Abstract
Purpose
Blastocysts contain a large amount of fluid in the blastocoel, which may pose a risk for ice crystal formation during vitrification. This study aimed to evaluate the effectiveness of laser-induced artificial shrinkage of blastocoel before vitrification on clinical outcome.
Methods
Patients were divided into two groups: a control group with untreated, expanded blastocysts (n = 115) and a study group with blastocoel artificially eliminated by a laser pulse prior to vitrification (n = 309). Blastocyst survival, clinical pregnancy, and implantation rates were compared.
Result(s)
The survival rate was significantly higher in the study group compared with the control group (97.3 and 74.9 %, respectively; p > 0.01). The clinical pregnancy and implantation rates of the study group were significantly higher (p < 0.01) than that of the control group (clinical pregnancy, 67.2 vs. 41.1 %; implantation, 39.1 vs. 24.5 %.
Conclusion(s)
This study demonstrated that the removal of blastocoel fluid before vitrification by laser pulse of in vitro-produced human blastocysts significantly improves blastocyst survival, clinical pregnancy, and implantation rates.
Keywords: Vitrification, Artificial shrinkage, Blastocyst, Laser-pulse, Collapse, Blastocoel
Introduction
The first successful pregnancy obtained from frozen-thawed embryo transfer was reported by Trounson and Mohr in 1983 [1]. A year later, the first child was born after embryo freezing [2]. Since then, embryo cryopreservation has become a clinically established standard procedure in assisted reproductive technology (ART) [3].
Nowadays, approximately 31 % of embryo transfers utilize cryopreserved/warmed transfer cycles [4], and about 8 % of all ART babies are born from cryopreserved embryos [5]. Cryopreservation of human embryos has been employed in assisted human reproduction [3] using three primary methods: slow freezing, ultra rapid freezing, and vitrification [6]. Vitrification has many advantages over slow freezing and ultra-rapid freezing, including the total elimination of ice crystals [7], shorter exposure time to cryoprotectant agents, reduced chilling injuries, and cost-effectiveness [8]. Vitrification is also [3, 6] associated with a significantly lower cellular trauma [9] and higher post-warming survival, pregnancy, and implantation rates than with other cryopreservation techniques [10].
Many factors can influence the results of vitrification [11], including technical and embryonic parameters. Cryopreservation at different stages of embryonic development has contributed to variability in clinical outcome [12–15]. Zygotes [16–19], cleavage stage embryos [20, 21], and blastocysts [22–24] have been successfully vitrified, but superior pregnancy rate has been observed with blastocysts, possibly due to their size or multicellular structure [25]. However, expanded blastocysts with a large amount of fluid in the blastocoel have been shown to have lower survival rates than less mature blastocysts or morula-stage embryos [26]. The blastocoelic fluid may cause inadequate vitrification, less permeability to cryoprotectants, ice crystal formation, and cryodamage [26–28]. The volume of the blastocoel prior to vitrification negatively affects the outcome measures, [29] specifically re-expansion, cell proliferation, and DNA integrity [30]. To overcome this problem, pre-vitrification artificial shrinkage (AS) of the blastocyst to reduce the fluid volume in the blastocoelic cavity is performed with a micro-needle [26, 31], laser-pulse [27], repeated micropipetting with a hand-drawn Pasteur pipette [32], or microsuction of the blastocoelic contents [29, 33].
This study evaluated the survival rate and clinical outcome of vitrified blastocysts when AS of the blastocoel was applied prior to vitrification using a laser pulse.
Materials and methods
Patients
This retrospective study was conducted from June 2012 to May 2015 at a specialized fertility and gynecology center. The study was approved by the Institutional Review Board (IRB) of the center.
Four hundred and twenty-four vitrification–warm cycles were evaluated, and only patients with blastocysts vitrified on day 5 and subsequently warmed for transfer were included. All vitrified blastocysts were left over after a fresh transfer cycles, “freeze all” cycles were excluded. Patients were divided into two groups: a control group with untreated, expanded blastocysts (n = 115) and a study group with blastocoeles artificially collapsed by a laser pulse prior to vitrification (n = 309). Survival rate (percentage of surviving embryos among all frozen/thawed embryos), clinical pregnancy rate (percentage of clinical pregnancies, pregnancies identified by fetal cardiac activity on ultrasound examination 4 weeks after embryo transfer, and the number of patients undergoing embryo transfer), and implantation rate (the number of intrauterine gestational sacs over the total number of embryos transferred) were compared between the two groups.
Stimulation protocol for ICSI
Ovaries were stimulated by a gonadotropin-releasing hormone (Lucrin®, Abbott, France) in the mid-luteal phase of the previous cycle. The administration of recombinant follicle-stimulating hormone (FSH; Gonal-F®, Serono, Switzerland) started on day 3 of the cycle, accompanied by human menopausal gonadotropin (hMG; Menogon®, Ferring Pharmaceuticals, Wittland, Germany) according to the follicle size and hormonal levels. Ovulation was induced by injection of 250 μg/0.5 mL of recombinant human chorionic gonadotropin (rhCG; Ovitrelle®, Serono, Switzerland).
Embryo culture and grading of blastocysts
Oocyte retrieval was performed 36 h after rhCG administration and oocytes were inseminated by intracytoplasmic sperm injection (ICSI). Fertilization was assessed 15–18 h after insemination by the presence of two pronuclei. At 46–48 h post-injection, cleaved embryos were graded and transferred to blastocyst culture media (Multiblast Medium®, Irvine Scientific®, USA). After day 5 of embryo transfer, all of the patient’s remaining blastocysts were vitrified. Blastocyst freeze criteria included AA, AB, and BA embryos that reached stage 3 or 4 on day 5 of culture according to the Gardner and Schoolcraft grading system [34]. Only day 5, blastocysts were included in this study, and all day 6 blastocysts were excluded.
AS of expanded blastocysts
Laser pulse shrinkage of expanded blastocysts was done using Mukaida’s method [27]. Using an OCTAX 1.48 μM laser (MTG, Germany), a single laser pulse (300 μs) created an opening in the zona pellucida at the cellular junction of the trophectoderm cells located far away from the inner cell mass (ICM). After 5–8 min, the blastocoel completed shrinkage and the collapsed blastocyst was immediately vitrified and stored in a liquid nitrogen (LN2) tank at −196 °C.
Vitrification procedure
Vitrification was performed with a Vitrification Freeze Kit (Vit Kit®-Freeze, Irvine Scientific, USA) according to the manufacturer’s instructions.
Two to three blastocysts were placed into a 50 μL drop of equilibration solution (ES) for 6–10 min at room temperature, and then were transferred into a 50 μL drop of vitrification solution (VS) for 30 s before loading. The blastocysts were loaded onto a McGill Cryoleaf™ (Origio, Denmark) with a minimal volume of VS and immediately plunged into LN2. The Cryoleaf was then placed into the LN2 tank for long-term storage.
Warming procedure
Warming was performed with the Vitrification Thaw Kit (Vit Kit®-Thaw, Irvine Scientific, USA) according to the manufacturer’s instructions. The Cryoleaf contents were dispensed in a 50 μL warmed thawing solution (TS) drop at 37 °C for 1 min. Then the blastocysts were transferred to a 50 μL dilution solution (DS) drop for 4 min, and finally, blastocysts were washed in two drops of washing solution (WS) for 4 min each.
Culture and transfer of post-warm blastocysts
Blastocysts were transferred to a pre-equilibrated culture dish containing microdrops of MultiBlast Medium supplemented with 20 % (v/v) Serum Substitute Solution (SSS™, Irvine Scientific, USA). Embryo transfer was performed 30–60 min after warming using an embryo transfer catheter (Labotect, Göttingen, Germany) under ultrasound guidance.
Statistics
The data were tabulated and analyzed using the computer program SPSS (Statistical package for social science) version 16. The statistical comparison between the two groups was tested using the student’s t test. A p value of <0.05 was considered statistically significant.
Results
A total of 424 vitrification–warm cycles, containing 1110 human blastocysts, were performed in this investigation. AS of 663 blastocysts from 309 vitrification–warm cycles by laser pulse prior to vitrification was compared to 447 untreated control blastocysts from 115 vitrification–warm cycles.
Table 1 summarizes the patient demographic characteristics, the survival rate, and the clinical outcomes of vitrified human blastocysts with AS (study group) and vitrified human blastocysts without AS (control group). No statistically significant differences were found in the demographic data between the two groups, including age, BMI, and number of previous ICSI attempts (p > 0.05). The post-warming survival rate of embryos in the study group was significantly higher compared to the control group (p < 0.01).
Table 1.
A comparison of retrospective data of vitrified day 5 blastocysts without AS (control group) and with AS (study group)
| Study group (n = 309) | Control group (n = 115) | t | p value | |
|---|---|---|---|---|
| Female age | 30.8 ± 4.43 | 30.4 ± 5.04 | 0.795 | >0.05 |
| BMI | 25.5 ± 2.23 | 25.4 ± 1.74 | 0.45 | >0.05 |
| No. of previous ICSI attempts | 2.1 ± 0.77 | 2.09 ± 0.78 | 0.118 | >0.05 |
| No. of blastocyst vitrified | 663 | 447 | – | – |
| Survival rate (%) | 97.28 ± 9.1 | 74.9 ± 18.7 | 16.47 | <0.01 |
| Mean No. of blastocyst transferred | 2.07 ± 0.84 | 2.10 ± 0.76 | 0.339 | >0.05 |
| Clinical pregnancies (%) | 67.21 ± 23.4 | 39.13 ± 21.6 | 11.21 | <0.01 |
| Implantation rate/embryos transferred (%) | 41.08 ± 11.8 | 24.477 ± 8.99 | 13.68 | <0.01 |
AS artificial shrinkage, BMI body mass index, ICSI intracytoplasmic sperm injection
P < 0.05 was considered to be significant when compared with the control group
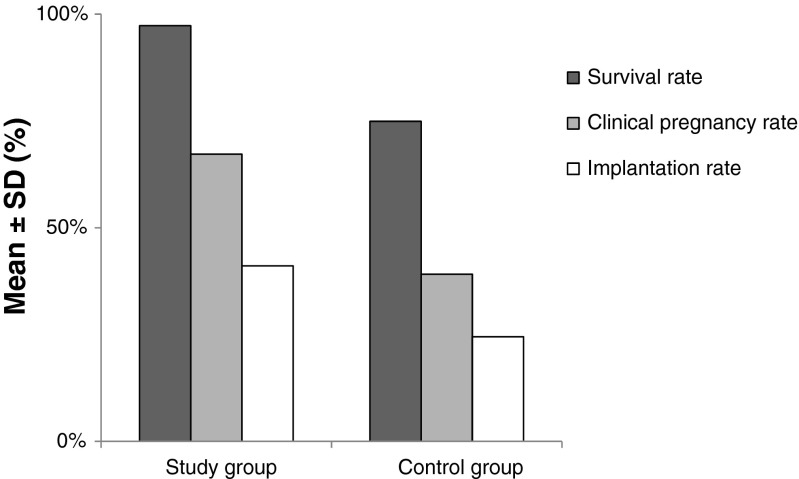
The mean number of blastocysts transferred was similar between the two groups (study group 2.07 ± 0.84; control group 2.09 ± 0.78). Figure 1 shows the clinical outcome of vitrified blastocysts transferred in the study. The clinical pregnancy rate of vitrified-warmed blastocysts in the study group (67.2 %) was significantly higher than the control group (39.1 %; p < 0.01). Also, the implantation rate of the study group (41.1 %) was statistically higher than the control group (24.5 %, p < 0.01).
Fig. 1.
Effect of artificial shrinkage by laser pulse treatment prior to vitrification of human blastocysts on survival, clinical pregnancy, and implantation rates (p < 0.01)
Discussion
Laser technology is a simple, efficient, and precise cellular microsurgery [35, 36] tool that has been used in ART in recent years. In this study, a short duration laser pulse was directed once at the junction between the two trophectoderm cells in a region away from the ICM to reduce the blastocoelic fluid before vitrification. During laser pulsing, the laser beam generates a heat effect [37, 38] when it hits the trophectoderm and may cause local damage and direct injury [39]. However, this damage may be less than the damage from not using AS. Desai et al. [30] showed that blastocysts vitrified without AS had significantly more damage (13 % dead cells) compared to blastocysts with AS (5 % dead cells); this may be due to insufficient dehydration of the blastocoelic cavity and a slower rate of recovery/re-expansion of non-collapsed control blastocysts. This is in agreement with several studies with encouraging data showing significant improvements in embryo survival, pregnancy, and implantation rates after applying several AS methods prior to vitrification [26, 31–33].
Only a few studies have been reported focusing on the laser pulse method of AS to date. Mukaida et al. [27] reported significant improvement in survival rate and pregnancy rates in 40 blastocysts collapsed by laser pulse prior to vitrification compared to a retrospective control group; additionally, the delivery of healthy babies confirmed the safety of the procedures. Data collected by Iwayama et al. [40] after vitrification found a significant increase in implantation rate from 34.2 to 59.7 % following AS treatment by laser pulse. Cao et al. [39] found that survival rates and pregnancy rates of 208 blastocysts subjected to laser pulse AS pre-vitrification were improved, and that the laser pulse group was associated with a significantly higher hatching rate and lower premature birth rate compared with the 29-gauge needle AS group.
Our data from 424 vitrification–warm cycles showed that the survival rate and the clinical outcome measures were significantly higher in the study group compared to the control group, confirming minimal deleterious effect of blastocyst vitrification combined with laser pulse AS. Our data also confirms that the high survival rate following warming (97.28 %) following pre-vitrification AS by laser pulse of expanded blastocysts did not adversely affect the vitality of human embryos. The high pregnancy rate and high implantation capacity of survived embryos proved that there was no impact from trophectoderm cell damage caused by laser pulse prior to vitrification. In agreement with the previous studies, this study showed that blastocoelic fluid reduction prior vitrification could enhance the efficacy of vitrification. Our findings also provide further support for the ease and safety of laser pulse AS.
In conclusion, this study provides evidence that vitrification of artificially collapsed blastocyst by laser pulse is safe, effective, and more efficient than vitrification of non-collapsed blastocyst in terms of survival, clinical pregnancy, and implantation rates. However, the confirmation of these findings requires further prospective studies to adequately judge the efficiency of AS as a routine protocol prior vitrification.
Acknowledgments
The authors would like to thank TopLab Company for financial support (Grant no. 001005).
Footnotes
Capsule This study demonstrated that the removal of blastocoel fluid before vitrification by laser pulse of in vitro-produced human blastocysts significantly improves blastocyst survival, clinical pregnancy, and implantation rates.
References
- 1.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;26:707–9. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 2.Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril. 1984;42:293–6. doi: 10.1016/s0015-0282(16)48029-5. [DOI] [PubMed] [Google Scholar]
- 3.Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–93. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan EA, Zegers-Hochschild F, Mansour R, Ishihara O, Mouzon J, Nygren KG, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod. 2013;28(5):1375–90. doi: 10.1093/humrep/det036. [DOI] [PubMed] [Google Scholar]
- 5.Camus M. Human embryo cryopreservation: review of clinical issues related to the success rate. Proceedings of Symposium on “Cryobiology and Cryopreservation on Human Gametes and Embryos” ESHRE Campus 2004. Brussels, Belgium, 12th to 13th March 2004; 24–26.
- 6.AbdelHafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online. 2010;20(2):209–22. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65:236–44. doi: 10.1016/j.theriogenology.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12(6):779–96. doi: 10.1016/S1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 9.Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23(9):1976–82. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- 10.Kasai M, Mukaida T. Cryopreservation of animal and human embryos by vitrification. Reprod Biomed Online. 2004;9:164–70. doi: 10.1016/S1472-6483(10)62125-6. [DOI] [PubMed] [Google Scholar]
- 11.Kader AA, Choi A, Orief Y, Agarwal A. Factors affecting the outcome of human blastocyst vitrification. Reprod Biol Endocrinol. 2009;7:99. doi: 10.1186/1477-7827-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavone ME, Innes J, Hirshfeld-Cytron J, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Hum Reprod Sci. 2011;4:23–8. doi: 10.4103/0974-1208.82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salumets A, Tuuri T, Mäkinen S, Vilska S, Husu L, Tainio R, et al. Effect of developmental stage of embryo at freezing on pregnancy outcome of frozen–thawed embryo transfer. Hum Reprod. 2003;18:1890–5. doi: 10.1093/humrep/deg339. [DOI] [PubMed] [Google Scholar]
- 14.Noyes N, Reh A, McCaffrey C, Tan O, Krey L. Impact of developmental stage at cryopreservation and transfer on clinical outcome of frozen embryo cycles. Reprod Biomed Online. 2009;19:9–15. doi: 10.1016/S1472-6483(10)60279-9. [DOI] [PubMed] [Google Scholar]
- 15.Surrey E, Keller J, Stevens J, Gustofson R, Minjarez D, Schoolcraft W. Freeze-all: enhanced outcomes with cryopreservation at the blastocyst stage versus pronuclear stage using slow-freeze techniques. Reprod Biomed Online. 2010;21:411–7. doi: 10.1016/j.rbmo.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hasani S, Ozmen B, Koutlaki N, Schoepper B, Diedrich K, Schultze-Mosgau A. Three years of routine vitrification of human zygotes: is it still fair to advocate slow-rate freezing? Reprod Biomed Online. 2007;14:288–93. doi: 10.1016/S1472-6483(10)60869-3. [DOI] [PubMed] [Google Scholar]
- 17.Liebermann J, Tucker MJ, Graham JR, Han T, Davis A, Levy MJ. Blastocyst development after vitrification of multipronucleate zygotes using the flexipet denuding pipette (FDP) Reprod Biomed Online. 2002;4:146–50. doi: 10.1016/S1472-6483(10)61932-3. [DOI] [PubMed] [Google Scholar]
- 18.Jelinkova L, Selman HA, Arav A, Strehler E, Reeka N, Sterzik K. Twin pregnancy after vitrification of 2-pronuclei human embryos. Fertil Steril. 2002;77:412–4. doi: 10.1016/S0015-0282(01)02992-2. [DOI] [PubMed] [Google Scholar]
- 19.Hoover L, Baker A, Check JH, Lurie D, Summers D. Clinical outcome of cryopreserved human pronuclear stage embryos resulting from intracytoplasmic sperm injection. 1997; 67(4): 621–624 [DOI] [PubMed]
- 20.El-Danasouri I, Selman H. Successful pregnancies and deliveries after a simple vitrification protocol for day 3 human embryos. Fertil Steril. 2001;76:400–2. doi: 10.1016/S0015-0282(01)01907-0. [DOI] [PubMed] [Google Scholar]
- 21.Mukaida T, Wada S, Takahashi K, Pedro PB, An TZ, Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13:2874–9. doi: 10.1093/humrep/13.10.2874. [DOI] [PubMed] [Google Scholar]
- 22.Liebermann J. Vitrification of human blastocysts: an update. Reprod Biomed Online. 2009;19:105–14. doi: 10.1016/S1472-6483(10)61073-5. [DOI] [PubMed] [Google Scholar]
- 23.Liebermann J, Tucker MJ. Comparison of vitrification and conventional cryopreservation of day 5 and day 6 blastocysts during clinical application. Fertil Steril. 2006;86(1):20–6. doi: 10.1016/j.fertnstert.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Reed ML, Lane M, Gardner DK, Jensen NL, Thompson J. Vitrification of human blastocysts using the Cryoloop method: successful clinical application and birth of offspring. J Assist Reprod Genet. 2002;19:304–6. doi: 10.1023/A:1015789532736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Azawia T, Tavukcuoglua S, Khakib AA, Al HS. Current data on the vitrification of human embryos: which one is the best; zygote, cleavage or blastocyst stage? Middle East Fertil Soc J. 2013;18:223–32. doi: 10.1016/j.mefs.2012.10.008. [DOI] [Google Scholar]
- 26.Vanderzwalmen P, Bertin G, Debauche C, Standaert V, van Roosendaal E, Vandervorst M, et al. Births after vitrification at morula and blastocyst stages: effect of artificial reduction of the blastocoelic cavity before vitrification. Hum Reprod. 2002;17:744–51. doi: 10.1093/humrep/17.3.744. [DOI] [PubMed] [Google Scholar]
- 27.Mukaida T, Oka C, Goto T, Takahashi K. Artificial shrinkage of blastocoeles using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod. 2006;21:3246–52. doi: 10.1093/humrep/del285. [DOI] [PubMed] [Google Scholar]
- 28.Cho HJ, Son WY, Yoon SH, Lee SW, Lim JH. An improved protocol for dilution of cryoprotectants from vitrified human blastocysts. Hum Reprod. 2002;17:2419–22. doi: 10.1093/humrep/17.9.2419. [DOI] [PubMed] [Google Scholar]
- 29.Chen SU, Lee TH, Lien YR, Tsai YY, Chang LJ, Yang YS. Microsuction of blastocoelic fluid before vitrification increased survival and pregnancy of mouse expanded blastocysts, but pretreatment with the cytoskeletal stabilizer did not increase blastocyst survival. Fertil Steril. 2005;84:1156–62. doi: 10.1016/j.fertnstert.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 30.Desai N, Szeptycki J, Scott M, AbdelHafez FF, Goldfarb J. Artificial collapse of blastocysts before vitrification: mechanical vs. laser technique and effect on survival, cell number, and cell death in early and expanded blastocysts. Cell Preserv Technol. 2008;6:181–90. doi: 10.1089/cpt.2008.0007. [DOI] [Google Scholar]
- 31.Son WY, Yoon SH, Yoon HJ, Lee SM, Lim JH. Pregnancy outcome following transfer of human blastocysts vitrified on electron microscopy grids after induced collapse of the blastocoele. Hum Reprod. 2003;18:137–9. doi: 10.1093/humrep/deg029. [DOI] [PubMed] [Google Scholar]
- 32.Hiraoka K, Kinutani M, Kinutani K. Blastocoele collapse by micropipetting prior to vitrification gives excellent survival and pregnancy outcomes for human day 5 and 6 expanded blastocysts. Hum Reprod. 2004;19(12):2884–8. doi: 10.1093/humrep/deh504. [DOI] [PubMed] [Google Scholar]
- 33.Kader A, Sharma RK, Falcone T, Agarwal A. Mouse blastocyst previtrification interventions and DNA integrity. Fertil Steril. 2010;93(5):1518–25. doi: 10.1016/j.fertnstert.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Toward reproductive certainty: fertility and genetics beyond 1999. UK: Parthenon Publishing London; 1999. pp. 378–88. [Google Scholar]
- 35.Taylor T, Gilchrist J, Hallowell S, Hanshew K, Orris J, Glassner M, et al. The effects of different laser pulse lengths on the embryo biopsy procedure and embryo development to the blastocyst stage. J Assist Reprod Genet. 2010;27:663–7. doi: 10.1007/s10815-010-9461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelmassih S, Cardoso J, Abdelmassih V, Dias JA, Abdelmassih R, Nagy ZP. Laser-assisted ICSI: a novel approach to obtain higher oocyte survival and embryo quality rates. Hum Reprod. 2002;17(10):2694–9. doi: 10.1093/humrep/17.10.2694. [DOI] [PubMed] [Google Scholar]
- 37.Berns M, Salet C. Laser microbeams for partial cell irradiation. Int Rev Cytol. 1972;33:131–54. doi: 10.1016/S0074-7696(08)61450-3. [DOI] [PubMed] [Google Scholar]
- 38.Berns M. A possible two-photon effect in vitro using a focused laser beam. Biophys J. 1976;16:973–7. doi: 10.1016/S0006-3495(76)85747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao S, Zhao C, Zhang J, Wu X, Guo X, Ling X. Retrospective clinical analysis of two artificial shrinkage methods applied prior to blastocyst vitrification on the outcome of frozen embryo transfer. J Assist Reprod Genet. 2014;31(5):577–81. doi: 10.1007/s10815-014-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwayama H, Hochi S, Yamashita M. In vitro and in vivo viability of human blastocysts collapsed by laser pulse or osmotic shock prior to vitrification. J Assist Reprod Genet. 2011;28(4):355–61. doi: 10.1007/s10815-010-9522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]