.111, n = 5/5; and change in strength × neurogenesis: HRT/LRT, r s = 0.600/0.700, P = 0.285/0.188, n = 5/5). To conclude, endurance training enhanced AHN compared with resistance training independent of the inherited predisposition for low or high response to aerobic exercise.
Experiment 3: adult hippocampal neurogenesis in rats subjected to resistance training did not differ from that observed in their sedentary counterparts
Eight weeks of resistance training in young adult Sprague–Dawley male rats increased strength by ∼250 ± 8% (see Fig. 3 B; Student's paired t test: t(9) = 58.55, P < 0.001). Note that the 10 animals subjected to resistance training increased absolute carrying capacity to a similar extent to the HRT rats but performed within a much narrower range both before and after training (see Fig. 3 B). Compared with sedentary control rats, rats subjected to resistance training gained less body weight during the 8 week training period (repeated‐measures ANOVA: interaction of group and time, F 1,18 = 10.44, P = 0.005; main effect of group, F 1,18 = 4.33, P = 0.052; main effect of time, F 1,18 = 444.26, P < 0.001). The mean body weight in rats subjected to resistance training increased from 293 ± 2 to 386 ± 7 g, whereas in the sedentary control group the body weight increased from 295 ± 4 to 421 ± 12 g.
The effects of resistance training on proliferation (Ki67), maturation (doublecortin) and survival (BrdU) of new adult‐born hippocampal neurons are presented in Fig. 3 C, D and E, respectively. Analysis of Ki67‐positive cell counts indicated no difference in cell proliferation between the sedentary and the trained group (one‐way ANOVA: F 1,18 = 0.12, P = 0.736). The same was true for the number of immature doublecortin‐positive neurons (F 1,18 = 0.71, P = 0.412). This result was corroborated by the fact that there was also no difference in the number of hippocampal BrdU‐positive cells between the groups (F 1,16 = 0.35, P = 0.563), indicating no effect of resistance training on the survival of adult‐born new cells in the hippocampus. Note that one animal in each group had to be excluded from the BrdU‐analysis because of non‐existent staining. Similar to what was reported in experiment 2, there was no consistent correlation between the change in strength (expressed as a percentage) and neurogenesis (Ki67/doublecortin/BrdU: r = −0.262/−0.036/−0.253, P = 0.465/0.922/0.512, n = 10/10/9). As a result of the very small variation in absolute strength after training (see Fig. 3 B), correlations between it and neurogenesis were not calculated. To summarize, compared with sedentary control animals, 8 weeks of anaerobic resistance exercise on a vertical ladder had no effect on AHN in young adult male Sprague–Dawley rats.
Discussion
Adult hippocampal neurogenesis is a continuous process that contributes to a variety of adaptive behaviours, such as learning (for review, see Aimone et al. 2014). A well‐demonstrated means of promoting AHN in rodents is aerobic exercise, namely running (van Praag et al. 1999). Here, we studied whether the effects of HIT or resistance training are comparable to aerobic exercise for promoting AHN in male rats. In line with previous work, our studies showed that the number of adult‐born hippocampal neurons was higher in animals subjected to sustained aerobic exercise, namely voluntary wheel running or treadmill endurance running, compared with sedentary control animals. Contrary to previous findings (Novaes Gomes et al. 2014), resistance training did not promote AHN compared with sedentary control animals, whereas HIT had a smaller than expected effect on AHN, which did not reach statistical significance. Our development of a rat model system for low (LRT) and high (HRT) response to aerobic exercise training (Koch et al. 2013) also provided us with an opportunity to study whether a genetic predisposition for adaptive capacity for aerobic trainability is related to the beneficial effects of physical exercise on AHN. Our data indicate the highest numbers of adult‐born hippocampal neurons in rats selectively bred for a high response to aerobic exercise that ran voluntarily on running wheels. That is, AHN is highest in animals born with a tendency for a higher response to exercise training and that engage in a large amount of voluntary aerobic activity.
A rat model system of low and high response to training
In experiment 1, summarized in Fig. 1, we discovered that when provided with unlimited access to voluntary wheel exercise the HRT rats increased running distance and improved running speed more than the LRT rats. Accordingly, the amount of AHN was significantly higher in HRT rats compared with LRT rats. This was expected based on the inherently differential responses to aerobic exercise training in the two rat lines (Koch et al. 2013). The LRT and HRT rats were not different in their capacity to complete HIT training or in the effects of HIT on AHN. Experiment 2 (see Fig. 2) shows that with 8 weeks of endurance treadmill training using a standard absolute speed‐ramped aerobic protocol, the HRT rats gain significantly more capacity for exercise compared with the LRT rats. Furthermore, when resistance trained using a load of 50–90% relative to maximal load, performed 3 days a week for 6 weeks, the HRT rats gained significantly more strength than the LRT rats. Interestingly, an equivalent increase in maximal running capacity in both rat lines was observed in response to relative endurance training on a treadmill performed at ∼60% of maximal running capacity and conducted following the initial 8 week training period. This suggests that animals with an inherently low response to training are capable of improving their performance if aerobic training is continued for an extended period of time and accommodated to their performance level. With this training set‐up, more AHN was observed in rats subjected to sustained endurance training vs. those performing resistance training. Thus, we demonstrate that the HRT‐LRT rat model system provides a unique substrate for investigating the physiological and molecular connections between variation in response to exercise training and AHN.
High‐intensity interval training had a smaller than expected effect on AHN
To our knowledge, our study is the first to address whether an exercise increasing in popularity among humans, namely HIT (see, for example, Gibala et al. 2012), has effects on AHN. Although HIT is shown in some cases to be superior for increasing cardiovascular fitness over moderate endurance training (Haram et al. 2009; Afzalpour et al. 2015), HIT had a smaller than expected effect on AHN in the present study. Despite HIT improving the running capacity of both LRT and HRT rats, it did not affect AHN to a statistically significant degree, although Fig. 1 E suggests a trend towards an increase in the number of new neurons for both LRT and HRT rats. This is somewhat surprising given that in experiment 2 we did find increased AHN in animals subjected to endurance training on a treadmill when compared with that observed in animals subjected to anaerobic resistance training (please see next section). It might be that if we had used a longer training session during HIT, we would also have observed bigger improvements in AHN. For example, Haram et al. (2009) used 60 min sessions of HIT and 2 h sessions of continuous running on a treadmill in their study, reporting greater benefits of HIT compared with continuous exercise in reducing a number of cardiovascular risk factors.
Our present results on the (lack of) effects of HIT on AHN comply with a recent report of greater improvements in AHN in terms of maturation of new neurons in response to 6 weeks (60 min day−1, 5 days a week) of mild (speed, 15 m min−1) rather than intense (40 m min−1) forced running on a treadmill in adult male Wistar rats (Inoue et al. 2015). Inoue and colleagues (2015) propose that their results might be explained by differences between the two forms of exercise in activating certain genes related to lipid metabolism, protein synthesis and inflammation in the hippocampus and also by the higher level of stress induced by intense exercise compared with mild exercise. In a recent study, the same group studied the effects of mild (15 m min−1) vs. intense (30 m min−1) running on a treadmill (30 min day−1, 5 days a week) in adult male C57BL/6J mice and found elevated plasma corticosterone concentrations in the intensely trained group compared with a sedentary control group (Okamoto et al. 2015). More importantly, the same group reports observations of increased hippocampal BDNF transcription (Soya et al. 2007) and AHN (Okamoto et al. 2015) in response only to mild but not to intense exercise. Interestingly, blocking mineralocorticoid and glucocorticoid receptor function seems to attenuate the increase in AHN induced by mild exercise, suggesting that some level of corticosterone action is needed to support elevations in AHN (Okamoto et al. 2015). This is in agreement with an observation of enhanced AHN in response to long‐term predictable mild stress (Parihar et al. 2011).
Based on the above discussion, stress might have dampened the effects of forced treadmill training, especially HIT, on AHN in our study (for a recent review, see Lucassen et al. 2015). In fact, the number of cells positive for doublecortin in our present experiments overall was relatively low compared with other reports of neurogenesis in adult rats using the same primary antibody and counting protocol (see, for example, Winocur et al. 2014). To summarize, it seems that sustained (mild) aerobic exercise rather than HIT increases AHN in HRT and LRT rats, and the role of stress warrants further investigation.
Resistance training does not promote AHN
Our findings using the LRT and HRT rat lines as well as our results testing commercially available Sprague–Dawley rats are contrary to a previous finding (Novaes Gomes et al. 2014) indicating that resistance training promotes AHN. Our present data indicate that resistance training does not affect the proliferation, neuronal maturation or survival of adult‐born new cells in the hippocampus, although an improvement in strength is evident. This is in direct contrast to a recent study reporting a higher number of proliferating cells in the hippocampi of male Wistar rats subjected to 4 weeks of resistance training according to a regimen similar to that used in our present study (Novaes Gomes et al. 2014). It is possible that the effects of resistance training are transient in nature, perhaps increasing cell proliferation only early in training. This could explain why no effects were evident when neurogenesis was evaluated after 6 (experiment 2) or 8 weeks (experiment 3) of training in our present study. Note that in our present experiments we did not use any external motivators to encourage rats to perform resistance training. Thus, it is unlikely that stress alone would explain why we did not observe an increase in AHN in response to resistance training (for a recent review, see Lucassen et al. 2015). This applies especially to experiment 2, where comparisons were made between rats trained on a treadmill (with shocks) and those trained on a vertical ladder (without shocks). It may in fact be that mild stress during the forced treadmill training may have contributed towards an increase in AHN (Parihar et al. 2011). The role of stress should be investigated further in future experiments of a similar kind.
Our results on the effects of resistance training on AHN comply with and could also in part be explained by the previously reported effects of anaerobic exercise on neural growth factors. Resistance training on a vertical ladder five times per week (eight climbs per session) did not increase BDNF expression in the hippocampus compared with sedentary control animals as measured after 8 weeks of training (Cassilhas et al. 2012 a). As it turns out, BDNF has a crucial role in regulating AHN (Sairanen et al. 2005; Scharfman et al. 2005), and normal BDNF function appears to be a requisite for the exercise‐induced increase in AHN (Li et al. 2008). In our present study (experiment 2), 30 min of forced aerobic endurance training on a treadmill three times a week led to higher numbers of new immature hippocampal neurons compared with that observed in animals engaged in resistance training, according to the same schedule. Thus, it seems that aerobic exercise is more efficient in persistently increasing AHN than anaerobic exercise, at least when carried out for extended periods.
All in all, and in accordance with previous knowledge, our results imply that in order to promote AHN maximally, exercise should be aerobic and sustained. Previous studies reviewed above further suggest that the exercise‐induced increases in AHN are dependent on enhanced BDNF function. In the future, to clarify exercise effects on AHN further, comparable dose–response studies with different training regimens should be performed.
The amount of running is correlated with AHN
In accordance with several previous reports on the beneficial effects of running on AHN and cognition in rodents (for a review, see Vivar et al. 2013), in our present study forced endurance training on a treadmill as well as voluntary running in a running wheel led to a higher number of immature adult‐born hippocampal neurons compared with that observed in animals not engaged in aerobic exercise. Furthermore, we found that daily voluntary running on a running wheel increased AHN considerably more than 30 min of forced endurance training on a treadmill three times a week. This is not surprising considering the positive correlation between distance run and AHN (Allen et al. 2001), a link also evident in our present data (experiment 1; see Fig. 4). Note that running on a treadmill at a steady speed of 40 m min−1 (the maximal speed for some of the best runners in our experiments) for 30 min amounts to 1200 m, whereas animals typically run several kilometres daily when given free access to a running wheel. To summarize, the amount of aerobic exercise may be crucial to its effect on AHN.
The correlation between running distance and AHN might be explained by considering the consequences of running (in a more naturalistic setting). The further an individual travels, the more likely it is to encounter new environments and stimuli from which it must make sense rapidly. It could be that the act of running per se, even if performed in a wheel or on a treadmill, primes the brain to take on the changes in external environment by, for example, increasing AHN. This notion is supported by findings indicating that new hippocampal neurons are especially important for learning (Shors et al. 2001) and pattern separation (Clelland et al. 2009), with animals performing worse in these tasks if AHN is disrupted.
One should also note that the positive effects of prolonged aerobic exercise on adult neurogenesis are not limited to the hippocampus alone, because increases in adult neurogenesis have also been reported in the subventricular zone (Bednarczyk et al. 2009; Chae et al. 2014) and in the hypothalamus (Niwa et al. 2015). These anatomically widespread increases in adult neurogenesis in response to sustained aerobic exercise suggest that in addition to mechanisms local to the hippocampus, such as increased androgenic function (Okamoto et al. 2012), a common mediator, the most obvious candidate being increased blood flow, must exist to support AHN. A large proportion of proliferating hippocampal cells is located in the vicinity of small capillaries (Palmer et al. 2000). In addition, aerobic exercise increases blood flow specifically in the dentate gyrus both in humans and in mice (Pereira et al. 2007). This increase in blood flow is likely to increase both metabolic and trophic support to the neurogenic niche. In fact, previous studies on the hippocampus indicate that in rodents the running distance is also correlated positively with BDNF expression (see, for example, Johnson et al. 2003), and anaerobic exercise fails to upregulate BDNF (Cassilhas et al. 2012 a; see also Soya et al. 2007). Furthermore, BDNF expression and function are also elevated by increased neuronal activity (for a recent review, see Rothman & Mattson, 2013). On a related matter, the effect of learning on the survival of new adult‐born hippocampal neurons (Gould et al. 1999) appears to depend on the amount of training; generally, the more effort learning a certain task takes and, presumably, the longer the hippocampal engagement in the learning process, the more new hippocampal neurons survive in response to learning (for a review, see Shors et al. 2012; see also Sisti et al. 2007).
Taken together, it seems that training (whether cognitive or physical) should be sustained in order to promote AHN most effectively. However, the underlying fundamental biological mechanism of how exercise enhances AHN is controversial. The precise regulatory mechanisms whereby the gene variants and exercise inputs interact are unknown but are likely to involve networks of modifier gene interactions that link increases in blood flow, signalling pathways, neuronal activity and neurotrophic action.
Limitations
Some limitations of our present study warrant further discussion. First, we conducted studies exclusively on adult male rats. However, as previous studies in females (van Praag et al. 1999; Marlatt et al. 2012) and in aged animals (van Praag et al. 2005) indicate an enhancement of both AHN and cognition in response to running, our results could be assumed to apply to both sexes and to older rodents as well. Our decision to test males only was a direct result of the high workload required for animal exercise training studies and our interest in conducting a comparable study for AHN in response to resistance training that was reportedly carried out in adult male rats. Thus, in future studies it would be preferable for both sexes and animals of different ages to be tested. Second, in terms of interspecies applicability, several studies have reported larger hippocampal volume and better cognitive ability in physically active/fit older adult humans (Erickson et al. 2009; Erickson et al. 2011; Varma et al. 2015). In a recent study conducted in mice, the volume of hippocampal grey matter showed the strongest correlation with the number of hippocampal doublecortin‐positive cells (Biedermann et al. 2014), suggesting that larger hippocampal volume might be, for the most part, a product of enhanced AHN. Tentatively, it would seem that physical exercise affects hippocampal structure and function in humans in a manner resembling that observed in laboratory rodents (see also Hauser et al. 2009). Third, although we did not directly measure motivation to exercise in our present experiments, its role in mediating the beneficial effects of physical activity on the brain should not be overlooked. In terms of forced training and testing maximal performance capacity, we did not observe any difference between the HRT and LRT rat lines in their motivational behaviour on the treadmill or the vertical ladder.
Conclusions
Our results add to the body of literature indicating that sustained aerobic exercise increases AHN and advances this field of study in several ways. First, we tested several different forms of physical exercise to study their effects on AHN. We also took advantage of a newly developed genetically heterogeneous contrasting rat model system that we selectively bred for low and high response to aerobic training to take into account genetic variation in training responsiveness. According to our findings, anaerobic resistance training does not affect AHN in the studied animals, despite its overall positive effects on physical fitness. Second, the effects of exercise on AHN depend, at least to some extent, on sustained aerobic activity, as HIT did not have statistically significant effect on AHN. Third, the highest numbers of adult‐born hippocampal neurons were observed in rats selectively bred for a high response to aerobic exercise that ran voluntarily on running wheels. Thus, for all reasons combined, AHN is highest in animals born with a tendency for a higher response to exercise training ,engaging in a large amount of voluntary aerobic activity.
Additional information
Competing interests
None declared.
Author contributions
The experiments were performed at the Department of Psychology and Department of Biology of Physical Activity at the University of Jyväskylä. M.S.N. acquired, analysed and interpreted the data and wrote the report. S.L. designed the work, acquired and analysed the data and revised the report. J.P.A. designed the work, acquired the data and revised the report. P.P.J. acquired and analysed the data and revised the report. L.G.K. designed the animal model, interpreted the data and wrote the report. S.L.B. designed the animal model, interpreted the data and revised the report. H.K. designed the work and revised the report. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Academy of Finland (grant no. 274098 to H.K., and grant nos 137783 and 275954 to M.S.N.), the Finnish Cultural Foundation (H.K.), METAPREDICT within the European Union Seventh Framework Program (HEALTH‐F2‐2012‐277936 to H.K.) and the National Institutes of Health (R24OD010950 to L.G.K. and S.L.B., and 3P60DK020572‐32S2 and 5P60 DK20572‐P/FS to L.G.K.).
Acknowledgements
The authors would like to thank Heikki Tanila, Pasi Miettinen, Kaisa‐Leena Tulla and Paavo Rahkila for help with optimizing immunohistochemical staining procedures and Satu Koskinen for helping with the microscope. The authors would also like to thank the following persons for their contribution to animal care, data collection and analysis: Josephine Adwrubi, Marje Harri, Kristiina Heiskanen, Katri Isohannu, Juho Hyödynmaa, Eliisa Kiukkanen, Jevgenia Lasmanova, Roland Loh, Niina Lonka, Niina Lukkarila, Mervi Matero, Bernardo M. S. Oliveira, Janne Paajala, Konstantin Papaioannou and Tiina Saapunki. We acknowledge the expert maintenance of the rat breeding colony provided by Molly Kalahar and Lori Heckenkamp and thank Eric Wall for coordinating the shipping of animals. Contact L.G.K. (lgkoch@med.umich.edu) or S.L.B. (brittons@umich.edu) for information on the LRT and HRT rats; these rat models are maintained as a resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, MI, USA.
References
- Afzalpour ME, Chadorneshin HT, Foadoddini M & Eivari HA (2015). Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol Behav 147, 78–83. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W & Gage FH (2014). Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94, 991–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, Gage FH & Barlow C (2001). Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev 15, 554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk MR, Aumont A, Decary S, Bergeron R & Fernandes KJ (2009). Prolonged voluntary wheel‐running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus 19, 913–927. [DOI] [PubMed] [Google Scholar]
- Biedermann SV, Fuss J, Steinle J, Auer MK, Dormann C, Falfán‐Melgoza C, Ende G, Gass P & Weber‐Fahr W (2014). The hippocampus and exercise: histological correlates of MR‐detected volume changes. Brain Struct Funct doi: 10.1007/s00429-014-0976-5 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Hakkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, Rao DC, Sarzynski MA, Skinner JS, Slentz CA & Rankinen T (2012). Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One 7, e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C & Rankinen T (2001). Individual differences in response to regular physical activity. Med Sci Sports Exerc 33, S446–S451; discussion S452–S453. [DOI] [PubMed] [Google Scholar]
- Cameron HA & McKay RD (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435, 406–417. [DOI] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S & Torres‐Aleman I (2000). Circulating insulin‐like growth factor I mediates effects of exercise on the brain. J Neurosci 20, 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Lee KS, Fernandes J, Oliveira MG, Tufik S, Meeusen R & de Mello MT (2012. a). Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 202, 309–317. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Lee KS, Venancio DP, Oliveira MG, Tufik S & de Mello MT (2012. b). Resistance exercise improves hippocampus‐dependent memory. Braz J Med Biol Res 45, 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla‐Ortega E, Rosell‐Valle C, Pedraza C, Rodríguez de Fonseca F, Estivill‐Torrús G & Santin LJ (2014). Voluntary exercise followed by chronic stress strikingly increases mature adult‐born hippocampal neurons and prevents stress‐induced deficits in ‘what–when–where’ memory. Neurobiol Learn Mem 109, 62–73. [DOI] [PubMed] [Google Scholar]
- Chae CH, Jung SL, An SH, Park BY, Kim TW, Wang SW, Kim JH, Lee HC & Kim HT (2014). Swimming exercise stimulates neuro‐genesis in the subventricular zone via increase in synapsin I and nerve growth factor levels. Biol Sport 31, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH & Bussey TJ (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H & Bussey TJ (2010). Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA 107, 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS & Shors TJ (2009). Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc Natl Acad Sci USA 106, 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E & Kramer AF (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19, 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E & Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Shah JD & Johnston IN (2012). Cognitive impairments caused by oxaliplatin and 5‐fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl) 220, 183–193. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH & Christie BR (2004). Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague–Dawley rats in vivo . Neuroscience 124, 71–79. [DOI] [PubMed] [Google Scholar]
- Fischer TJ, Walker TL, Overall RW, Brandt MD & Kempermann G (2014). Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front Neurosci 8, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, Macdonald MJ & Hawley JA (2012). Physiological adaptations to low‐volume, high‐intensity interval training in health and disease. J Physiol 590, 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A & Shors TJ (1999). Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2, 260–265. [DOI] [PubMed] [Google Scholar]
- Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al‐Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM & Wisløff U (2009). Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81, 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser T, Klaus F, Lipp HP & Amrein I (2009). No effect of running and laboratory housing on adult hippocampal neurogenesis in wild caught long‐tailed wood mouse. BMC Neurosci 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA Jr & Farrar RP (2004). Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol 29, 16–31. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R & Soya H (2015). Long‐term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One 10, e0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Rhodes JS, Jeffrey SL, Garland T Jr & Mitchell GS (2003). Hippocampal brain‐derived neurotrophic factor but not neurotrophin‐3 increases more in mice selected for increased voluntary wheel running. Neuroscience 121, 1–7. [DOI] [PubMed] [Google Scholar]
- Koch LG & Britton SL (2008). Aerobic metabolism underlies complexity and capacity. J Physiol 586, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch LG & Britton SL (2001). Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5, 45–52. [DOI] [PubMed] [Google Scholar]
- Koch LG, Pollott GE & Britton SL (2013). Selectively bred rat model system for low and high response to exercise training. Physiol Genomics 45, 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Inoue K, Okamoto M, Liu YF, Matsui T, Yook JS & Soya H (2013). Voluntary resistance running induces increased hippocampal neurogenesis in rats comparable to load‐free running. Neurosci Lett 537, 6–10. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Alves‐Wagner AB, Hirshman MF, Gallagher IJ, Constantin‐Teodosiu D, Atkins R, Greenhaff PL, Qi NR, Gustafsson T, Fielding RA, Timmons JA, Britton SL, Koch LG & Goodyear LJ (2013). Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise‐regulated signaling networks. Diabetes 62, 2717–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER & Gould E (2010). Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One 5, e11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel‐Duby R & Parada LF (2008). TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Oomen CA, Naninck EF, Fitzsimons CP, van Dam AM, Czeh B & Korosi A (2015). Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation. Cold Spring Harb Perspect Biol 7, a021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ & van Praag H (2012). Running throughout middle‐age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol 72, 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton O, Koltai E, Takeda M, Koch LG, Britton SL, Davies KJ, Boldogh I & Radak Z (2015). Mitochondrial biogenesis‐associated factors underlie the magnitude of response to aerobic endurance training in rats. Pflugers Arch 467, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa A, Nishibori M, Hamasaki S, Kobori T, Liu K, Wake H, Mori S, Yoshino T & Takahashi H (2015). Voluntary exercise induces neurogenesis in the hypothalamus and ependymal lining of the third ventricle. Brain Struct Funct doi: 10.1007/s00429-015-0995-x [DOI] [PubMed] [Google Scholar]
- Nokia MS, Anderson ML & Shors TJ (2012). Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci 36, 3521–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaes Gomes FG, Fernandes J, Vannucci Campos D, Cassilhas RC, Viana GM, D'Almeida V, de Moraes Rêgo MK, Buainain PI, Cavalheiro EA & Arida RM (2014). The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 50, 106–117. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Hojo Y, Inoue K, Matsui T, Kawato S, McEwen BS & Soya H (2012). Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci USA 109, 13100–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Yamamura Y, Liu Y, Min‐Chul L, Matsui T, Shima T, Soya M, Takahashi K, Soya S, McEwen BS & Soya H (2015). Hormetic effects by exercise on hippocampal neurogenesis with glucocorticoid signaling. Brain Plasticity 1, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR & Gage FH (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425, 479–494. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B & Shetty AK (2011). Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry 16, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GWC (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press, Inc, San Diego, CA, USA. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR & Small SA (2007). An in vivo correlate of exercise‐induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 104, 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM & Mattson MP (2013). Activity‐dependent, stress‐responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience 239, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M & Castren E (2005). Brain‐derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25, 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C & Croll S (2005). Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192, 348–356. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM Jr & Nokia MS (2012). Use it or lose it: how neurogenesis keeps the brain fit for learning. Behav Brain Res 227, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T & Gould E (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376. [DOI] [PubMed] [Google Scholar]
- Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN & Church TS (2009). Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc 41, 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Glass AL & Shors TJ (2007). Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn Mem 14, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J & Cameron HA (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS & Nishijima T (2007). BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun 358, 961–967. [DOI] [PubMed] [Google Scholar]
- Timmons JA (2011). Variability in training‐induced skeletal muscle adaptation. J Appl Physiol 110, 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E & Torres‐Aleman I (2001). Circulating insulin‐like growth factor I mediates exercise‐induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21, 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2, 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C & Gage FH (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25, 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Chuang Y, Harris GC, Tan EJ & Carlson MC (2015). Low‐intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus 25, 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC & van Praag H (2013). All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 15, 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Huang J & Tannock IF (2014). Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy‐treated rats. Psychopharmacology (Berl) 231, 2311–2320. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Bye A, Stølen T, Kemi OJ, Pollott GE, Pande M, McEachin RC, Britton SL & Koch LG (2015). Blunted cardiomyocyte remodeling response in exercise‐resistant rats. J Am Coll Cardiol 65, 1378–1380. [DOI] [PubMed] [Google Scholar]


