Abstract
The study aims to discuss the effects of aging on the male reproductive system. A systematic review was performed using PubMed from 1980 to 2014. Aging is a natural process comprising of irreversible changes due to a myriad of endogenous and environmental factors at the level of all organs and systems. In modern life, as more couples choose to postpone having a child due to various socioeconomic reasons, research for understanding the effects of aging on the reproductive system has gained an increased importance. Paternal aging also causes genetic and epigenetic changes in spermatozoa, which impair male reproductive functions through their adverse effects on sperm quality and count as, well as, on sexual organs and the hypothalamic-pituitary-gonadal axis. Hormone production, spermatogenesis, and testes undergo changes as a man ages. These small changes lead to decrease in both the quality and quantity of spermatozoa. The offspring of older fathers show high prevalence of genetic abnormalities, childhood cancers, and several neuropsychiatric disorders. In addition, the latest advances in assisted reproductive techniques give older men a chance to have a child even with poor semen parameters. Further studies should investigate the onset of gonadal senesce and its effects on aging men.
Keywords: Aging, Gonads, Hormone, Oxidative stress, Telomeres
Introduction
Aging is a combination of irreversible changes over time observed in all eukaryotic organisms at the level of molecules, cells, tissues, organs, and systems. Both male and female reproductive capacities decline with age. Compared to women, the decline in male reproductive capacity with age is less pronounced and men are considered to be able to have a child throughout their lifespan. In literature, the role and the importance of maternal age in fertilization has been extensively studied; however, the effect of the paternal age is poorly understood [1–5]. Now, especially in developed countries, the fact that couples postpone having a child due to economic reasons, high standards of living, or career planning has substantially increased the significance of research for investigating the effects of aging on reproductive systems [1]. It is not well understood how increasing paternal age may influence the probability of pregnancy or the risk of the newborn developing any genetic or other types of abnormalities [4]. In this review, we aim to discuss the structural and functional changes in the male reproductive system during the course of aging.
The effect of aging on sexual organs
Despite individual differences, testicular morphology is one of the effects of aging on the male reproductive system. Mean testicular volume tends to increase between 11 and 30 years of age, remains constant between 30 and 60 years of age, and decreases gradually every year after age 60 [6]. The mean testicular volume in men over 75 years has been reported to be 31 % less than in men between 18 and 40 years of age. This difference is associated with significantly higher mean serum levels of gonadotropins and lower serum free testosterone (T) [7]. In testicular disorders or failures, levels of serum gonadotropins increase. Different age-related changes in testicles including decrease in the number of Leydig cells act on feedback mechanisms and cause increased secretion of gonadotropins. Age-related increase in gonadotropins is mainly due to primary testicular failure. Testicular metabolism increases between 11 and 40 years of age, and it gradually decreases between the ages of 40 and 90 [8, 9].
Histomorphological studies have clearly demonstrated a decrease in the number of germ cells and Sertoli cells with aging. During the course of aging, the thickness of the tunica propria of the basal membrane of seminiferous tubuli increases while the seminiferous epithelium reduces and the testes vascularize, which altogether results in the narrowing of the tubules [10]. In a study where young men with obstructive azoospermia but normal histologic spermatogenesis were used as controls, the thickness of the basal membrane of the older men with preserved spermatogenesis was shown to be similar to that of the control group [11]. The decline of seminiferous tubules with age is characterized by a decrease in the number of Sertoli cells and germ cells. During the course of aging, a portion of Sertoli cells display multinucleation and a high number of mitochondria with tubular cristae, while another portion displays immature nuclei representative of dedifferentiation and sparse cytoplasmic organelles. Furthermore, abnormal germ cells phagocytized by Sertoli cells lead to vacuolization in Sertoli cell cytoplasm and accumulation of lipid droplets. Vascular changes play a role in testicular fibrosis. The progression of fibrosis with age causes separation of the germinal epithelium from the blood supply. The development of tubular involution with age is thought to have an essential role in testicular atrophy [12].
Despite personal differences, the number of Leydig cells also decreases with age [13]. Leydig cells in aging men have many cytoplasmic or intranuclear Reinke crystals and paracrystalline inclusions, and contain a large number of vacuoles and lipid droplets. The number of cytoplasmic organelles in these cells is lesser, and, compared to normal cells, their nuclei are darker and more irregular in shape. In addition, Leydig cells may also contain an underdeveloped endoplasmic reticulum (ER) and a number of lipofuscin granules. At later stages of dedifferentiation, the nucleolus shrinks or disappears completely [12].
The prostate gland located at the base of the urethra is an endocrine organ under the control of androgens and produces about 30 % of the seminal fluid [9, 14]. Prostate enlargement in older men is one of the most common age-related diseases and is defined as benign prostatic hyperplasia (BPH). Although any DNA mutation associated with BPH has not been reported to date, in general DNA methylation has been shown to decrease in BPH [15, 16]. Prakash et al. have identified 511 differentially expressed genes that can distinguish symptomatic BPH from asymptomatic BPH and most of these genes encode epithelium/stroma-derived growth factors [17]. In autopsy studies, 80 % of the male population above 80 was found to have histologic BPH. Age-related prostate enlargement is known to be caused by hyperplasia of basal cells and stromal cells, such as muscle and fibroblasts, located in the transitional zone around the urethra. Stromal volume has been observed to increase in patients with symptomatic BPH along with a decrease in apoptosis of stromal cells [18]. Pre-malignant dysplastic lesions (prostatic intraepithelial neoplasia, PIN) are characterized by enlarged nuclei, decline in basal epithelial layer, and proliferation and abnormal differentiation of secretory cells. PIN is regarded as pre-invasive stage of prostatic adenocarcinoma [16, 19].
The impact of aging on semen parameters
Semen parameters are known to change with age. Daily sperm production, total sperm count, and sperm viability are negatively correlated with age. Daily sperm production decreases more than 30 % in men over the age of 50 and is negatively correlated with age in men in general [13, 20, 21]. In a study where semen values of men above 45 years of age were analyzed, four measured parameters (semen volume, sperm concentration, sperm motility, and sperm morphology) and one derived parameter (total sperm count) were calculated according to the age range, and these values were compared to the reference values of the World Health Organization. After the age of 45, semen volume gradually decreases due to functional decline of accessory glands. In addition, sperm morphology is also affected with aging and the percentage of sperms with normal morphology begins to decrease after the age of 40 [22]. Recently, a meta-analysis was conducted to evaluate the effects of aging on semen parameters including semen volume, sperm concentration, total sperm count, morphology, total motility, progressive motility, and DNA fragmentation. The results have demonstrated that paternal aging leads to a decrease in sperm parameters except for sperm concentration; however, compared to more commonly used parameters, increased sperm DNA fragmentation and progressive motility may be better diagnostic parameters to be considered during fertility treatment of older men [4].
In conclusion, parameters such as sperm volume, sperm motility, and sperm morphology are all known to decrease with aging [23]. The effect of aging on semen parameters is summarized in Table 1.
Table 1.
WHO reference values for human semen characteristics and summary of studies investigating effects of aging on semen parameters
| Semen volume | Sperm count | Sperm concentration | Viability | Motility | Morphologically normal forms | Study population (age) | Reference |
|---|---|---|---|---|---|---|---|
| 1.5 ml (1.4–1.7) |
39 million (33–46 × 106) | 15 million/ml (12–16 × 106) | 58 % (55–63) |
32 % progressive motility (31–34) 40 % total (progressive + non-progressive) motility (38–42) |
4.0 % (3.0–4.0) | 4500 fertile men | WHO (2010) Cooper et al. [24] |
| ↓ | ↓ | ↓ | ↓ | Head: width ratio ↓ | 4822 men (16.5–72.3) |
Stone et al. [22] | |
| ↓ | ↑ | ↓ | 3729 men with normal sperm count (22–62) 498 men (21–66) |
Mukhopadhyay et al. [25] | |||
| Progressive motility ↓ | Elzanaty [26] | ||||||
| Youngest group (45–48): 2.8 ml Oldest group (56–80): 1.95 ml |
↓ | No significant change | ↓ 55 % ↓ in youngest group 50 % ↓ in oldest group |
↓ | 1174 men with no or mild erectile dysfunction (45–80) | Hellstrom et al. [23] | |
| 0.8 % ↓/year motility 0.9 % ↓/year progressive motility 0.4 % ↓/year rapid motility 0.2 % ↓/year linearity 0.2 % ↓/year straight line velocity 0.3 % ↓/year average path velocity |
97 healthy men (22–80) | Sloter et al. [27] | |||||
| 0.01 ml ↓/year | ↓ | 0.27 % ↓/year | %0.039 ↓/year | 889 fertile men (24–>45) | Pasqualotto et al. [28] | ||
| 0.03 ml ↓/year | 4.7 % ↓/year total progressivity motile sperm count | 0.7 % ↓ /year motility 3.1 %↓ /year progressive motility | 97 healthy men (20–80) | Eskenazi et al. [29] | |||
| 29 % ↓ Older group | No significant change | No significant change | 32 % ↓ progressive motility in older group | 40 % ↓ morphologically normal forms Tail defects 27 % ↑ in older group |
66 older men (≥50) and 134 young men (21–25), in both groups proven fertile and men in barren couples | Jung et al. [34] | |
| 2.6 × 106 ↓/year | 441 couples underwent IVF (male age 22–64) | Paulson et al. [30] | |||||
| ↓ | Total and motile sperm count ↓ | ↓ | 20,411 non-azoospermic infertile men (15–74) | Andolz et al. [31] | |||
| No significant change |
302 fertile men (20–45) | Bujan et al. [32] | |||||
| Microsephalic, macrosephalic, duplicate heads and coiled tails ↑ | 214 fertile men | Bujan et al. [33] |
The effect of aging on endocrine system
The hypothalamic-pituitary-gonadal (HPG) axis in men functions to control the release of sex hormones and to ensure the formation and maturation of spermatogenic cells. The axis is composed of three essential parts, including the hypothalamus, anterior pituitary, and the testes. In this axis, gonadotropin-releasing hormone (GnRH) secreted from the hypothalamus reaches the anterior pituitary gland via the hypophyseal portal system and stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the bloodstream by the gonadotropic cells. LH induces the production of testosterone by the Leydig cells, whereas FSH induces the Sertoli cells to secrete androgen-binding protein (ABP) and inhibin and plays an essential role in initiation and progression of spermatogenesis. In a GnRH-independent manner, FSH release is also controlled by the inhibin-activin-follistatin system. Inhibin acts as an FSH inhibitor secreted from the Sertoli cells, while activin is secreted by the Sertoli cells and the pituitary gland and stimulates FSH secretion. Follistatin is an activin-binding protein secreted from the gonads and the pituitary gland, and can inhibit activin-stimulated FSH release [35].
Serum testosterone levels are known to decrease with age particularly due to the decrease in the number of the Leydig cells, deterioration of testicular perfusion, and disturbance in diurnal rhythm of GnRH and chorionic gonadotropin secretion [36–38]. Testosterone deficiency due to a testicular defect is known as primary hypogonadism, while gonadotropin deficiency is called secondary hypogonadism [18]. Age-related changes in the male reproductive system may, in part, be due to the reduction in the amount of oxygen reaching the testes [39]. Studies conducted with spermatic vein plasmas and testicular tissues from older men have shown a clear decrease in the levels of testosterone and its precursors (pregnenolone, progesterone, 17 alpha- hydroxyprogesterone, 17 alpha-hydroxypregnenolone, androstenedione, androstenediol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate) [39, 40].
Testosterone is locally converted to estrogen by the enzyme aromatase in many tissues [35]. Estrogen in men plays key roles in the regulation of gonadotropin feedback, many brain functions, bone maturation and resorption, and lipid metabolism [41]. Estrone (E1) and estradiol (E2) have been shown to decrease with aging [40]. In a study where plasma levels of total testosterone, bioavailable testosterone, and E2 were measured in 810 men between the ages of 24 and 90, total E2 and bioavailable E2 were reported to decrease by 0.03 and 0.12 pg/mL, respectively [37].
Research has extensively shown that levels of many hormones vary with age (Table 2). In addition, other parameters such as body weight, lifestyle, and acute and chronic diseases play a role in the alteration of the HPG axis [45, 46]. Changes in testosterone and sex-hormone binding globulin (SHBG) are inversely proportional to body weight [42, 46]. Although testosterone is the main source of plasma E2, decline in testosterone levels with aging is poorly reflected in plasma E2 levels. This discrepancy can be explained by increases in aromatase activity and body fat mass observed during aging, as E2 is highly positively correlated with body fat index [46].
Table 2.
Summary of studies investigating the alterations of hormone levels with age
| Hormone | Study design | Hormone levels | Ratio or measurement | Age group (sample sizes) | Reference |
|---|---|---|---|---|---|
| Total T | Cross-sectional | ↓ | 0.4 %/year | 39–70 (1709) | Gray et al. [42] |
| Free T | ↓ | 1.2 %/year | |||
| Albumin-bound T | ↓ | 1 %/year | |||
| SHBG | ↑ | 1.2 %/year | |||
| Total T | Longitudinal study with two cohorts | ↓ | 1.3 %/year | 18–90 (480) | Liu et al. [43] |
| SHBG | ↑ | 2.3 %/year | |||
| Total T | ↓ | 0.9 %/year | 60–90 (370) | ||
| SHBG | ↑ | 2.5 %/year | |||
| Androstenediol | Cross-sectional | ↓ | 0.8 %/year | 39–70 (1709) | Gray et al. [42] |
| Androstenedione | ↓ | 1.3 %/year | |||
| Androstanediol glucuronide | ↓ | 0.6 %/year | |||
| E2 | Cross-sectional | ↓ | 0.03 %/year | 24–90 | Ferrini and Barrett-Connor [37] |
| Bioavailable E2 | ↓ | 0.12 %/year | |||
| DHEA | Cross-sectional | ↓ | 3.1 %/year | 39–70 (1709) | Gray et al. [42] |
| DHEAS | ↓ | 2.2 %/year | |||
| FSH | ↑ | 1.9 %/year | |||
| LH | ↑ | 1.3 %/year | |||
| PRL | ↓ | 0.4 %/year | |||
| Activin A | Cross-sectional | ↑ | Youngest (age 30–50, n = 20): 0.47 ng/ml Oldest (age 90–101 n = 9): 0.74 ng/ml |
30–101 (73) |
Baccarelli et al. [44] |
| Inhibin B | ↓ | Youngest (age 30–50, n = 20): 198 ng/ml Oldest (age 90–101 n = 9): 78 pg/ml |
|||
| Inhibin B/FSH | Cross-sectional | ↓ | Young: 57.8 ng/mU Older: 18 ng/mU |
18–40 (42) and 75–90 (115) |
Mahmoud et al. [7] |
| T/LH | ↓ | Young: 5.5 nmol/U Older: 2.5 nmol/U |
Apart from primary testicular defects, secondary mechanisms such as changes in the hypothalamic-pituitary axis also have an effect on reproductive aging in men. Aging causes a reduction in the secretion of GnRH, which in turn leads to smaller LH and testosterone pulses. Compared to young men, LH pulses in older men are more frequent and smaller. Aging impairs the gonadotropin response to exogenous GnRH, increases LH pulse size, and/or reduces in vitro LH bioactivity [47]. In a study where age-related changes in pulsatile LH secretions in rats were investigated, LH pulse intervals and amplitudes as well as the total area of LH pulses were all found to be reduced in older rats [48]. Aging attenuates the inhibitory effect of free testosterone on GnRH release and equally suppresses the feedback by bioavailability and total testosterone concentrations. Although hypothalamopituitary androgen and estrogen receptor density may decline in the course of aging, the deterioration of negative feedback by testosterone has a plain effect on GnRH release [49].
Free radical theory of aging and apoptosis
Free radicals are reactive chemical species containing unpaired valence electron(s) in their outer orbit. These unpaired electrons make free radicals highly reactive towards carbohydrates, lipids, proteins, nucleic acids, and other cellular molecules [50, 51]. Free radicals arise from normal cell metabolism or can be produced by exogenous sources including radiation, herbicides, cigarette smoking, chronic stress, alcohol abuse, some drugs, and air pollution [52, 53]. In mitochondria, oxygen is converted to highly reactive oxygen molecules called reactive oxygen species (ROS). Low and medium concentrations of ROS are involved in cellular defense against infection agents, signal transduction, and response against mitogens, carboxylation, peroxidation, and reduction of ribonucleotides; however, high levels of ROS lead to various cellular damage [54–59]. ROS induced DNA damage involves base modifications, abasic sites, single strand (ss) and double strand (ds) DNA breaks, and DNA protein cross-links [60–62].
mtDNA is more vulnerable to ROS generated by electron transport chain (ETC) than nuclear DNA since mtDNA is not packed with histone proteins and there is no DNA repair mechanism in the mitochondria [63]. Consequently, mutations accumulate in mtDNA and lead to mitochondrial dysfunction, which in turn leads to an increase in ROS production and oxidative damage and decrease in ATP/ADP ratio [64–66]. Increased ROS production, decreased ATP production, and apoptosis are three features of dysfunctional mitochondria disrupted by aging. Finally, all these alterations lead to mitochondrial aberrations and activation of cellular death pathways [63, 67].
ROS production in ejaculate is a normal physiological process. ROS are significant mediators in signal transduction mechanisms and have function in sperm capacitation and acrosome reaction, and in regulation of spermatozoa and oocyte joining [68–70]. In addition, biological agents, radiation, excess heat, some medications, radiation, exposure to heavy metals, and smoking lead to increased ROS production in the testes and epididymis [71]. Human spermatozoal membrane is comprised of a high quantity of polyunsaturated fatty acids, which are the primary targets of oxidative stress. After spermatozoal membrane, other targets of oxidative stress include biomolecules, such as structural proteins and nucleic acids [52, 71]. Spermatozoa contain a small amount of cytoplasm, and therefore are poor in antioxidant enzymes like superoxide dismutase, glutathione peroxidase, and catalase. In addition, residual sperm cytoplasm is concentrated at the center of spermatozoa, which makes it unlikely for any antioxidant to protect spermatozoal membrane from head to tail [72, 73].
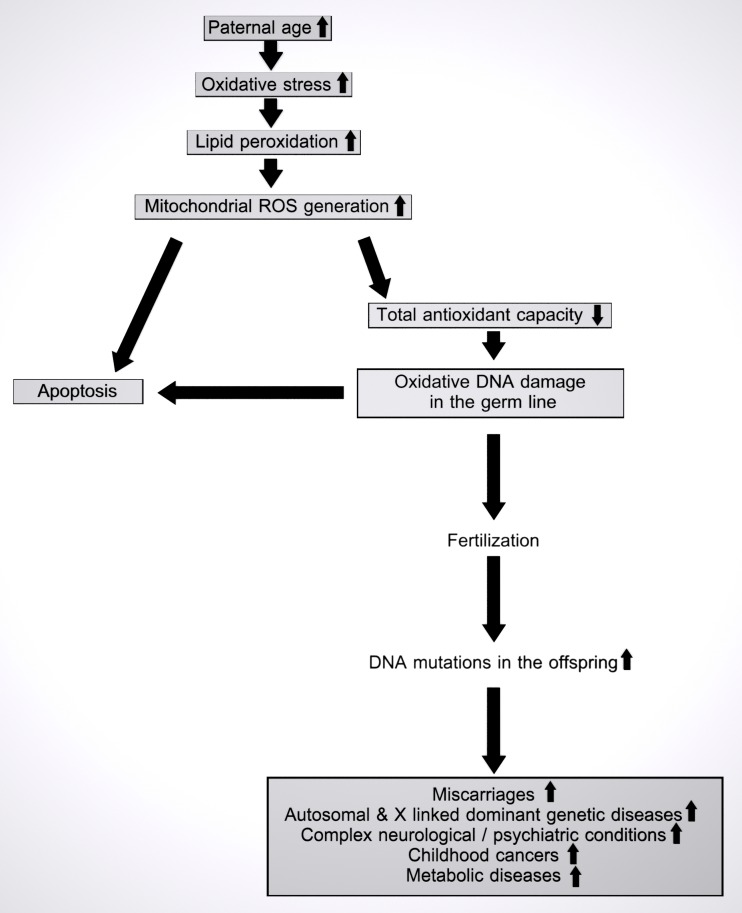
Overaccumulation of ROS leads to increased oxidative stress. High oxidative stress causes an increase in lipid peroxidation, DNA damage and apoptosis, which ultimately culminates in loss of sperm motility and vitality [60–62, 70, 74] (Fig. 1). Recent studies have indicated that increased ROS levels may be a major cause of idiopathic male infertility [74, 75]. Increased ROS production is inversely correlated with sperm function [76]. Several studies have shown an association between sperm motility and leukocyte contamination in ejaculate, ROS production, lipid peroxidation, and sperm oxidative DNA damage [72, 77, 78]. Some studies have indicated higher levels of dsDNA breaks and higher DNA fragmentation index (DFI) in older men [79–82] (Table 4). An imbalance between antioxidant defence mechanisms and ROS production eventually results in poor quality DNA, lipids, and proteins in the course of aging [88]. Weir and Robaire have shown an increased generation of ROS along with a decreased capacity of glutathione peroxidase and superoxide dismutase enzymes in aging spermatozoa of Brown Norway rats [89]. Sperm DNA integrity degenerates with age as exemplified by increased sperm DNA fragmentation, abnormal chromatin packaging, and protamination [90]. Sperm DFI is positively correlated with sperm paternal aging (p < 0.00001). Furthermore, high oxidative stress leads to increased apoptosis and spermatozoal DNA damage [79].
Fig. 1.
Accumulation of oxidative damage with paternal aging and its consequences in the offspring. Aging causes oxidative stress due to accumulation of ROS in male germ cells over time. Oxidative stress in spermatozoal mitochondria induces lipid peroxidation and further ROS generation. Excessive amounts of ROS and decreased antioxidant capacity in the course of aging may induce apoptosis or oxidative damage to DNA. If left unrepaired, oxidatively damaged paternal DNA may proceed through fertilization into the offspring, causing a variety of diseases in the offspring
Table 4.
The effects of advanced paternal aging on embryo development and embryo morphology
| Parameter/condition | Study population (age range) |
PA effect | Reference |
|---|---|---|---|
| Chromosomal aberrations | |||
| Numerical abnormalities | |||
| Autosomes | 225.846 sperm from 10 donors (21–52) |
Significant increase in the frequency of disomy chromosome 1 in spermatozoa (p = 0.01) | Templado et al. [115] |
| Gonosomes | 38 fathers of boys with Klinefelter syndrome (24–57) |
Significant correlation with the frequency of XY sperm (p = 0.02) with a 10-year period (p = 0.006) | Lowe et al. [116] |
| Structural abnormalities | 27 translocations | Double the risk for balanced translocations with every 10-year increase in age odds ratios (OR = 2.19; 95 % CI 1.23–3.90) | Thomas et al. [83] |
| DNA fragmentation | 1974 normozoospermic men, Mean age: 37 ± 6 |
Positive correlation with DFI (p ˂ 0.00001). | Belloc et al. 2014 [79] |
| De novo DNA mutations | 97 men (22–80) |
Significant association between DFI and FGFR3 mutations associated with achondroplasia (p ˂ 0.001) | Wyrobek et al. [81] |
| Congenital anomalies | >15,000 children, father’s Age groups: ˂24, 25–34, and ˃35 |
Significant association with cleft palate (p = 0.037), diaphragmatic hernia (p = 0.001), RVOTO (p = 0.002), and PVS (p = 0.011) Elevated ORs for each year of PA |
Green et al. [84] |
| Neurological disorders | |||
| ASD | 209 trios | Positive correlation in children to develop ASD | O’Roak et al. [85] |
| Schizophrenia | 14.211 people born in Denmark and diagnosed with schizophrenia | Association between PA when first child was born | Petersen et al. [117] |
| Telomere length | 135 men (18–68) |
Sperm telomere length have got longer by 57 bp/year (p = 0.0002) Male germ cells were estimated to gain 2.48 bp per replication |
Aston et al. [86] |
| Apoptosis | 25 fertile men (20–68) |
Significantly association with the presence of early apoptotic markers in spermatozoa (increase in translocation of plasma membrane phosphatidylserine) (p ˂ 0.01) | Colin et al. [87] |
| DNA methylation | 17 fertile men, when the first sample analyzed, mean group age, 37.7 ± 2.12 when the last sample analyzed; group age, 50.3 ± 2.1 |
Significant hypomethylation in 139 regions and hypermethylation in 8 regions in sperm DNA (p = 0.012 and p = 0.07, respectively) Age age-related alterations in sperm DNA was found to be localized at genes associated with bipolar disorder and schizophrenia |
Jenkins et al. [5] |
| miRNA expression | 10 fertile men with normal seminogram and standard karyotype | No significant correlation with expression levels of 736 miRNAs using Taqman arrays | Salas-Huetos et al. [118] |
Although apoptosis is essential for spermatogenesis under normal conditions, the balance between proliferation of spermatogonia and apoptosis of different germ cell types appears to be disturbed with aging [91]. In spermatogonia of aged men, both expression of Ki-67, a marker of cell proliferation activity, and apoptotic rate were found to be decreased compared to young controls [92]. In contrast to spermatogonia, apoptotic rate of primary spermatocytes, as shown by TUNEL positivity, was significantly elevated compared to controls, which might, in part, explain the mechanism of germ cell loss in aged men [92]. The discrepancy might be due to an age-related deterioration of spermatogonial stem cell niche accompanied by a compensatory decrease in apoptotic spermatogonia [91]. In supporting evidence, a recent histological and ultrastructural study has shown increased apoptosis along with a reduced proliferation in germ cells of the aging testes [93]. Although the significant decrease in the number of germ cells was found at the late spermatid level, primary spermatocytes did show a numerical decrease in the elderly men compared with the young controls. Interestingly, the same study also utilized transmission electron microscopy to reveal deterioration of Sertoli cell organelles and reduction in intercellular tight junctions in the aged cases, which has been proposed by the authors as yet another likely mechanism for age-associated decline in spermatogenic potential [93]. As the effect of age on sperm DNA single- and double-strand breaks is well documented [94, 95], presence of DNA damage repair-associated proteins such as poly(ADP-ribose) polymerase 1 (PARP-1) were also investigated in testicular tissue samples from older men [96]. Statistically significant differences in the expression of DNA repair proteins as well as apoptosis markers, such as active caspase-3 and cleaved PARP-1, were found most markedly in aging spermatocytes. The immunohistochemical co-existence of DNA repair and apoptotic markers in aging testicular germ cells can be explained by overactivated PARP-1 leading to initiation of apoptosis [96, 97]. Membrane translocation of phosphatidyl serine is one of the early apoptotic biomarker and increases with paternal aging (p < 0.01) [87] (Table 4). Consequently, increased oxidative stress in semen correlates with increased male age and, reduced sperm motility, and increased sperm DNA fragmentation.
Fertilization capacity
A trend toward delaying childbirth and increasing spacing between children is common in advanced Western countries, reflecting an increase in life expectancy and the changing position of women in society. The adverse effects of advanced maternal aging on reproductive potential have been well documented [98]; however, studies examining the effect of paternal aging on fertilization capacity are insufficient and the results are conflicting. Although motility, vitality, and normal morphology appear to decrease with aging, there is no clinical finding for an alteration in fertilization capacity in older men [99].
A study examining paternal age effect on 3287 couples with natural fertilization demonstrated an increased delay in pregnancy onset and conception difficulties in cases where men were older than 40 years [100]. The effect of paternal aging has also been studied in the setting of assisted reproductive technologies (ART). When in vitro fertilization (IVF) was examined, lower pregnancy rates and live birth rates were found in couples where the mean age of men was 38.4 [101]. Additionally, Robertshaw et al. found a significantly low live birth rate with IVF for elder men (decreased by 26 % for each 5-year increase in paternal age) [102]. Two studies have indicated that fertilization rates and blastocyst formation rates significantly decreased, and pregnancy loss increased with ART in men older than 50 years [103, 104]. A retrospective analysis of 4887 oocyte donation cycles has demonstrated no differences in biochemical, clinical, and ongoing pregnancies, miscarriage, and live birth rates among different male age groups [105]. A study examining 1024 couples with intracytoplasmic sperm injection has demonstrated decreased implantation rate for oligozoospermic men (decreased by 5 % for each year of paternal age) yet not for normozoospermic men [106]. A recent study has demonstrated that there was no difference in fertilization rates and quality of embryos at day 3 among different paternal age groups. However, aneuploidy rates in embryos from donated oocytes increased significantly with paternal age for patients older than 50 years concurrent with increased sperm DNA fragmentation [107]. The effects of advanced paternal age on reproductive outcomes including live birth, pregnancy loss, miscarriages and implantation rates, fertilization, and embryo development and morphology are summarized in Table 3.
Table 3.
The effects of advanced paternal aging on reproductive outcomes including live birth rate, pregnancy loss rate, miscarriage rate, implantation rate, fertilization, embryo development, and embryo morphology
| Fertilization type | Study population | Observed effects of paternal age | Paternal age limits | Reference |
|---|---|---|---|---|
| Natural | 3287 couples | Delay in pregnancy onset ↑ Conceptional difficulties ↑ |
≥40 | de La Rochebrochard and Thonneau [100] |
| IVF/GIFT | 221 couples | Pregnancy rate ↓ Live birth rate ↓ |
Mean: 38.4 | Klonoff-Cohen and Natarajan [101] |
| IVF, ICSI, CI | 1023 donor oocytes (donor age; ≤35) | Live birth rate ↓ Pregnancy loss rate ↑ Blastocyst formation rate ↓ No significant difference in implantation and pregnancy rate or early embryo development |
>50 | Frattarelli et al. [99] |
| CI, ICSI | 672 ovum donor cycles (donor age; <35) | Fertilization rate ↓ Day 3 embryos with >7 cells ↓ Blastocyst formation rate ↓ Implantation rate ↓ Pregnancy loss rate ↑ |
<40, 40–49, >50 (subgroup >60) | Luna et al. [104] |
| ICSI | 1024 couples | For couples in which the men are oligozoospermic implantation rate ↓ (decreased by 5 % for each year of paternal age) No such difference was observed for normozoospermic men In both groups, paternal age did not influence miscarriage outcomes. |
Mean: 36.85 for oligozoospermic and 37.18 for normozoospermic | Ferreira et al. [106] |
| IVF | 237 oocyte donation cycles (donor age, 21–31) |
Live birth rate ↓ (decreased by 26 % for each 5 years of paternal age) |
25–66 | Robertshaw et al. [102] |
| ICSI | 4887 oocyte donation cycles (donor age, 18–35) | No differences were found in biochemical, clinical and ongoing pregnancy, miscarriage, and live birth in different male age groups | 22–81 | Begueria et al. [105] |
IVF in vitro fertilization, IVF-ET in vitro fertilization and embryo transfer, GIFT gamete intrafallopian transfer, CI conventional insemination, ICSI intracytoplasmic sperm injection
DNA mutations and paternal aging
Mutations are permanent changes in the nucleotide sequence of DNA that originate during DNA replication or other cell cycle phases. These changes may be de novo or a result of various exogenous factors. Male germ cells undergo continuous DNA replication and cell division during the entire reproductive lifespan. The number of DNA replication cycles is 25 times higher in 40-year-old male germ cells than that in female germ cells [108].
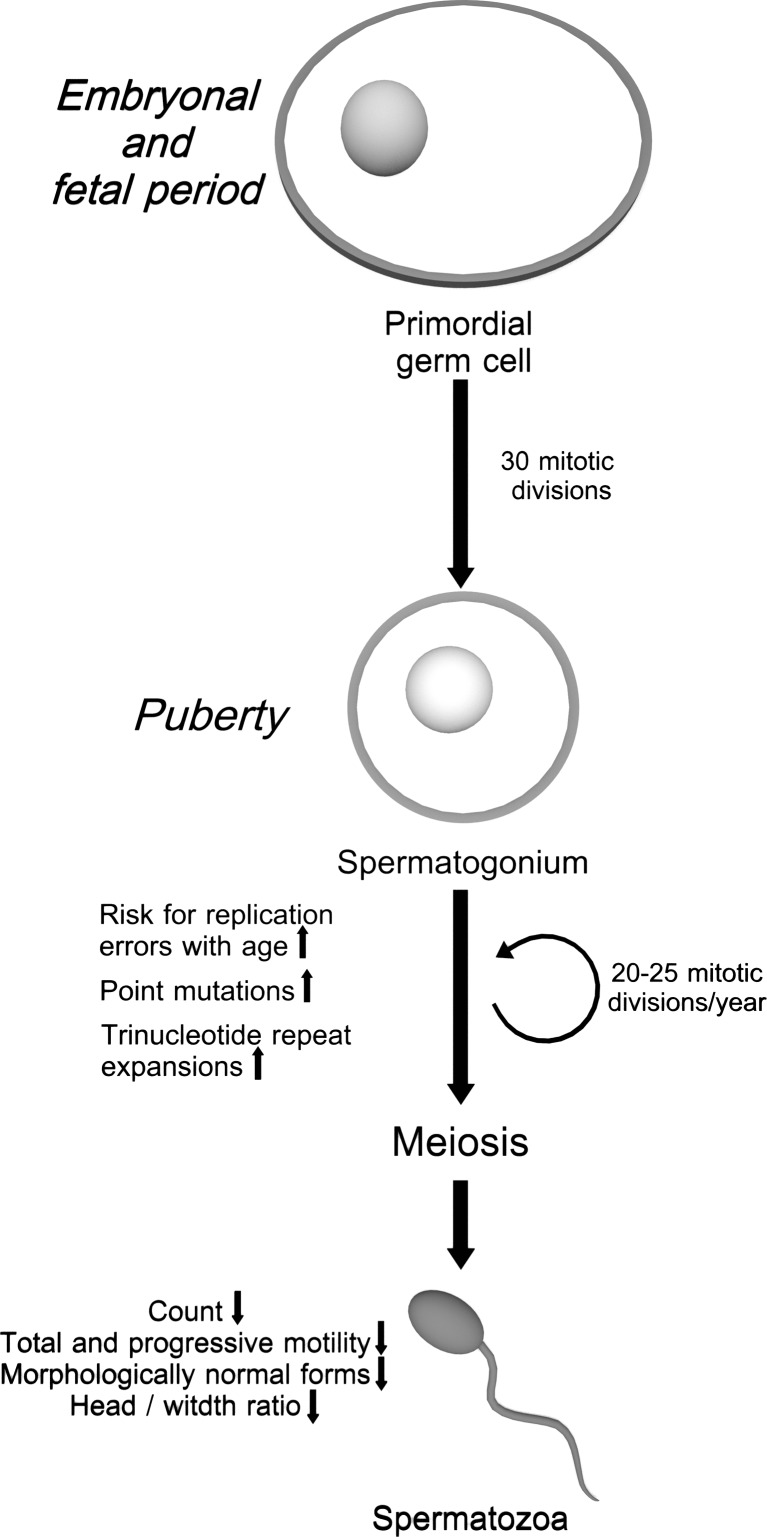
However, continuous division of male germ cell line during the entire reproductive lifespan causes an increase in the frequency of mutations in spermatozoa [109] (Fig. 1). Several mechanisms underlying the accumulation of mutations have been proposed for aging spermatozoa. Accumulation of mutations caused by internal and external sources increases with aging. Chromosomal rearrangements, decrease in fidelity of proofreading during DNA replication and DNA repair mechanisms, increase in apoptosis in germ cell line, accumulation of endogenous or exogenous mutagens, and increased oxidative stress enhance mutation rates [108, 110]. Base substitutions are the most frequent paternal mutations and the result of faulty DNA polymerase, leading to mismatching of nucleotides during mitotic cell divisions [110]. Kong et al. studied the genome-wide mutation rates by sequencing the entire genomes of 78 Icelandic parent offspring trios. The authors have demonstrated paternal mutations doubling every 16.5 years with an average increase of two mutations per year. Finally, they have suggested the importance of paternal age on the risk of diseases, such as schizophrenia and autism [111]. Similarly, DNA repeat extension mutations increase with increasing paternal age through replication errors during proliferative stages of spermatogenesis (Fig. 2) [112]. Based on this data, paternal age effect (PAE) disorders are associated with a higher risk of being born with single gene disorders. These de novo mutations can affect fibroblast growth factor receptors (FGFR), tyrosine kinase receptors, and genes having function in Rat Sarcoma Viral Oncogene Homolog (RAS)/mitogen activated protein kinases (MAPK) signaling pathways and cause gain-of-function mutations [113]. PAE causes impairment in RAS signaling and alterations in growth and differentiation properties of spermatogonial stem cells (SSCs) [114]. Additionally, mutations of SSCs may give selective growth advantage to mutant stem cells. Dark type A (Adark) and pale type A (Apale) spermatogonia are the two types of SSCs where the Adark spermatogonia are considered as reserve stem cells of spermatogenesis and proliferate rarely, whereas, Apale spermatogonia proliferate regularly and give rise to Apale and finally mature type B spermatogonia. It is considered that while wild-type SSCs undergo asymmetric cell division, mutant SSCs divide symmetrically once per 100 cell divisions. The selfish spermatogonial division of mutant SSCs gives selective growth advantage to mutant spermatogonial cells and causes clonal expansion of mutant spermatogonial cells over time, which consequently results in increase of mutation frequency in aged human testes [113].
Fig. 2.
Cell divisions and errors in spermatogenesis in the course of aging. A continuous series of DNA replication and mitosis take place during spermatogenesis. Primordial germ cells undergo about 30 mitotic divisions during intrauterine development. After puberty, DNA replication and mitosis continue and 20–25 mitotic divisions occur each year during adulthood. Errors during DNA replication are the primary source of point mutations in spermatozoa
The most common PAE disorders are autosomal dominant disorders caused by mutations in five genes (FGFR2, FGFR3, HRAS, PTPN11, RET), including Apert, Crouzon, Pfeiffer, Muenke syndromes, achondroplasia, Costello and Noonan syndromes, and multiple endocrine neoplasia type 2A and type 2B [113, 114]. Interestingly, these mutations are point mutations and originate from the unaffected father during spermatogenesis [34]. All of these disorders are characterized by congenital skeletal deformities, growth retardation, cardiac disorders, skin hyperpigmentation and cancer susceptibility. Apert syndrome is one of the most studied PAE disorders that results from 755C>G or 758C>G mutations in FGFR2 gene, and the frequency of these two mutations increases with advanced paternal age [81, 108, 109] (Table 4).
The promoter regions of genes contain CpG islands and methylation of these cytosines is correlated with inactivation or silencing of the associated promoter, whereas hypomethylation usually leads to activation of gene expression [119, 120]. Silencing of gene expression is either due to inhibition of transcription factor binding to methylated cytosines or repression mediated by methyl-CpG-binding proteins [119, 121]. A recent research has shown that 139 regions are hypomethylated and 8 regions are hypermethylated in spermatozoal DNA with aging, and these regions contain genes associated with schizophrenia and bipolar disorder (p = 0.012 and p = 0.07, respectively) [122] (Table 4). Several studies have reported higher incidence of neurodevelopmental disorders in children with older fathers. Similarly, Miller et al. reported a significant association between advanced paternal age and schizophrenia among the offspring by meta-analysis [123]. Advanced paternal age is a risk factor for development of bipolar disorder in children and the highest risk has been reported for children whose fathers were equal and above 55 years old [123].
Effect of paternal age on other diseases
Although base substitutions are the most frequent paternal genetic abnormalities, it is reported that 80 % of structural chromosomal abnormalities may originate paternally [83, 124] (Table 4). Several studies have suggested that chromosomal deletions and duplications increase with paternal aging [124]. Similarly, the possibility of balanced translocations in spermatozoa doubles in 10 years with advanced paternal age [83].
Some studies have reported that congenital abnormalities including heart defects, tracheo-esophageal fistula, and muscle-skeletal-skin abnormalities are frequent in offspring of older fathers [125, 126]. In addition, advanced paternal age has been shown to be associated not only with childhood cancers but also with certain sporadic nervous system cancers [127, 128]. Furthermore, using exome sequencing, O’Roak et al. have reported that 39 % of paternally originated de novo mutations are associated with autism candidate genes [85]. Additionally, Green and colleagues have found a significant association between schizophrenia, low birth weight, stillbirth, preterm delivery, cleft palate, diaphragmatic hernia, right ventricular outflow tract obstruction, pulmonary valve stenosis, multiple complex defects, and advanced paternal age [84] (Table 4).
D’Onofrio et al. have reported a significant association between advanced paternal aging (>45) and increased risk of psychiatric and academic morbidity including autism, attention deficit-hyperactivity disorder, schizophrenia, bipolar disorder, suicidal behavior, substance abuse problems, and academic problems compared to offspring born to fathers 20 to 25 years old [129]. A recent study conducted in mice has shown a significant loss of DNA methylation at CGI regions in sperm of old fathers. These aberrations in DNA methylation were transmitted to the offspring although some of them are known to be reset during prenatal life. The results have shown that both CGI regions and splice junctions are abnormally methylated in older fathers’ offspring and these genomic regions correspond to neurodevelopmental genes implicated in autism and schizophrenia [130].
Telomeres
Human telomeres are repetitive (TTAGGG)n hexameric sequences of DNA located at the ends of chromosomes and are about 5–12 kilobases (kb) long. Telomeres and the associated proteins protect the integrity of chromosomes and genetic information from enzymatic degradation and fusion by neighboring chromosomes, and are associated with senescence and apoptosis [131, 132]. Additionally, telomeres specify the location of chromosomes in the nucleus and have an effect on pairing of the homologous chromosomes and movement of chromosomes during cell division [131].
During DNA replication, DNA polymerase cannot completely duplicate 5′ terminal ends of the chromosomes, which causes shortening of the chromosomal telomeres [133, 134]. Critical shortening of telomeres causes an arrest of the cell division cycle and induce cellular senescence or apoptosis [135, 136]. Although in many proliferating tissues telomeres shorten with each cell division, telomere length unusually increases with aging in spermatozoa [133]. High expression of testicular telomerase leads to extension of telomeres of spermatozoal chromosomal ends with advanced age. Leukocyte telomere length is a biological marker of aging, and the telomere length of leukocytes of children of older fathers is higher than that of children of younger fathers. [86, 137–139]. However, the exact mechanism of longer offspring’s leukocyte telomere length depending on paternal aging is not clear. Previous studies have suggested two mechanisms to explain the longer telomere length in spermatozoa of older men [86, 139] (Table 4). The former explanation is high expression of testicular telomerase leading to extension of telomeres of spermatozoa chromosomal ends with advanced age [140]. The latter suggests germ stem cells with long telomeres are favored with replicative advantage compared to ones with short telomeres [141]. Indeed, recently, a study was conducted with monozygotic and dizygotic twins to elucidate the potential mechanism of increased telomere length with paternal aging. Their results demonstrated increased similarity in leukocyte telomere length in dizygotic twins of older fathers and this similarity may represent an age-dependent selection of germ stem cells with longer telomeres [140]. The genetic and molecular pathways that are associated with aging in regards to telomeres are summarized in Table 4.
Conclusion
Multiple genetic and environmental factors accelerate aging in somatic cells, as well as in male reproductive cells, and these alterations increase the death risk and the susceptibility to a number of diseases. Aging detrimentally affects a woman’s reproductive potential, including a high risk of spontaneous abortion, chromosomal abnormalities especially aneuploidies in the offspring, intrauterine growth retardation, and preterm delivery [142]. However, the effects of aging on men’s fertility and offspring remain poorly defined. Age-related alterations lead to gradual changes in hormone levels and spermatogenesis in men. Consequently, these progressive changes result in decrease in both quality and quantity of spermatozoa. On the other hand, a few studies have demonstrated that obesity, lack of exercise, and age-related comorbidities are more effective than chronological aging in declining testosterone levels in aging men [143]. The effects of aging on male reproductive system are quite complex driven by both normal physiological process and environmental factors. Further studies are required to investigate the onset of gonadal senescence and its regulation in aging men.
Rigorous living conditions and desire for a better lifestyle cause postponing of parenthood until older ages, yet the effects of advanced age on disease risk in offspring remain unknown. Advanced paternal age is widely accepted as 40 years or older at the time of the conception [144]. Recent studies on reproduction propose that men can maintain their fertility until older ages. Although paternal age does not affect fecundity directly as an independent factor, it may be significant in combination with the maternal age [145]. On the other hand, recent studies have indicated that advanced paternal age has a significant influence on the risk of certain diseases and correlates with a number of complications in the offspring. The offspring of older fathers show high prevalence of some genetic abnormalities, childhood cancers, and several neuropsychiatric disorders [85, 127]. In addition, the latest advances in assisted reproductive techniques give chance to have a child to older men even with poor semen parameters. Although the studies provide link between advanced paternal age and certain disorders, there is yet no diagnostic or screening test panels to detect these disorders using patient gametes [144]. The genetic abnormalities and diseases risk of offspring should be assessed by parent-offspring trios studies for base substitutions. Additionally, the epigenetic statute of spermatozoa may change with aging and it is not completely clear whether these changes are transmitted and affect the health of the offspring. Whole genome sequencing and new technologies in epigenetics may be useful in understanding the effects of aging on male reproductive system and offspring.
Acknowledgments
We would like to thank Alaaddin Hekim for his help in drawing of the graphical figures
Footnotes
Capsule
The study aims to discuss the effects of aging on the male reproductive system. Paternal aging causes genetic and epigenetic changes in spermatozoa, which impair male reproductive functions through their adverse effects on sperm quality and count, as well as, sexual organs and hypothalamic-pituitary-gonadal axis. As a result, the offspring of older fathers show high prevalence of a few genetic abnormalities, childhood cancers, and several neuropsychiatric disorders.
Contributor Information
Sezgin Gunes, Phone: +90 362 312 19 19/3164, Email: sgunes@omu.edu.tr.
Gulgez Neslihan Taskurt Hekim, Phone: +90 362 312 19 19/3245, Email: gntkurt@gmail.com.
Mehmet Alper Arslan, Phone: +90 362 312 19 19/2278, Email: alpera55@gmail.com.
Ramazan Asci, Phone: +90 362 312 19 19/2255, Email: rasci@omu.edu.tr.
References
- 1.Cedars MI. Introduction: childhood implications of parental aging. Fertil Steril. 2015;103(6):1379–80. doi: 10.1016/j.fertnstert.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Belloc S, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008;17(3):392–7. doi: 10.1016/S1472-6483(10)60223-4. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod. 2008;23(3):538–42. doi: 10.1093/humrep/dem431. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SL, et al. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins TG, et al. The sperm epigenome, male aging, and potential effects on the embryo. Adv Exp Med Biol. 2015;868:81–93. doi: 10.1007/978-3-319-18881-2_4. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, et al. The effects of aging on testicular volume and glucose metabolism: an investigation with ultrasonography and FDG-PET. Mol Imaging Biol. 2011;13(2):391–8. doi: 10.1007/s11307-010-0341-x. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud AM, et al. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J Clin Endocrinol Metab. 2003;88(1):179–84. doi: 10.1210/jc.2002-020408. [DOI] [PubMed] [Google Scholar]
- 8.Well D, et al. Age-related structural and metabolic changes in the pelvic reproductive end organs. Semin Nucl Med. 2007;37(3):173–84. doi: 10.1053/j.semnuclmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Zenzmaier C, Untergasser G, Berger P. Aging of the prostate epithelial stem/progenitor cell. Exp Gerontol. 2008;43(11):981–5. doi: 10.1016/j.exger.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Zitzmann M. Effects of age on male fertility. Best Pract Res Clin Endocrinol Metab. 2013;27(4):617–28. doi: 10.1016/j.beem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Dakouane M, et al. A histomorphometric and cytogenetic study of testis from men 29–102 years old. Fertil Steril. 2005;83(4):923–8. doi: 10.1016/j.fertnstert.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Paniagua R, et al. Ultrastructure of the aging human testis. J Electron Microsc Tech. 1991;19(2):241–60. doi: 10.1002/jemt.1060190209. [DOI] [PubMed] [Google Scholar]
- 13.Neaves WB, et al. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59(4):756–63. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- 14.Untergasser G, et al. Proliferative disorders of the aging human prostate: involvement of protein hormones and their receptors. Exp Gerontol. 1999;34(2):275–87. doi: 10.1016/S0531-5565(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 15.Bedford MT, van Helden PD. Hypomethylation of DNA in pathological conditions of the human prostate. Cancer Res. 1987;47(20):5274–6. [PubMed] [Google Scholar]
- 16.Sampson N, et al. The ageing male reproductive tract. J Pathol. 2007;211(2):206–18. doi: 10.1002/path.2077. [DOI] [PubMed] [Google Scholar]
- 17.Prakash K, et al. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A. 2002;99(11):7598–603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermann M, et al. Aging of the male reproductive system. Exp Gerontol. 2000;35(9-10):1267–79. doi: 10.1016/S0531-5565(00)00159-5. [DOI] [PubMed] [Google Scholar]
- 19.Bostwick DG, et al. High-grade prostatic intraepithelial neoplasia. Rev Urol. 2004;6(4):171–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson L, Petty CS, Neaves WB. Influence of age on sperm production and testicular weights in men. J Reprod Fertil. 1984;70(1):211–8. doi: 10.1530/jrf.0.0700211. [DOI] [PubMed] [Google Scholar]
- 21.Homonnai ZT, et al. Semen quality and sex hormone pattern of 29 middle aged men. Andrologia. 1982;14(2):164–70. doi: 10.1111/j.1439-0272.1982.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 22.Stone BA, et al. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100(4):952–8. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrom WJ, et al. Semen and sperm reference ranges for men 45 years of age and older. J Androl. 2006;27(3):421–8. doi: 10.2164/jandrol.05156. [DOI] [PubMed] [Google Scholar]
- 24.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45. [DOI] [PubMed]
- 25.Mukhopadhyay D, Varghese AC, Pal M, Banerjee SK, Bhattacharyya AK, Sharma RK, et al. Semen quality and age-specific changes: a study between two decades on 3,729 male partners of couples with normal sperm count and attending an andrology laboratory for infertility-related problems in an Indian city. Fertil Steril. 2010;93(7):2247–54. [DOI] [PubMed]
- 26.Elzanaty S. Association between age and epididymal and accessory sex gland function and their relation to sperm motility. Arch Androl. 2007;53(3):149–56. [DOI] [PubMed]
- 27.Sloter E, Schmid TE, Marchetti F, Eskenazi B, Nath J, Wyrobek AJ. Quantitative effects of male age on sperm motion. Hum Reprod. 2006;21(11):2868–75. [DOI] [PubMed]
- 28.Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Sperm concentration and normal sperm morphology decrease and follicle-stimulating hormone level increases with age. BJU Int. 2005;96(7):1087–91. [DOI] [PubMed]
- 29.Eskenazi B, Wryobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–54. [DOI] [PubMed]
- 30.Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol. 2001;184:818–22. [DOI] [PubMed]
- 31.Andolz P, Bielsa MA, Vila J. Evolution of semen quality in North-eastern Spain: a study in 22,759 infertile men over a 36 year period. Hum Reprod. 1999;14:731–5. [DOI] [PubMed]
- 32.Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. BMJ. 1996;312(7029):471–2. [DOI] [PMC free article] [PubMed]
- 33.Bujan L, Mieusset R, Mondinat C, Mansat A, Pontonnier F. Sperm morphology in fertile men and its age related variation. Andrologia. 1988;20(2):121–8. [DOI] [PubMed]
- 34.Jung A, Schuppe HC, Schill WB. Comparison of semen quality in older and younger men attending an andrology clinic. Andrologia. 2002;34(2):116–22. doi: 10.1046/j.0303-4569.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 35.Araujo AB, Wittert GA. Endocrinology of the aging male. Best Pract Res Clin Endocrinol Metab. 2011;25(2):303–19. doi: 10.1016/j.beem.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammar M. Impaired in vitro testicular endocrine function in elderly men. Andrologia. 1985;17(5):444–9. doi: 10.1111/j.1439-0272.1985.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147(8):750–4. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 38.Morley JE, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. doi: 10.1016/S0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 39.Pirke KM, Sintermann R, Vogt HJ. Testosterone and testosterone precursors in the spermatic vein and in the testicular tissue of old men. Reduced oxygen supply may explain the relative increase of testicular progesterone and 17 alpha-hydroxyprogesterone content and production in old age. Gerontology. 1980;26(4):221–30. doi: 10.1159/000212418. [DOI] [PubMed] [Google Scholar]
- 40.Feldman HA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 41.Vermeulen A, et al. Estradiol in elderly men. Aging Male. 2002;5(2):98–102. doi: 10.1080/tam.5.2.98.102. [DOI] [PubMed] [Google Scholar]
- 42.Gray A, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73(5):1016–25. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 43.Liu PY, Beilin J, Meier C, Nguyen TV, Center JR, Leedman PJ, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab. 2007;92(9):3599–603. [DOI] [PubMed]
- 44.Baccarelli A, Morpurgo PS, Corsi A, Vaghi I, Fanelli M, Cremonesi G, et al. Activin A serum levels and aging of the pituitary-gonadal axis: a cross-sectional study in middle-aged and elderly healthy subjects. Exp Gerontol. 2001;36(8):1403–12. [DOI] [PubMed]
- 45.Kaufman JM, Vermeulen A. Declining gonadal function in elderly men. Baillieres Clin Endocrinol Metab. 1997;11(2):289–309. doi: 10.1016/S0950-351X(97)80302-3. [DOI] [PubMed] [Google Scholar]
- 46.Camacho EM, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 47.Veldhuis JD, et al. The aging male hypothalamic-pituitary-gonadal axis: pulsatility and feedback. Mol Cell Endocrinol. 2009;299(1):14–22. doi: 10.1016/j.mce.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonavera JJ, et al. In the male Brown-Norway (BN) male rat, reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl. 1997;18(4):359–65. [PubMed] [Google Scholar]
- 49.Keenan DM, Veldhuis JD. Age-dependent regression analysis of male gonadal axis. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1215–27. doi: 10.1152/ajpregu.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013;14(4):158–72. [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aitken RJ, et al. Oxidative stress and male reproductive health. Asian J Androl. 2014;16(1):31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moller P, et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res Rev Mutat Res. 2014;762:133–66. doi: 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59(4):459–69. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 55.Valko M, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Victor VM, Rocha M, De la Fuente M. Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol. 2004;4(3):327–47. doi: 10.1016/j.intimp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molteni CG, Principi N, Esposito S. Reactive oxygen and nitrogen species during viral infections. Free Radic Res. 2014;48(10):1163–9. doi: 10.3109/10715762.2014.945443. [DOI] [PubMed] [Google Scholar]
- 60.Aitken RJ, et al. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil. 1992;94(2):451–62. doi: 10.1530/jrf.0.0940451. [DOI] [PubMed] [Google Scholar]
- 61.Tamburrino L, et al. Mechanisms and clinical correlates of sperm DNA damage. Asian J Androl. 2012;14(1):24–31. doi: 10.1038/aja.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma R, Masaki J, Agarwal A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol Biol. 2013;927:121–36. doi: 10.1007/978-1-62703-038-0_12. [DOI] [PubMed] [Google Scholar]
- 63.Vajapey R, et al. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol. 2014;5:439. doi: 10.3389/fphys.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21(10):569–76. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang YC, Van Remmen H. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44(4):256–60. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Lopez-Otin C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr Opin Clin Nutr Metab Care. 2007;10(6):688–92. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 68.Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14(3):367–81. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 69.de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46(4):502–10. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Koksal IT, et al. Potential role of reactive oxygen species on testicular pathology associated with infertility. Asian J Androl. 2003;5(2):95–9. [PubMed] [Google Scholar]
- 71.Lavranos G, et al. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol. 2012;34(3):298–307. doi: 10.1016/j.reprotox.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Aitken RJ, et al. Superoxide dismutase in human sperm suspensions: relationship with cellular composition, oxidative stress, and sperm function. Free Radic Biol Med. 1996;21(4):495–504. doi: 10.1016/0891-5849(96)00119-0. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal A, Prabakaran SA, Said TM. Prevention of oxidative stress injury to sperm. J Androl. 2005;26(6):654–60. doi: 10.2164/jandrol.05016. [DOI] [PubMed] [Google Scholar]
- 74.Durairajanayagam D, et al. Lycopene and male infertility. Asian J Androl. 2014;16(3):420–5. doi: 10.4103/1008-682X.126384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El-Taieb MA, et al. Oxidative stress and epididymal sperm transport, motility and morphological defects. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S199–203. doi: 10.1016/j.ejogrb.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 76.Shi TY, et al. Effects of reactive oxygen species from activated leucocytes on human sperm motility, viability and morphology. Andrologia. 2012;44(Suppl 1):696–703. doi: 10.1111/j.1439-0272.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- 77.Meseguer M, et al. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89(5):1191–9. doi: 10.1016/j.fertnstert.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura H, et al. Detection of oxidative stress in seminal plasma and fractionated sperm from subfertile male patients. Eur J Obstet Gynecol Reprod Biol. 2002;105(2):155–60. doi: 10.1016/S0301-2115(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 79.Belloc S, et al. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil Steril. 2014;101(6):1588–93. doi: 10.1016/j.fertnstert.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Cohen-Bacrie P, et al. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009;91(5):1801–5. doi: 10.1016/j.fertnstert.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 81.Wyrobek AJ, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103(25):9601–6. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmid TE, et al. Micronutrients intake is associated with improved sperm DNA quality in older men. Fertil Steril. 2012;98(5):1130–1137 e1. doi: 10.1016/j.fertnstert.2012.07.1126. [DOI] [PubMed] [Google Scholar]
- 83.Thomas NS, et al. De novo apparently balanced translocations in man are predominantly paternal in origin and associated with a significant increase in paternal age. J Med Genet. 2010;47(2):112–5. doi: 10.1136/jmg.2009.069716. [DOI] [PubMed] [Google Scholar]
- 84.Green RF, et al. Association of paternal age and risk for major congenital anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann Epidemiol. 2010;20(3):241–9. doi: 10.1016/j.annepidem.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–50. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aston KI, et al. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol Hum Reprod. 2012;18(11):517–22. doi: 10.1093/molehr/gas028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colin A, et al. The effect of age on the expression of apoptosis biomarkers in human spermatozoa. Fertil Steril. 2010;94(7):2609–14. doi: 10.1016/j.fertnstert.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 88.Kao SH, et al. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008;89(5):1183–90. doi: 10.1016/j.fertnstert.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 89.Weir CP, Robaire B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J Androl. 2007;28(2):229–40. doi: 10.2164/jandrol.106.001362. [DOI] [PubMed] [Google Scholar]
- 90.Ozkosem B, et al. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015;5:15–23. doi: 10.1016/j.redox.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pastor LM, et al. Proliferation and apoptosis in aged and photoregressed mammalian seminiferous epithelium, with particular attention to rodents and humans. Reprod Domest Anim. 2011;46(1):155–64. doi: 10.1111/j.1439-0531.2009.01573.x. [DOI] [PubMed] [Google Scholar]
- 92.Kimura M, et al. Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J Androl. 2003;24(2):185–91. doi: 10.1002/j.1939-4640.2003.tb02661.x. [DOI] [PubMed] [Google Scholar]
- 93.Jiang H, et al. Quantitative histological analysis and ultrastructure of the aging human testis. Int Urol Nephrol. 2014;46(5):879–85. doi: 10.1007/s11255-013-0610-0. [DOI] [PubMed] [Google Scholar]
- 94.Schmid TE, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22(1):180–7. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 95.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80(6):1420–30. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 96.El-Domyati MM, et al. Deoxyribonucleic acid repair and apoptosis in testicular germ cells of aging fertile men: the role of the poly(adenosine diphosphate-ribosyl)ation pathway. Fertil Steril. 2009;91(5 Suppl):2221–9. doi: 10.1016/j.fertnstert.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 97.Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31(6):446–54. doi: 10.1016/S0301-472X(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 98.Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95(1):1–8. doi: 10.1016/j.fertnstert.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 99.Frattarelli JL, et al. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90(1):97–103. doi: 10.1016/j.fertnstert.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 100.de La Rochebrochard E, Thonneau P. Paternal age > or =40 years: an important risk factor for infertility. Am J Obstet Gynecol. 2003;189(4):901–5. doi: 10.1067/S0002-9378(03)00753-1. [DOI] [PubMed] [Google Scholar]
- 101.Klonoff-Cohen HS, Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol. 2004;191(2):507–14. doi: 10.1016/j.ajog.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 102.Robertshaw I, et al. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014;21(5):590–3. doi: 10.1177/1933719113506497. [DOI] [PubMed] [Google Scholar]
- 103.Frattarelli JL, et al. A luteal estradiol protocol for expected poor-responders improves embryo number and quality. Fertil Steril. 2008;89(5):1118–22. doi: 10.1016/j.fertnstert.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 104.Luna M, et al. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril. 2009;92(5):1772–5. doi: 10.1016/j.fertnstert.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 105.Begueria R, et al. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod. 2014;29(10):2114–22. doi: 10.1093/humrep/deu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferreira RC, et al. Negative influence of paternal age on clinical intracytoplasmic sperm injection cycle outcomes in oligozoospermic patients. Fertil Steril. 2010;93(6):1870–4. doi: 10.1016/j.fertnstert.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 107.Garcia-Ferreyra J, et al. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of sperm DNA fragmentation. Clin Med Insights Reprod Health. 2015;9:21–7. doi: 10.4137/CMRH.S32769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1(1):40–7. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 109.Yoon SR, et al. The ups and downs of mutation frequencies during aging can account for the Apert syndrome paternal age effect. PLoS Genet. 2009;5(7):e1000558. doi: 10.1371/journal.pgen.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gregoire MC, et al. Male-driven de novo mutations in haploid germ cells. Mol Hum Reprod. 2013;19(8):495–9. doi: 10.1093/molehr/gat022. [DOI] [PubMed] [Google Scholar]
- 111.Kong A, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–5. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pearson CE, Nichol Edamura K, Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005;6(10):729–42. doi: 10.1038/nrg1689. [DOI] [PubMed] [Google Scholar]
- 113.Maher GJ, Goriely A, Wilkie AO. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology. 2014;2(3):304–14. doi: 10.1111/j.2047-2927.2013.00175.x. [DOI] [PubMed] [Google Scholar]
- 114.Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Templado C, Vidal F, Estop A. Aneuploidy in human spermatozoa. Cytogenet Genome Res. 2011;133(2-4):91–9. [DOI] [PubMed]
- 116.Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, Wyrobek AJ. Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet. 2001;69(5):1046–54. [DOI] [PMC free article] [PubMed]
- 117.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry. 2011;168(1):82–8. [DOI] [PubMed]
- 118.Salas-Huetos A, Blanco J, Vidal F, Mercader JM, Garrido N, Anton E. New insights into the expression profile and function of micro-ribonucleic acid in human spermatozoa. Fertil Steril. 2014;102(1):213–22. e4. [DOI] [PubMed]
- 119.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25(10):1010–22. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 121.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31(2):89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Jenkins TG, et al. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;10(7):e1004458. doi: 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miller B, et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull. 2011;37(5):1039–47. doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas NS, et al. Parental and chromosomal origin of unbalanced de novo structural chromosome abnormalities in man. Hum Genet. 2006;119(4):444–50. doi: 10.1007/s00439-006-0157-6. [DOI] [PubMed] [Google Scholar]
- 125.McIntosh GC, Olshan AF, Baird PA. Paternal age and the risk of birth defects in offspring. Epidemiology. 1995;6(3):282–8. doi: 10.1097/00001648-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 126.Zhu JL, et al. Paternal age and congenital malformations. Hum Reprod. 2005;20(11):3173–7. doi: 10.1093/humrep/dei186. [DOI] [PubMed] [Google Scholar]
- 127.Hemminki K, Kyyronen P. Parental age and risk of sporadic and familial cancer in offspring: implications for germ cell mutagenesis. Epidemiology. 1999;10(6):747–51. doi: 10.1097/00001648-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 128.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35(6):1495–503. doi: 10.1093/ije/dyl177. [DOI] [PubMed] [Google Scholar]
- 129.D’Onofrio BM, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71(4):432–8. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Milekic MH, et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry. 2015;20(8):995–1001. doi: 10.1038/mp.2014.84. [DOI] [PubMed] [Google Scholar]
- 131.Gancarcikova M, et al. The role of telomeres and telomerase complex in haematological neoplasia: the length of telomeres as a marker of carcinogenesis and prognosis of disease. Prague Med Rep. 2010;111(2):91–105. [PubMed] [Google Scholar]
- 132.Nussey DH, et al. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol Evol. 2014;5(4):299–310. doi: 10.1111/2041-210X.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eisenberg DT. An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23(2):149–67. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- 134.Oeseburg H, et al. Telomere biology in healthy aging and disease. Pflugers Arch. 2010;459(2):259–68. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Else T. Telomeres and telomerase in adrenocortical tissue maintenance, carcinogenesis, and aging. J Mol Endocrinol. 2009;43(4):131–41. doi: 10.1677/JME-08-0189. [DOI] [PubMed] [Google Scholar]
- 136.Lobetti-Bodoni C, et al. Telomeres and telomerase in normal and malignant B-cells. Hematol Oncol. 2010;28(4):157–67. doi: 10.1002/hon.937. [DOI] [PubMed] [Google Scholar]
- 137.Kalmbach KH, et al. Telomeres and human reproduction. Fertil Steril. 2013;99(1):23–9. doi: 10.1016/j.fertnstert.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sebastian C, et al. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol. 2009;183(4):2356–64. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 139.Kimura M, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hjelmborg JB, et al. Paternal age and telomere length in twins: the germ stem cell selection paradigm. Aging Cell. 2015;14(4):701–3. doi: 10.1111/acel.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Momand JR, Xu G, Walter CA. The paternal age effect: a multifaceted phenomenon. Biol Reprod. 2013;88(4):108. doi: 10.1095/biolreprod.112.103440. [DOI] [PubMed] [Google Scholar]
- 142.Balasch J, Gratacos E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Fetal Diagn Ther. 2011;29(4):263–73. doi: 10.1159/000323142. [DOI] [PubMed] [Google Scholar]
- 143.Grossmann M. Diagnosis and treatment of hypogonadism in older men: proceed with caution. Asian J Androl. 2010;12(6):783–6. doi: 10.1038/aja.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Toriello HV, et al. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10(6):457–60. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Myrskyla M, Fenelon A. Maternal age and offspring adult health: evidence from the health and retirement study. Demography. 2012;49(4):1231–57. doi: 10.1007/s13524-012-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]