Abstract
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has become widely accepted as an effective method of treating peritoneal metastases (PM) from various cancers. CRS performed with the goal of removing all the macroscopic disease and comprises of peritonectomy procedures and visceral resections. CRS is a technically challenging surgery that requires a considerable amount of skill and appropriate patient selection. This article is a review of the techniques and current recommendations for performing CRS.
Keywords: Cytoreductive surgery, Peritonectomy, Techniques of cytoreductive surgery, Pelvic peritonectomy, Subphrenic peritonectomy, Multi-organ resection
Introduction
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has become widely accepted as an effective method of treating peritoneal metastases (PM) from various cancers. [1] However this is a complex treatment that is difficult to implement and careful patient selection is needed for obtaining good results. Cytoreductive surgery is performed with the goal of complete removal of all macroscopic disease. This is achieved by peritonectomy procedures and en-bloc resection of the viscera where required. Their use depends on the extent of PM. Normal peritoneum is not excised, only that which is affected is removed [2].
Patterns of Peritoneal Cancer Spread
An understanding of the mechanisms and patterns of peritoneal cancer spread is required for treating PM. The peritoneum is a complex organ comprising of the parietal peritoneum lining the abdominal wall and the visceral peritoneum covering the abdominal and pelvic organs. It reflects and folds as it covers the visceral organs, forming potential spaces between its lining along the abdominal and pelvic walls. Additionally, the ligaments, mesenteries and omentum are also formed from its reflections over the abdominal and pelvic viscera. [3].
The network of reflections, folds, boundaries and potential spaces provides a pathway for disease spread within the peritoneal cavity and between the peritoneal and retroperitoneal spaces. [4] Primary tumors arising from the peritoneum are rare and cancer spread to the peritoneum, PM is caused by a primary tumor arising elsewhere. [5] There are four pathways of peritoneal cancer spread. [6]
Direct spread First, contiguous spread of tumor from one organ to another can occur directly via the serosa. Tumors of the stomach, colon and pancreas spread in that manner to contiguous and non-contiguous organs. [7]
Lymphatic spread: Tumor can also spread directly from one noncontiguous organ to another through the subperitoneal lymphatics along the ligaments, mesenteries and omenta. This pattern of spread accounts for a small percentage of PM and is seen is lymphomas especially non-Hodgkins lymphomas. [8]
Along the flow of ascitic fluid (Redistribution phenomenon) This is characteristic of pseudomyxoma peritonei and ovarian cancer [9, 10]. The tumor cells follow the movement of intraperitoneal fluid. Gravity causes the fluid to collect in the pelvis. The negative pressure created by respiratory movement causes an upward movement of the fluid along the paracolic gutter to the subdiaphragmatic area from where it is redistributed via the falciform ligament to the lesser and greater omenta and over the bowel surfaces. The commonest sites of PM are the pelvis, the lower end of the small bowel mesentry, the right paracolic gutter, the undersuface of the diaphragm and the greater and lesser omenta. Besides, PM tend to involve the visceral peritoneum in greater volumes at 3 definite sites, where the bowel is anchored to the retroperitoneum. These are the rectosigmoid colon where it emerges from the pelvis (this site is also dependent and tends to be more often heavily involved), the region of the ileocaecal valve and the region of the pylorus. Small bowel sparing is characteristic and is due to the constant peristaltic activity
Hematogenous route: This route is employed by both intra and extra- abdominal tumors and PM arising from breast cancer, lung cancer and melanoma spread by this route. [11]
Basic Principles
CRS comprises of peritonectomy procedures and visceral resections. The goal is to remove all macroscopic disease leaving no residual disease or for some pathologies like pseudomyxoma peritonei or peritoneal mesothelioma, disease that is not greater than 2.5 mm in size. The rationale being that intraperitoneal chemotherapy is not effective in eradicating tumor nodules larger than 2.5 mm in size. [12] CRS aims at removal of macroscopic disease while HIPEC acts on the microscopic disease. The various peritonectomy procedures required to achieve complete tumor removal are listed in Table 1 [13].
Table 1.
Peritonectomy procedures and resections that are combined to achieve a complete cytoreduction (reproduced from Ref 13 with permission)
| Peritonectomy procedures | Resections |
|---|---|
| Anterior parietal peritonectomy | Old abdominal incisions, umbilicus, epigastric fat pad |
| Left upper quadrant peritonectomy | Greater omentum and spleen |
| Right upper quadrant peritnectomy | Glissons capsule deposits |
| Pelvic peritonectomy | Uterus, ovaries and rectosigmoid colon |
| Omental bursectomy | Gall bladder and lesser omentum |
Complete tumor removal may necessitate removal of adjacent viscera. For example, a complete pelvic peritonectomy usually requires stripping of the pelvic side-walls, the peritoneum overlying the urinary bladder, the cul-de-sac and resection of the rectosigmoid with or without a panhysterectomy [14]. Metastases in the region of the ileocaecal valve may necessitate a removal of the terminal ileum and limited part of the right colon to achieve a complete resection. Disease in the subpyloric space is often confluent with the tumor coming in from the foramen of Winslow and sometimes the tumor involving the lesser omentum. [15] This may necessitate a distal or total gastrectomy to achieve a complete cytoreduction. Layering of cancer on a peritoneal surface and a portion of the intestine may require en-bloc peritonectomy and bowel resection to facilitate faster and complete removal [16].
Patient Selection for CRS and HIPEC
The greatest criticism of CRS and HIPEC has been the morbidity and mortality. Not all patients benefit from extensive CRS [17]. Rapid recurrence of peritoneal metastases with or without lymph node or systemic metastases occurs in patients with extensive disease leading to morbidity without benefit in survival. Quantitative prognostic indicators have been defined to measure the outcomes of CRS and HIPEC and these should be used in selecting patients for the procedure. [18] These indicators are histopathology, imaging findings, peritoneal cancer index (PCI) and the completeness of cytoreduction score (CCR). [18, 19] Apart from the general health and fitness for the procedure, these indicators should be taken into consideration before taking up a patient for surgery. All patients need evaluation by a multidisciplinary team comprising of surgeon, medical oncologist, anesthesiologist, intensivist, radiologist and pathologist.
Histopathology
In appendiceal tumors and pseudomyxoma peritonei, the grade of the tumor has an impact on the outcome regardless of the completeness of cytoreduction. [19] Low grade tumors do better than high grade tumors and signet ring cell tumors. [20] Patients with extensive disease also benefit from the combined modality treatment. The same is not seen in other GI cancers like colorectal cancer and gastric cancer where the histologic subtype has a lesser impact on survival [21]. In peritoneal mesotheliomas, histopathology has a strong impact on survival results and the epitheloid subtype do better than the biphasic or sarcomatoid sub-type [22].
Imaging
A contrast enhanced CT scan of the thorax abdomen and pelvis is the standard investigation used for evaluating patients prior to surgery. It rules out major distant metastases and may predict the extent of disease [23, 24]. The sensitivity of helical CT for peritoneal tumors less than 1 cm was found to be only 25–50 % compared with 85–95 % for larger tumor deposits [25]. In a multi-institutional study, Esquivel et al. found that the preoperative CT PCI score underestimated the extent of carcinomatosis in 33 % of patients [26]. In recent studies MRI has been reported to be more accurate for detecting <1 cm nodules by some authors, while others have found no difference [27, 28]. MRI results are also dependent on the expertise of the interpreter [28]. PET or PET-CT scans may add some more information in this direction by detecting extraabdominal (mediastinal or supraclavicular) lymphadenopathy. However, regarding evaluation of peritoneal disease volume and distribution, PET or PET-CT scans does not give additional information compared with a regular, good-quality CT scan [29]. For mucinous carcinomatosis, CT scan with two distinctive radiologic criteria (segmental obstruction of the small bowel and presence of tumor nodules greater than 5 cm in diameter on small bowel surfaces or directly adjacent to small bowel mesentery) may distinguish patients with resectable disease from those with non-resectable malignancy. But sensitivity of CT scan for malignant nodules less than 5 mm, especially on small bowel surfaces, remains low [30]. Carcinomatosis with implants less than 5 mm would not be imaged or would be underestimated in their distribution, especially in patients with postoperative changes [31].
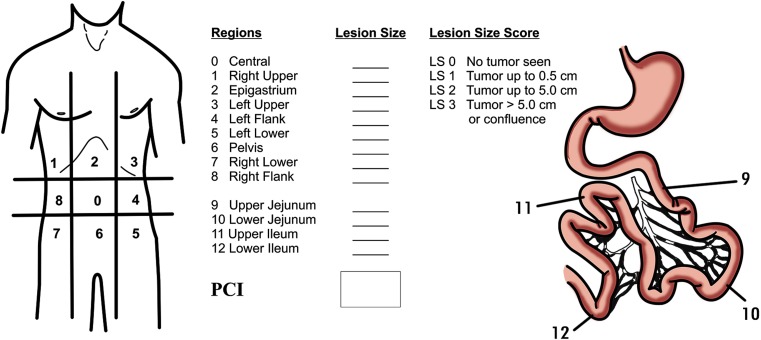
Peritoneal Cancer Index (PCI) (Fig. 1)
Fig. 1.
Peritoneal carcinomatosis index (PCI)
The PCI is a quantitative assessment of cancer distribution throughout the abdomen and the pelvis [32]. The PCI involves integration of both peritoneal implant size & distribution of nodules on the peritoneal surface. To assess the distribution of peritoneal surface disease, the abdominopelvic regions are examined, and for each of these 13 regions, a Lesion Size (LS) score is determined. The LS score grades lesions as LS-0 score when no malignant deposits are visualised. LS-1 score signifies tumor nodules <0.5 cm; LS-2 score indicates tumour nodules between 0.5 to 5.0 cm; and an LS-3 score signifies tumour nodules >5.0 cm in any dimension or confluent nodules or layering of tumour. The number of nodules is not scored, and only the size of the largest nodule is considered. The summation of LS score in each of the 13 abdominopelvic region is the PCI for that patient. Thus, a minimum score of 1 and a maximum of 39 (3 × 13) is possible [32].
The PCI has been found to be an independent prognostic factor for morbidity and survival in various disease types like colorectal peritoneal metastases, gastric peritoneal metastases and in ovarian cancer [33–35]. In patients with colorectal PM when the predicted PCI is >17–20, CRS and HIPEC should not be offered, similarly for gastric cancer this cut off is a predicted PCI of 12 [33, 34]. Exceptions to the prognostic value of PCI include non-invasive diseases like low grade pseudomyxoma peritonei and peritoneal mesothelioma. In these disease types, so long as a complete cytoreduction is achieved, the initial PCI does not impact the survival as much as the completeness of cytoreduction score [36]. The second exception is the presence of invasive tumor deposits at crucial anatomic sites like the common bile duct, the base of the bladder or pelvic side wall. The presence of residual unresectable disease at crucial anatomic sites overrides the favorable effect on the prognosis of low PCI score [37].
Completeness of Cytoreduction Score (CC score)
In order to describe more precisely the type of cytoreduction performed, Sugarbaker reported the CC score [38]. A CC-0 score indicates that no visible peritoneal seeding exists following the cytoreduction; a CC-1 score indicates that tumor nodules persisting after cytoreduction are <2.5 mm, which is a nodule size thought to be penetrable by intracavity chemotherapy and would, therefore, be designated a complete cytoreduction; a CC-2 score indicates tumor nodules between 2.5 mm and 2.5 cm; and a CC-3 score indicates tumor nodules >2.5 cm or a confluence of unresectable tumor nodules at any site within the abdomen or pelvis. CC-2 and CC-3 cytoreductions are considered incomplete. Stricter criteria for complete cytoreduction are required for high-grade non-mucinous neoplasms; a complete cytoreduction is restricted to resection to absolutely no visible evidence of disease. The CC score is a major prognostic indicator for PM from colorectal cancer, ovarian cancer, gastric cancer, pseudomyxoma peritonei and peritoneal mesotheliomas as shown by several large multiinstitutional studies [33, 35, 36, 39].
This information is of less value to the surgeon in planning treatments than the PCI; the CC score is not available until after the cytoreduction is complete, whereas the PCI is available at the time of abdominal exploration [37]. Based on the pre-operative evaluation the surgeon must be able to reasonably predict the probability of a complete cytoreduction and in cases where it is not deemed feasible, surgery should not be undertaken. Though in certain cases, a palliative debulking can be done to provide symptomatic relief and/or prolong survival, the goal needs to be defined before undertaking the procedure [40, 41].
Contra-Indications to CRS and HIPEC
CRS and HIPEC is a major surgery for which the patient needs to have a good performance status and all other systemic illness (cardiac, pulmonary etc.) should be under control. Absence of any of these would be a contraindication for the procedure [42]. Age is not an absolute contraindication to the combined treatment if the patient is fit to undergo major surgery under general anesthesia, although surgical efforts and chemotherapy dosages may have to be modified [44]. There are also disease specific contraindications as discussed, for e.g. a predicted PCI of >17–20 for colorectal cancer and >12 for gastric cancer would preclude a curative surgery [33, 34]. For patients with colorectal cancer and ovarian cancer, progression on neoadjuvant chemotherapy is not a contraindication to CRS and HIPEC [35, 43]. Involvement of the urinary tract is also not an absolute contraindication provided complete tumor clearance can be attained and does not enhance the morbidity from the procedure [44]. Multiple extra-abdominal metastasis or massive suprarenal retroperitoneal lymph nodes involvement are absolute contraindications [45, 46]. Liver metastases may also be a contraindication except for colorectal carcinomatosis. Several studies have shown that the presence of few (one to three) liver metastases did not influence survival if they could be surgically removed for PC from colorectal cancer [47, 48]. For conditions where there is no cut off for PCI, the contraindications are
Extensive bowel resection that is likely to compromise the future quality of life e.g. 2 or more sites of segmental small bowel obstruction, patients requiring a total gastrectomy with a total colectomy
Involvement of pancreas head, bladder triagone, porta hepatis.
Massive or diffuse involvement of pleural space [49]
Technique of CRS and HIPEC
Electro-Evaporative Surgery
It is imperative that the surgeon dealing with PSM must develop the necessary technical skills and must be proficient in dissection with electrosurgery. High-voltage electrosurgery leaves a margin of heat necrosis that is devoid of viable malignant cells [50, 51]. Peritonectomies and visceral resections using traditional scissor and knife dissection will unnecessarily disseminate a large number of tumor cells within the abdomen. This minimizes the likelihood of persistent disease and decreases blood loss, which is extremely important in these long duration surgeries. Besides, hemostasis has to be absolute before starting the process of HIPEC, since during that period, no hemostasis can be achieved and if there is persistent bleeding, it may be necessary to abort the HIPEC. The commonest method of dissection is using the ball-tip electrocautry as described by Dr. Sugarbaker [50]. However, several surgeons have their preferences, including the bipolar scissors, the ultrasonic scalpel or a combination of any of the above.
Positioning of the Patient, Incision and Exposure
Patient is placed in a supine position with the gluteal fold at the end of the table to allow full access to the perineum. Either a modified lithotomy or a leg-split position is used with utmost care to prevent pressure points and myonecrosis of the calf muscles [52]. Skin preparation is from the mid-chest to mid-thigh with preparation of the genitalia and catheterization.
Abdominal cavity is opened through a midline incision from the xiphoid to the pubis. The old abdominal incision is often excised, and so is the umbilicus. Quite often, these patients have had previous surgeries and caution needs to be exercised to prevent injury to intestines while opening the abdomen. Generous abdominal exposure using a self-retaining retractor system is essential. Pre-operative assessment using imaging or laparoscopy usually gives a roadmap to the steps involved in the surgery. If diaphragmatic stripping needs to be done, a xiphoidectomy will help in better exposure and placements of retractor blades [53]. When disease is extensive, a through exploration is performed to look for contraindication for CRS and no bowel should be resected till the surgical plan is finalized.
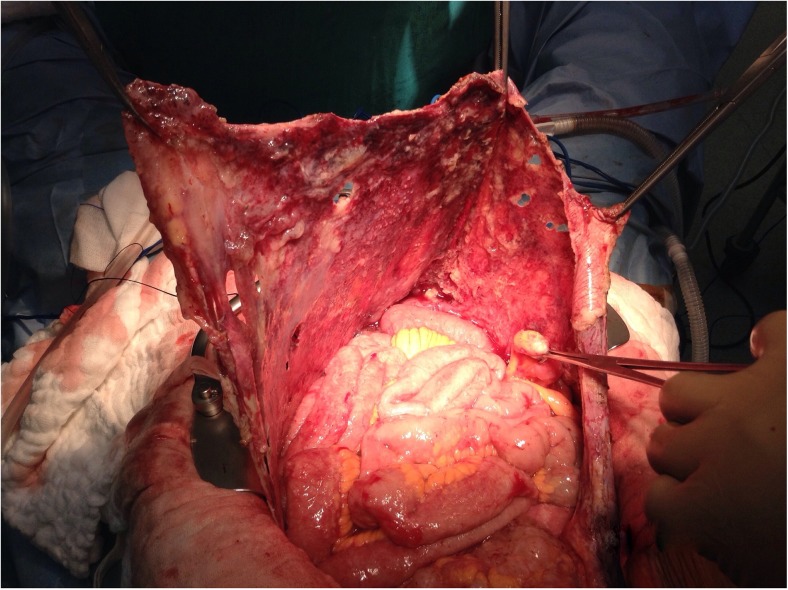
Anterolateral Parietal Peritonectomy (Fig. 2)
Fig. 2.
Anterolateral parietal peritonectomy
If an anterolateral parietal peritonectomy is contemplated based on either the pre-operative imaging or laparoscopy, an extra-peritoneal approach may facilitate dissection and save time. This extra-peritoneal dissection can be carried into the upper abdomen to continue into the sub-diaphragmatic plane to perform the diaphragmatic peritonectomy [37] Once a part of the peritoneum along the costal margin is dissected off the diaphragmatic muscles, the costal retractor blades can be placed to elevate the parities and this greatly facilitates the dissection. At this stage, it is wise to make a small window in the peritoneum to palpate the peritoneal surface. If there are extensive deposits over the anterior parietal peritoneum, a bilateral anterolateral parietal peritonectomy can be performed by the extra-peritoneal approach. During this procedure, constant traction on the abdominal wall and the specimen is important to expose the planes, where high voltage electrocautery current is applied to dissect off the peritoneum of the parities. When the dissection reaches the paracolic region, the dissection turns medially, facilitated by the medial traction on the colon, and the dissection can proceed in the plane of the fascia of Toldt. Superiorly, this dissection can blend into the right and left subphrenic peritonectomy and inferiorly it can continue into the complete pelvic peritonectomy [54].
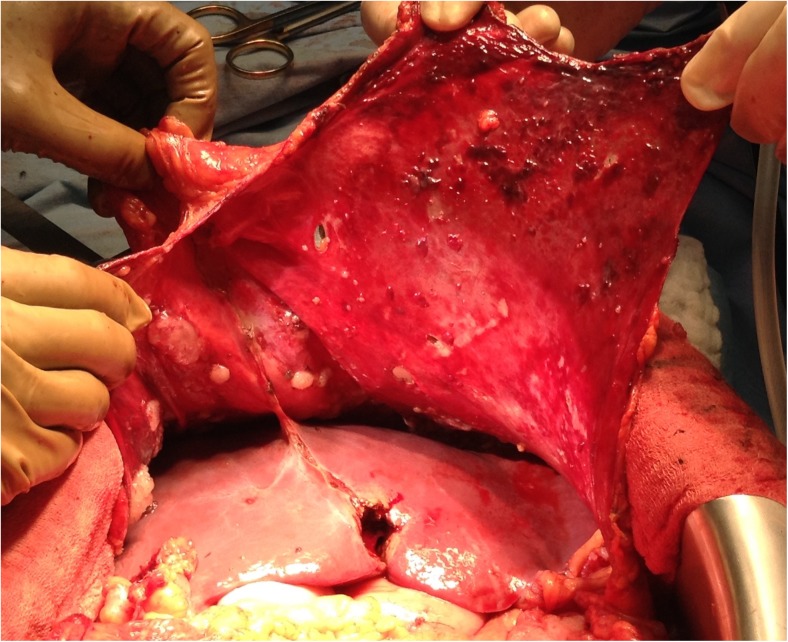
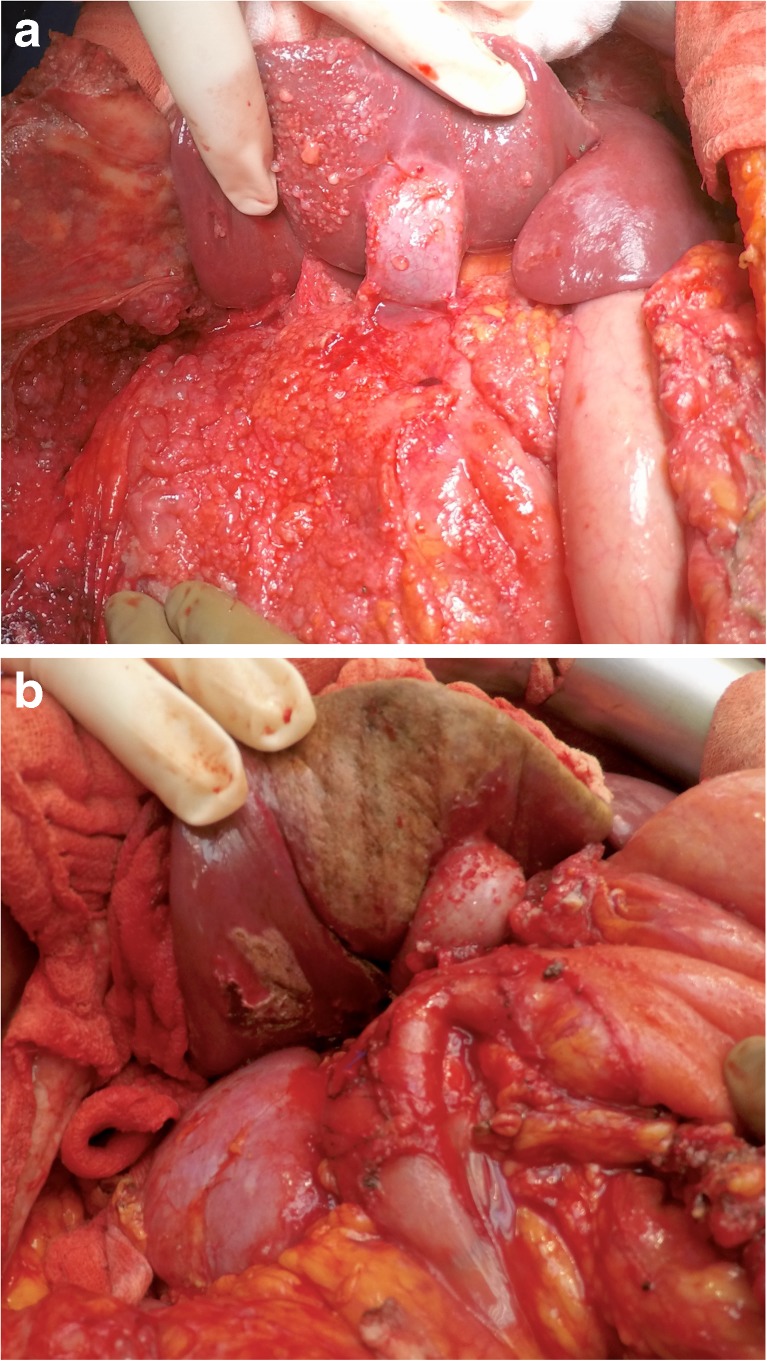
Right Subphrenic Peritonectomy (Figs. 3 and 4) and Stripping of the Glisson’s Capsule
Fig. 3.
Subphrenic peritonectomy – peritoneum dissected off the diaphragm on either sides
Fig. 4.
Completed right subphrenic peritonectomy
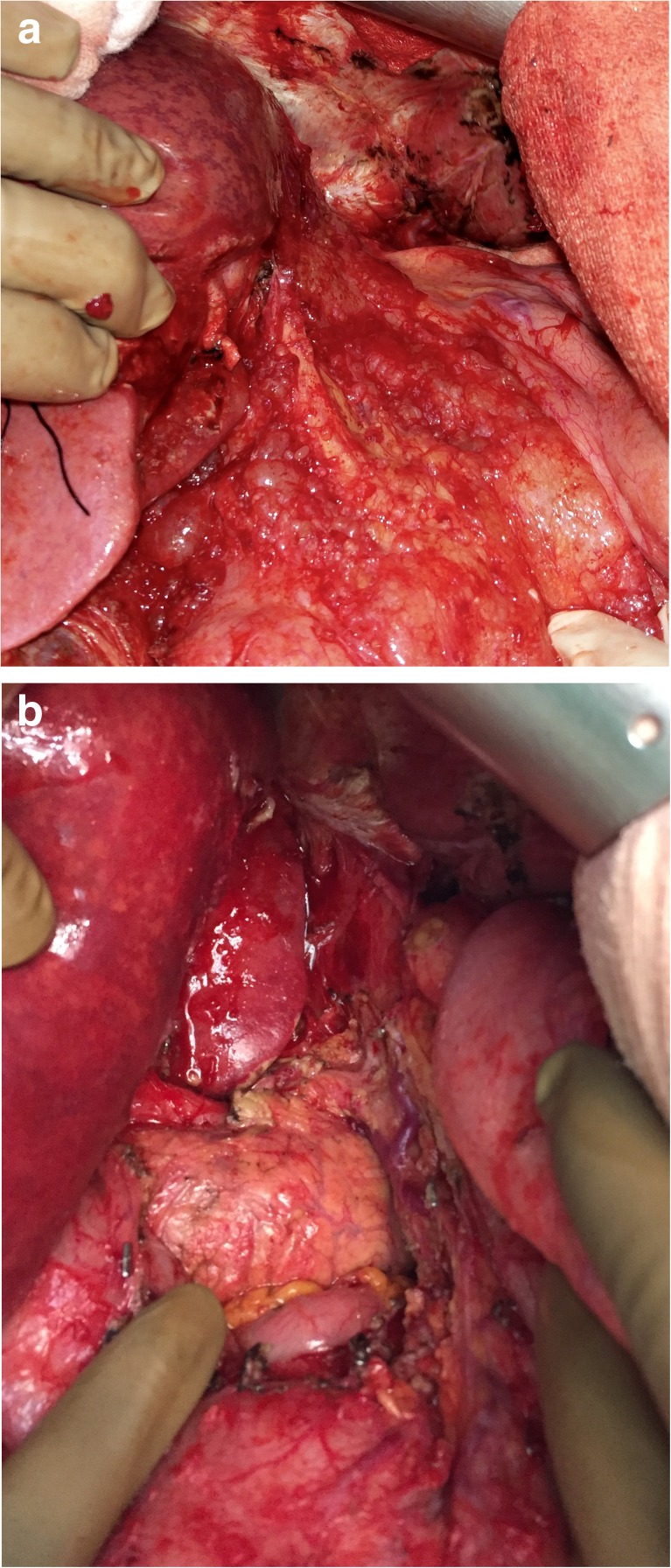
A firm traction on the peritoneal specimen helps to get the diaphragmatic muscle into view of the abdominal incision and the plane exposed is then dissected to proceed with the subphrenic peritonectomy. There are several small vessels from the diaphragmatic muscles to the peritoneum and these need to be coagulated to minimize blood loss. The diaphragmatic vessels will be encountered just before the tendinous portion of the diaphragm, and if possible, they should be preserved. In invasive diseases, the tumor deposits may be infiltrative and involve the diaphragmatic muscle, especially in the region of the tendinous portion. This may require resection of a part of the diaphragm which can be sutured with continuous or interrupted non-absorbable monofilament suture. This suturing may be done immediately or can be deferred to after the HIPEC to let the chemotherapy circulate in the chest as well. Caution must be exercised to avoid injury to the right hepatic vein and the IVC. Posterolaterally, the dissection proceeds over the upper part of the Gerota’s fascia and the adrenal, which constitute the base of the dissection (Fig. 5a,b).
Fig. 5.
Base of the right subphrenic peritonectomy and subhepatic space; 5a – before; 5b - after
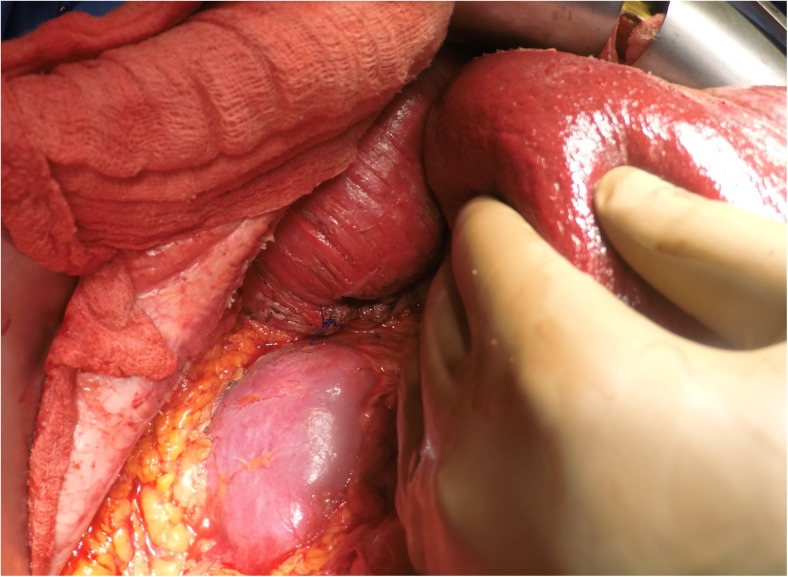
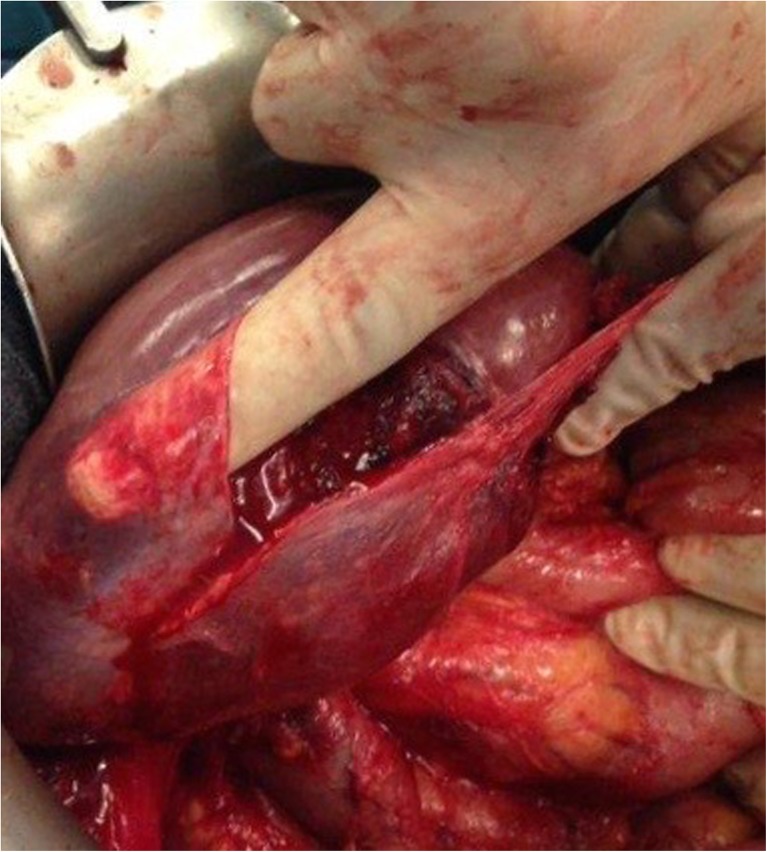
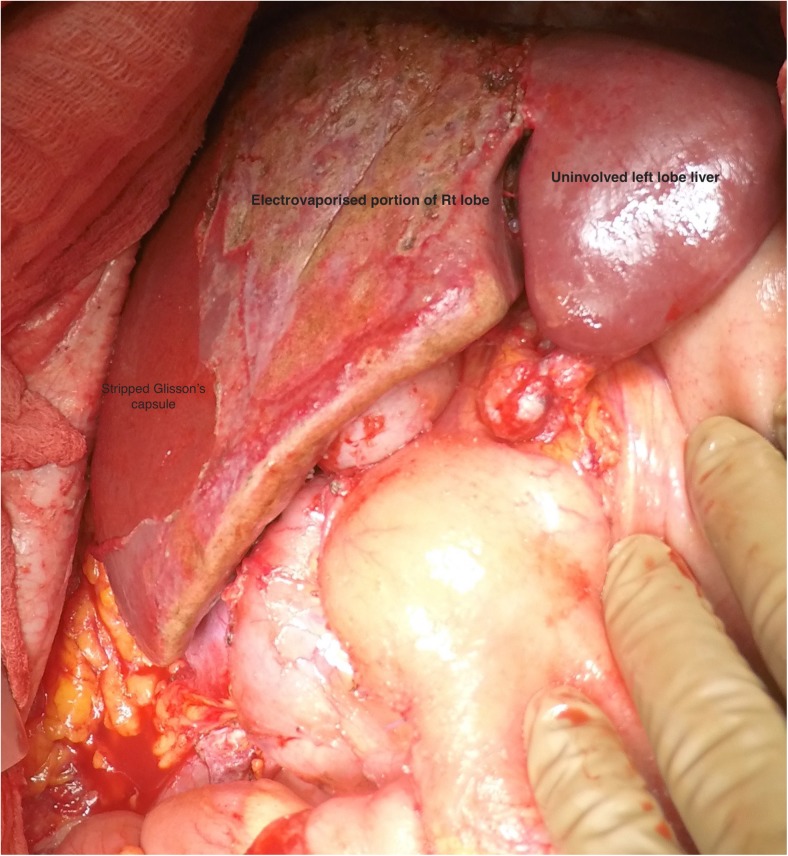
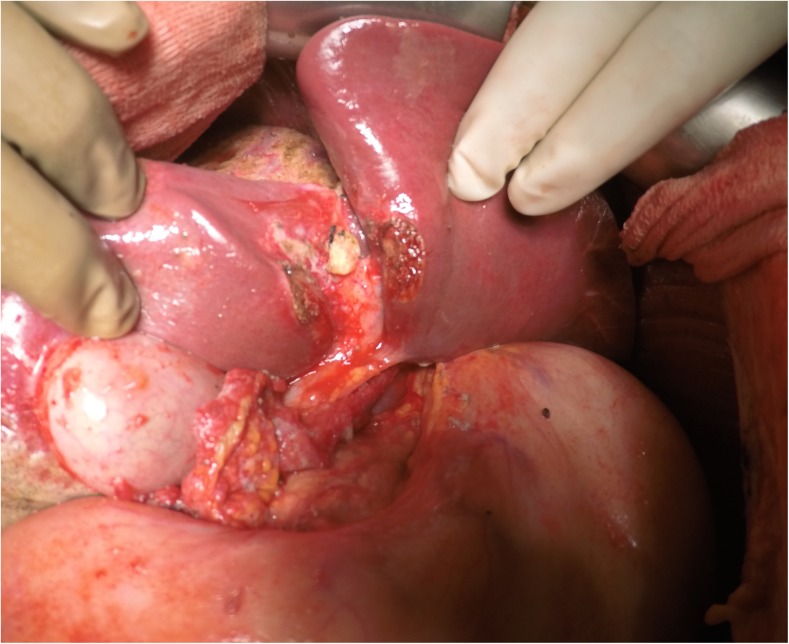
Once the bare area of the liver is encountered superiorly, the diaphragmatic peritoneum turns onto and becomes continuous with the Glisson’s capsule. In diseases like pseudomyxoma peritonei, it is not uncommon to encounter heavy disease over the liver, which can form a thick layer over the Glisson’s capsule. Glehen et al. have described a very effective method of removing this disease [55]. Using either sharp or electrocautery, the sub-Glissonian space is entered and then by bluntly moving the fingers in this plane, the Glisson’s capsule along with the disease can be effectively and swiftly lifted off the liver surface (Figs. 6 and 7). This dissection is greatly facilitated if the tumor specimen is maintained intact. Isolated deposits of tumour can be electrovaporated or dissected off. Homeostasis can be achieved by placing a surgical pad over the liver surface while the dissection proceeds to some other area of the abdomen. Planning this part of the dissection in the earlier part of the cytoreduction ensures that adequate time is given for the hemostasis [55]. The dissection continues laterally on the right to encounter the perirenal fat and the adrenal. As the peritoneal reflection at the posterior aspect of the liver is divided, there is a risk of traumatising the vena cava or the caudate lobe veins that pass between the vena cava and the segment 1 of the liver. Care should be exercised to avoid injury to these structures, which can cause significant bleeding.
Fig. 6.
Finger dissection to strip off the Glisson’s capsule
Fig. 7.
Completed Glisson’s capsule stripping
Left Subphrenic Peritonectomy (Fig. 8)
Fig. 8.
Left subphrenic peritonectomy
The epigastric fat and peritoneum at the edge of the abdominal incision is placed in firm traction and dissected of the posterior rectus and as the dissection proceeds cephalad in the left upper quadrant, the diaphragmatic muscle is visualised. The dissection proceeds posterolaterally to separate the peritoneum off the entire diaphragmatic surface, the left adrenal and the superior half of the perirenal fat. The splenic flexure of the colon is severed from the left parabolic gutter and retracted medially by dividing the peritoneum along the Toldt’s line. Again, most of the dissection must be done with electrosurgery to ensure minimal blood loss and adequate hemostasis. When the base of the dissection is reached, the left adrenal gland, pancreas and left perinephric fat along with the anterior surface of the transverse mesocolon is visualized [37].
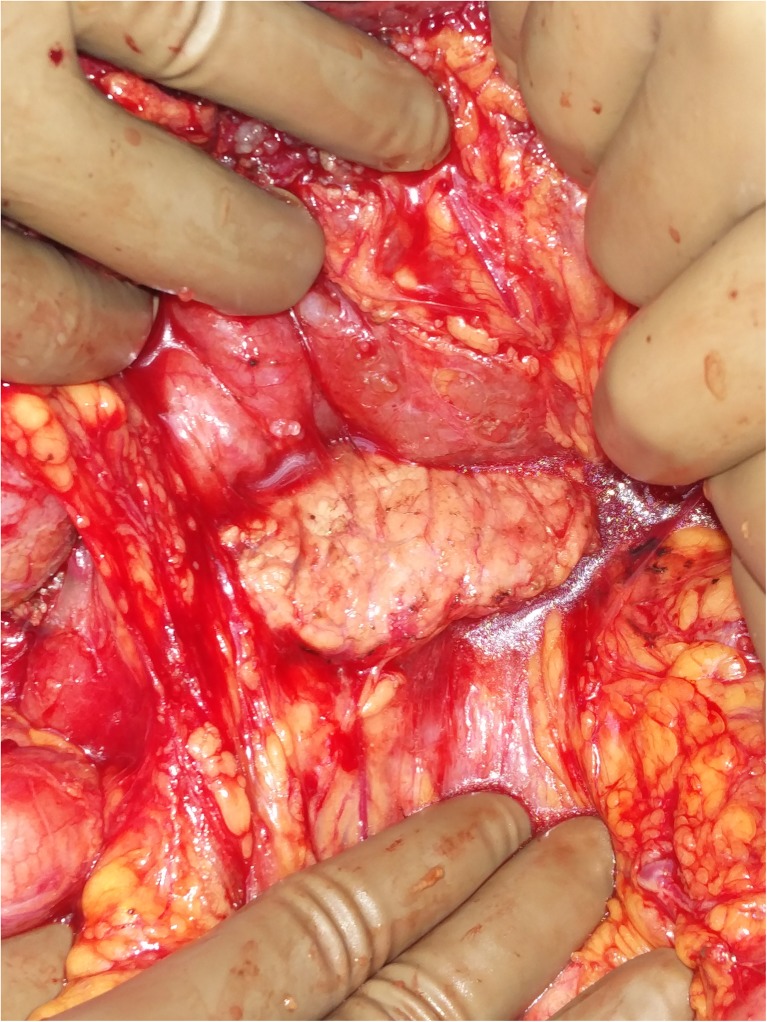
Greater Omentectomy and Splenectomy
The greater omentum is dissected off the transverse colon and dissection proceeds below the layer of peritoneum covering the transverse mesocolon to reach the lower border of the pancreas (omental bursectomy). Occasionally, the pancreatic capsule may need to be dissected, especially in recurrent cases where a previous omentectomy has been performed and there are deposits in the lesser sac and pancreatic capsule (Fig. 9). The greater omentectomy is completed by dividing the branches of the gastroepiploic arcade to the greater curvature. If the omental disease is not significant, the gastroepiploic arcade may be preserved. When the left upper quadrant peritonectomy has been performed, the structures beneath the left hemidiaphragm can be elevated and the short gastric vessels can be divided under direct vision. If a splenectomy needs to be performed, great care needs to be taken while ligating and dividing the splenic vessels to avoid traumatising the tail and body of pancreas. Often, when there is significant disease in the hilum of the spleen, the posterior approach where the splenic vessels are approached and dissected posterolaterally after the spleen has been completely mobilised, is safer and can help in avoiding injury to the pancreas. Splenectomy should not be performed in cases where the spleen is not involved by tumor [56]. Some authors have suggested that splenectomy ameliorates the hematologic toxicity of HIPEC and reduces the requirement of growth factors and platelets. [57].
Fig. 9.
Lesser sac dissection – note the pancreatic capsule being stripped off to clear the deposits in the lesser sac following a previous attempt at cytoreduction with recurrent lesions in the lesser sac
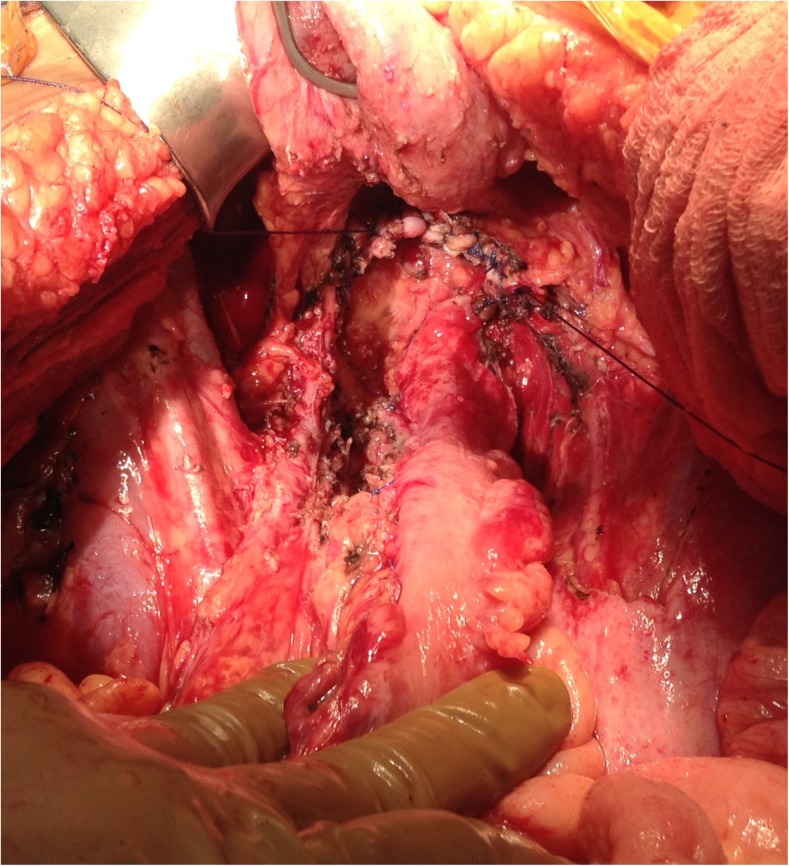
Lesser Omentectomy and Hepatoduodenal Ligament Clearance (Fig. 10 a and b)
Fig. 10.
Lesser omentectomy and hepatoduodenal ligament clearance; 10a – before; 10b – after
The gallbladder is removed in the routine fashion by the funds first approach with dissection and ligation of the cystic artery and the cystic duct. The hepatoduodenal ligament can be heavily layered with tumour and dissection of the cystic duct helps identify the bile duct and the plane between the tumour and the portal structures. The plane is further developed by combination of blunt and bipolar dissection towards the duodenum and across the porta to the lesser omentum. Attempt should be made to preserve the right gastric artery going into the lesser omental arcade. Posteriorly, the tumour layer along the posterior aspect of the porta hepatis is dissected off bluntly towards the lesser omentum to connect it to the layer dissected off anteriorly. Maintaining the tumour layer in one piece helps in traction and complete removal. To continue the resection of the lesser omentum, the surgeon must separate the gastrohepatic ligament from the fissure that divides the liver segments 2 and 3 from segment 1. Tumour deposits over the caudate lobe may be dissected off or electrovaporated. Care must be taken to identify and avoid injury to the accessory or replaced left hepatic artery that may arise from the left gastric artery. If the artery is embedded in the tumour and or its preservation prevents clear exposure if the omental bursa, the artery can be divided close to the liver [37].
The left lateral segment of the liver is mobilised by dividing the left triangular ligament (if it is not already performed as a part of left sub diaphragmatic peritonectomy), and the segment is retracted to the right to facilitate exposure of the entire hepatogastric ligament. The lesser omental bursa is circumferentially dissected from the hepatic fissure, the the caudate lobe and the arcade of the right and left gastric arteries. This is done with a combination of electrosurgery and digital dissection, with every attempt being made to preserve the arcade, the anterior vagus and the left gastric vein, especially if the gastroepiploic arcade has been divided and a splenectomy has been done.
The caudate lobe often overhangs the floor of the omental bursa and retraction using a deep retractor is required to adequately expose the area. Using a combination of blunt and bipolar dissection, this layer of peritoneum is stripped off the crus of diaphragm and the sub hepatic vena cava. In this way, the entire omental bursectomy is completed and removed, if possible, in continuity with the hepatoduodenal ligament clearance. If there is large volume tumour in the sub pyloric space, the lesser omentectomy specimen can be left in continuity with the antral region and a distal gastrectomy may be performed to achieve complete clearance of this region.
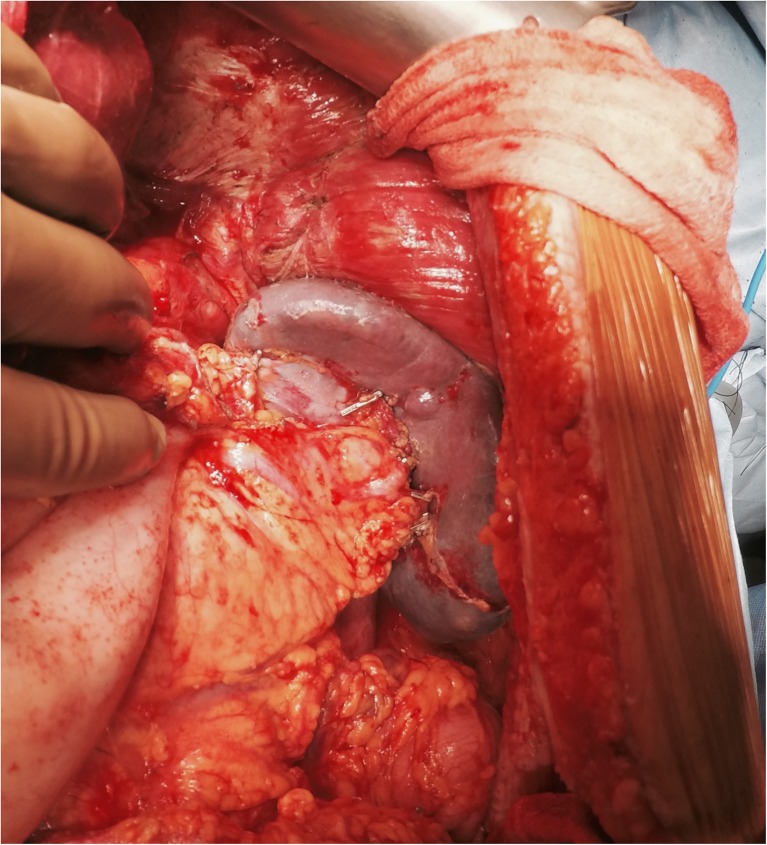
A mention needs to be made regarding the resection of the umbilical ligament. The umbilical ligament may be surrounded by a variable amount of hepatic parenchyma in the umbilical fissure. Sugarbaker has referred to this bridge as ‘pont hepatique’ [58]. When there are deposits on the peritoneum surrounding the umbilical ligament, it becomes imperative to divide the pont hepatique covering the umbilical fissure to expose the umbilical ligament in its entirety (Fig. 11). The left hepatic artery or one of its branches may be at risk of injury during the stripping of the peritoneum in the umbilical fissure and special care needs to be taken to avoid it.
Fig. 11.
Divided Pont Hepatique exposing the umbilical ligament
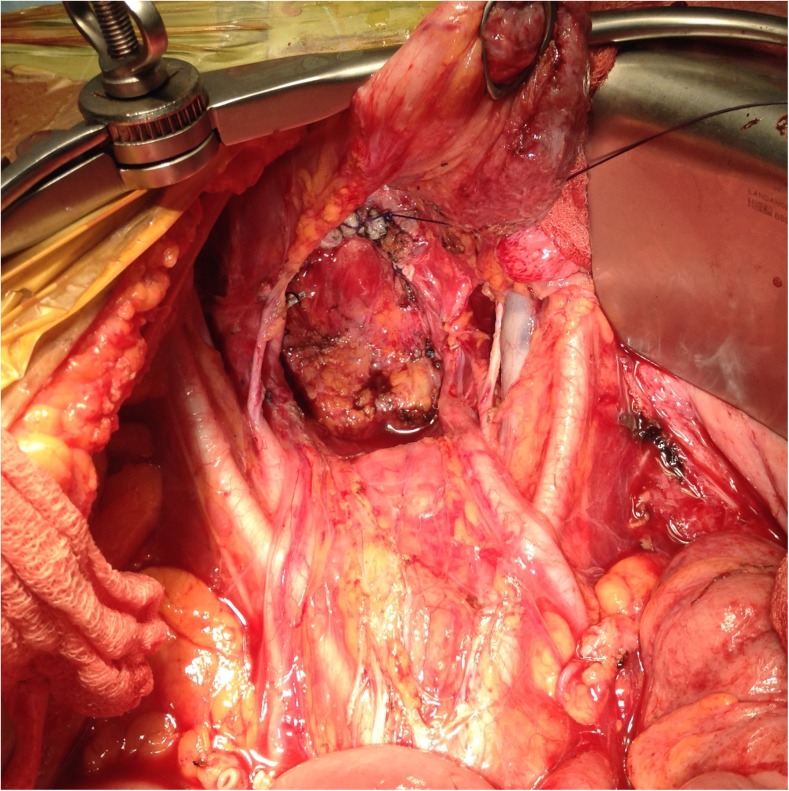
Pelvic Peritonectomy (Figs. 12 and 13)
Fig. 12.
Rectum preserving pelvic peritonectomy
Fig. 13.
Pelvic peritonectomy with anterior resection below the reflection of the pouch of Douglas
Pelvic peritoneum is quite often involved in peritoneal spread of malignancies. To perform a pelvic peritonectomy, the peritoneum from the posterior surface of the lower anterior abdominal wall muscles is stripped from the midline to expose the rectus abdomens muscles. After generously dissecting the peritoneum on either sides to reach the psoas muscles, the peritoneum is dissected on either side of the urinary bladder. The urachus is identified and held and the peritoneum is stripped off the surface of the urinary bladder. The inferior limit of the dissection is the cervix in the females and the seminal vesicles in males. In the females, the round ligaments are divided extraperitoneally on either side and the ureters are dissected away from the peritoneum. The ovarian vessels are ligated at the lower pole of the kidney and left on the pelvic peritoneum. At this point, if a rectosigmoid resection is contemplated, the dissection can proceed along the plane of Toldt’s and the mesolectal fascia to mobilise the rectum. The inferior mesenteric artery is ligated and divided and the sigmoid colon can be stapled off at the required level. The rest of the bowel can be packed off into the upper abdomen to give adequate space for dissection in the pelvis [37].
In females, the deeper dissection in the pelvis proceeds extraperitoneally till the uterine vessels are exposed, which are ligated above the ureters [59]. The bladder is dissected away from the cervix and the vagina and the vagina is entered. The vaginal cuff is divided circumferentially and the rectovaginal septum is exposed. The perirectal fat is divided beneath the peritoneal reflection of the pouch of Douglas (POD) so that all the tumour in this region is removed intact with the specimen. When a rectal resection is being performed, the rectal musculature is skeletonized and stapler is fired to divide the rectum and complete the pelvic peritonectomy. If the rectal wall and serosa is spared of metastases, the pelvic peritoneum can be divided along the lateral border of the mesorectum and along the reflection onto the anterior surface of the rectum to complete the pelvic peritonectomy.
The vaginal stump is closed at this stage (to prevent leakage of chemotherapy solution from the vagina during the HIPEC phase) but the colorectal anastomosis is left to after completion of HIPEC. However, it may be beneficial to mobilise the left colon and take down the splenic flexure to facilitate a tension-free colorectal anastomosis after completion of HIPEC [37].
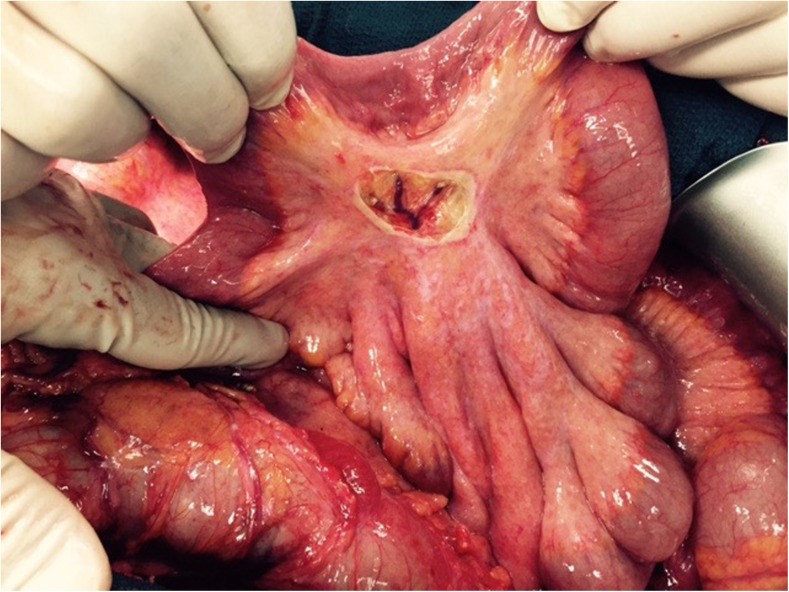
Small Bowel and Mesentry
The mesentery of the small intestine is a frequent site of peritoneal metastases and this is amenable to treatment by electrovaporisation or careful dissection to avoid injury to the underlying vessels (Fig. 14). However, use of high-voltage electrocautry on the small bowel serosa can lead to small bowel fistulae. Sugarbaker et al. have classified small bowel involvement into 5 types based on the extent of invasion, the size of the tumor nodule and its anatomic location on the bowel wall [60].
Type 1: Non-invasive nodules that can be removed with a curved Mayo scissors.
Type 2: Small invasive nodules on the anti-mesenteric portion of the small bowel. These involve only the seromuscular layer and require partial thickness bowel resection
Type 3: Moderately sized invasive nodules on the anti-mesenteric portion of the small bowel which require a full thickness elliptical resection of the bowel wall
Type 4: Small invasive nodules at the junction of the small bowel and its mesentry, if possible can be removed without damaging the vascular supply and segmental bowel resection could be avoided. Other require a segmental resection
Type 5: Large invasive nodules which require a segmental resection with a generous margin of bowel and mesentry on either side.
Fig. 14.
Localized peritonectomy over mesentry to remove a mesenteric deposit
Resection of Contiguous Structures and Viscera
Full Thickness Diaphragm Resection
When there is tumor infiltration of the diaphragmatic muscle, a full thickness resection is needed. This can be performed with a bipolar scissors or electrocautery or Mayo scissors. When the tumor is close to the vascular structures on the right side, complete vascular control of the hepatic vessels should be taken prior to a subphrenic peritonectomy [61].
Resection of the Rectosigmoid Colon
Resection of the rectosigmoid colon is often necessary to achieve a complete cytoreduction. Hertel et al. showed that in patients with advanced ovarian cancer, with suspected rectal serosal invovlment, 73 % of the patients had residual disease when a recto-sigmoid resection was not performed with a pelvic peritonectomy [62]. Several studies have shown that it does not increase the morbidity of CRS and not all cases require a diverting ileostomy. [63–66] When 10–15 cm of the rectum is preserved, an ileostomy can be avoided. Sugarbaker has described the technique of inverting the stapled anatamosis with a layer of interrupted silk sutures [67]. Care should be taken to avoid the left ureter and the vaginal stump in females while performing a stapled anastomosis.
Partial/total Gastrectomy
Presence of tumor around the stomach/and or involvement of the left gastric artery may necessitate a total or a partial gastrectomy. Sugarbaker initially used a staged procedure performing a high jejunostomy to drain the enteric secretions followed by a Roux-en-Y anastomosis few months later [68]. Recent studies have shown that in experienced centres, immediate restoration of gastrointestinal continuity is feasible and safe [69, 70].
Colectomy, Distal Pancreatectomy, Hepatic Resection
Resection of the right or transverse colon is often required in patients with extensive omental deposits or bowel surface/mesenteric deposits. In cases of more extensive disease, a total or subtotal colectomy maybe required. A right hemicolectomy may not be necessary for dealing with an appendiceal primary in patients with pseudomyxoma peritonei [71]. A distal pancreatectomy maybe required in patients with involvement of the distal pancreas with or without splenic hilar involvement, pancreatic capsule involvement or due to iatrogenic injuries [72]. In experienced centres this procedure has shown to be safe when performed as a part of CRS and HIPEC, though it increases the morbidity, mortality is not increased [73, 74]. In patients with ovarian cancer, morbidity caused by pancreatic fistula may cause a delay in starting adjuvant chemotherapy [75]. Similarly, non- anatomic liver resection may be required in patients with extensive deposits on the liver surface. In patients with ovarian cancer, synchronous resection of intraparenchymal liver metastases can be performed with CRS, especially in patients with solitary liver metastases [76, 77].
Morbidity of Multivisceral Resection
Several studies have shown that 2 or more bowel anastomosis has a significant impact on morbidity of patients undergoing CRS and HIPEC [78–80]. Increasing number of peritonectomies also increase the morbidity. Only the number of anastomoses seem to have an impact on morbidity not the number of organs resected [81].
Conclusions
CRS is a complex procedure associated with a prolonged learning curve. A surgeon perfoming CRS must be comfortable in operating on all areas of the abdominal cavity and must be familiar with the anatomy of various regions. Patients selection is as important as the technical skill required for performing this procedure. Teamwork and a multidisciplinary approach is also essential for obtaining optimal results: potentially curative procedures with controlled postoperative mortality-morbidity and adequate impact of long-term quality of life.
References
- 1.Lambert LA. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65(4):284–298. doi: 10.3322/caac.21277. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Management of peritoneal metastases - Basic concepts. J BUON. 2015;20(Suppl 1):S2–11. [PubMed] [Google Scholar]
- 3.Healy JC, Reznek RH. The peritoneum, mesenteries and omenta: normal anatomy and pathological processes. Eur Radiol. 1998;8:886–900. doi: 10.1007/s003300050485. [DOI] [PubMed] [Google Scholar]
- 4.Meyers MA, Oliphant M, Berne AS, Feldberg MA. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593–604. doi: 10.1148/radiology.163.3.3575702. [DOI] [PubMed] [Google Scholar]
- 5.Levy AD, Arnáiz J, Shaw JC, Sobin LH. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008;28:583–607. doi: 10.1148/rg.282075175. [DOI] [PubMed] [Google Scholar]
- 6.Sheth S, Horton KM, Garland MR, Fishman EK. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003;23:457–473. doi: 10.1148/rg.232025081. [DOI] [PubMed] [Google Scholar]
- 7.Oliphant M, Berne AS. Computed tomography of the subperitoneal space: demonstration of direct spread of intraabdominal disease. J Comput Assist Tomogr. 1982;6:1127–1137. doi: 10.1097/00004728-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Karaosmanoglu D, Karcaaltincaba M, Oguz B, Akata D, Ozmen M, Akhan O. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. Eur J Radiol. 2009;71:313–317. doi: 10.1016/j.ejrad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219(2):109–111. doi: 10.1097/00000658-199402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lengyel E. Ovarian Cancer Development and Metastasis. Am J Pathol. 2010;177(3):1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le O. Patterns of peritoneal spread of tumor in the abdomen and pelvis. World J Radiol. 2013;5(3):106–112. doi: 10.4329/wjr.v5.i3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15(3):204–211. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22:465–472. doi: 10.1023/A:1023791229361. [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker PH. The subpyloric space: an important surgical and radiologic feature in pseudomyxoma peritonei. Eur J Surg Oncol. 2002;28:443–446. doi: 10.1053/ejso.2001.1238. [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker P. Cytoreductive surgery using peritonectomy and visceral resections for peritoneal surface malignancy. Transl Gastrointest Cancer. 2013;2(2):54–74. [Google Scholar]
- 17.Mohamed F, Cecil T, Moran B, Sugarbaker P. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol. 2011;18(2):e84–e96. doi: 10.3747/co.v18i2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH. Epithelial appendiceal neoplasms. Cancer J. 2009;15:225–235. doi: 10.1097/PPO.0b013e3181a9c781. [DOI] [PubMed] [Google Scholar]
- 20.Ronnett BM, Shmookler BM, Sugarbaker PH, Kurman RJ. Pseudomyxoma peritonei: new concepts in diagnosis, origin, nomenclature, and relationship to mucinous borderline (low malignant potential) tumors of the ovary. Anat Pathol. 1997;2:197–226. [PubMed] [Google Scholar]
- 21.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2009;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 22.Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 23.Verwaal VJ, Kusamura S, Baratti D, et al. The eligibility for local-regional treatment of peritoneal surface malignancy. J Surg Oncol. 2008;98:220–223. doi: 10.1002/jso.21060. [DOI] [PubMed] [Google Scholar]
- 24.Yan TD, Morris DL, Kusamura S, et al. Preoperative investigations in the management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy. J Surg Oncol. 2008;98:224–227. doi: 10.1002/jso.21069. [DOI] [PubMed] [Google Scholar]
- 25.Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002;223:495–499. doi: 10.1148/radiol.2232011081. [DOI] [PubMed] [Google Scholar]
- 26.Esquivel J, Chua TC, Stojadinovic A, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol. 2010;102:565–570. doi: 10.1002/jso.21601. [DOI] [PubMed] [Google Scholar]
- 27.Low RN, Barone RM, Lee MJ. Surveillance MR imaging is superior to serum tumor markers for detecting early tumor recurrence in patients with appendiceal cancer treated with surgical cytoreduction and HIPEC. Ann Surg Oncol. 2013;20(4):1074–1081. doi: 10.1245/s10434-012-2788-2. [DOI] [PubMed] [Google Scholar]
- 28.Torkzad MR, Casta N, Bergman A, Ahlström H, Påhlman L, Mahteme H. Comparison between MRI and CT in prediction of peritoneal carcinomatosis index (PCI) in patients undergoing cytoreductive surgery in relation to the experience of the radiologist. J Surg Oncol. 2015;111(6):746–751. doi: 10.1002/jso.23878. [DOI] [PubMed] [Google Scholar]
- 29.Dromain C, Leboulleux S, Auperin A, et al. Staging of peritoneal carcinomatosis: Enhanced CT vs. PET/CT. Abdom Imaging. 2008;33:87–89. doi: 10.1007/s00261-007-9211-7. [DOI] [PubMed] [Google Scholar]
- 30.Jacquet P, Jelinek JS, Chang D, et al. Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg. 1995;181:530–538. [PubMed] [Google Scholar]
- 31.Yan TD, Haveric N, Carmignani CP, et al. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005;103:839–849. doi: 10.1002/cncr.20836. [DOI] [PubMed] [Google Scholar]
- 32.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: Sugarbaker PH, editor. Peritoneal carcinomatosis: principles of management. Boston: Kluwer Academic Publishers; 1996. pp. 359–374. [DOI] [PubMed] [Google Scholar]
- 33.Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22(9):2958–2964. doi: 10.1245/s10434-015-4387-5. [DOI] [PubMed] [Google Scholar]
- 34.Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, Montori G, Ansaloni L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol. 2015;41(7):911–919. doi: 10.1016/j.ejso.2015.03.231. [DOI] [PubMed] [Google Scholar]
- 35.Bakrin N, JM B, Decullier E, JM C, Msika S, Lorimier G, Abboud K, Meeus P, Ferron G, Quenet F, Marchal F, Gouy S, Morice P, Pomel C, Pocard M, Guyon F, Porcheron J, Glehen O, FROGHI (FRench Oncologic and Gynecologic HIPEC) Group Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435–1443. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 37.Sugarbaker PH. Cytoreductive surgery and perioperative chemotherapy: Textbook and video atlas. Connecticut: Cine-Med Publishing; 2013. [Google Scholar]
- 38.Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43:15–25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 39.Glehen O, FN G, Arvieux C, Cotte E, Boutitie F, Mansvelt B, JM B, Lorimier G, Quenet F, Elias D, Association Française de Chirurgie Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 40.Glehen O, Mohamed F, Sugarbaker PH. Incomplete Cytoreduction in 174 Patients with Peritoneal Carcinomatosis From Appendiceal Malignancy. Ann Surg. 2004;240(2):278–285. doi: 10.1097/01.sla.0000133183.15705.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dayal S, Taflampas P, Riss S, Chandrakumaran K, Cecil TD, Mohamed F, Moran BJ. Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved. Dis Colon Rectum. 2013;56(12):1366–1372. doi: 10.1097/DCR.0b013e3182a62b0d. [DOI] [PubMed] [Google Scholar]
- 42.González-Moreno S, Ortega-Pérez G, González-Bayón L. Indications and patient selection for cytoreductive surgery and perioperative intraperitoneal chemotherapy. J Surg Oncol. 2009;100(4):287–292. doi: 10.1002/jso.21325. [DOI] [PubMed] [Google Scholar]
- 43.Passot G, Vaudoyer D, Cotte E, You B, Isaac S, Noël Gilly F, Mohamed F, Glehen O. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256(1):125–129. doi: 10.1097/SLA.0b013e318255486a. [DOI] [PubMed] [Google Scholar]
- 44.Honoré C, Souadka A, Goéré D, Dumont F, Deschamps F, Elias D. HIPEC for peritoneal carcinomatosis: does an associated urologic procedure increase morbidity? Ann Surg Oncol. 2012;19(1):104–109. doi: 10.1245/s10434-011-1820-2. [DOI] [PubMed] [Google Scholar]
- 45.Glehen O, Beaujard AC, Arvieux C, Huber O, Gilly FN. Peritoneal carcinomatosis. Surgical treatment, peritonectomy and intraperitoneal chemohyperthermia. Gastroenterol Clin Biol. 2002;26:210–215. [PubMed] [Google Scholar]
- 46.Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am. 2001;10:915–933. [PubMed] [Google Scholar]
- 47.Elias D, Benizri E, Pocard M, Ducreux M, Boige V, Lasser P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol. 2006;32:632–636. doi: 10.1016/j.ejso.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, Hay JM, Flamant Y, Msika S. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cotte E, Passot G, Gilly F-N, Glehen O. Selection of patients and staging of peritoneal surface malignancies. World J Gastrointest Oncol. 2010;2(1):31–35. doi: 10.4251/wjgo.v2.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugarbaker PH. Dissection by electrocautery with a ball tip. J Surg Oncol. 1994;56:246–248. doi: 10.1002/jso.2930560409. [DOI] [PubMed] [Google Scholar]
- 51.Sugarbaker PH. Laser-mode electrosurgery. Cancer Treat Res. 1996;82:375–385. doi: 10.1007/978-1-4613-1247-5_24. [DOI] [PubMed] [Google Scholar]
- 52.Zappa L, Sugarbaker PH. Compartment syndrome of the leg associated with lithotomy position for cytoreductive surgery. J Surg Oncol. 2007;96:619–623. doi: 10.1002/jso.20884. [DOI] [PubMed] [Google Scholar]
- 53.de Lima VV, Sugarbaker PH. Xiphoidectomy. Gastric Cancer. 2003;6:127–129. doi: 10.1007/s10120-003-0237-4. [DOI] [PubMed] [Google Scholar]
- 54.Vazquez Vde L, Sugarbaker PH. Total anterior parietal peritonectomy. J Surg Oncol. 2003;83:261–263. doi: 10.1002/jso.10277. [DOI] [PubMed] [Google Scholar]
- 55.Dagbert F, Passot G, Glehen O, Bakrin N. Glisson capsulectomy for extensive superficial liver involvement in peritoneal carcinomatosis (with video) J Visc Surg. 2015;152(5):332–333. doi: 10.1016/j.jviscsurg.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Deslauriers N, Olney H, Younan R. Splenectomy revisited in 2011: Impact on hematologic toxicities while performing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Gastrointest Oncol. 2011;2(2):61–63. doi: 10.3978/j.issn.2078-6891.2011.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becher RD, Shen P, Stewart JH, Russell G, Bradley JF, Levine EA. Splenectomy ameliorates hematologic toxicity of hyperthermic intraperitoneal chemotherapy. J Gastrointest Oncol. 2011;2(2):70–76. doi: 10.3978/j.issn.2078-6891.2011.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugarbaker PH. Pont hepatique (hepatic bridge), an important anatomic structure in cytoreductive surgery. J Surg Oncol. 2010;101:251–252. doi: 10.1002/jso.21484. [DOI] [PubMed] [Google Scholar]
- 59.Chang S-J, Bristow RE. Surgical technique of en bloc pelvic resection for advanced ovarian cancer. J Gynecol Oncol. 2015;26(2):155. doi: 10.3802/jgo.2015.26.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bijelic L, Sugarbaker PH. Cytoreduction of the small bowel surfaces. J Surg Oncol. 2008;97:176–179. doi: 10.1002/jso.20912. [DOI] [PubMed] [Google Scholar]
- 61.Pathiraja PNJ, Garruto-Campanile R, Tozzi R. Diaphragmatic Diaphragmatic peritonectomy versus full thickness diaphragmatic resection and pleurectomy during cytoreduction in patients with ovarian cancer. Int J Surg Oncol. 2013;2013:876150. doi: 10.1155/2013/876150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hertel H, Diebolder H, Herrmann J, Köhler C, Kühne-Heid R, Possover M, Schneider A (2001) Is the decision for colorectal resection justified by histopathologic findings: a prospective study of 100 patients with advanced ovarian cancer. Gynecol Oncol; 83(3):481–484. PMID:11733959 [DOI] [PubMed]
- 63.Obermair A, Hagenauer S, Tamandl D, Clayton RD, Nicklin JL, Perrin LC, Ward BG, Crandon AJ. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. Gynecol Oncol. 2001;83(1):115–120. doi: 10.1006/gyno.2001.6353. [DOI] [PubMed] [Google Scholar]
- 64.Yildirim Y, Ertas IE, Nayki U, Ulug P, Nayki C, Yilmaz I, Gultekin E, Dogan A, Aykas A, Ulug S, Ozdemir A, Solmaz U. En-bloc pelvic resection with concomitant rectosigmoid colectomy and immediate anastomosis as part of primary cytoreductive surgery for patients with advanced ovarian cancer. Eur J Gynaecol Oncol. 2014;35(4):400–407. [PubMed] [Google Scholar]
- 65.Mourton SM, Temple LK, Abu-Rustum NR, Gemignani ML, Sonoda Y, Bochner BH, Barakat RR, Chi DS. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2005;99(3):608–614. doi: 10.1016/j.ygyno.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 66.Sonnendecker EW, Beale PG. Rectosigmoid resection without colostomy during primary cytoreductive surgery for ovarian carcinoma. Int Surg. 1989;74(1):10–12. [PubMed] [Google Scholar]
- 67.Sugarbaker PH (2015) Avoiding Diverting Ileostomy in Patients Requiring Complete Pelvic Peritonectomy. Ann Surg Oncol 2015 [DOI] [PubMed]
- 68.Sugarbaker PH. Cytoreduction including total gastrectomy for pseudomyxoma peritonei. Br J Surg. 2002;89(2):208–212. doi: 10.1046/j.1365-2168.2002.01967.x. [DOI] [PubMed] [Google Scholar]
- 69.Piso P, Slowik P, Popp F, Dahlke MH, Glockzin G, Schlitt HJ. Safety of gastric resections during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2009;16(8):2188–2194. doi: 10.1245/s10434-009-0478-5. [DOI] [PubMed] [Google Scholar]
- 70.Deraco M, Baratti D, Kusamura S, Laterza B, Balestra MR. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J Surg Oncol. 2009;100(4):321–328. doi: 10.1002/jso.21388. [DOI] [PubMed] [Google Scholar]
- 71.Foster JM, Gupta PK, Carreau JH, Grotz TE, Blas JV, Gatalica Z, Nath S, Loggie BW. Right hemicolectomy is not routinely indicated in pseudomyxoma peritonei. Am Surg. 2012;78(2):171–177. [PubMed] [Google Scholar]
- 72.Doud AN, Randle RW, Clark CJ, Levine EA, Swett KR, Shen P, Stewart JH, Votanopoulos KI. Impact of distal pancreatectomy on outcomes of peritoneal surface disease treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2015;2(5):1645–1650. doi: 10.1245/s10434-014-3976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarz L, Votanopoulos K, Morris D, Yonemura Y, Deraco M, Piso P, Moran B, Levine EA, Tuech JJ. Is the Combination of Distal Pancreatectomy and Cytoreductive Surgery With HIPEC Reasonable?: Results of an International Multicenter Study. Ann Surg. 2016;263(2):369–375. doi: 10.1097/SLA.0000000000001225. [DOI] [PubMed] [Google Scholar]
- 74.Mahdi H, Rose PG, Gonzalez S, DeBernardo R, Knight J, Michener C, Moselmi-Kebria M. Postoperative Complications After Distal Pancreatectomy Performed During Cytoreductive Surgery for Gynecologic Malignancies. Int J Gynecol Cancer. 2015;25(6):1128–1133. doi: 10.1097/IGC.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 75.Bacalbasa N, Dima S, Brasoveanu VDavid L, et al. Liver resection for ovarian cancer liver metastases as part of cytoreductive surgery is safe and may bring survival benefit. World J Surg Oncol. 2015;13:235. doi: 10.1186/s12957-015-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gasparri ML, Grandi G, Bolla D, Gloor B, Imboden S, Panici PB, Mueller MD, Papadia A (2015) Hepatic resection during cytoreductive surgery for primary or recurrent ovarian cancer. J Cancer Res Clin Oncol 1–12 [DOI] [PubMed]
- 77.Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–869. doi: 10.1245/ASO.2003.01.018. [DOI] [PubMed] [Google Scholar]
- 78.Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FAN. Toxicity of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2004;85:61–67. doi: 10.1002/jso.20013. [DOI] [PubMed] [Google Scholar]
- 79.Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106:1144–1153. doi: 10.1002/cncr.21708. [DOI] [PubMed] [Google Scholar]
- 80.Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–796. doi: 10.1007/s10434-999-0790-0. [DOI] [PubMed] [Google Scholar]
- 81.Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, HJ Z, 3rd, DL B. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15(11):3065–3072. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]