Abstract
Purpose
The aim of this work was to develop a microbioreactor using liquid marble (LM) as a novel system for oocyte in vitro maturation (IVM) in small volumes.
Methods
Cumulus-oocyte complexes (COCs) obtained from slaughterhouse sheep ovaries were in vitro matured in a LM system prepared by placing a drop (30 μl containing 10 COCs) suspended in TCM 199 supplemented with 10 % (v/v) oestrus sheep serum (OSS) and 0.1 IU FSH and LH onto a polytetrafluoroethylene (PTFE) particle bed (LM group). As a control group (CTRL group), COCs were in vitro matured in standard volume and conditions (600 μl of IVM medium in a four-well dish). After 24-h culture at 38.5 °C in 5 % CO2 in air, COCs were released from LM and the following parameters were evaluated: (a) percentage of MII oocytes, (b) oocyte developmental competence following in vitro fertilization (IVF) or parthenogenetic activation (PA) and embryo culture for 8 days in synthetic oviductal fluid (SOF) medium at 38.5 °C in 5 % O2, 5 % CO2, and 90 % N2.
Results
The results indicated similar percentage of MII oocytes in LM and CTRL groups (88.0 vs. 92.0 %). No differences were observed in blastocyst rate after IVF (LM 47.5 % vs. CTRL 50.2 %, P=0.637) or PA (LM 44.4 % vs. CTRL 48.3 %, P=0.426).
Conclusions
The results indicate that LM microbioreactor is a viable technique that provides a suitable microenvironment to induce oocyte in vitro maturation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0666-8) contains supplementary material, which is available to authorized users.
Keywords: Liquid marble, Bioreactor, Oocyte, In vitro maturation, Embryo development
Introduction
In vitro oocyte maturation is a well-established technique largely applied to in vitro embryo production in the livestock field [1]. However, in most mammalian species, even if high rates of in vitro meiotic maturation of cumulus-oocyte complexes (COCs) are obtained (range 60 to 90 %) [2], the developmental competence of the in vitro matured oocyte is still suboptimal as indicated by the relative low development up to blastocyst stage and the poor viability to term after transfer into recipient animals.
Several attempts have been made to increase the developmental competence of embryos derived from in vitro matured oocytes; these include the formulation of specific maturation media [3], length of in vitro culture [4, 5], addition of growth factors and molecules during in vitro culture [6, 7], and the support action exerted by the addition of different somatic cells in the co-culture systems [8].
Liquid marbles (LM) are a form of 3D bioreactor that have been previously shown to support the growth of living microorganisms [9], tumor spheroids [10], fibroblasts [11], red blood cells [12], and embryonic stem cells [13]. LM, first described by Aussillous and Quere [14], consists of a drop of liquid encapsulated by hydrophobic powder particles. The particles adhere to the surface of the liquid drop, isolating the liquid core from the supporting surface, while allowing gas exchange between the interior liquid and the surrounding environment. The coating material acts as a confined space which is non-adhesive and allows the cells to freely interact with each other.
This new technical procedure could provide a novel 3D in vitro culture system that (1) allows oocyte in vitro maturation in a reduced medium volume, (2) maintains the gaseous in vitro culture environment, and (3) avoids the exchange with other solid and liquid surfaces that could interfere with the physiological processes.
The aim of this work was to develop a microbioreactor using LM for the in vitro maturation of sheep oocytes. The feasibility of the system was tested by evaluation of oocyte in vitro meiotic competence and embryo development up to blastocyst stage following in vitro fertilization or parthenogenetic activation.
Materials and methods
All chemicals in this study were purchased from Sigma-Aldrich S.r.l. (Milan, Italy) unless stated otherwise.
Source of oocytes and in vitro maturation
Ovaries of adult Sarda sheep (4–6 years old) were collected from a local slaughterhouse in PBS solution (Dulbecco’s phosphate-buffered saline) containing penicillin (100 μg ml−1) and streptomycin (100 μg ml−1) at 37 °C. Cumulus-oocytes complexes (COCs) were recovered by slashing in sterile Petri dishes containing dissection medium (20 mM Hepes-buffered TCM 199 supplemented with 0.1 % (w/v) polyvinyl alcohol (PVA) and antibiotics. COCs with a uniform cytoplasm and several layers of unexpanded cumulus cells [15] were selected and randomly divided between two different in vitro maturation systems as outlined below.
A- control group (CTRL)
Groups of 30~35 COCs were matured in 600 μl of TCM 199 supplemented with 10 % (v/v) oestrus sheep serum (OSS), 0.1 IU ml−1 FSH and 0.1 IU ml−1 LH (Pergonal, Serono Italy), 8 mg/ml of pyruvate and 100 mM cysteamine (in vitro maturation (IVM) medium). COCs were cultured in four-well Petri dishes (Nunclon; Nalge Nunc International, Roskilde, Denmark) covered with 300 μl pre-equilibrated mineral oil for 24 h under 5 % CO2 in air at 38.5 °C.
B- liquid marble group (LM)
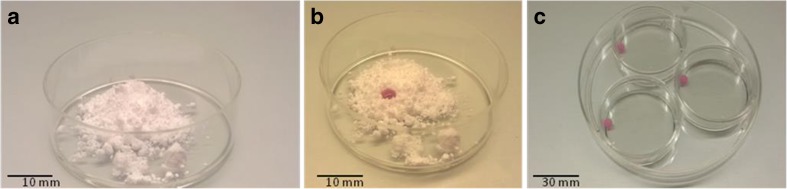
LM microbioreactor was created inside a Petri dish by preparing a polytetrafluoroethylene (PTFE) powder bed with particle size of 1 μm; a spatula was used to gently make a curved gully at the centre of the powder bed (Fig. 1a). A micropipette was used to dispense the required volume (30 μl) of IVM medium, containing a predetermined number of COCs (10 COCs for each drop) on the PTFE powder bed (Fig. 1b). The Petri dish was then gently shaken in a circular motion to ensure that the powder particles completely covered the surface of the liquid drop. LM drops were incubated in 35-mm Petri dishes for 24 h at 38.5 °C in 5 % CO2 in air (All procedures are shown in the supplement video). To increase humidity and avoid dehydration, the Petri dish was placed in a larger Petri dish containing sterile water and both Petri dishes were capped (Fig. 1c). All experiments were performed in three replicates.
Fig. 1.
Preparation of liquid marble containing COCs. a A hydrophobic PTFE powder bed is prepared in a 35-mm Petri dish. b 30 μl of IVM medium, containing 10 COCs, is dispensed over the hydrophobic PTFE powder bed. To fully coat the IVM drop by the PTFE powder, the IVM drop is gently rolled over the PTFE powder. c The resulting LM drop is placed in a 35-mm Petri dish placed within a bigger Petri dish containing sterile water to prevent evaporation
In vitro fertilization and parthenogenetic activation
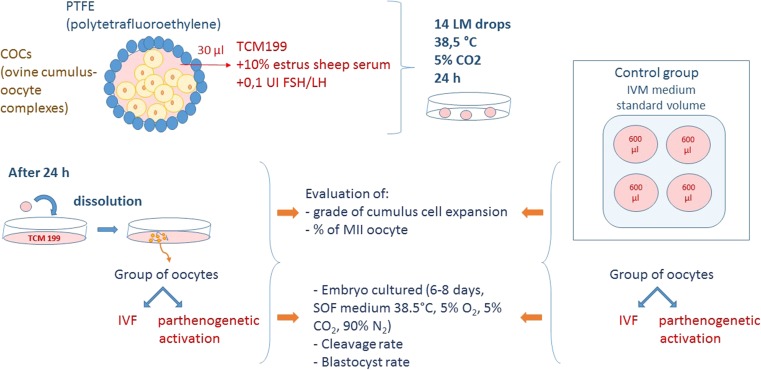
After 24 h, in vitro matured oocytes from the CTRL and LM systems were divided into two groups (within treatment) and either in vitro fertilized (IVF) or parthenogenetically activated (PA). COCs from the LM drops were released by the addition of IVM culture volume (200 μl) over the LM drops. In vitro fertilization (IVF) was performed as previously described by Bebbere et al. [15], in synthetic oviductal fluid (SOF, [16]) + 2 % OSS + 1 μg ml−1 heparin + 1 μg ml−1 hypotaurine for 22 h at 38.5 °C and under a 5 % CO2, 5 % O2, and 90 % N2 atmosphere in four-well Petri dishes with frozen/thawed spermatozoa selected by swim-up technique (1 × 106 spermatozoa/ml−1). Parthenogenetic activation (PA) was performed by incubation of COCs with 5 μM ionomycin for 5 min, followed by 3-h culture in 2 mM of 6-dimethyl amino purine (6-DMAP) [17] (Fig. 2).
Fig. 2.
Schematic steps leading to in vitro maturation in liquid marble (LM) microbioreactor and control (CTRL) group and subsequent in vitro fertilization or parthenogenetic activation and embryo culture
In vitro embryo development
IVF and PA presumptive zygotes were cultured (within treatment) for 8 days in SOF + essential and non-essential amino acids at oviductal concentration [18] + 0.4 % bovine serum albumin (BSA) under mineral oil, in four-well Petri dishes in maximum humidified atmosphere with 5 % CO2, 5 % O2, and 90 % N2 at 38.5 ° C. Cleavage rates were recorded 40–48 h after the start of fertilization or parthenogenetic activation. Blastocyst development was recorded on day 8 (day 0 = day of IVF or PA).
Statistical analysis
In vitro maturation and embryonic development rates were analyzed by chi-square test at each time point. The differences were considered significant when P < 0.05.
Results
After 24 of IVM culture, COCs derived from both the LM and CTRL groups showed similar expansion of cumulus cells (personal communication). The percentage of COCs that reached MII did not differ between LM and CTRL groups (88.0 vs. 92.0 %, P = 0.212, respectively), and no statistical differences were observed in the blastocyst rate after IVF (LM 47.5 % vs. CTRL 50.2 %, P = 0.637) or PA (LM 44.4 % vs. CTRL 48.3 %, P = 0.426), Table 1.
Table 1.
In vitro maturation and developmental competence of sheep oocytes cultured in liquid marble (LM) microbioreactor and control (CTRL) systems
| Culture system | No. of oocytes | No. of M II oocytes (%) | No. of cleaved embryos (40–48 h post IVF) | No. of blastocyst (8 days post IVF/PA) | ||
|---|---|---|---|---|---|---|
| IVF (%) | PA (%) | IVF (%) | PA (%) | |||
| CTRL | 200 | 184 (92.0) | 76/102 (74.5) | 61/82 (74.4) | 45/76 (59.2) | 29/61 (47.5) |
| LM | 141 | 124 (88.0) | 60/84 (71.4) | 27/40 (67.5) | 32/60 (53.3) | 12/27 (44.4) |
Discussion
In this work, we present, for the first time, a reliable method for a microbioreactor of liquid marble (LM) for in vitro maturation of sheep oocytes. Our data demonstrate that the LM system does not affect oocyte in vitro maturation and developmental competence after IVF or PA. Reports indicate a number of different systems have been employed to in vitro mature oocytes and culture embryos in small volumes of media. The mini drops covered by oil (ranging between 6 and 50 μl, [19]), the hanging drops system [20], the micromultiwell (MW) plates [21], and the Well of the Well (WOW) system [22] are the most experimented systems used for the COCs and embryo culture in a small volume. These systems offer some advantages, but also have limitations.
The two main reasons to culture COCs and embryos in small medium volumes are (1) to benefit from the autocrine and paracrine secretion that may be produced by the somatic cells, the oocyte, or other supporting cells and (2) to monitor individual COCs or embryo development, thereby providing a new perspective on nutrient uptake and utilization.
However, culturing COCs or embryos in a small volume of medium can present technical problems. Uncovered culture medium droplets are impractical because of (1) the rapid rise in salt and substrate concentration resulting from evaporation and (2) the increased sensitivity to temperature changes. The commonly used oil overlay may minimize these problems, but the high surface/volume ratio of oil to medium may result in high diffusion of lipid solvable materials necessary for oocyte and or embryo development [23]. In this regard, it has been reported that progesterone and estrogens diffuse into the mineral oil during oocyte culture [24]. A delay of nuclear maturation and reduction in developmental competence of pig oocytes has also been found after mineral oil overlay of in vitro maturation media [25].
To avoid the use of oil overlay, an alternative system for vitro maturation of COCs in a small volume is the hanging drop culture system. The hanging drop monoculture has been used for screening substances that affect oocyte culture, for example testing the addition of antioxidants during in vitro culture of porcine oocytes [20], or to measure the consumption of energy substrates in vitro culture of mouse and canine oocytes [26, 27]. The main limitations of the hanging drop system are the following: (1) it is not suitable for microscopic tracking and (2) it is not practical for media exchange or for the addition of specific molecules. Moreover, in both systems (mini drops cover by oil and hanging drops), the droplet volume should not be less than 10 μl. In less than 10 μl medium droplets, development is lower than that observed in droplets with a larger volume size [21]. This decrease in development may be attributed to the accumulation of toxic substances in the fluid volume surrounding the COCs.
Micromultiwell plates (MW) have also been used for in vitro oocyte maturation or embryo culture without oil overlay, but the few studies performed with commercial available tools have not significantly improved the developmental rates. The results of IVM system of individual bovine oocytes using the MW showed that the acquisition of developmental competence was higher compared with oocyte matured in small drops cover by mineral oil but the same when compared with the conventional group IVM culture [21].
“The Well-of-the-Well (WOW) culture system” cultures embryos in microwells formed on the bottom of a four-well culture dish [22]. Compared with culturing in drops, the WOW system resulted in significant improvements in developmental competence for in vitro matured and parthenogenetically activated porcine oocytes and for in vivo derived mouse zygotes [28]. In human studies, using a sibling oocyte design, embryos cultured in WOW developed to blastocyst stage in a significantly higher proportion than embryos cultured in a conventional system [28]. However, although the WOW system appears superior to the drop overlay by oil and to the hanging drop systems, it does not allow for the precise measurement of cellular needs or the addition of specific molecules. This is because the content of the well is openly connected with the large amount of medium above. On the other hand, the contact of the COC or embryo with a larger volume of medium could overcome the accumulation of excessive toxic substances.
In this study, we are proposing an alternative system for in vitro culture of COCs in small volume. The LM microbioreactor system offers several advantages:
Liquid marble is a realistic scaffold-free 3D bioreactor providing a maximum potential contact for aggregation of cells and could better simulate the follicular environment during meiotic maturation. The material coating LM is non-adhesive and acts as a confined space that allows suspended cells to freely interact with each other and self-assemble. Studies based on the use of liquid marbles for the multicellular growth have shown their ability to keep cells in suspension avoiding the problem of cell adhesion to the base of a cell culture dish. Increase in the efficiency of cell culture has been reported culturing tumor cells, fibroblasts, and embryonic stem cells in LM microbioreactor [10–13].
The culture of LM offers the possibility to easily perform uptake of medium to be analyzed with the scope of evaluating COC requirements and performing medium changes in programmed time. It is evident that the identification of metabolites during assisted reproductive technology (ART) procedures could have relevant clinical implications. In fact, the discovery and measurement of biomarkers in ART could define gametes’ quality and embryo viability, thus helping embryologists to achieve selection criteria alternative, or complementary, to the standard morphological assessments. Moreover, metabolomic profiling of culture media could be used to evaluate the health of the oocyte during in vitro maturation (IVM) and could facilitate selection of oocytes to be frozen in cryopreservation programs [29]. The LM system for IVM excludes the need to overlay small media volumes with mineral oil and hence could be a good system to perform precise measurements of different metabolic fingerprints in the spent medium and may also provide predictive information on subsequent embryo development. However, larger prospective studies are indeed required to further validate these methodologies in order to fully optimize their value as predictors of gamete and/or embryo quality.
The size of LM droplets is variable and can be adjusted according to the number of COCs. This maintains the advantage of culturing COCs in groups but can facilitate the incubation of single COC by reducing the drops size. However, as development is compromised in droplets with less than 10 μl medium [21, 30], we recommend 30 μl for in vitro oocyte maturation in LM microbioreactors. This size could be sufficient to dilute the possible accumulation of toxic substances such as ammonia [31, 32] or oxygen-derived free radicals [33], which may be harmful for oocyte maturation and lead to reduced developmental rates.
The easy access to the LM microbioreactor facilitates the addition of novel candidate drugs. By using small volumes, the protocol is “cost saving” because it limits the amount of reagents, such as growth factors or test compounds, needed for the assays. The dimensions of LM microbioreactors are modular according to the needs of cells and thus are also likely to culture separately cumulus cells and oocyte to monitor, in a fine-tuning manner, levels of nutrient and/or drugs.
In addition, the LM microbioreactor system could be adapted for use in culturing embryos in groups or individually, thereby offering the same potential benefits to evaluate embryos as outlined for COCs above. The small dimensions of LM are also suitable for the development of other ART techniques when a reduced volume is required as in vitro follicular growth and oocyte/embryo vitrification.
Conclusions
This technique could be used in a variety of applications including evaluating individual COC requirements. The potential application areas of LM microbioreactor system are not restricted to in vitro maturation. The system can offer advantages also for certain forms of oocyte fertilization and individual embryo culture. In addition, it reduces reagent consumption and decreases the potential for contamination, as a result of the indirect contact between the liquid core and the supporting surface.
Further application fields in the areas of reproductive biology are also conceivable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video illustrate the preparation of liquid marble drops for in vitro maturation of sheep cmulus oocyte complexes and their release (MP4 79 mb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Capsule
The results indicate that liquid marble microbioreactors provide a microenvironment capable of supporting mammalian oocyte in vitro maturation conducive to blastocyst development.
References
- 1.Paramio MT, Izquierdo D. Current status of in vitro embryo production in sheep and goats. Reprod Domest Anim. 2014;49(4):37–48. doi: 10.1111/rda.12334. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67:6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Farsi MM, Kamali N, Pourghasem M. Embryological aspects of oocyte in vitro maturation. Int J Mol Cell Med. 2013;2(3):99–109. [PMC free article] [PubMed] [Google Scholar]
- 4.You J, Lee E, Bonilla L, Francis J, Koh J, Block J, et al. Treatment with the proteasome inhibitor MG132 during the end of oocyte maturation improves oocyte competence for development after fertilization in cattle. PLoS One. 2012;7(11):e48613. doi: 10.1371/journal.pone.0048613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnay I, Faerge I, Grøndahl C, Verhaeghe B, Sayoud H, Ponderato N, et al. Effect of prematuration, meiosis activating sterol and enriched maturation medium on the nuclear maturation and competence to development of calf oocytes. Theriogenology. 2004;62(6):1093–107. doi: 10.1016/j.theriogenology.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Ledda S, Bogliolo L, Leoni G, Calvia P, Naitana S. Influence of vasoactive intestinal peptide (VIP), atrial natriuretic peptide (ANP) and insulin-like growth factor-I (IGF-I) on in vitro maturation of prepubertal and adult sheep oocytes. Zygote. 1996;4:343–348. doi: 10.1017/s0967199400003361. [DOI] [PubMed] [Google Scholar]
- 7.Uhm SJ, Gupta MK, Yang JH, et al. Epidermal growth factor can be used in lieu of follicle-stimulating hormone for nuclear maturation of porcine oocytes in vitro. Theriogenology. 2010;73:1024–36. doi: 10.1016/j.theriogenology.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Lin YH, Hwang JL Seow KM, Huang LW, Chen HJ, Tzeng CR. Effects of growth factors and granulosa cell co-culture on in-vitro maturation of oocytes. Reprod BioMed Online. 2009;19(2):165–170. doi: 10.1016/S1472-6483(10)60068-5. [DOI] [PubMed] [Google Scholar]
- 9.Tian J, Fu N, Chen XD, Shen W. Respirable liquid marble for the cultivation of microorganisms. Colloids Surf B: Biointerfaces. 2013;106:187–190. doi: 10.1016/j.colsurfb.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Arbatan T, Al-Abboodi A, Sarvi F, Chan PP, Shen W. Tumor inside a pearl drop. Adv Healthc Mater. 2012 doi: 10.1002/adhm.201200050. [DOI] [PubMed] [Google Scholar]
- 11.Serrano MC, Nardecchia S, Gutierrez MC, Ferrer ML, del Monte F. Mammalian cell cryopreservation by using liquid marbles. ACS Appl Mater Interfaces. 2015;7:3854–3860. doi: 10.1021/acsami.5b00072. [DOI] [PubMed] [Google Scholar]
- 12.Arbatan T, Li L, Tian J, Shen W. Liquid marbles as micro-bioreactors for rapid blood typing. Adv Healthc Mater. 2012;1:80–83. doi: 10.1002/adhm.201100016. [DOI] [PubMed] [Google Scholar]
- 13.Sarvi F, Jain K, Arbatan T, Verma PJ, Hourigan K, Thompson MC, et al. Cardiogenesis of embryonic stem cells with liquid marble micro-bioreactor. Adv Healthc Mater. 2015;4:77–86. doi: 10.1002/adhm.201400138. [DOI] [PubMed] [Google Scholar]
- 14.Aussillous P, Quéré D. Liquid marbles. Nature. 2001;411:924–927. doi: 10.1038/35082026. [DOI] [PubMed] [Google Scholar]
- 15.Bebbere D, Bogliolo L, Ariu F, Fois S, Leoni GG, Tore S, et al. Expression pattern of zygote arrest 1 (ZAR1), maternal antigen that embryo requires (MATER), growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) genes in ovine oocytes and in vitro-produced Preimplantation embryos. Reprod Fertil Dev. 2008;20(8):908–15. doi: 10.1071/RD08095. [DOI] [PubMed] [Google Scholar]
- 16.Tervit HR, Whittingham DG, Rowson LE. Successful culture in vitro of sheep and cattle ova. J Reprod Fertil. 1972;30(3):493–497. doi: 10.1530/jrf.0.0300493. [DOI] [PubMed] [Google Scholar]
- 17.Loi P, Ledda S, Fulka J, Jr, Cappai P, Moor RM. Development of parthenogenetic and cloned ovine embryos: effect of activation protocols. Biol Reprod. 1998;58(5):1177–87. doi: 10.1095/biolreprod58.5.1177. [DOI] [PubMed] [Google Scholar]
- 18.Walker SK, Hill JL, Kleemann DO, Nancarrow CD. Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations. Biol Reprod. 1996;55(3):703–8. doi: 10.1095/biolreprod55.3.703. [DOI] [PubMed] [Google Scholar]
- 19.Dai SJ, Xu CL, Wang J, Sun YP, Chian RC. Effect of culture medium volume and embryo density on early mouse embryonic development: tracking the development of the individual embryo. J Assist Reprod Genet. 2012;29(7):617–23. doi: 10.1007/s10815-012-9744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa S, Machida R, Hiraga K, Hiradate Y, Suda Y, Tanemura K. Hanging drop monoculture for selection of optimal antioxidants during in vitro maturation of porcine oocytes. Reprod Domest Anim. 2014;49(2):26–30. doi: 10.1111/rda.12289. [DOI] [PubMed] [Google Scholar]
- 21.Nagano M, Kang SS, Koyama K, Huang W, Yanagawa Y, Takahashi Y. In vitro maturation system for individual culture of bovine oocytes using micro-volume multi-well plate. Jpn J Vet Res. 2013;61(4):149–154. [PubMed] [Google Scholar]
- 22.Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, et al. New method for culture of zona-included or zona-free embryos: the well of the well (WOW) system. Mol Reprod Dev. 2000;55:256–264. doi: 10.1002/(SICI)1098-2795(200003)55:3<256::AID-MRD3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki J, Nagai Y, Chiba K. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil Steril. 2009;91:1745–1749. doi: 10.1016/j.fertnstert.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Reinsberg J, Ackermann D, Van der Ven H. Pitfalls in assessment of progesterone production by granulosa cells cultured in contact with silicone rubber or paraffin oil. Arch Gynecol Obstet. 2004;270:174–178. doi: 10.1007/s00404-003-0539-0. [DOI] [PubMed] [Google Scholar]
- 25.Shimada M, Kawano N, Terada T. Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media. Reproduction. 2002;124:557–564. doi: 10.1530/rep.0.1240557. [DOI] [PubMed] [Google Scholar]
- 26.Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60(6):1446–52. doi: 10.1095/biolreprod60.6.1446. [DOI] [PubMed] [Google Scholar]
- 27.Songsasen N, Spindler RE, Wildt DE. Requirement for, and patterns of, pyruvate and glutamine metabolism in the domestic dog oocyte in vitro. Mol Reprod Dev. 2007;74(7):870–7. doi: 10.1002/mrd.20667. [DOI] [PubMed] [Google Scholar]
- 28.Vajta G, Korösi T, Du Y, Nakata K, Ieda S, Kuwayama M, et al. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod BioMed Online. 2008;17(1):73–81. doi: 10.1016/S1472-6483(10)60296-9. [DOI] [PubMed] [Google Scholar]
- 29.Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, et al. Metabolomic assessment of oocyte viability. Reprod BioMed Online. 2009;18(2):219–25. doi: 10.1016/S1472-6483(10)60259-3. [DOI] [PubMed] [Google Scholar]
- 30.Lane M, Gardenr DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod. 1992;7:558–562. doi: 10.1093/oxfordjournals.humrep.a137690. [DOI] [PubMed] [Google Scholar]
- 31.Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 1993;48:377–385. doi: 10.1095/biolreprod48.2.377. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair KD, McEvoy TG, Carolan C, Maxfield EK, Maltin CA, Young LE, et al. Conceptus growth and development following in vitro culture of ovine embryos in media supplemented with bovine sera. Theriogenology. 1998;49:218. doi: 10.1016/S0093-691X(98)90571-4. [DOI] [Google Scholar]
- 33.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod BioMed Online. 2009;18(6):864–80. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video illustrate the preparation of liquid marble drops for in vitro maturation of sheep cmulus oocyte complexes and their release (MP4 79 mb)