Abstract
Purpose
The purpose of this study was to evaluate the serum estradiol (E2) per oocyte ratio (EOR) as a function of selected embryology events and reproductive outcomes with IVF.
Methods
This retrospective analysis included all IVF cycles where oocyte collection and fresh transfer occurred between January 2001 and November 2012 at a single institution. Patients were divided by three age groups (<35, 35–39, and ≥40 years) and further stratified into nine groups based on EOR (measured in pmol/L/oocyte). Terminal serum E2 (pmol/mL) was recorded on day of hCG trigger administration, and fertilization rate, cleavage rate, number of good quality embryos, and reproductive outcomes were recorded for each IVF cycle.
Results
During the study interval, 9109 oocyte retrievals were performed for 5499 IVF patients (mean = 1.7 cycles/patient). A total of 63.4 % of transfers were performed on day 3 (n = 4926), while 36.6 % were carried out on day 5 (n = 2843). Clinical pregnancy rates were highest in patients with EOR of 250–750 and declined as this ratio increased, independent of patient age. While the odds ratio (OR) for clinical pregnancy where EOR = 250–750 vs. EOR > 1500 was 3.4 (p < 0.001; 95 % CI 2.67–4.34), no statistically significant correlation was seen in fertilization, cleavage rates or number of good quality embryos as a function of EOR.
Conclusions
Predicting reproductive outcomes with IVF has great utility both for patients and providers. The former have the opportunity to build realistic expectations, and the latter are better able to counsel according to measured clinical parameters. A better understanding of follicular dynamics and ovarian response to gonadotropin stimulation could optimize IVF treatments going forward.
Keywords: Estradiol:oocyte ratio, Outcome, Advanced reproductive technology, IVF
Introduction
Controlled ovarian hyperstimulation is the standard of care in assisted conception units. Two of the principal clinical parameters when following follicle development are serum estradiol (E2) level and follicular diameter as measured by ultrasound. It is of interest to both clinicians and patients alike to predict the response to (and, ultimately, live birth rate following) ovarian hyperstimulation and IVF. Parameters that are known to correlate positively with success in IVF include endometrial thickness and the number of oocytes retrieved [1–3]. Conversely, negative predictors include advanced maternal age, elevated basal FSH, and low antral follicular count [4–7].
While the role of E2 is well documented in the follicular phase, controversy persists regarding its role in the luteal phase. Previous research has suggested that both low [8, 9] and supraphysiologic [10, 11] serum levels of E2 are negative predictors of clinical pregnancy after embryo transfer in IVF cycles.
It has been hypothesized that lower serum E2 levels represent hormonally dysfunctional developing follicles, which, in turn, luteinize poorly when exposed to hCG. Oocytes derived from such follicles often have poor fertilization [12]. In oocyte donation, it has been shown that high E2 levels are associated with impaired endometrial receptivity without affecting embryo quality [13]. Loumaye et al. [14] studied serum E2 level on day of hCG administration and the number of oocytes retrieved in patients undergoing long GnRH-agonist suppression. They found the estradiol:oocyte ratio (EOR) to be a reliable indicator for IVF success, with the highest pregnancy rates observed among patients with EOR of 70–140 pg/mL. Yang et al. [15] showed that patients undergoing the flare GnRH-agonist protocol with elevated EOR demonstrated lower pregnancy and implantation rates.
Orvieto et al. [16] found that while EOR cannot predict success of a GnRH-agonist protocol, for patients undergoing a GnRH-antagonist protocol EOR within 100–200 pg/mL range offers the best likelihood of a positive outcome.
While it is clear that elevated EOR denotes disordered folliculogenesis, the limited sampling in prior studies has made meaningful clinical conclusions difficult to develop. In the current investigation evaluating more than 9000 IVF cycles performed at a single institution, we aimed to examine IVF outcome as a function of EOR to contribute an original dataset to the study of this issue.
Methods
This retrospective analysis reviewed data on all patients who underwent IVF at a major European referral center (The HARI Unit/Rotunda Hospital; Dublin, Ireland) between January 2001 and December 2012. As this research was a retrospective medical record audit and did not involve any direct patient contact, it was considered exempt from review by the Ethics Committee of Rotunda Hospital, Dublin. Patient records were divided into three categories: age <35, 35–39, and ≥40 years old, and further sub-divided into nine groups based on EOR:
<250 pmol/L/oocyte
250–500 pmol/L/oocyte
500–750 pmol/L/oocyte
750–1000 pmol/L/oocyte
1000–1250 pmol/L/oocyte
1250–1500 pmol/L/oocyte
1500–1750 pmol/L/oocyte
1750–2000 pmol/L/oocyte
>2000 pmol/L/oocyte
Database accuracy was validated by multiple sources of ascertainment for key variables, and a review of individual medical records was performed if inconsistencies arose.
As previously described [16, 17], all IVF patients received pituitary downregulation for 12–14 days with either buserelin acetate (Suprecur, Aventis; Dublin, Ireland) 1.2 mg/6 h daily starting on day 1 or 2 of menses, or triptorelin (Decapeptyl SR, Ipsen; Dublin, Ireland) in a dose of 3 mg IM × 1. Pituitary suppression was confirmed by serum E2 levels (<150 pmol/L) and ultrasound scan. Real-time transvaginal ultrasound was performed using the GE LOGIC 200 ProSeries (Samsung GE Medical Systems, Korea) with a 6.5 MHz vaginal probe. Follicular diameters were calculated from the mean of two perpendicular diameters.
Serum oestradiol E2 levels were measured by time-resolved fluoroimmunoassay on an AutoDELFIA 1235 Automatic Immunoassay System (Turku, Finland). If the patient was not adequately suppressed, GnRH-a administration was continued × 1 week at double the dose and oestradiol E2 level repeated. After confirmation of pituitary down-regulation, gonadotrophin-controlled ovarian stimulation with either follitropin beta (Puregon, Organon; Oss, Netherlands) or urinary-derived product (Menopur, Ferring; Dublin, Ireland) was commenced. Starting dose was determined according to the day 3 follicle-stimulating hormone (FSH) level. For patients on their first cycle where their basal day 3 FSH was <8.5 U/L, 200 IU rFSH or 225 IU human menopausal gonadotropin (hMG) were used for initial dosing, whereas if the day 3 FSH was ≥8.5 U/L, 400 IU rFSH or 450 IU hMG was administered. For patients in subsequent cycles of treatment, the maximum dose in the previous cycle was used. These regimens were prescribed × 6 days, and on day 7 the ovarian response was assessed by ultrasound scan and E2 levels.
Periodic ultrasound and serum E2 monitoring was used to determine the timing of hCG (Pregnyl, Organon; Oss, Netherlands) administration. Cycles were cancelled on d8 if serum E2 levels were <300 pmol/L, or if <3 follicles were present. When the ovarian response was deemed adequate, the same daily dosage was continued with a monitoring visit on day 10, and daily visits from then onwards. If excessive response was evident on day 7 (E2 > 3000 pmol/L or more than six follicles in each ovary), the gonadotrophin dose was reduced and daily monitoring with transvaginal ultrasound and E2 levels was instituted. In some cases where rapid elevation of levels occurred, coasting was employed.
Once the leading three follicles reached an average diameter of 18 mm or more, hCG 10,000 IU IM was administered. In cases where coasting was employed, hCG was administered after E2 levels plateaued or started to fall. Transvaginal oocyte recovery was performed 34 h later under midazolam (Hypnovel, Roche; London, UK) and fentanyl citrate (Sublimaze, Janssen-Cilag; High Wycombe, UK) i.v.-monitored sedation. All surgeons performing oocyte retrievals were carefully audited and trained to the same standard; follicular flushing was used to reduce the risk of failure of oocyte retrieval. Regarding fertilization method, criteria for ICSI included non-ejaculated sperm, total sperm count <2.5 M/mL, motility <20 %, and normal forms morphology <30 % (WHO criteria). ICSI was also performed when fertilization failure was documented in a prior cycle. All serum E2 measurements were recorded from the same laboratory using uniform equipment where assay sensitivity was 5 pmol/L and mean intra-assay CV was 9 %.
Fresh transfer of two embryos (otherwise three embryos if available at age >35 years) was performed routinely on day 3 or day 5 after oocyte retrieval. This review did not include any frozen embryo transfer cycles.
Criteria for ICSI included non-ejaculated sperm, total sperm count <2.5 M/mL, motility <20 %, normal forms morphology <30 % (WHO criteria). ICSI was also performed when fertilization failure was documented in a prior cycle. All serum E2 measurements were recorded from the same laboratory using uniform equipment where assay sensitivity was 5 pmol/L and mean intra-assay CV was 9 %.
When transfer did not occur, no luteal support was given. Luteal support used vaginal progesterone pessaries 200 mg, p.v., twice daily (Cyclogest, Actavis; Devon, UK) or gel 1.125 g, p.v., twice daily (Crinone 8 %, Serono; London, UK) for 14 days post transfer. If the number of oocytes was less than 10, hCG (Pregnyl, Organon; Oss, Netherlands) 5000 IU, IM × 2 (day of transfer and 2 days after) was given. When more than 10 oocytes were collected, luteal phase hormonal support was with progesterone only.
The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and WHO revised glossary of ART terminology for IVF/ICSI outcomes were used, such that clinical pregnancy was defined as a pregnancy diagnosed by ultrasound visualization of one or more gestational sacs or definitive clinical signs of pregnancy, including ectopic pregnancies, with multiple gestational sacs counting as a single clinical pregnancy.
The following variables were recorded from all records for analysis: fertilization rate, cleavage rate, number of embryos, and outcome (no pregnancy vs. clinical pregnancy). Individual associations between demographic and clinical characteristics of patients according to E2 response were evaluated by Student’s t test (for parametric data), Mann-Whitney U test (for continuous non-parametric variables), and chi-square test for categorical variables, as appropriate. Univariate analysis was performed to identify significant individual associations between variables and clinical outcomes. Multiple logistic regression analysis was employed to control for potential confounders, where appropriate. Data were analyzed using SPSS v21.0; statistical significance was assigned to any p value <0.05.
Results
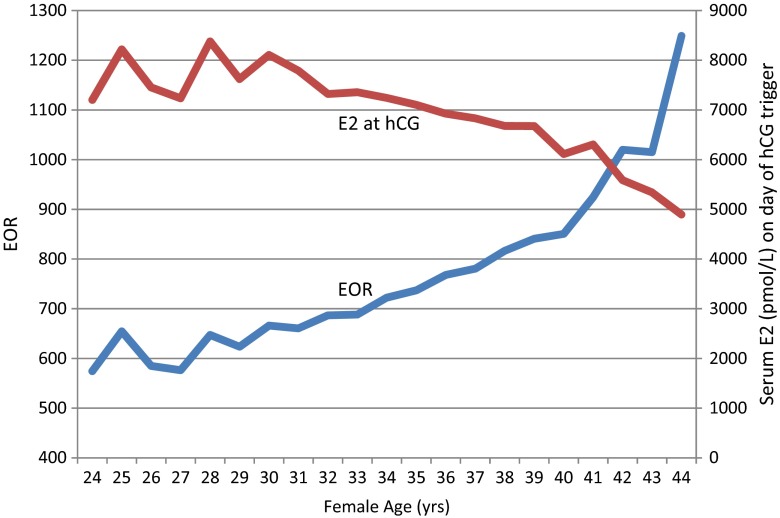
A total of 9,109 oocyte retrievals were performed for 5,499 patients during the study interval. The median (±SD) patient age was 35 ± 2.9 (range 18–47) years, mean serum E2 on day of hCG administration was 6950.7 (±3,865) pmol/L, and mean number of oocytes retrieved was 10.7. Of 9109 cycles, clinical pregnancy was achieved in approximately 32 %. Table 1 summarizes demographic and cycle characteristics according to EOR. In this population 63.4 % of transfers were performed on day 3 (n = 4926), while 36.6 % were carried out on day 5 (n = 2843). EOR increased dramatically for IVF patients above age 40. Furthermore, mean peak serum E2 level at hCG administration was inversely related to patient age; this was most notable for patients above age 33 (see Figs. 1 and 2).
Table 1.
Selected clinical characteristics and serum estradiol:oocyte ratio (EOR) as recorded in patients (n = 5499) completing treatment at Rotunda IVF, 2001–2012
| Age (years) | Day 3 FSH (IU/L) | Follicles punctured (n) | Oocytes recovered (n) | Gonadotropin dose (IU) | EOR |
|---|---|---|---|---|---|
| 34.40 ± 4.27 | 7.32 ± 2.66 | 13.99 ± 8.16 | 12.02 ± 7.54 | 2618.79 ± 1690.86 | <250 |
| 34.48 ± 4.01 | 7.57 ± 11.70 | 14.96 ± 6.98 | 13.28 ± 6.35 | 2396.12 ± 1836.71 | 250–500 |
| 34.97 ± 3.99 | 7.64 ± 3.36 | 13.85 ± 6.46 | 11.81 ± 5.64 | 2630.05 ± 1660.24 | 500–750 |
| 35.44 ± 4.00 | 8.44 ± 12.61 | 11.84 ± 5.77 | 9.67 ± 4.82 | 3066.75 ± 2344.01 | 750–1000 |
| 36.20 ± 3.83 | 8.76 ± 3.98 | 10.15 ± 5.42 | 7.98 ± 4.30 | 3648.54 ± 3093.69 | 100–1250 |
| 36.53 ± 4.09 | 9.48 ± 7.42 | 8.18 ± 4.68 | 5.86 ± 3.19 | 4182.06 ± 1920.17 | 1250–1500 |
| 36.99 ± 3.76 | 11.80 ± 27.04 | 7.33 ± 4.03 | 4.89 ± 2.39 | 4182.06 ± 1920.17 | 1500–1750 |
| 37.55 ± 4.02 | 10.99 ± 4.38 | 6.33 ± 4.27 | 3.85 ± 2.56 | 4290.50 ± 1694.87 | 1750–2000 |
| 37.50 ± 3.70 | 11.66 ± 5.33 | 5.63 ± 3.93 | 3.02 ± 2.57 | 4960.05 ± 1569.32 | >2000 |
Day 3 serum FSH measured on cycle day three
Fig. 1.
Serum E2 measured on day of hCG (red) and EOR (blue) as correlated with IVF patient age from 9109 oocyte retrievals, 2001–2012
Fig. 2.
Relationships among estradiol:oocyte ratio (EOR), patient age, and clinical pregnancy rates following 9109 oocyte retrievals, 2001–2012
Oocyte maturation
The percentage of oocytes at metaphase II was lowest (72.0 %) among patients with EOR < 250, and was highest in the group where EOR was greatest (81.8 % when EOR = 1750–2000, 81.9 % when EOR > 2000) as summarized in Table 2. Of note, the proportion of germinal vesicle stage oocytes was maximal in the group where EOR was lowest (11.2 % when EOR < 250).
Table 2.
Distribution of oocyte maturity as a function of serum estradiol:oocyte ratio (EOR), recorded from IVF retrievals (n = 9109), 2001–2012
| Oocyte maturity (%) | |||
|---|---|---|---|
| EOR | M II | M I | GV |
| <250 | 72.0 | 9.0 | 11.2 |
| 250–500 | 78.8 | 7.6 | 8.2 |
| 500–750 | 80.2 | 7.3 | 7.7 |
| 750–1000 | 80.8 | 6.8 | 6.9 |
| 1000–1250 | 80.5 | 7.0 | 7.5 |
| 1250–1500 | 79.6 | 7.5 | 7.6 |
| 1500–1750 | 80.0 | 7.9 | 7.5 |
| 1750–2000 | 81.8 | 7.6 | 10.1 |
| >2000 | 81.9 | 9.4 | 8.7 |
| p | <0.001 | <0.001 | <0.001 |
GV germinal vesicle, MI metaphase I, MII metaphase II
Fertilization rates
When fertilization rates were assessed with respect to EOR, a direct relationship was apparent: the lowest fertilization rates were found in the group where EOR was lowest (see Table 3), and this parameter increased with increasing EOR—until the EOR reached > 2000, at which point the fertilization rate declined. This pattern was also apparent for ICSI cases, with lowest fertilization measured when EORs were at the extremes of our reference groups (i.e., <250 and >2000). Moreover, the fertilization failure rate was highest when EOR was maximal >2000 (15.2 %).
Table 3.
Distribution of fertilization outcome as a function of serum estradiol:oocyte ratio (EOR), recorded from IVF retrievals (n = 9109), 2001–2012
| Fertilization outcome | |||
|---|---|---|---|
| EOR | IVF | ICSI | No 2pn |
| <250 | 51.6 | 68.4 | 6.7 |
| 250–500 | 58.2 | 70.5 | 1.3 |
| 500–750 | 59.9 | 71.0 | 1.4 |
| 750–1000 | 61.5 | 69.4 | 2.1 |
| 1000–1250 | 61.8 | 70.4 | 3.2 |
| 1250–1500 | 57.9 | 67.7 | 5.0 |
| 1500–1750 | 59.6 | 69.5 | 6.3 |
| 1750–2000 | 62.3 | 72.7 | 8.7 |
| >2000 | 54.4 | 67.7 | 15.2 |
| p | <0.001 | <0.001 | <0.001 |
IVF conventional insemination, ICSI intracytoplasmic sperm injection, No 2pn fertilization failure
Clinical pregnancy
As shown in Table 4, clinical pregnancy rates per egg retrieval in this population reached a peak at 36.1 % when EOR was 250–500 (p < 0.05, OR 1.19) and were lowest when EOR was highest (12.6 and 11.9 %) when serum E2 levels were 1750–2000 and >2000 pmol/L, respectively (p < 0.05, OR 1.37).
Table 4.
Reproductive outcome as a function of serum estradiol:oocyte ratio (EOR), recorded from IVF retrievals (n = 9109). During the study interval (2001–2012), overall clinical pregnancy rate for this population was 32 %
| EOR | CPR | OR | 95 % CI | p |
|---|---|---|---|---|
| <250 | 32.1 | 1.00 | 0.80–1.25 | 0.99 |
| 250–500 | 36.1 | 1.19 | 1.08–1.31 | 0.0003 |
| 500–750 | 35.6 | 1.17 | 1.08–1.28 | 0.0004 |
| 750–1000 | 31.7 | 1.00 | 0.91–1.14 | 0.75 |
| 1000–1250 | 27.4 | 0.78 | 0.68–0.94 | 0.0056 |
| 1250–1500 | 19.9 | 0.52 | 0.41–0.67 | <0.0001 |
| 1500–1750 | 17.2 | 0.43 | 0.39–0.62 | <0.0001 |
| 1750–2000 | 12.6 | 0.31 | 0.17–0.55 | <0.0001 |
| >2000 | 11.9 | 0.29 | 0.19–0.42 | <0.0001 |
CPR clinical pregnancy rate, OR odds ratio, CI confidence interval
EOR 250–750 vs. >1000
When reproductive outcome was evaluated as a function of EOR of 250–750 vs. EOR >1000, it was observed that, irrespective of age, the former ratio was more likely to result in clinical pregnancy with OR’s of 1.66, 1.94, and 1.82 for age groups <35, 35–39, and >40 years, respectively (see Table 5). Overall, patients in our study group with an EOR of 250–750 were twice as likely to achieve clinical pregnancy compared to patients with an EOR > 1000 (OR 2.04, 95% CI 1.80–2.31). Moreover, patients with an EOR of 250–750 were much less likely to encounter fertilization failure vs. those with EOR > 1000 irrespective of age, with rates of 1.4 vs. 6.4 % (p < 0.05, OR 0.22, 95 % CI 0.16–0.30). Follicular measurements and EOR observed on the day of hCG administration are summarized in Tables 6 and 7, while observed endometrial thickness measurements are presented in Table 8.
Table 5.
Comparison of reproductive outcome and fertilization efficiency as a function of serum estradiol:oocyte ratio (EOR) from 9109 IVF retrievals
Data for entire study population (not stratified by patient age) is shown in shaded cells
OR odds ratio, CI confidence interval
Table 6.
Selected treatment characteristics among patients completing IVF, 2001–2012
| Patient age (years) | ||||
|---|---|---|---|---|
| <35 n = 3779 |
35–39 n = 4022 |
≥40 n = 1308 |
||
| IVF | 2213 | 2303 | 758 | |
| IVF + ICSI | 1566 | 1718 | 550 | |
| Stimulation protocol | GnRH (long) agonist | 2862 | 3033 | 986 |
| GnRH antagonist | 497 | 585 | 187 | |
| GnRH flare | 380 | 347 | 116 | |
| Other | 40 | 42 | 14 | |
ICSI intracytoplasmic sperm injection, GnRH gonadotropin releasing hormone
Table 7.
Follicular measurements and EOR observed on day of hCG administration among IVF study patients, 2001–2012
| EOR | Follicles with mean diameter ≥17 mm, n (±SD) | Mean follicular diameter (mm)/oocyte, n (±SD) |
|---|---|---|
| <250 | 12.02 (7.54) | 0.87 (0.20) |
| 250–500 | 13.28 (6.35) | 0.90 (0.16) |
| 500–750 | 11.81 (5.64) | 0.86 (0.15) |
| 750–1000 | 9.67 (4.82) | 0.83 (0.16) |
| 1000–1250 | 7.98 (4.30) | 0.80 (0.17) |
| 1250–1500 | 5.86 (3.19) | 0.74 (0.18) |
| 1500–1750 | 4.89 (2.39) | 0.71 (0.20) |
| 1750–2000 | 3.85 (2.56) | 0.68 (0.25) |
| >2000 | 3.02 (2.57) | 0.55 (0.22) |
EOR estradiol:oocyte ratio, hCG human chorionic gonadotropin
Table 8.
Observed relation between endometrial measurements and EOR on day of hCG administration among IVF study patients, 2001–2012.
| EOR | Mean endometrial thickness (mm) on hCG day (±SD) |
|---|---|
| <250 | 10.84 (6.62) |
| 250–500 | 11.22 (4.72) |
| 500–750 | 11.04 (4.28) |
| 750–1000 | 11.33 (4.02) |
| 1000–1250 | 10.43 (2.87) |
| 1250–1500 | 10.44 (2.81) |
| 1500–1750 | 10.25 (3.32) |
| 1750–2000 | 10.01 (3.01) |
| >2000 | 10.07 (6.46) |
EOR estradiol:oocyte ratio
Discussion
The success of ART depends on several factors including female age, embryo quality, and endometrial receptivity. While E2 levels can serve as a marker of granulosa cell function and modulates endometrial development, studies of the relationship between serum E2 and pregnancy rates from IVF have yielded inconsistent results [8, 16–18].
In this retrospective study, the largest of its kind ever undertaken in Ireland, several observations are notable and provide support for previous investigations. We have demonstrated that serum E2 levels measured on the day of hCG administration are inversely proportional to patient age, and that the most marked decline is seen after the age of 33 years. Likewise, with increasing patient age, the quantity of oocytes retrieved decreases. In addition, as maternal age increased, a linear increase in EOR was observed as the number of oocytes retrieved declined (in contrast to simple E2 level measured on day of hCG administration).
Our research also contributes further evidence supporting what may be predicted during IVF: relatively low quality oocytes are more likely to be retrieved as EOR rises. Indeed, the frequency of immature oocytes is greatest when EOR is <250, where the highest percentage of oocytes at germinal vesicle stage maturity and the lowest percentage of oocytes reaching metaphase II are present. Our study also illustrates that the proportion of germinal vesicle stage cells retrieved during IVF increases when EOR is >1750 (although in this group, the percentage of oocytes reaching metaphase II stage did not similarly correlate, e.g., 81.8 % when EOR 1750–2000, and 81.9 % when EOR >2000). These data also show that, independent of patient age, clinical pregnancy rates correlate with the EOR. We suggest that an optimal EOR is in the range of 250–750 which resulted in a CPR of 35.8 %, compared to the mean clinical pregnancy rate of ∼32 % overall.
Early follicular phase E2 levels have been used to predict the outcome of COH cycles, such that serum E2 levels <100 pg/mL appear to have worse outcomes than those >100 [9]. While some authors have shown that EOR is important in a successful COH cycle [14, 18–20], generalizations have been tempered by limited sampling. Thus, it is not particularly surprising that an “ideal” or “target” EOR during IVF remains elusive. In the first study describing EOR in this setting, Loumaye et al. [14] suggested that the optimal EOR was 70–140 pg/mL per oocyte (250–500 pmol/oocyte).
Pena et al. [21] reported improved embryo quality with increasing E2 levels, resulting in improved pregnancy rates with IVF. Ozdegirmenci et al. [22] examined serum E2 level with respect to number of mature follicle and, while the results were not statistically significant, higher clinical pregnancy rates were observed when the serum E2:follicle ratio exceeded 540 pg/mL, while pregnancy results were impaired when the serum E2:follicle ratio was <250 pg/mL.
There are some weaknesses associated with our research, which should be acknowledged. For example, although this work reports on the largest IVF patient cohort ever tabulated from a single institution in Ireland (and possibly worldwide), it is limited in being a retrospective analysis. Also, due to constraints in data recording, how specific COH protocols may have affected serum E2 and various embryology parameters could not be assessed in this investigation. Moreover, it would have been interesting to correlate outcomes with serum E2 values throughout the follicular recruitment interval (not just on day of hCG administration), but unfortunately these data points were not uniformly recorded for all study patients. While we were able to capture data on endometrial dynamics near the time of fresh embryo transfer, other characteristics (e.g., homogeneous vs. trilaminar configuration) were not specifically tabulated. Finally, having either morphokinetic or cytogenetic data (i.e., from preimplantation screening) on embryos would also have added a further dimension to our research, but this technology was not available during the entire study interval.
In summary, we believe that EOR data can be a useful adjunct in predicting success rates of an IVF cycle. Additional observational research is needed to better characterize the relationships among serum E2 measurements, ultrasound data, embryo development, and reproductive outcome in IVF.
Compliance with ethical standards
As this research was a retrospective medical record audit and did not involve any direct patient contact, it was considered exempt from review by the Ethics Committee of Rotunda Hospital, Dublin.
Authors’ contributions
DAV was the principal investigator. He collected all data and was responsible for the study concept; CH was the research associate and contributed to the statistical analysis; ESS and EVM supervised the project and assisted with the manuscript development and revisions. All authors read and approved the final version of the work.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Capsule In the context of clinical IVF, EOR can provide useful information about ovarian response and follicular dynamics.
References
- 1.Padilla SL, Garcia JE. Effect of maternal age and number of in vitro fertilization procedures on pregnancy outcome. Fertil Steril. 1989;52:270–3. doi: 10.1016/s0015-0282(16)60854-3. [DOI] [PubMed] [Google Scholar]
- 2.Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87:53–9. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 3.Sharma V, Allgar V, Rajkhowa M. Factors influencing the cumulative conception rate and discontinuation of in vitro fertilization treatment for infertility. Fertil Steril. 2002;78:40–6. doi: 10.1016/S0015-0282(02)03160-6. [DOI] [PubMed] [Google Scholar]
- 4.Chuang CC, Chen CD, Chao KH, Chen SU, Ho HN, Yang YS. Age is a better predictor of pregnancy potential than basal follicle-stimulating hormone levels in women undergoing in vitro fertilization. Fertil Steril. 2003;79:63–8. doi: 10.1016/S0015-0282(02)04562-4. [DOI] [PubMed] [Google Scholar]
- 5.van Loendersloot LL, van Wely M, Limpens J, Bossuyt PM, Repping S, van der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–89. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 6.Hendriks DJ, Kwee J, Mol BW, te Velde ER, Broekmans FJ. Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87:764–75. doi: 10.1016/j.fertnstert.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Khairy M, Clough A, El-Toukhy T, Coomarasamy A, Khalaf Y. Antral follicle count at down-regulation and prediction of poor ovarian response. Reprod Biomed Online. 2008;17:508–14. doi: 10.1016/S1472-6483(10)60237-4. [DOI] [PubMed] [Google Scholar]
- 8.Phelps JY, Levine AS, Hickman TN, Zacur HA, Wallach EE, Hinton EL. Day 4 estradiol levels predict pregnancy success in women undergoing controlled ovarian hyperstimulation for IVF. Fertil Steril. 1999;69:1015–9. doi: 10.1016/S0015-0282(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 9.Khalaf Y, Taylor A, Brade P. Low serum estradiol concentrations after five days of controlled ovarian hyperstimulation for in vitro fertilization are associated with poor outcome. Fertil Steril. 2000;74:63–6. doi: 10.1016/S0015-0282(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 10.Garcia JE, Acosta AA, Hsiu JG, Jones HW., Jr Advanced endometrial maturation after ovulation induction with menopausal gonadotropin/human chorionic gonadotropin for in vitro fertilization. Fertil Steril. 1984;41:31–5. doi: 10.1016/s0015-0282(16)47536-9. [DOI] [PubMed] [Google Scholar]
- 11.Hadi FH, Chantler E, Anderson E, Nicholson R, McClelland RA, Seif MW. Ovulation induction and endometrial steroids receptors. Hum Reprod. 1994;9:2405–10. [PubMed] [Google Scholar]
- 12.Balasch J, Miró F, Burzaco I, Casamitjana R, Civico S, Ballescá JL, et al. The role of luteinizing hormone in human follicle development and oocyte fertility: evidence from in-vitro fertilization in a woman with long-standing hypogonadotrophic hypogonadism and using recombinant human follicle stimulating hormone. Hum Reprod. 1995;10:1678–83. doi: 10.1093/oxfordjournals.humrep.a136154. [DOI] [PubMed] [Google Scholar]
- 13.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Implantation; clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–7. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 14.Loumaye E, Engrand P, Howles CM, O’Dea L. Assessment of the role of serum luteinizing hormone and estradiol response to follicle stimulating hormone on in vitro fertilization treatment outcomes. Fertil Steril. 1997;67:889–99. doi: 10.1016/S0015-0282(97)81402-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang JH, Chen HF, Lien YR, Chen SI, Ho HN, Yang YS. Elevated E2:oocyte ratio in women undergoing IV and tubal ET. Correlation with a decrease in the implantation rate. J Reprod Med. 2001;46:434–8. [PubMed] [Google Scholar]
- 16.Orvieto R, Zohav E, Scharf S, Rabinson J, Meltcer S, Anteby EY, et al. The influence of estradiol/follicle and estradiol/oocyte ratios on the outcome of controlled ovarian stimulation for in vitro fertilization. Gynecol Endocrinol. 2007;23:72–5. doi: 10.1080/09513590601137137. [DOI] [PubMed] [Google Scholar]
- 17.Mocanu E, Redmond ML, Hennelly B, Collins C, Harrison R. Odds of ovarian hyperstimulation syndrome (OHSS)—time for reassessment. Hum Fertil (Camb) 2007;10:175–81. doi: 10.1080/14647270701194143. [DOI] [PubMed] [Google Scholar]
- 18.Sharara F, McClamrock HD. High estradiol levels and high oocyte yield are not detrimental to clinical outcome of in vitro ferilisation. Fertil Steril. 1999;72:401–5. doi: 10.1016/S0015-0282(99)00293-9. [DOI] [PubMed] [Google Scholar]
- 19.Ng EH, Lau EY, Yeung WS, Ho PC. Oocyte and embryo quality in patients with excessive ovarian response during in vitro fertilization treatment. J Assist Reprod Genet. 2003;20:186–91. doi: 10.1023/A:1023670010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of HCG administration and pregnancy achievement in IVF: a systematic review. Hum Reprod. 2004;19:2446–53. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]
- 21.Peña JE, Chang PL, Chan LK, Zeitoun K, Thornton MH, 2nd, Sauer MV. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod. 2002;17:83–7. doi: 10.1093/humrep/17.1.83. [DOI] [PubMed] [Google Scholar]
- 22.Ozdegirmenci O, Dilbaz S, Cinar O, Aydin S, Beydilli G, Cakir L, et al. Can serum oestradiol be a predictor of quality of oocytes and embryos, maturation of oocytes and pregnancy rate in ICSI cycles? Gynecol Endocrinol. 2011;27:279–85. doi: 10.3109/09513590.2010.491168. [DOI] [PubMed] [Google Scholar]