Abstract
An integrated treatment strategy using peritonectomy procedures plus hyperthermic intraperitoneal chemotherapy (HIPEC) is now a clinical standard of care in selected patients with peritoneal metastases and primary peritoneal tumors. This comprehensive approach can offer many patients, who hitherto had no hope of cure, a good quality of life and survival despite limited morbidity. The increasingly successful results and chance of interfering in the natural history of disease has prompted research to develop for some clinical conditions a therapeutic strategy designed to prevent malignant peritoneal dissemination before it becomes clinically evident and treat it microscopically (tertiary prevention). The main factor governing successful cytoreductive surgery and predicting outcome is the extent of peritoneal spread assessed with the peritoneal cancer index (PCI). In peritoneal metastases from colorectal and gastric cancer the PCI score acquires a specific role acting as the cut-off between patients who can undergo curative surgery or palliation. Long-term results show that the only group enjoying favorable results are patients with limited disease (a statistical minority). By applying to appropriately selected patients with primary malignancies a proactive management strategy including HIPEC we can treat patients with microscopic peritoneal dissemination and therefore at PCI 0. Among treated conditions pseudomyxoma peritonei enjoys the best results. But a major future advance comes from identifying among lesions at major risk of pseudomyxoma.
Keywords: Peritoneal metastases, Proactive management, Gastric cancer, Colorectal cancer, Appendiceal cancer
Until the 1980s consensus considered endoperitoneal spread from an intraabdominal neoplasm and a primary tumor developing within the peritoneum conditions for which no therapy existed apart from palliative procedures able to guarantee only a few months of life [1]. Thanks to Paul Sugarbaker’s pioneering efforts [2, 3], advances over the past 30 years now offer these patients a standardized integrated (multimodal therapy) combining cytoreductive surgery (CRS) (peritonectomy procedures) with hyperthermic intraperitoneal chemotherapy (HIPEC), an approach that has in selected cases allowed hitherto unhoped for survival [4–8]. Owing to these results the previously used term carcinomatosis, which implies a terminal condition, has been abandoned and cancer spread into the peritoneal space is now referred to as peritoneal metastases, a term that more intuitively implies a chance for cure similar to that for disease in other currently treatable localizations, such as hepatic and lung metastases [9]. At the 9th International Congress on Peritoneal Surface Malignancies the Peritoneal Surface Oncology Group International (PSOGI) guidelines recommend CRS combined with HIPEC as the preferred treatment for pseudomyxoma peritonei, for appendiceal neoplasia with peritoneal metastases and for selected patients with peritoneal mesothelioma or peritoneal metastases with moderate spread from colorectal cancer. Although this strategy may be helpful also in patients who have ovarian or advanced peritoneal metastases from gastric cancer, additional evidence is needed from ongoing collaborative studies at experienced treatment centers [10]. Collectively the literature to date suggests that the currently generalized use of proper selection criteria, advanced surgical techniques and lack of substantially new drugs in the pipeline argue, at least in the short term, against a further improvement in the results for the aforementioned peritoneal dissemination or in the available therapeutic strategies. For this reason, research in this field seems now to move in two different directions. The first consists in using these integrated procedures to treat peritoneal spread from cancers hitherto left untreated (breast, small bowel, and endometrial cancer) [11–13].
The second research direction, on which this review focuses, concerns developing proactive management for peritoneal metastases for specific primary malignancies (gastric, colorectal and appendiceal tumors). Consensus opinion among those who treat these diseases affirms that the good results obtained with integrated treatment (CRS + HIPEC) for peritoneal metastases from gastric and colorectal cancer correlate closely with the extent of peritoneal spread. The higher the peritoneal cancer index (PCI) [14] the less likely are we to achieve a good therapeutic outcome and acceptable long-term results. In these cases, the amount of peritoneal spread negatively influences the patient’s outcome to such an extent that the PCI score seems to acquire a specific role as a cut-off value for selecting candidates for CRS plus HIPEC [7, 15–18]. Ample evidence shows that in these patients whether we can intervene to interrupt a frequently unfortunate clinical history depends exclusively on applying the same therapeutic procedures used to treat peritoneal spread but intervening at an earlier time-point, when peritoneal spread remains microscopic (tertiary prevention). The preventive approach originated from the concept originally expressed by Benjamin Franklin “An ounce of prevention is worth a pound of cure” and later underlined by Paul Sugarbaker in a well-known article that expresses the underlying therapeutic rationale right from the title “It’s what the surgeon does not see that kills the patient” [19]. Analogously, though in another clinical setting, others adapted the concepts for “proactive management” to treat appendiceal neoplasms in preventing pseudomyxoma peritonei (PMP). PMP is a clinical condition caused by mucin accumulating within the peritoneum secondary to mucinous epithelial neoplasia. Mucinous neoplastic epithelium most often spreads to the peritoneum from a low-grade appendiceal mucinous neoplasm (LAMN) or mucinous adenocarcinoma [20]. In about 20 % of the patients with a mucinous tumor in the appendix PMP subsequently develops. Even though multimodal treatment (CRS + HIPEC) for peritoneal metastases obtains its best results in PMP, several investigators in recent years suggest that using proactive management on a presumably initial lesion such as an appendiceal mucinous neoplasm tumor could prevent PMP from developing [21, 22].
Proactive Management for Peritoneal Metastases from Gastric Cancer
An estimated 951,600 new gastric cancer cases and 723,100 deaths occurred in 2012 worldwide. Even though the incidence of gastric cancer has steadily declined in the more developed countries in Northern America and Europe since the mid-20th century, these data suggest that treatment results remain disappointing [23]. From 5 to 20 % of patients already have peritoneal metastases at diagnosis and metachronous peritoneal metastases onset during a 5-year follow-up in a percentage ranging from 29 to 38 % of the patients who underwent resection with curative intent [24–26]. Even though the past 10 years have witnessed advances in systemic chemotherapy and novel targeted drugs, no phase III study has yet proved that any therapeutic regimen has really benefitted disease progression [27]. As in other clinical conditions characterized by peritoneal metastases, our literature review identified several studies reporting the results obtained with CRS + HIPEC (usually always preceded by systemic or endoperitoneal neoadjuvant chemotherapy or both) in gastric cancer [6, 18, 28, 29] (Table 1). Among these, the study conducted by Yang et al. [6] – one of the few randomized control studies focusing on cytoreductive surgery for peritoneal metastases – has shown that in patients with peritoneal spread from gastric cancer CRS + HIPEC achieves better results than CRS alone.
Table 1.
Cytoreduction + HIPEC in gastric cancer with peritoneal metastases
| Gastric cancer with peritoneal metastases | |||||
|---|---|---|---|---|---|
| Results of cytoreduction + HIPEC | |||||
| Author/year | n/pts | Morbidity % | Mortality % | Survival | |
| Median (mo) | Survival rate % | ||||
| Glehen 2010 | 159 | 20 | 6.5 | 9 | 23 |
| Yang 2011 | 34 | 14.7 | – | 11 | 15 |
| Canbay 2014* | 152 | 23.6 | 3.9 | 15.8 | 10.7 |
| Magge 2014 | 22 | 52 | 4.3 | 9.5 | 18 |
*Bidirectional neoadjuvant chemotherapy prior to surgery
In general, therefore, outcomes after integrated treatment for peritoneal metastases from gastric cancer, though better than those after systemic chemotherapy alone, remain exceedingly disappointing. Ample data confirm as the main independent factors indicating a worsening prognosis, a PCI >6 and the presence of metachronous peritoneal metastases [6, 18]. These observations clearly support a proactive approach to peritoneal metastases in advanced gastric cancer so as to treat microscopic endoperitoneal spread before it becomes clinically evident. In gastric carcinoma lengthy debate questions whether the various diagnostic techniques can reliably ascertain a positive endoperitoneal cytologic finding, what this means for prognosis and how it influences the therapeutic strategy [30, 31]. A systematic review published in recent years confirms that the various diagnostic techniques have intrinsic limitations related to reliability, to the cost-benefit ratio and last to the time needed to obtain a response able to influence therapeutic strategies [32]. The limitations of the perioperative cytological diagnosis have prompted many, almost exclusively Asian investigators, to conduct numerous studies addressing HIPEC understood as hyperthermic perioperative adjuvant chemotherapy done with curative intent in patients with no signs indicating peritoneal spread but with gastric carcinoma invading the serosa. Three meta-analyses conducted in recent years show that this strategy especially when combined with R0 resection can reduce the onset of peritoneal recurrence more efficiently than standard treatment and improve the outcome without increasing morbidity [33–35]. Although these results remain important even today, perioperative chemotherapy regimens still need standardizing especially given the systemic or endoperitoneal neoadjuvant chemotherapy regimens that patients often undergo. Besides, these studies typically conducted in Asian countries receive scarce support from those in Western countries where stomach cancer also owing to its low epidemiological incidence finds it hard to fit into a homogenous therapeutic organization.
Proactive Management of Peritoneal Metastases from Colorectal Cancer
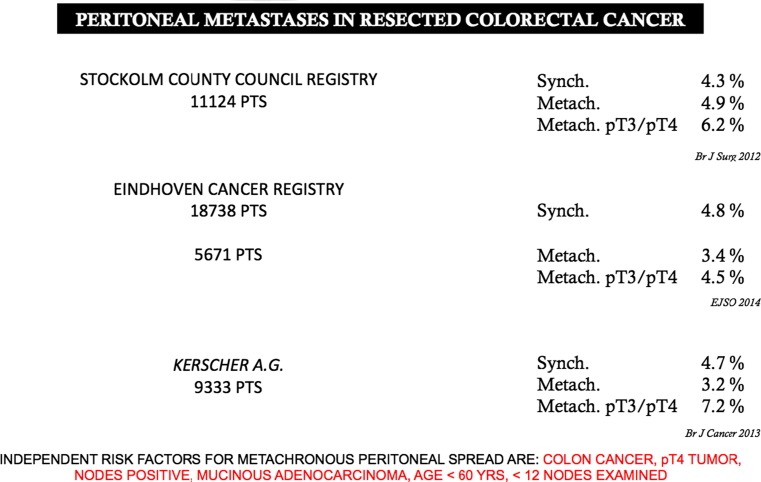
Colorectal cancer is one of the leading causes of cancer death in developed countries. Despite recent advances in understanding the molecular pathogenesis and improvements in diagnosis and treatment, more than 1,2 million new cases and 600,000 deaths occur annually worldwide and cure rates remain low for patients with metastatic or recurrent disease [36]. According to reports from the National Cancer Institute, colon cancer is a highly treatable disease, and when confined to the bowel is often curable. Primary treatment, surgery, results in a cure in about 50 % of the patients. A major problem, however, and often the ultimate cause of death, is recurrence after surgery [37]. Recurrence remains a frequent cause of mortality after the surgical treatment of colorectal cancer with curative intent. Epidemiological studies show that the site involved by recurrent disease (liver, lung, locoregional sites) can vary according to the site of the primary tumor and its stage [38, 39]. Specifically for colorectal cancers a cumulative analysis addressing disease recurrence is made more complex, especially for locoregional recurrence, by the different approaches used in treating rectal tumors and colon tumors. In this scenario, major influential factors are the primary tumor site (colon vs. rectum) and treatment variables. In the past, survival was from 5 % to 10 % higher for colon than for rectal cancer [40, 41]. Over the decades the widespread usage in rectal surgery of total mesorectal excision (TME) procedures popularized by Heald and Ryall [42], together with neoadjuvant chemo-radiotherapy protocols in advanced cases, have lowered local recurrence rates and improved survival [43–45]. Similar trends remain unobserved in colon cancer, and patients with colon cancer now have a worse prognosis than those with rectal cancer even though they more frequently undergo adjuvant chemotherapy [46–49]. Cancer statistics in the United States as well as in Europe show that in the past 20 years the survival rates for rectal cancer have overtaken those for colon cancer [50, 51]. Yet the criteria for defining and quantifying endoperitoneal recurrence in colon cancer remain unclear, some proposed classifications that leave the problem unsolved [52] and some investigators surprisingly considered peritoneal seeding or ovarian involvement after colonic resection as distant metastases [53]. If we accept the term “locoregional recurrence” in resected colonic cancer defined in an aspecific way, data from 27,000 patients resected for cure yield a recurrence rate ranging between from 5.6 to 12.8 % of the cases [54–63] (Table 2). Conversely, when published data refer specifically to peritoneal metastases (and in these cases include also those from rectal cancer) the rates for metachronous spread approach the previously cited figures. The rate for metachronous peritoneal spread increases in pT3/pT4 tumors, namely 60–70 % of the patients usually treated in surgical centers. Equally important, metachronous spread rates would be even higher if they referred only to patients with colonic cancer [64–66] (Fig. 1). Strategies for treating locoregional recurrence in colon cancer are disappointing for two reasons: first because only 30 % of patients can be surgically treated and second because from this 30 % only 30 % survive 5 years, leaving only 10 % of patients with a chance of being cured [67]. For the aforementioned reasons, the results of surgical treatment for these patients are difficult to analyze because some papers specifically report the results obtained for so-called locoregional recurrence whereas others refer to cytoreductive surgery (usually combined with intraoperative chemotherapy) for peritoneal metastases insofar as the two clinical conditions usually coexist. Two of the largest series describing attempted salvage surgery for locoregional recurrences from colon cancer reported by the Memorial Sloan Kettering Cancer Center and by the Netherland Cancer Registry [57, 68], showed a Kaplan-Meier 5-year survival rate between 25 and 40 %.
Table 2.
Rate of locoregional recurrence in colon cancer patients resected for cure
| 27,111 Colon cancer patients resected for cure | ||
|---|---|---|
| Study | PTS (N) | Locoregional recurrence (%) |
| Manfredi s. (54) | 2657 | 12,8 |
| Stockholm colorectal cancer study group (55) | 1856 | 11,5 |
| Color trial (56) | 1076 | 8 |
| Netherlands cancer registry (57) | 2282 | 6,4 |
| Classic trial (58) | 413 | 5 LEFT 14,7 RIGHT |
| Digestive cancer registry cote d’or (59) | 3375 | 8,2 |
| German research group oncology of gastrointestinal tumor (60) | 904 | 8,4 |
| Danish colorectal cancer group (61) | 9333 | 12,2 |
| Korean national cancer center (62) | 1632 | 4,1 LEFT 8,5 RIGHT |
| Japanese society for colorectal cancer (63) | 3583 | 5,6 |
Fig. 1.
Peritoneal metastases in resected colorectal cancer
Peritoneal spread from colorectal cancer has long been regarded as a terminal condition carrying a dismal prognosis. Only during the past 10 years has a new approach combining CRS + HIPEC yielded encouraging results [7, 69, 70]. A French multicenter study [7] showed that in the surgical approach to colorectal peritoneal metastases the determinant factor in predicting the likelihood of achieving optimal cytoreduction and a major prognostic indicator is the PCI. When the PCI is low, long-term results and postoperative morbidity improve. The PCI score is a critical issue in evaluating patients to undergo CRS and HIPEC for peritoneal metastases from colorectal cancer. Some suggest that 20 is a limit over which the surgical approach should be excluded [71]. Yet even if the Uppsala group report that treatment for high–volume peritoneal disease (PCI >20) seldom results in long-term survival [72], in their multivariate analysis Goéré et al. found as the only independent factor predicting cure a PCI of 10 or less (73): the median PCI in long-term survivors was 4, a value we rarely find in our clinical practice.
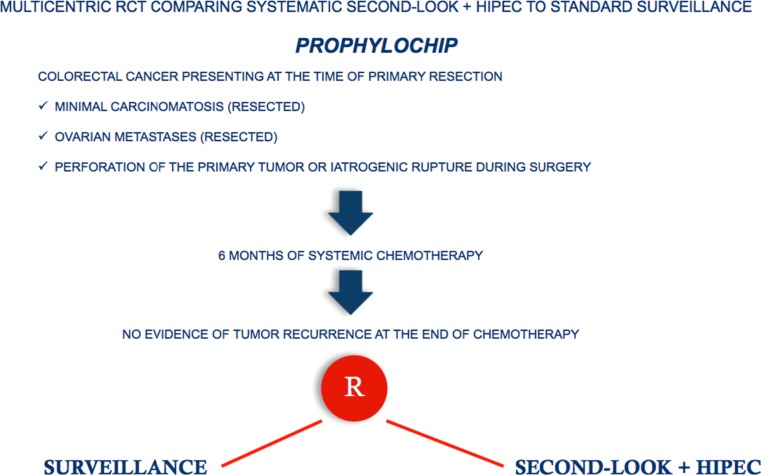
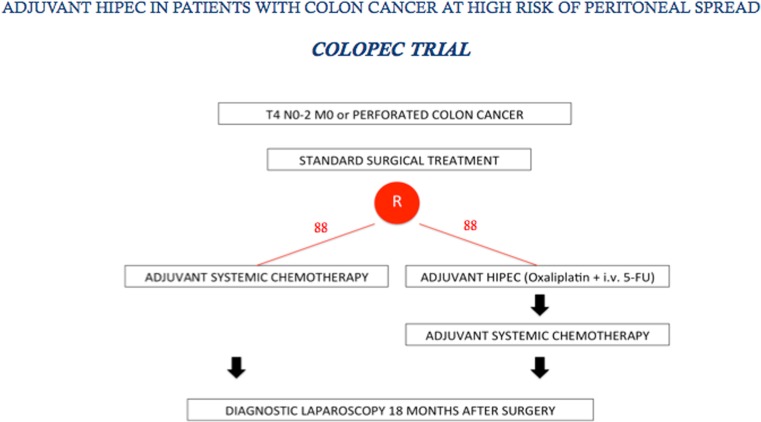
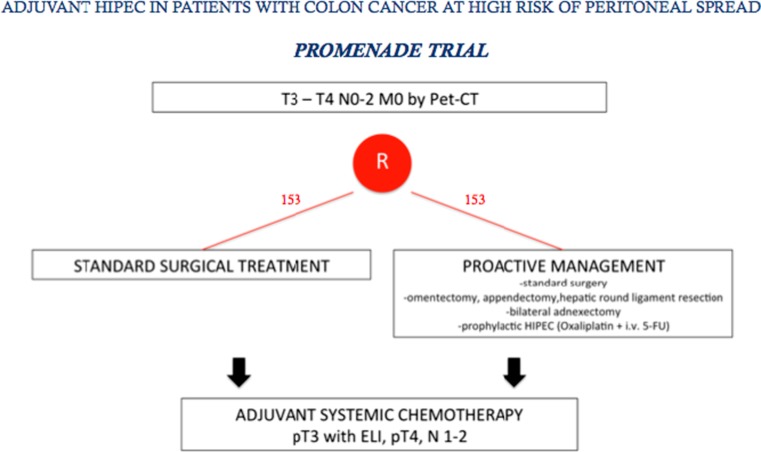
Among the first to suggest using preventive perioperative intraperitoneal chemotherapy in colorectal cancer were Jayne et al. several years ago [74]. Various investigators have re-proposed this strategy particularly in patients with free endoperitoneal cancer cells [75, 76]. In 2014 Sloothaak et al. [77] published a systematic review evaluating the main experiences in preventing endoperitoneal spread in advanced colorectal cancer with proactive endoperitoneal chemotherapy. Unfortunately the various studies evaluated used different selection criteria, drugs and timing for intraperitoneal therapy. Despite these drawbacks, collectively these studies suggest that proactive intraperitoneal chemotherapy (including HIPEC) after primary resection is feasible, well-tolerated and possibly reduces metachronous peritoneal spread. What we now need are data from well-designed randomized control trials addressing two fundamental issues: which patients these trials should include and the optimal timing for HIPEC. Published studies so far focus their efforts in preventing peritoneal metastases in colorectal cancer on second-look surgery plus HIPEC, a strategy proposed by Elias et al. [78]. Based on these preliminary results, a multicenter randomized trial was designed in France in 2010 (Prophylochip) [71] comparing, in asymptomatic patients at high risk of colorectal peritoneal metastases, systematic second-look + HIPEC (oxaliplatin intraperitoneally + intravenous 5-FU) with standard surveillance. Patient accrual for this trial has recently completed (Fig. 2). This trial nevertheless raises questions regarding patient selection because the main study group comprises patients whose malignancies had already spread to the peritoneum at the first operation, hence were incorrectly defined as “patients at risk of peritoneal spread” [79]. A trial more closely addressing the concept of proactive management is the COLOPEC trial started last April in The Netherlands and involving nine Dutch HIPEC centers. The target is patients with colon cancer pT4 any N M0 selected before or after primary surgery with HIPEC (oxaliplatin +5-FU) given simultaneously or eventually delayed in the immediate postoperative period. The trial randomizes 88 patients per arm to receive adjuvant systemic chemotherapy only versus adjuvant HIPEC plus adjuvant systemic chemotherapy. At 18 months staging laparoscopy is done in both treatment arms in patients considered disease-free [80] (Fig. 3). Even though the rationale underlying the decision to limit preventive measures to avoid peritoneal recurrence only to patients with pT4 tumors seems correct, in clinical practice identifying pT4 tumors preoperatively with the current diagnostic tools seems an almost impossible task. Multidetector computed tomography (MDCT) is highly accurate in distinguishing pT1–2 from pT3–4 tumors, but differentiating between pT3 and pT4 tumors remains a challenging task [81–84]. A correct differential diagnosis between pT3 and pT4 tumors is even difficult macroscopically unless tumor filtrates clearly into adjacent organs, and often requires a thorough pathologic assessment [85]. These observations underline that because correctly identifying pT4 tumors preoperatively and perioperatively remains difficult, patients selected for the COLOPEC trial will inevitably undergo a two-stage procedure, primary resection followed by HIPEC. Equally important, pathology studies also showed that, as in lung cancer [86], pT3 tumors invading the peritoneal elastic lamina (30 % of the cases) and pT4 cancers have the same outcome [87–90]. Studies designed to decide on the therapeutic strategy before the definitive pathological assessment are therefore unable to consider pT3 and pT4 tumors separately. For this reason, the FOXTROT trial, aimed to investigate the feasibility, safety and efficacy of preoperative systemic chemotherapy for locally-advanced but operable colon cancer, considered pT3 (with extramural depth > 5 mm) and pT4 tumors together [91]. Based on previous studies conducted in our Institution [92, 93], a new trial (PROMENADE trial) will start next year in four high-volume centers for colon cancer surgery in Italy. The target is patients with pT3, pT4 tumors any N, M0, selected by MDCT (in patients with suspected systemic disease combined with functional positron-emission tomography (PET)), who will be randomized (153 patients in each arm) to undergo standard surgical treatment versus proactive management (standard surgery plus complete greater omentectomy, appendectomy, resection of the round ligament of the liver and, in post-menopausal women, a bilateral adnexectomy) including HIPEC (oxaliplatin +5 FU). In both groups adjuvant systemic chemotherapy will be given in patients with pT3 tumors invading the peritoneal elastic lamina, in pT4 tumors and in patients with lymph node metastases (Fig. 4). Even though the COLOPEC and PROMENADE trials have almost the same primary and secondary endpoints (including rate of metachronous peritoneal spread and outcome) other factors in the two trials differ. In the PROMENADE trial we include patients with pT3 tumors and have several reasons for doing so. First consensus experience from surgeons treating peritoneal metastases from colorectal cancer suggests that peritoneal spread correlates with the same rate in pT3 and in pT4 tumors [65, 74, 94]. Second, correctly assessing serosal invasion in colorectal cancer is difficult and can require extensive tissue sampling. The studies conducted by several far eastern authors show that the subserosal elastic lamina is an anatomic landmark for stratifying pT3 colorectal cancer. When a pT3 tumor invades the subserosal elastic lamina, as it does in 30 % of the cases currently classified as pT3, the clinical outcome almost matches that in patients with pT4 cancer [87–90]. Other differences involve the timing for HIPEC, the decision as to whether to include other surgical procedures and last, including in the trial surgical quality measures. The PROMENADE trial foresees compulsory HIPEC given as soon as the surgical procedure ends whereas COLOPEC – because they use it only in patients with pT4 tumors and definitive histopathological findings – often waits for some days after surgery. Another difference is that unlike COLOPEC the PROMENADE trial envisages ancillary surgical procedures such as omentectomy and adnexectomy. The COLOPEC investigators [80] underline that no evidence exists to justify these procedures especially in patients who undergo HIPEC, a procedure explicitly aimed to eradicate microscopic residual disease. Even though we might agree that no clinical evidence exists for justifying these ancillary resections we underline that removing these anatomic structures known at high risk of harboring tumor cells (omentum or appendix) is included in the guidelines for staging initial ovarian cancers [95]. Hence these procedures might seem reasonable in patients presenting with a large colon tumor infiltrating the peritoneal serosa despite HIPEC. Last, in designing study protocols for preventing locoregional or diffuse peritoneal recurrence our experience suggests that we need to establish criteria for surgical quality intended to leave uninfluenced the meaning of the awaited results. For this reason, we consider that the PROMENADE trial should include only patients resected with curative intent and whose surgical specimen contains, according to the criteria stated by the American Joint Committee on Cancer Staging (7th edition), a congruent lymph node count established in our protocol as a minimum number of 12 [96, 97].
Fig. 2.
PROPHYLOCHIP trial
Fig. 3.
COLOPEC trial
Fig. 4.
PROMENADE trial
Proactive Management of Peritoneal Metastases from Appendiceal Tumors
PMP has an estimated incidence of 1–2 in a million [98] and is listed by the National Organization for Rare Disorders as a rare disease [99]. Appendiceal mucinous neoplasms are considered equally rare tumors with an age-adjusted incidence of 0–12 cases per 1 million individuals per year [100]. As many as 50 % of these patients present with mucinous ascites. The main clinical and pathological prognostic factors for PMP developing are stage at diagnosis and pathologic features of primary tumor [101]. Data from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) showed that the overall incidence of PMP and disease diagnosis in younger patients increased and survival improved from 1973 to 2006 mainly because patients with appendiceal mucinous tumors with advanced stage disease underwent CRS + HIPEC [102]. The finding that three-quarters of the patients with appendiceal mucinous tumors presented with symptoms of acute appendicitis or right iliac fossa pain suggests that many attend general surgical services. Hence at least at disease onset most of these patients are managed by non-specialist centers who lack the necessary know-how and technical equipment needed for CRS + HIPEC. And notwithstanding an interesting contribution showing that among patients who present to a general hospital with symptoms of acute appendicitis, criteria exist that raise a suspicion of appendiceal cancer (patient’s age, disease onset with perforation) [103], most cases are diagnosed only after anatomopathological operative specimen analysis. Hence the onset of PMP can be preventively managed only as second-look surgery [22]. Of great interest in this regard is the study conducted by McDonald et al. at Manchester University, UK, who reviewed patients who had a LAMN and disease limited to the appendix or immediate peri-appendiceal tissues, and identified two LAMN subtypes, LAMN I (disease confined to the appendiceal lumen) and LAMN II (mucin or neoplastic epithelium or both in the appendiceal submucosa, wall or peri-appendiceal tissue or both, with or without perforation) that differed in pathological features and risk for dissemination towards PMP [21]. Patients with LAMN II lesions are therefore at increased risk for dissemination and even those with no clinical signs of spread should undergo second-look with preventive HIPEC. In their series, second-look disclosed mucin in the peritoneum or microscopic spread in 47 % of the patients [21]. Identifying a LAMN class at risk therefore opens the way to minimal access laparoscopic CRS combined with HIPEC [104].
Closing Remarks
The idea of preventing peritoneal metastases before they arise is now among the most interesting though speculative concepts in this fascinating oncologic surgical field. Tertiary prevention, to which proactive management is inter-connected, is ideally suited to advanced colorectal and gastric M0 tumors for which the treatment of metachronous peritoneal spread fails to achieve satisfactory results. In these patients, the ideal therapeutic aim is to treat microscopic peritoneal spread (PCI = 0), a clinical condition that is probably frequently present in patients with advanced tumors, but is exceedingly hard to diagnose. Of fundamental importance in establishing which patients should undergo proactive management are selection criteria, criteria that still today rely on anatomopathological features. Further studies, above all concerning genetic data able to illustrate the changing molecular dynamics underlying malignant progression, are indispensable to integrate present knowledge [105]. In other conditions such as appendiceal tumors, most being low-grade lesions, the decision to undertake proactive management for PMP (for these patients second-look) depends on how the histopathological features of the primary lesion are interpreted. Interpretative variability can run the risk of undertreatment or overtreatment.
Compliance with Ethical Standards
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- 1.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi: 10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Peritoneal carcinomatosis: principle of management. Boston: Kluwer Academic; 1996. [Google Scholar]
- 3.Sugarbaker PH (2013) Cytoreductive surgery and perioperative chemotherapy for peritoneal surface malignancy: textbook and video atlas. Cine-Med Publishing Inc.
- 4.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;10;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 5.Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435–1443. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 8.Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27(36):6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH (2015) Prevention and management of peritoneal metastases from gastrointestinal cancer: a short history of a paradigm for peritoneal surface malignancies. In: Di Giorgio A, Pinto E, Sammartino P, Roviello F (eds) In Treatment of peritoneal surface malignancies: state of art and perspectives, Editors edn. Springer Verlag, pp. 93–105
- 10.Yan L, Yang Y, Yang L. Report of the 9th international congress on peritoneal surface malignancies. Cancer Biol Med. 2014;11(4):281–284. doi: 10.7497/j.issn.2095-3941.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardi M, Sammartino P, Framarino ML, et al. Treatment of peritoneal carcinomatosis from breast cancer by maximal cytoreduction and HIPEC: a preliminary report on 5 cases. Breast. 2013;22(5):845–849. doi: 10.1016/j.breast.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 12.van Oudheusden TR, Lemmens VE, Braam HJ. Peritoneal metastases from small bowel cancer: results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in The Netherlands. Surgery. 2015;157(6):1023–1027. doi: 10.1016/j.surg.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Delotte J, Desantis M, Frigenza M, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of endometrial cancer with peritoneal carcinomatosis. Eur J Obstet Gynecol Reprod Biol. 2014;172:111–114. doi: 10.1016/j.ejogrb.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 15.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Elias D, Mariani A, Cloutier AS, et al. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol. 2014;40(11):1467–1473. doi: 10.1016/j.ejso.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Goéré D, Souadka A, Faron M, et al. (2015) Extent of Colorectal Peritoneal Carcinomatosis: Attempt to Define a Threshold Above Which HIPEC Does Not Offer Survival Benefit: A Comparative Study. Ann Surg Oncol 29[Epub ahead of print] [DOI] [PubMed]
- 18.Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol. 2014;21(4):1147–1152. doi: 10.1245/s10434-013-3443-2. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH. It's what the surgeon doesn't see that kills the patient. J Nippon Med Sch. 2000;67(1):5–8. doi: 10.1272/jnms.67.5. [DOI] [PubMed] [Google Scholar]
- 20.Misdraji J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod Pathol. 2015;28(Suppl 1):S67–S79. doi: 10.1038/modpathol.2014.129. [DOI] [PubMed] [Google Scholar]
- 21.McDonald JR, O'Dwyer ST, Rout S, et al. Classification of and cytoreductive surgery for low-grade appendiceal mucinous neoplasms. Br J Surg. 2012;99(7):987–992. doi: 10.1002/bjs.8739. [DOI] [PubMed] [Google Scholar]
- 22.Honoré C, Caruso F, Dartigues P, et al. Strategies for preventing pseudomyxoma peritonei after resection of a mucinous neoplasm of the appendix. Anticancer Res. 2015;35(9):4943–4947. [PubMed] [Google Scholar]
- 23.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 24.D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240(5):808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasako M, Sano T, Yamamoto S, et al. Japan clinical oncology group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 26.Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250(2):242–246. doi: 10.1097/SLA.0b013e3181b0c80e. [DOI] [PubMed] [Google Scholar]
- 27.Boku N. Gastrointestinal oncology study group of Japan clinical oncology group. Chemotherapy for metastatic disease: review from JCOG trials. Int J Clin Oncol. 2008;13(3):196–200. doi: 10.1007/s10147-008-0784-0. [DOI] [PubMed] [Google Scholar]
- 28.Glehen O, Gilly FN, Arvieux C, et al. Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 29.Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol. 2014;21(5):1448–1455. doi: 10.1245/s10434-013-3327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15(10):2684–2691. doi: 10.1245/s10434-008-0055-3. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzen S, Panzram B, Rosenberg R, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol. 2010;17(10):2733–2739. doi: 10.1245/s10434-010-1090-4. [DOI] [PubMed] [Google Scholar]
- 32.Leake PA, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2011;15(Suppl 1):S27–S37. doi: 10.1007/s10120-011-0071-z. [DOI] [PubMed] [Google Scholar]
- 33.Huang JY, Xu YY, Sun Z, et al. Comparison different methods of intraoperative and intraperitoneal chemotherapy for patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2012;13(9):4379–4385. doi: 10.7314/APJCP.2012.13.9.4379. [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Song Y, Wang Z, et al. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials. BMC Cancer. 2012;12:526. doi: 10.1186/1471-2407-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mi DH, Li Z, Yang KH, et al. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: a systematic review and meta-analysis of randomised controlled trials. Int J Hyperth. 2013;29(2):156–167. doi: 10.3109/02656736.2013.768359. [DOI] [PubMed] [Google Scholar]
- 36.Jemal A, Bray F, Mm C, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 37.http://www.cancer.gov/cancertopics/pdq/treatment/colon/HealthProfessional
- 38.Pugh SA, Shinkins B, Fuller A et al (2015) Site and Stage of Colorectal Cancer Influence the Likelihood and Distribution of Disease Recurrence and Postrecurrence Survival: Data From the FACS Randomized Controlled Trial. Ann Surg. [Epub ahead of print]. [DOI] [PubMed]
- 39.Augestad KM, Bakaki PM, Rose J, et al. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer Epidemiol. 2015;39(5):734–744. doi: 10.1016/j.canep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Gall FP, Hermanek P. Change and current status of surgical treatment of colorectal cancer. Report of experiences of the Erlangen Surgical University Clinic. Chirurg. 1992;63(4):227–234. [PubMed] [Google Scholar]
- 41.West NP, Sutton KM, Ingeholm P, et al. Improving the quality of colon cancer surgery through a surgical education program. Dis Colon Rectum. 2010;53(12):1594–1603. doi: 10.1007/DCR.0b013e3181f433e3. [DOI] [PubMed] [Google Scholar]
- 42.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–1482. doi: 10.1016/S0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 43.Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the county of Stockholm. Stockholm colorectal cancer study group, Basingstoke bowel cancer research project. Lancet. 2000;356(9224):93–96. doi: 10.1016/S0140-6736(00)02469-7. [DOI] [PubMed] [Google Scholar]
- 44.Kapiteijn E, Putter H, van de Velde CJ. Cooperative investigators of the Dutch ColoRectal cancer group. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89(9):1142–1149. doi: 10.1046/j.1365-2168.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 45.Wibe A, Møller B, Norstein J, et al. Norwegian rectal cancer group. A national strategic change in treatment policy for rectal cancer–implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45(7):857–866. doi: 10.1007/s10350-004-6317-7. [DOI] [PubMed] [Google Scholar]
- 46.Birgisson H, Talbäck M, Gunnarsson U, et al. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol. 2005;31(8):845–853. doi: 10.1016/j.ejso.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Iversen LH, Nørgaard M, Jepsen P, et al. Northern Danish cancer quality assessment group. Trends in colorectal cancer survival in northern Denmark: 1985-2004. Color Dis. 2007;9(3):210–217. doi: 10.1111/j.1463-1318.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 48.Talbäck M, Stenbeck M, Rosén M, et al. Cancer survival in Sweden 1960-1998–developments across four decades. Acta Oncol. 2003;42(7):637–659. doi: 10.1080/02841860310013391. [DOI] [PubMed] [Google Scholar]
- 49.Lemmens V, van Steenbergen L, Janssen-Heijnen M, et al. Trends in colorectal cancer in the south of The Netherlands 1975-2007: rectal cancer survival levels with colon cancer survival. Acta Oncol. 2010;49(6):784–796. doi: 10.3109/02841861003733713. [DOI] [PubMed] [Google Scholar]
- 50.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 51.Joern F, Gunter H, Thomas J, et al. Outcome for stage II and III rectal and colon cancer equally good after treatment improvement over three decades. Int J Color Dis. 2015;30(6):797–806. doi: 10.1007/s00384-015-2219-5. [DOI] [PubMed] [Google Scholar]
- 52.Harji DP, Sagar PM, Boyle K, et al. Surgical resection of recurrent colonic cancer. Br J Surg. 2013;100(7):950–958. doi: 10.1002/bjs.9113. [DOI] [PubMed] [Google Scholar]
- 53.Yun JA, Yun SH, Park YA et al (2015) Oncologic Outcomes of Single-incision Laparoscopic Surgery Compared With Conventional Laparoscopy for Colon Cancer. Ann Surg. [Epub ahead of print] [DOI] [PubMed]
- 54.Manfredi S, Bouvier AM, Lepage C, et al. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well Defined population. Br J Surg. 2006;93(9):1115–1122. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 55.Sjövall A, Granath F, Cedermark B, et al. Loco-regional recurrence from colon cancer: a population-based study. Ann Surg Oncol. 2007;14(2):432–440. doi: 10.1245/s10434-006-9243-1. [DOI] [PubMed] [Google Scholar]
- 56.Colon Cancer Laparoscopic or Open Resection Study Group Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10(1):44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 57.Elferink MA, Visser O, Wiggers T, et al. Prognostic factors for locoregional recurrences in colon cancer. Ann Surg Oncol. 2012;19(7):2203–2211. doi: 10.1245/s10434-011-2183-4. [DOI] [PubMed] [Google Scholar]
- 58.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the medical research council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100(1):75–82. doi: 10.1002/bjs.8945. [DOI] [PubMed] [Google Scholar]
- 59.Cortet M, Grimault A, Cheynel N, et al. Patterns of recurrence of obstructing colon cancers after surgery for cure: a population-based study. Color Dis. 2013;15(9):1100–1106. doi: 10.1111/codi.12268. [DOI] [PubMed] [Google Scholar]
- 60.Kornmann M, Staib L, Wiegel T, et al. Long-term results of 2 adjuvant trials reveal differences in chemosensitivity and the pattern of metastases between colon cancer and rectal cancer. Clin Colorectal Cancer. 2013;12(1):54–61. doi: 10.1016/j.clcc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, et al. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259(5):930–938. doi: 10.1097/SLA.0b013e3182a6f2fc. [DOI] [PubMed] [Google Scholar]
- 62.Park JH, Kim MJ, Park SC, et al. Difference in time to locoregional recurrence between patients with right-sided and left-sided colon cancers. Dis Colon Rectum. 2015;58(9):831–837. doi: 10.1097/DCR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe T, Itabashi M, Shimada Y, et al. Japanese society for cancer of the colon and rectum. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Gestel YR, Thomassen I, Lemmens VE, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40(8):963–969. doi: 10.1016/j.ejso.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Kerscher AG, Chua TC, Gasser M, et al. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer. 2013;108(7):1432–1439. doi: 10.1038/bjc.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Segelman J, Granath F, Holm T, et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(5):699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 67.Chang GJ, Kaiser AM, Mills S, et al. Standards practice task force of the American society of colon and rectal surgeons. Practice parameters for the management of colon cancer. Dis Colon Rectum. 2012;55(8):831–843. doi: 10.1097/DCR.0b013e3182567e13. [DOI] [PubMed] [Google Scholar]
- 68.Bowne WB, Lee B, Wong WD, et al. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon Rectum. 2005;48(5):897–909. doi: 10.1007/s10350-004-0881-8. [DOI] [PubMed] [Google Scholar]
- 69.Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. 2008;98(4):263–267. doi: 10.1002/jso.21053. [DOI] [PubMed] [Google Scholar]
- 70.Chua TC, Morris DL, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(5):1330–1336. doi: 10.1245/s10434-009-0866-x. [DOI] [PubMed] [Google Scholar]
- 71.Maggiori L, Elias D. Curative treatment of colorectal peritoneal carcinomatosis: current status and future trends. Eur J Surg Oncol. 2010;36(7):599–603. doi: 10.1016/j.ejso.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Cashin PH, Dranichnikov F, Mahteme H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: cohort analysis of high volume disease and cure rate. J Surg Oncol. 2014;110(2):203–206. doi: 10.1002/jso.23610. [DOI] [PubMed] [Google Scholar]
- 73.Goéré D, Malka D, Tzanis D, et al. (2013) Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 257(6):1065–1071 [DOI] [PubMed]
- 74.Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 75.Hompes D, Tiek J, Wolthuis A, et al. HIPEC in T4a colon cancer: a defendable treatment to improve oncologic outcome? Ann Oncol. 2012;23(12):3123–3129. doi: 10.1093/annonc/mds173. [DOI] [PubMed] [Google Scholar]
- 76.Noura S, Ohue M, Shingai T, et al. Effects of intraperitoneal chemotherapy with mitomycin C on the prevention of peritoneal recurrence in colorectal cancer patients with positive peritoneal lavage cytology findings. Ann Surg Oncol. 2011;18(2):396–404. doi: 10.1245/s10434-010-1319-2. [DOI] [PubMed] [Google Scholar]
- 77.Sloothaak DA, Mirck B, Punt CJ, et al. Intraperitoneal chemotherapy as adjuvant treatment to prevent peritoneal carcinomatosis of colorectal cancer origin: a systematic review. Br J Cancer. 2014;111(6):1112–1121. doi: 10.1038/bjc.2014.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elias D, Honoré C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254(2):289–293. doi: 10.1097/SLA.0b013e31822638f6. [DOI] [PubMed] [Google Scholar]
- 79.Sammartino P, Sibio S, Accarpio F, et al. Prevention of peritoneal carcinomatosis from colorectal cancer: a critical issue. Ann Surg. 2014;259(3):e51. doi: 10.1097/SLA.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 80.Klaver CE, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428. doi: 10.1186/s12885-015-1430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith NJ, Bees N, Barbachano Y, et al. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer. 2007;96(7):1030–1036. doi: 10.1038/sj.bjc.6603646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dighe S, Blake H, Koh MD, Swift I, et al. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg. 2010;97(9):1407–1415. doi: 10.1002/bjs.7096. [DOI] [PubMed] [Google Scholar]
- 83.Sibileau E, Ridereau-Zins C, Vanel D, et al. Accuracy of water-enema multidetector computed tomography (WE-MDCT) in colon cancer staging: a prospective study. Abdom Imaging. 2014;39(5):941–948. doi: 10.1007/s00261-014-0150-9. [DOI] [PubMed] [Google Scholar]
- 84.Wiegering A, Kunz M, Hussein M, et al. Diagnostic value of preoperative CT scan to stratify colon cancer for neoadjuvant therapy. Int J Color Dis. 2015;30(8):1067–1073. doi: 10.1007/s00384-015-2265-z. [DOI] [PubMed] [Google Scholar]
- 85.Stewart CJ, Hillery S, Platell C, et al. Assessment of serosal invasion and criteria for the classification of pathological (p) T4 staging in colorectal carcinoma: confusions, controversies and criticisms. Cancers (Basel) 2011;3(1):164–181. doi: 10.3390/cancers3010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gallagher B, Urbanski SJ. The significance of pleural elastica invasion by lung carcinomas. Hum Pathol;21(5):512–517. [DOI] [PubMed]
- 87.Shinto E, Ueno H, Hashiguchi Y et al (2004) The subserosal elastic lamina: an anatomic landmark for stratifying pT3 colorectal cancer. Dis Colon Rectum;47(4):467–473. [DOI] [PubMed]
- 88.Kojima M, Nakajima K, Ishii G, et al. Peritoneal elastic laminal invasion of colorectal cancer: the diagnostic utility and clinicopathologic relationship. Am J Surg Pathol. 2010;34(9):1351–1360. doi: 10.1097/PAS.0b013e3181ecfe98. [DOI] [PubMed] [Google Scholar]
- 89.Liang WY, Chang WC, Hsu CY, et al. Retrospective evaluation of elastic stain in the assessment of serosal invasion of pT3N0 colorectal cancers. Am J Surg Pathol. 2013;37(10):1565–1570. doi: 10.1097/PAS.0b013e31828ea2de. [DOI] [PubMed] [Google Scholar]
- 90.Yokota M, Kojima M, Nomura S, et al. Clinical impact of elastic laminal invasion in colon cancer: elastic laminal invasion-positive stage II colon cancer is a high-risk equivalent to stage III. Dis Colon Rectum. 2014;57(7):830–838. doi: 10.1097/DCR.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 91.Foxtrot Collaborative Group Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13(11):1152–1160. doi: 10.1016/S1470-2045(12)70348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract. 2012;2012:141585. doi: 10.1155/2012/141585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sammartino P, Sibio S, Biacchi D, et al. Long-term results after proactive management for locoregional control in patients with colonic cancer at high risk of peritoneal metastases. Int J Color Dis. 2014;29(9):1081–1089. doi: 10.1007/s00384-014-1929-4. [DOI] [PubMed] [Google Scholar]
- 94.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 95.Ledermann JA, Raja FA, Fotopoulou C, et al. ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 96.Nelson H, Petrelli N, Carlin A, et al. National cancer institute expert panel. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93(8):583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 97.AJCC Cancer Staging Manual 7th Edition . American joint committee on cancer. New York: Springer; 2010. [Google Scholar]
- 98.Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34(2):196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 99.Amini A, Masoumi-Moghaddam S, Ehteda A, et al. Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects. Orphanet J Rare Dis. 2014;9:71. doi: 10.1186/1750-1172-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 101.Pai RK, Beck AH, Norton JA, et al. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425–1439. doi: 10.1097/PAS.0b013e3181af6067. [DOI] [PubMed] [Google Scholar]
- 102.Shaib WL, Goodman M, Chen Z et al (2015) Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol [Epub Ahead of Print] [DOI] [PubMed]
- 103.Wright GP, Mater ME, Carroll JT, et al. Is there truly an oncologic indication for interval appendectomy? Am J Surg. 2015;209(3):442–446. doi: 10.1016/j.amjsurg.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 104.Fish R, Selvasekar C, Crichton P, et al. Risk-reducing laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasm: early outcomes and technique. Surg Endosc. 2014;28(1):341–345. doi: 10.1007/s00464-013-3189-8. [DOI] [PubMed] [Google Scholar]
- 105.Lenz HJ, Stintzing S (2014) So much effort, so little progress? J Natl Cancer Inst 106(10) [DOI] [PubMed]