Abstract
Occurring either synchronously or metachronously to the primary tumor, peritoneal metastases (PM) are diagnosed in 8 to 20 % of the patients with colorectal cancer (CRC). Prognosis of these patients appears to be worse than those with other sites of metastases. While systemic therapy has shown significant prolongation of survival in patients with stage IV colorectal cancer, the outcomes in the subset of patients with PM has been much inferior. Over the last 2 decades, cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) have been effective in substantially prolonging survival in patients with colorectal PM and have the potential to cure certain patients as well. This article reviews the current evidence for CRS and HIPEC to treat colorectal PM as well as future research going on in this form of locoregional treatment.
Keywords: Cytoreductive surgery, HIPEC, Colorectal Cancer, Peritoneal Metastases
Introduction
Approximately one-fourth of newly diagnosed colorectal cancer (CRC) patients presents with disseminated disease, the liver being the most commonly affected. Besides the liver, a common site of synchronous metastases is the peritoneum [1]. Epidemiological data indicate that peritoneal metastases (PM) from CRC is an event that involves 5 to 10 % of the patients at the time of primary cancer treatment and about 15–30 % of patients with recurrent disease, generally leading to death within weeks or months [2, 3]. Importantly, the peritoneal cavity is thought to be the only site of spread in 15 to 20 % of patients with recurrent CRC [4]. With modern combination of systemic chemotherapy like oxaliplatin and irinotecan in addition to 5-FU and targeted agents like bevacizumab and cetuximab, the median overall survival (OS) of patients with stage IV disease ranges from 7 months to over 24 months [5]. However, in patients with PM alone, Morris et al. reported a median survival of 9 months when treated with modern systemic chemotherapy alone [6].
Though colorectal PM is considered to be a stage IV metastatic disease, Sugarbaker et al., have proposed that it is still locoregional disease and have introduced and popularized the use of the aggressive locoregional combined modality of treatment comprising of cytoreductive surgery (CRS) and hyperthermic Intraperitoneal chemotherapy (HIPEC) for the same [7].
Over the last 2 decades this treatment has gained popularity worldwide and the number of centers offering this treatment has increased. For e.g., in France, the number of centers increased from 3 in 1994 to 25 in 2009 [8].
This article provides an overview of the diagnosis and management strategies and current evidence supporting this treatment of colorectal PM.
Clinical Presentation and Diagnostic Evaluation
In around 8 % of patients undergoing surgery for removal of the primary tumor, PM is an incidental finding [9]. The identified risk factors for PM are female sex, patients with primary mucinous adenocarcinomas, tumor stage T4, lymph node stage N2, a colonic primary, emergency surgery and patients with positive resection margins [9, 10]. In a recent Swedish registry, which analyzed 11, 124 patients with CRC treated between 1995 and 2007, PM was diagnosed in 8.3 % [10], the prevalence of synchronous PM being 4.3 % and that of metachronous PM, 4.2 %. In this analysis, peritoneal disease was the first and only site of metastases in 4.8 % of the patients [10], with median time to recurrence around 14–16 months [10, 11].
On a clinical basis, no symptoms are fully specific of PM, which is atop of that usually symptomatic at a very advanced stage [12, 13]. The main signs which, in combination with the possibly associated symptoms of the primary tumor, can lead to suspicion are the presence of an ascites occurring in 28 to 30 % of patients with synchronous PM and/or an associated small bowel obstruction which concerns 8 to 20 % of the patients at the time of diagnosis [3, 14].
Although regular follow-up and serial imaging is the rule in patients with resected primary tumors, early diagnosis of small-volume PM is rarely possible: definitely, there are no symptoms or signs for small volume progression on imaging. Computed tomography remains the standard for the diagnosis of PM, although its sensitivity is moderate, ranging from 23 to 76 % [6, 7]. Intravenous injection of contrast media and multiplanar reconstructions (especially in the coronal plan) are mandatory, allowing distinguishing PM from small bowel loops. Current recommendation is to do a contrast enhanced CT or MRI and PET-CT for the high risk cases to look for the presence of peritoneal disease. A diagnostic laparoscopy may be used as required. The use of these investigations needs to be individualized, keeping in mind that the PM is usually more extensive than predicted by one or more of these investigations [15].
The current AJCC system (2012) divides stage IV into IVA (comprising of metastatic disease confined to one organ like the liver, lungs, no regional nodes) and IVB (comprising of metastatic disease to more than one organ site of to the peritoneum). Esquivel suggested that this system is flawed with respect to PM as it puts a patient with 3 small peritoneal implants in one region of the peritoneal cavity in the same category as those with multiple liver and lung metastases [16]. The extent of disease is very important in planning the treatment and for this the Peritoneal Cancer Index of Sugarbaker is a more efficient tool [16]. Thus, since almost half of the colorectal PM is diagnosed at the time of surgery for the primary colorectal malignancy, it is imperative for the operating surgeon to note and describe the extent of PM in the operative notes.
Treatment Options for Colorectal PM
The conventional treatment for colorectal PM is systemic chemotherapy. Since two decades, radical treatment combining complete cytoreductive surgery (CRS) followed by intraperitoneal chemotherapy, either HIPEC or early postoperative intraperitoneal chemotherapy, has strongly changed the prognosis of patients with PM from CRC. However, this aggressive locoregional therapy, can be proposed only to patients highly selected on their general status, the extension of the peritoneal disease and the absence of extra peritoneal disease.
Systemic Therapy for Colorectal PM
Systemic therapy in the form of combination chemotherapy with or without targeted therapy has been the backbone of treating colorectal PM.
Two decades ago the only chemotherapy used for colorectal cancer was 5FU and leucovorin, resulting in a median survival rarely exceeded 12 months. The FOLFOX regimen showed a median survival of 19.5 months in previously untreated patients with advanced metastatic disease [17]. Irinotecan based chemotherapy has been shown to produce a median survival of 15.6 months as first line therapy in metastatic disease. The addition of Bevacizumab (Avastin) improved this survival to 20.3 months which was statistically significant [18]. Similarly its addition to FOLFOX produced a median survival of 21.3 months when used as first line therapy though this was not statistically different from that obtained with FOLFOX alone (19.9 months) [19]. The other monoclonal antibody cetuximab has produced a median survival of 19.9 months in combination with FOLFIRI as compared to 18.6 months with FOLFIRI alone [20]. Median survival was 22.8 months with FOLFOX and cetuximab as compared to 18.5 months with FOLFOX alone in K-Ras wild type tumors and 17.5 and 13.4 months respectively in the K-Ras mutant subgroup [21]. However, these studies were not carried out exclusively for patients with colorectal PM and a large proportion of the patients in these studies had liver only metastases which is a more favorable prognostic group.
Franko et al. reported the outcome of patients with colorectal PM from a pooled analysis of two large phase III trials from the North Central Cancer Treatment Group (NCCTG) that included 2101 patients treated with systemic chemotherapy [22]. The majority of the patients were included in the N9741 trial of first-line chemotherapy for metastatic colorectal cancer (n = 1646), and the remaining were in the N9841 trial of second-line chemotherapy (n = 455). Patients with colorectal PM as the sole presentation of metastatic colorectal cancer were uncommon in this patient population (n = 44, 2.1 %) which is in contrast to the reported incidence of 15–20 %. In this study, the prognosis of stage IV patients unable to surgery and treated with systemic chemotherapy (5-FU plus oxaliplatin or plus irinotecan) was worst in case of PM associated to others metastatic sites, with a median survival 12.7 months compared to 17.6 months when patients had no PM (HR = 1.32, 95 % CI, 1.15 to 1.50; P < .001). The presence of PM in patients with metastatic colorectal cancer was associated with a 30 % reduction in the overall survival (OS). This study also concluded that the presence or absence of PM should not affect the choice of chemotherapy regimen.
Klaver et al. reported the results of two similar studies from the Dutch Colorectal Cancer Group (DCCG) and came to the same conclusion as the North American group [23]. They analyzed the data from CAIRO study, in which 820 patients were randomized between sequential treatment (first-line: capecitabine, second-line: irinotecan, and third-line: oxaliplatin plus capecitabine, arm A) and combination treatment (first-line: irinotecan plus capecitabine, second-line: oxaliplatin plus capecitabine, arm B) [24]. In the CAIRO2 study, 755 patients were randomized between capecitabine, oxaliplatin, and bevacizumab (CB regimen), and the same regimen plus weekly cetuximab (CBC regimen) [25]. In these studies, the number of patients with metastatic CRC but no PM exceeded 90 %. In the CAIRO study, only 34 patients (4 %) had PM and of these 34 patients, only 4 had isolated PM. In the CAIRO2 study, only 47 patients (6 %) had PM and 5 of them had isolated PM. In the CAIRO study, median OS was 10.4 months for patients with PM vs 17.3 months for patients with no PM, (P < .001), and in CAIRO2, this was 15.2 months vs 20.7 months, respectively (P < .001). The authors concluded that their data demonstrate decreased efficacy of current standard chemotherapy with or without biologic agents in patients with PM of colorectal origin and that the poor outcome compared to those patients without PM could not be explained by under treatment or increased susceptibility to toxicity, but rather by a relative resistance to treatment secondary to a different biologic behavior of tumors that spread to the peritoneal cavity.
In comparison, patients with PM treated with cytoreductive surgery and HIPEC can reach a median survival of 63 months, and two- and five year survival rates of 81 and 51 %, respectively [26]. Though systemic chemotherapy is widely used to treat colorectal PM, there is no strong evidence showing it benefit in this subgroup of patients [13].
Evidence for CRS and HIPEC for PM from Colorectal Cancer
The combination of maximal cytoreductive surgery with HIPEC to treat peritoneal cancer was first described by Spratt in 1980 [27], but the main initiator of this combined treatment for peritoneal disease was P.H. Sugarbaker [28]. The purpose of surgery is to treat all the macroscopic i.e. visible disease and immediately after resection, and the purpose of HIPEC is to treat the remaining microscopic i.e. non visible residual disease. It is essential that surgery resects all the tumor implants exceeding 1 mm, as the drugs penetration in the tissue and in the tumor deposits is small, less than 1 to 2 mm [29, 30]. HIPEC must be performed immediately after surgery to avoid peritoneal adhesions in which cancer cells may be trapped and could constitute a tumor sanctuary [31–34]. In fact, the exact effect of HIPEC alone in this package is currently unknown in human beings. In an experimental study, animals treated with HIPEC survived longer than those treated with intraperitoneal chemotherapy alone or exclusively with intraperitoneal hyperthermia [35]. This was confirmed in another experimental study, with a reduced tumor load in rats which received intraperitoneal chemotherapy combined to hyperthermia, compared to those which had either chemotherapy or hyperthermia alone [36]. Regarding the potential beneficial effect on survival of HIPEC, until now, only one randomized study has been conducted, a multicentric French trial (PRODIGE 7 trial, NCT00769405), which compared CRS plus HIPEC to CRS without HIPEC. This trial has just closed for inclusion and final results on overall survival should be available in 2017. Two retrospective [37, 38] and one prospective [39] studies have reported survivals of patients who had a complete resection of the PM without intraperitoneal treatment; the 5–year overall survival ranged from 24 to 36 %, but their non-randomized manner and their small effective make conclusions difficult to do.
Most of the evidence for the combined modality treatment of CRS and HIPEC comes from single institution studies and some multicentric studies (Table 1).
Table 1.
Studies reporting outcomes in patients treated with Cytoreductive surgery with or without HIPEC for Colorectal PM
| Ref no | Type of study | No of Patients | Peritoneal Metastases alone | Other distant Metastases | CC-0/CC-1 | HIPEC | Drug | 5 year Disease free survival | 3 year OS | 5 year OS | Median OS | P-Value | Survival in CC-0/CC-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | Prospective | 125 | 41 | 84 | 24.8 % | - | - | - | - | 4.8 % | 12 M | - | 22 % |
| 38 | Retrospective | 153 | - | - | 31 | - | - | - | - | - | - | - | 36 % |
| 39 | Retrospective | 50 | 27 | 23 | - | - | - | - | 45.5 % | 29.64 % | 32.4 M | ||
| 40 | Prospective | 122 | 122 | 85.2 % | Yes | CDDP MMC OX |
25.8 % | 33.5 (3 year OS) | |||||
| 41 | Prospective Comparative |
48 (96) | 48 | - | 100 % | Yes | OX | - | - | 51 % | 62.7 M | <.05 | 51 % |
| 42 | Retrospective | 523 | 523 | 77 | 84 % | Yes (86 %) | MMC OX |
10 % | 41 % | 27 % | 30.1 M | 29 % | |
| 43 | Prospective Case Control |
67 | 67 | Not specified | - | Yes | MMC | 34.7 M | 0.001 | ||||
| 44 | Prospective RCT | 52 (105) | 52 | - | Yes | MMC | 22.3 M | 45 % |
RCT: Randomized controlled Trial; CDDP: Cisplatin; MMC: Mitomycin-C, OX: Oxaliplatin; OS: Overall Survival; M: Months
Cavaliere et al. reported the results of 120 patients treated at 6 different Institutions in Italy with the protocol designed by the Italian Society of Locoregional Treatment in Oncology (SITILO) [40]. Patients were treated with CRS and HIPEC with cisplatin (CDDP) and mitomycin-C (MMC). Eleven patients underwent HIPEC using intraperitoneal oxaliplatin and intravenous 5FU and leucovorin. Complete cytoreductive surgery CC-0 was achieved in 85.2 % of the patients. The three-year survival was 25.8 % and increased to 33.5 % in patients who had an optimal cytoreduction (CC-0) (p < 0001).
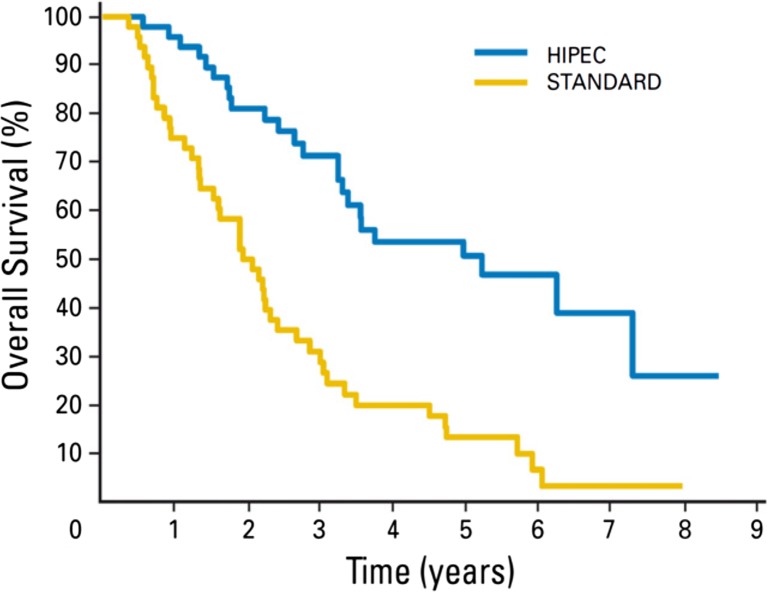
Elias et al. compared 48 patients from the French Multicentric Database with peritoneal carcinomatoses arising from CRC who received palliative systemic oxaliplatin or irinotecan based chemotherapy to 48 patients who underwent additional CRS and HIPEC with oxaliplatin [41]. There was no difference in systemic chemotherapy, with a mean of 2.3 lines per patient. Median follow-up was 95.7 months in the standard group versus 63 months in the HIPEC group. Two-year and 5-year overall survival rates were 81 % and 51 % for the HIPEC group, respectively, and 65 % and 13 % for the standard group, respectively. Median survival was 23.9 months in the standard group versus 62.7 months in the HIPEC group (P < .05, log-rank test). They concluded that though patients with isolated, resectable PM achieve a median survival of 24 months with modern chemotherapies, only surgical cytoreduction plus HIPEC is able to prolong median survival to roughly 63 months, with a 5-year survival rate of 51 % (Fig. 1).
Fig. 1.
Overall survival Cytoreductive Surgery and HIPEC versus Standard Systemic Chemotherapy in patients with Colorectal PM (Reproduced with permission from Ref 41)
Elias et al. reported the results of a study of that included 523 patients from 23 centers in four French-speaking countries that underwent CRS and HIPEC between 1990 and 2007 [42]. At a median follow-up of 45 months, the overall median survival was 30.1 months. The 5 year overall survival was 27 % and the 5 year disease free survival was 10 %. The 5 year survival was 29 % in patients with no residual disease, 14 % in patients with residual disease <2.5 mm and the group of patients with residual disease >2.5 mm had no 5 year survivors. Independent prognostic variables on multivariate analysis were completeness of CRS, extent of PM evaluated by the peritoneal cancer index from Sugarbaker (PCI), lymph node positivity and the use of adjuvant chemotherapy. They concluded that the combined modality of treatment has a low postoperative morbidity and mortality, and provides a good long-term survival in patients with peritoneal cancer index (PCI) scores lower than 20.
Franko et al. reported the results of a case control study in which the study group comprised of 67 patients with colorectal PM who were treated with CRS and HIPEC in addition to systemic chemotherapy and the control group comprised of 38 patients treated with systemic chemotherapy alone [43]. Again, median survival measured from the diagnosis of peritoneal disease was longer with CRS combined with HIPEC (34.7 months vs 16.8 months; P < .001).
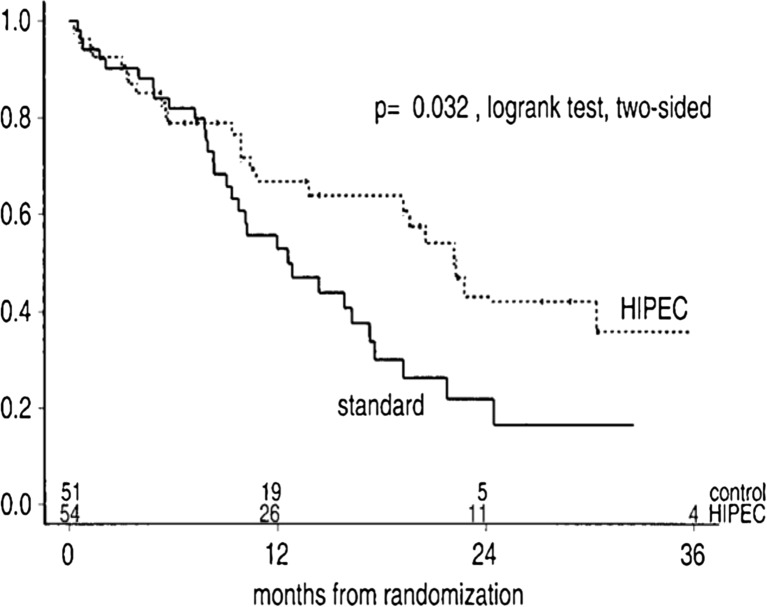
The benefit of the cytoreductive surgery combined with HIPEC above systemic chemotherapy (5-FU leucovorin) has been confirmed in a phase 3 randomized study [44]. One hundred and five patients of colorectal PM were randomized to CRS and HIPEC with mitomycin C followed by systemic chemotherapy with 5-FU and leucovorin or to systemic chemotherapy alone with the same agents. Palliative surgery for prophylaxis or therapy of tumor-related complications was allowed in the control group. The median overall survival was significantly improved from 12.6 to 22.2 months (p = 0.028). Moreover, these results were obtained despite the fact that half of the patients in the experimental arm were ultimately not good candidates for HIPEC because their PM could not be completely surgically resected. These results were confirmed after longer follow-up, at 8 years [45]. In the subgroup of patients with complete macroscopic cytoreduction (CCR-0/1), the median survival increased up to 48 months confirming that the CRS plays a pivotal role regarding the efficacy of the combined treatment. The 5-year survival rate of these patients was 45 % [46]. However, the main criticism of this trial has been that modern chemotherapeutic agents have not been used and that it fails to clarify whether the survival benefit that is gained is from CRS and HIPEC both or CRS alone [47], reason why the French study (NCT00769405), mentioned above, has been performed (Fig. 2).
Fig. 2.
Overall survival after Cytoreductive surgery and HIPEC or standard systemic chemotherapy. In patients with Colorectal PC (Reproduced with permission from Ref 44)
Concerns with CRS and HIPEC
Despite the large body of international publications there is still a lot of skepticism in the medical community regarding CRS and HIPEC. Some of the concerns are a lack of uniformity in the HIPEC methods, in the drugs used and the quiet favorable results obtained with early postoperative intraperitoneal chemotherapy (EPIC).
EPIC Methology and Results
EPIC is the infusion of chemotherapy directly into the abdominal cavity through ports or drains placed during surgery, and is continued for 3–5 days post CRS starting from day 1 following surgery. Some centers give multiple cycles of intraperitoneal chemotherapy and this treatment continues for a few months after surgery.
In 1995, a French group trial comparing CRS and EPIC to systemic chemotherapy had to be abandoned due to patient dissatisfaction in the control arm. In 1996, Elias et al. initiated a study comparing CRS and EPIC to CRS alone and this also had to be closed prematurely due to poor accrual. However, this trial showed that a complete cytoreduction CC-0 resulted in a 2 year survival of 60 % which is a substantial gain from 10 % survival gained from systemic chemotherapy and palliative surgery [48].
Glehen et al. reported results of a multi-institutional study of 506 patients who underwent CRS and HIPEC with or without EPIC from 28 institutions, in which 76 % of the patients had HIPEC, 46 % had EPIC and 22 % had HIPEC and EPIC both. A complete cytoreduction was obtained in 75 % of the patients. HIPEC was commonly performed with mitomycin C (71 %). Other regimens included mitomycin C with cisplatin (13 %) and oxaliplatin (8 %). The regimen of 5-FU with or without mitomycin C (96 %) was commonly used for EPIC. With a median follow-up of 53 months, the median overall survival was 19.2 months and the overall 1-, 3-, and 5-year survival rates were 72 %, 39 %, and 19 %, respectively. In patients with no residual disease after cytoreduction (i.e., CC0) or low burden of disease at initial exploration, the median overall survival could be as high as 32.4 or 34.8 months, respectively. However, no statistically significant difference was seen among patients treated with HIPEC, EPIC, or combined HIPEC/EPIC (overall survival, 19.2, 19.2, and 21.6 months, respectively) [49].
Currently the ICARuS trial (NCT01815359) which is a phase 2 trial is accruing patients in the United States at the Memorial Sloan Kettering Cancer Centre. In this trial HIPEC with mitomycin C will be compared to EPIC with FUDR in patients with colorectal and appendiceal primary tumors following complete cytoreduction.
HIPEC Methology
HIPEC techniques are heterogeneous but their elaboration is highly complex. The combination of drugs can be modified, as can their concentration, but also the composition as well as the volume of the perfusion, the duration, and the temperature. A high number of combinations of these six parameters are possible, and it is not possible to test all of them [50]. Each modification of one of these parameters implies conducting a new pharmacokinetic study. In a recent experimental study which compared the open to the closed technique, using intraperitoneal oxaliplatin at a temperature of 42 °C, the open technique had far higher systemic absorption and abdominal tissue penetration of oxaliplatin than the closed technique, but the closed technique achieved a higher temperature in the diaphragmatic regions, while the open technique was more effective in the other areas. Whatever, intraperitoneal hyperthermia could be achieved with both techniques [51]. And any difference in term of morbidity, mortality and long-term survival has been founded between these 2 techniques in larges studies [41]. At all, it is very important to obtain a high and homogeneous temperature throughout the abdominal cavity, to choose “his” technique and to routinely perform this technique, leading to analyze homogenous data, as no prospective comparison of open and closed techniques of HIPEC in terms of survival, morbidity, or pharmacokinetics as ever been reported [52].
Schematically, there are two main trends worldwide for HIPEC: one uses mitomycin C over 60 to 90 min at 41 °C with a closed-abdomen technique, and the other uses oxaliplatin (460 mg/m2 of oxaliplatin in 2 L/m2 of iso-osmotic 5 % dextrose) over 30 min (strictly 30 min as soon as the minimal temperature of 42 °C had been reached throughout the abdominal cavity, plus 5 to 8 min before to heat the infusate from 38° to 42 °C), at a homogeneous temperature of 43 °C (range: 42–44 °C) with an opened-abdomen technique [53]. A bidirectional (intraperitoneal + systemic) intraoperative chemotherapy which combines intraperitoneal oxaliplatin preceded by an intravenous infusion of 5-FU (400 mg/m2) with leucovorin (20 mg/m2) is now mostly used for PM from CRC [54]. Current evidence does not show that one is superior to the other. Mitomycin has been used due to its high molecular weight, tissue penetration up to 5 mm and a favorable pharmacokinetic profile that permits increased intraperitoneal concentration with limited systemic toxicity [55]. Elias et al. have shown the efficacy and safety of intraperitoneal oxaliplatin in pharmacological and clinical studies both [38]. Though the PRODIGE-7 trial will answer the question about the benefit of HIPEC in addition to CRS, it will not answer the question regarding which technique or drug is superior, as both a the techniques could be performed according to the choice of the surgeon.
Morbidity and Mortality
CRS and HIPEC is considered to be a procedure leading to a high morbidity and mortality. But, over the years, an improvement in the patient selection, surgical techniques and perioperative management has led to a reduction in the morbidity and mortality. Various studies report morbidity and mortality rates of 23 %-45 % and 0 %-12 % respectively [56–60]. In general, morbidity can be divided into surgery related complications such as anastomotic leakage, bleeding and wound infection, and chemotherapy-related complications such as neutropenia, cardiac arrhythmia, or renal insufficiency. There are other postoperative adverse events common to all surgical procedures such as thrombosis, lung embolism, or pneumonia [61]. In fact, in experienced centers, the morbidity and mortality is similar to that of other major abdominal surgery. A learning curve exists for this surgery as well as HIPEC and it’s both the surgeon’s as well as the institutional experience that has an impact on the morbidity and mortality [42, 62, , and 63]. Smeenk et al. also demonstrated that the peak of this learning curve was reached at 130 procedures [62].
Many predictors of postoperative complications have been described, including the operation length [64, 65], the age [66], the number of visceral resections [64], the stoma formation [67], the dose of chemotherapeutic agent [68], and recurrent cancer [69]. But? the most widely known factor is the extent of the peritoneal disease measured with the PCI, with an increased risk of grade IV morbidity (life threatening complications) when the PCI is greater than 12 [65, 69, 70]. In the study reported by Saxena et al., an extensive disease involvement in the left hemi diaphragm was the only significant predictor of severe morbidity on multivariate analysis, probably because this procedure results in respiratory complications, and in a higher risk of pancreatic leak, bleeding, intra-abdominal abscess, due to the dissection of the hilum of the spleen [71].
Is there a Possibility of Cure?
Finally, definitive cure of PM with HIPEC is possible. Goéré et al. recently followed up their patients who had no recurrence more than 5 years after their last treatment [72]. Among 107 patients treated between 1995 and 2005, and after a median follow up of 77 months, the 5 year and 10 year survival rates were 35 % and 15 % respectively. Patients were considered cured if the disease-free survival interval lasted at least 5 years after the treatment of CRC PM or its last recurrence. Patients who had died postoperatively, or from non-cancer-related deaths or patients with a follow-up of less than 5 years since the last curative treatment were excluded from the analysis. Out of 107 patients, seventeen patients (16 %) were considered cured after a disease-free interval of at least 5 years, of whom 14 never developed a recurrence. Cured patients had a significantly lower median peritoneal cancer index than patients who were not cured, respectively 4 (3–16) and 12 (2–36) (P = 0.0002). In multivariate analysis, a peritoneal cancer index of 10 or less was the only independent factor predicting cure. This rate of cure was comparable to that reported after resection of colorectal liver metastases [73, 74], suggesting that prognosis of selected patients who underwent CRS plus HIPEC could benefit from this aggressive treatment as well as patients operating on liver metastases. This was confirmed in a recent study, with a 5-year overall survival rates not statistically different between patients operated on liver metastases from CRC and those who had CCRS plus HIPEC (respectively, 38.5 % and 36.5 %) [75].
Selecting Patients for CRS and HIPEC
Patient selection is an extremely crucial aspect of planning for treatment of patients with colorectal PM. A consensus statement from representatives from the major peritoneal surface malignancy centers from around the world listed eight clinical and radiographic variables associated with increased chances of achieving a complete cytoreduction: Eastern Cooperative Oncology Group (ECOG) performance status one or less; no evidence of extra abdominal disease; up to three small, resectable parenchymal hepatic metastasis; no evidence of biliary obstruction; no evidence of ureteral obstruction; no evidence of intestinal obstruction at more than one site; small bowel involvement: no evidence of gross disease in the mesentery with several segmental sites of partial obstruction; small volume disease in the gastro-hepatic ligament [76].
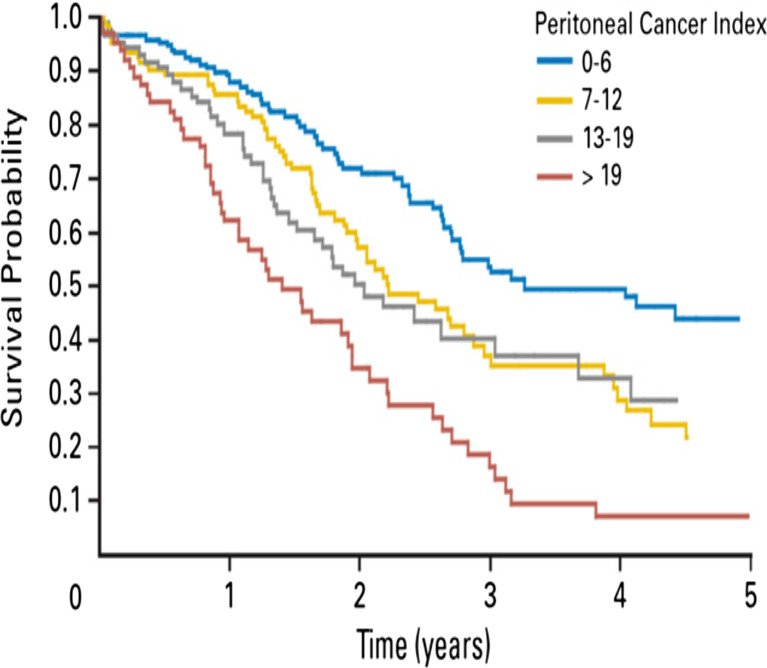
In the French study of 523 patients, the prognostic factors on multivariate analysis were a complete cytoreduction, limited PCI, absence of lymph node involvement and the use of systemic chemotherapy [42]. This study found that in patients with a PCI <20 had a good long term survival with CRS and HIPEC. In another retrospective study of 180 patients published by Goéré et al., the authors showed that when the PCI was >17, the combined modality treatment offered no significant survival benefit compared to palliative systemic chemotherapy alone [77]. Hence, in selecting patients for CRS and HIPEC, patients with a PCI of >17–20 should not be considered for the combined modality treatment (Fig. 3).
Fig. 3.
Overall survival after Cytoreductive Surgery and HIPEC in patients with Colorectal PM according to the Peritoneal Carcinomatosis Index (PCI) (Reproduced with permission from Ref 42)
Thus, indications for CRS plus HIPEC are based on absolute and relative contraindications. An absolute contraindication for CRS plus HIPEC is a poor general status, the presence of extra peritoneal metastases (except 3 liver metastases easily resectable) and huge and diffuse PM. Relative contraindications are: a sub occlusive syndrome due to more than one digestive stenosis, peritoneal disease progressing under systemic chemotherapy and the presence of more than 3 resectable liver metastases (LM are not contraindicated if there are <4 and they are easily resectable) [78, 79].
At all, eligibility criteria for CRS and HIPEC are as follows: a good general status and age below 65–70-70 years, no extra-abdominal disease, no occlusive disorders and no bulky clinical or radiological PM.
Role of HIPEC Is Prevention and Early Treatment of Peritoneal Metastases
In most of the cases, colorectal PM is diagnosed at an advanced stage [80, 81]. As the extent of the disease (PCI) and the completeness of resection are the main linked prognostic factors, survivals are far better in patient with low PCI. Hence, early diagnosis and treatment of PM is paramount.
Elias et al. reported a new policy consisting in a systematic second look surgery in patients at high risk of developing PM. This new strategy has been evaluated in 41 patients without evidence of recurrence (either clinical, radiological or biological), from 1999 to 2009 [82]. Patients were selected on their high risk of developing PC based on 3 criteria related to the primary tumor at the time of surgery: resected minimal synchronous macroscopic PM (n = 25), synchronous ovarian metastases (n = 8), and perforation of the colon (n = 8). PM was discovered and resected during the second-look surgery in 23 patients (55 %). The mean PCI was low (8 ± 6) and peritoneal deposits were resectable in all of the patients. Grade 3–4 morbidity rate was low (9.7 %). After a median follow up of 30 [9–109] months, overall and disease free survivals at 5 years were 90 % and 44 %, respectively. Peritoneal recurrences occurred in 7 patients (17 %), 6 of whom had macroscopic PM discovered during the second-look surgery (26 %). Therefore, this strategy is currently being tested in a randomized phase 3 study (NCT01226394) which has just been closed for inclusion. In this study, after 6 months of adjuvant systemic chemotherapy after resection of the primary, patients at high risk of peritoneal recurrence without any sign of recurrence were randomized in 2 arms: standard arm which consists in monitoring every 3 months the first 2 years and then every 6 months the 3 years later, and the experimental arm which consist in a systematic second-look surgery followed by HIPEC [83].
Another similar study is underway in the United States in which patients with CRC at high risk of developing PM who underwent curative surgery and subsequently received standard of care adjuvant chemotherapy will be evaluated. The patients who remain without evidence of disease by imaging, physical examination, and tumor markers for 12 months after the primary operation will be randomized to mandatory second look surgery or standard-of-care surveillance. At laparotomy, CRS and HIPEC will be performed with intraperitoneal oxaliplatin with concurrent systemic 5-fluorouracil and leucovorin. Up to 100 patients will be enrolled to allow for 35 evaluable patients in each arm; accrual is expected to last 5 years [84].
Another prospective case control study was carried out by Sammartino et al. in patients with advanced colonic cancer at high risk of PM (mucinous or signet-ring cell) without peritoneal or systemic spread in which patients were either treated with standard colectomy or a more aggressive combined surgical approach [85]. The study group comprised of 25 patients with mucinous or signet ring cell histology T3/T4, any N, M0 colonic cancer who underwent hemicolectomy, omentectomy, bilateral salpingo oophorectomy, hepatic round ligament resection and appendectomy followed by HIPEC during the resection of the primary, while the control group of 25 patients was treated by standard surgical resection during the same time period. Peritoneal recurrence occurred in 4 % of the patients in the experimental group compared to 22 % of them in the control, without increased morbidity (P < 0.05). Actuarial overall survival curves disclosed no significant differences, whereas actuarial disease-free survival curves showed a significant difference in favor for the experimental group (36.8 versus 21.9 months, P < 0.01). The authors concluded that this aggressive preventive surgical approach including HIPEC reduces the incidence of peritoneal recurrence in patients with advanced mucinous colonic cancer and also significantly increases disease-free survival compared with a homogeneous control group treated with a standard surgical approach, without increasing morbidity. It has the benefit of avoiding a second operation in asymptomatic patients at the risk of morbidity and the associated cost. However, these results require confirmation in randomized controlled trials.
The above studies highlight the fact that the best way to deal with PM is to treat it early or prevent it and will form the basis of future treatment for PM from CRC. At present, the focus should be early detection and treatment, prophylactic HIPEC and second look surgery with or without HIPEC remains investigational.
Conclusion
PM from colorectal cancer represents a poor prognostic subgroup of patients. Despite the advances in the systemic therapy, outcomes in patients with PM remain poor. The combined modality of treatment comprising of complete cytoreductive surgery and HIPEC has the potential to substantially prolong survival in a selected subgroup of patients with limited disease spread (PCI < 20) and should be considered the standard of care. Since most of the peritoneal extent is diagnosed at a late stage, the focus should be on early detection of peritoneal recurrence after resection of the primary in patients at high risk of developing PM. Preventive strategies comprising of aggressive initial surgery with HIPEC or second look surgery with HIPEC seem to be promising but require further evaluation in randomized controlled trials.
References
- 1.Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JWW, De Hingh IH. Predictors and survival of synchronous peritoneal carcinomatoses of colorectal origin: a population-based study. Int J Cancer. 2011;128(11):2717–2725. doi: 10.1002/ijc.25596. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi: 10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Zerhouni S, McCart A (2012) Peritoneal carcinomatosis of colorectal origin: Is there a role for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Oncology Exchange 11(2) May 2012
- 5.Gustavsson B, Carlsson G, Machover D, Petrelli P, Roth A, Schmoll H, Tveit K, Gibson F. A review of the evolution of systemic chemotherapy in the Management Of Colorectal Cancer. Clin Colorectal Cancer. 2015;14(1):1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Chua TC, Morris DL, Saxena A, et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011;18:1560–1567. doi: 10.1245/s10434-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH, VJ v, van tinteren H, SV R, et al. Re:Toxicity of Cytoreductive Surgery and Hyperthermic Intra-Peritoneal Chemotherapy. J Surg Oncol. 2004;88:276–278. doi: 10.1002/jso.20157. [DOI] [PubMed] [Google Scholar]
- 8.Maggiori L, Elias D. Curative treatment of colorectal peritoneal carcinomatosis: current status and future trends. Eur J Surg Oncol. 2010;36:599–603. doi: 10.1016/j.ejso.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ, Creemers GJ, de Hingh IH. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:963–969. doi: 10.1016/j.ejso.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 11.Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ, Pemberton JH, Wolff BG. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32. [PubMed] [Google Scholar]
- 12.Russell AH, Tong D, Dawson LE, Wisbeck WM, Griffin TW, Laramore GE, Luk KH. Adenocarcinoma of the retroperitoneal ascending and descending colon: sites of initial dissemination and clinical patterns of recurrence following surgery alone. Int J Radiat Oncol Biol Phys. 1983;9(3):361–365. doi: 10.1016/0360-3016(83)90297-3. [DOI] [PubMed] [Google Scholar]
- 13.Russell AH, Tong D, Dawson LE, Wisbeck W. Adenocarcinoma of the proximal colon. sites of initial dissemination and patterns of recurrence following surgery alone. Cancer. 1984;53(2):360–367. doi: 10.1002/1097-0142(19840115)53:2<360::AID-CNCR2820530232>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 15.Ihemelandu C, Shen P, Stewart J, Votanopoulos K, Levine A. Management Of Peritoneal Carcinomatosis from colorectal cancer. Semin Oncol. 2011;38(4):568–575. doi: 10.1053/j.seminoncol.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esquivel J. (2014) Current Status and Future Directions of Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Intervent Oncol 360;2(6):E45-E52
- 17.Goldberg RM, Sargent DJ, Morton RF. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 20.Cutsem V, Kohne C, Hitre E, Zaluski J, Chang Chien C, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Kyung Roh J, Folprecht G, Ruff R, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 21.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 22.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal cacinomatosis with systemic chemotherapy: A pool analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaver YL, Simkens LH, Lemmens VE, et al. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol. 2012;38(7):617–623. doi: 10.1016/j.ejso.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomized control trial. Lancet. 2007;370(9582):135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 25.Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO 2 study of the Dutch Colorectal Cancer Group. An interim analysis of toxicity. Ann Oncol. 2008;19(4):734–738. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 26.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 27.Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40(2):256–260. [PubMed] [Google Scholar]
- 28.Sugarbaker PH, Cunliffe WJ, Beliveau JF, de Bruin E, Graves T. Rationale for perioperative intraperitoneal chemotherapy as a surgical adjuvant for gastrointestinal malignancy. Reg Cancer Treatment. 1988;1:66–79. [Google Scholar]
- 29.Van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, Beijnen JH, Bartelink H, Begg AC. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34(1):148–154. doi: 10.1016/S0959-8049(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 30.Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7(2):108–115. doi: 10.1038/nrclinonc.2009.217. [DOI] [PubMed] [Google Scholar]
- 31.Zoetmulder FA. Cancer cell seeding during abdominal surgery: experimental studies. Cancer Treat Res. 1996;82:155–161. doi: 10.1007/978-1-4613-1247-5_10. [DOI] [PubMed] [Google Scholar]
- 32.Jacquet P, Averbach AM, Jacquet N.(1995) Abdominal wall metastasis and peritoneal carcinomatosis after laparoscopic-assisted colectomy for colon cancer. Eur J Surg Oncol 1995 Oct;21(5):568–70. Review [DOI] [PubMed]
- 33. Elias D, Di Pietrantonio D, Boulet T, Honore C, Bonnet S, Goere D, Kohneh-Shahri N, Raynard B. (2009) Natural history of complete cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2009 Apr;35(4):434–8. Epub 2008 Mar 28 [DOI] [PubMed]
- 34.Sugarbaker PH. Strategies for the prevention and treatment of peritoneal carcinomatosis from gastrointestinal Cancer. Cancer Investig. 2005;23(2):155–172. doi: 10.1081/CNV-50478. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T, Maeta M, Koga T. Influence of local hyperthermia on the healing of small intestinal anastomoses in the rat. Br J Surg. 1991;78(1):57–59. doi: 10.1002/bjs.1800780119. [DOI] [PubMed] [Google Scholar]
- 36.Pelz JO, Doerfer J, Dimmler A, et al. Histological response of peritoneal carcinomatosis after hyperthermic intraperitoneal chemoperfusion (HIPEC) in experimental investigations. BMC Cancer. 2006;6:162. doi: 10.1186/1471-2407-6-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulsow J, Merkel S, Agaimy A, Hohenberger W. Outcomes following surgery for colorectal cancer with synchronous peritoneal metastases. Br J Surg. 2011;98(12):1785–1791. doi: 10.1002/bjs.7653. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda K, Hotta T, Takifuji K, Yamamoto M, Nasu T, Togo N, Oka M, Tabuse K, Yamaue H. Clinical impact of a macroscopically complete resection of colorectal cancer with peritoneal carcinomatosis. Surgery. 2012;151(2):238–244. doi: 10.1016/j.surg.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Désolneux G, Mazière C, Vara J, Brouste V, Fonck M, Béchade D, Bécouarn Y, Evrard S. Cytoreductive surgery of colorectal peritoneal metastases: outcomes after complete cytoreductive surgery and systemic chemotherapy only. PLoS ONE. 2015;31:10(3). doi: 10.1371/journal.pone.0122816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavaliere F, Valle M, De Simone M, et al. 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra-abdominal chemohyperthermia: a S. I. T. I. L. O. multicentric study. In Vivo. 2006;20(6 A):747–750. [PubMed] [Google Scholar]
- 41.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2008;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 42.Elias D, Gilly F, Boutitie F, Quenet F, Bereder J, Mansvelt B, Lorimier G, D` D, Glehen O. Peritoneal colorectal Carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2009;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 43.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 44.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 45.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 46.Khatri P. (2009) Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer: A Panacea or Just an Obstacle Course for the Patient? JCO.25.5109 [DOI] [PubMed]
- 47.Elias D, Quenet F, Goere D. Current status and future directions in the treatment of peritoneal dissemination from colorectal carcinoma. Surg Oncol Clin N Am. 2012;21:611–623. doi: 10.1016/j.soc.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Elias D, Delperro JR, Sideris L, Benhamou E, Pocard M, Baton O, Giovannini M, Lasser P. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting ran domized trials. Ann Surg Oncol. 2004;11:518–521. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Glehen OKF, Sugarbaker PH, Elias D, et al. Cytoreductive surgery combined with perioperative intraperitonea chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Elias D, Antoun S, Goharin A, Otmany AE, Puizillout JM, Lasser P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig. 2000;1(5):431–439. [PubMed] [Google Scholar]
- 51.Ortega-Deballon P, Facy O, Jambet S, Magnin G, Cotte E, Beltramo JL, Chauffert B, Rat P. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann Surg Oncol. 2010;17(7):1957–1963. doi: 10.1245/s10434-010-0937-z. [DOI] [PubMed] [Google Scholar]
- 52.Sarnaik AA, Sussman JJ, Ahmad SA, McIntyre BC. Lowy AM Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. Recent Results Cancer Res. 2007;169:75–82. doi: 10.1007/978-3-540-30760-0_6. [DOI] [PubMed] [Google Scholar]
- 53.Elias D, Bonnay M, Puizillou JM, Antoun S, Dermirdjian S, El Otomany A, et al. Heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Surg Oncol. 2002;13:267–272. doi: 10.1093/annonc/mdf019. [DOI] [PubMed] [Google Scholar]
- 54.Elias D, Goere D, Blot F, Billard V, Pocard M, Kohneh-Shahri N, Raynard B. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol. 2007;14(6):1818–1824. doi: 10.1245/s10434-007-9348-1. [DOI] [PubMed] [Google Scholar]
- 55.Kuzuya T, Yamauchi M, Ito A, et al. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol. 1994;46:685–689. doi: 10.1111/j.2042-7158.1994.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 56.Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–796. doi: 10.1007/s10434-999-0790-0. [DOI] [PubMed] [Google Scholar]
- 57.Pilati P, Mocellin S, Rossi CR, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508–513. doi: 10.1245/ASO.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Kusamura S, Younan R, Baratti D, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106:1144–1153. doi: 10.1002/cncr.21708. [DOI] [PubMed] [Google Scholar]
- 59.Franko J, Gusani NJ, Holtzman MP, et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15:3065–3072. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 60.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Glockzin G, Ghali N, Lang S, Schlitt H, Piso P. ‘results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal Carcinomatosis from colorectal cancer. J Surg Oncol. 2009;100:306–310. doi: 10.1002/jso.21332. [DOI] [PubMed] [Google Scholar]
- 62.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 63.Levine SJH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J. Am. coll. Surg. 2007;204(5):943–953. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 64.Elias D, Goere D. Peritoneal carcinomatosis of colorectal origin: recent advances and future evolution toward a curative treatment. Recent Results Cancer Res. 2007;169:115–122. doi: 10.1007/978-3-540-30760-0_11. [DOI] [PubMed] [Google Scholar]
- 65.Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, François Y, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10(8):863–869. doi: 10.1245/ASO.2003.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Jacquet P, Stephens AD, Averbach AM, Chang D, Ettinghausen SE, Dalton RR, Steves MA, Sugarbaker PH. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. 1996;77(12):2622–2629. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2622::AID-CNCR28>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 67.Hansson J, Graf W, Påhlman L, Nygren P, Mahteme H. Postoperative adverse events and long-term survival after cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2009;35(2):202–208. doi: 10.1016/j.ejso.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, Deraco M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106(5):1144–1153. doi: 10.1002/cncr.21708. [DOI] [PubMed] [Google Scholar]
- 69.Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;85(2):61–67. doi: 10.1002/jso.20013. [DOI] [PubMed] [Google Scholar]
- 70.Saxena A, Yan TD, DL. M. A critical evaluation of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. World J Surg. 2010;34(1):70–78. doi: 10.1007/s00268-009-0206-0. [DOI] [PubMed] [Google Scholar]
- 71.Saxena A, Yan TD, Morris DL. Critical assessment of preoperative and operative risk factors for complications after iterative peritonectomy procedures. Eur J Surg Oncol. 2010;36(3):309–314. doi: 10.1016/j.ejso.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257(6):1065–1071. doi: 10.1097/SLA.0b013e31827e9289. [DOI] [PubMed] [Google Scholar]
- 73.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 74.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D. (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009 Apr 10;27(11):1829–35. doi: 10.1200/JCO.2008.19.9273. Epub 2009 Mar 9 [DOI] [PubMed]
- 75.Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D. (2015) Extent of colorectal peritoneal Carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: A comparative study. Ann Surg Oncol 2015 Sep;22(9):2958–64. Epub 2015 Jan 29 [DOI] [PubMed]
- 76.Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257(6):1065–1071. doi: 10.1097/SLA.0b013e31827e9289. [DOI] [PubMed] [Google Scholar]
- 77.Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society Of Surgical Oncology. Ann. surg. Oncologia. 2007;14(1):128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 78.Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D.’ (2015) Extent of Colorectal Peritoneal Carcinomatosis: Attempt to Define a Threshold Above Which HIPEC Does Not Offer Survival Benefit: A Comparative Study.’ Ann Surg Oncol Jan 29 [DOI] [PubMed]
- 79.Maggiori L, Goéré D, Viana B, Tzanis D, Dumont F, Honoré C, Eveno C, Elias D. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258(1):116–121. doi: 10.1097/SLA.0b013e3182778089. [DOI] [PubMed] [Google Scholar]
- 80.González-Moreno S (2006 Aug) Peritoneal surface Oncology: A progress report. Eur J Surg Oncol 32(6):593–596 [DOI] [PubMed]
- 81.de Bree E, Koops W, Kröger R, van Ruth S, Witkamp AJ. Zoetmulder FA Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol. 2004;86(2):64–73. doi: 10.1002/jso.20049. [DOI] [PubMed] [Google Scholar]
- 82.Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D. Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT Abdominal Imaging. 2008;33(1):87–93. doi: 10.1007/s00261-007-9211-7. [DOI] [PubMed] [Google Scholar]
- 83.Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, Burtin P, Dromain C, Goéré D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254(2):289–293. doi: 10.1097/SLA.0b013e31822638f6. [DOI] [PubMed] [Google Scholar]
- 84.R. T. Ripley, J. L. Davis, C. D. Kemp, S. M. Steinberg, M. A. Toomey, and I. Avital. (2010) Prospective randomized trial evaluating mandatory second look surgery with HIPEC and CRS vs. standard of care in patients at high risk of developing colorectal peritoneal metastases. Trials, vol. 11, article no. 62 [DOI] [PMC free article] [PubMed]
- 85.Sammartino P, Sibio S, Biacchi D, Cardi M, Accarpio F, Mingazzini P, Rosati MS, Cornali T, Di Giorgio A. Prevention of peritoneal metastases from Colon Cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Prac. 2012;2012:141585. doi: 10.1155/2012/141585. [DOI] [PMC free article] [PubMed] [Google Scholar]