Abstract
Purpose
The objective of this study was to test the hypothesis that ovarian kisspeptin (kiss1) and its receptor (kiss1r) expression are affected by age, obesity, and the age- and obesity-related chemokine monocyte chemoattractant protein-1 (MCP-1).
Methods
Ovaries from reproductive-aged and older C57BL/6J mice fed normal chow (NC) or high-fat (HF) diet, ovaries from age-matched young MCP-1 knockout and young control mice on NC, and finally, cumulus and mural granulosa cells (GCs) from women who underwent in vitro fertilization (IVF) were collected. Kiss1, kiss1r, anti-Mullerian hormone (AMH), and AMH receptor (AMHR-II) messenger RNA (mRNA) expression levels were quantified using real-time polymerase chain reaction (RT-PCR).
Results
In mouse ovaries, kiss1 and kiss1r mRNA levels were significantly higher in old compared to reproductive-aged mice, and diet-induced obesity did not alter kiss1 or kiss1r mRNA levels. Compared to young control mice, young MCP-1 knockout mice had significantly lower ovarian kiss1 mRNA but significantly higher AMH and AMHR-II mRNA levels. In human cumulus GCs, kiss1r mRNA levels were positively correlated with age but not with BMI. There was no expression of kiss1 mRNA in either cumulus or mural GCs.
Conclusion
These data suggest a possible age-related physiologic role for the kisspeptinergic system in ovarian physiology. Additionally, the inflammatory MCP-1 may be associated with kiss1 and AMH genes, which are important in ovulation and folliculogenesis, respectively.
Keywords: Kisspeptin, MCP-1, Ovary, Obesity, Aging
Introduction
The excitatory neuropeptide kisspeptin (kiss1) and its G protein-coupled receptor (kiss1r) are essential for the regulation of gonadotropin-releasing hormone (GnRH) neurons and puberty [1–3]. Additional actions of kiss1 at the level of the ovaries have been suggested [3–6] but remain scarcely studied. Recent data have shown that the intraovarian kiss1 system is required for properly coordinated ovarian function in humans and animals [3–6]. For example, kiss1r hypomorph (i.e., gene with reduced level of activity) mice displayed a premature decline in ovulatory rate, followed by progressive loss of antral follicles, oocyte loss, and a reduction in all categories of pre-antral follicles [3]. Additionally, in vivo administration of the kiss1 antagonist P234 to the ovarian bursa of 22- to 50-day-old rats delayed vaginal opening and disrupted estrous cyclicity [4]. It was also demonstrated that kisspeptin-10 peptide stimulates progesterone secretion in cultured chicken granulosa cells, a phenomenon that was associated with upregulation of the steroidogenic enzymes: steroidogenic acute regulatory protein (StAR), P450 cholesterol side-chain cleavage (P450scc), and 3 beta-hydroxysteroid dehydrogenase (3β-HSD). Altogether, these findings indicate that kiss1 is locally produced inside the ovaries and that it is involved in normal ovulatory process.
Reproductive senescence, in particular, ovarian aging, remains poorly understood in humans. We and others have documented that reproductive aging is characterized by reduced hypothalamic kiss1 expression [7, 8]. On the other hand, women with diminished ovarian reserve have a significant increase in ovarian sympathetic nerve fibers [9] whose stimulation with β-adrenergic agonist induces ovarian kiss1 expression [4]. Given this background, we hypothesized that ovarian kisspeptin expression is upregulated during ovarian aging.
There is mounting evidence that suggests that hypothalamic kiss1 could be affected by adiposity [10]. For instance, rats subjected to food deprivation demonstrated a significant decrease in kiss1 expression in the hypothalamus leading to a reduction in luteinizing hormone (LH) levels [11, 12]. Likewise, diet-induced obese female DBA/2J mice also showed a marked decrease in kiss1 messenger RNA (mRNA) levels in both the arcuate and the anteroventral periventricular nucleus areas of the hypothalamus compared with normal chow (NC)-fed controls [13]. However, given the newly discovered role of the kiss1 system in the ovaries, it remains to be determined whether ovarian kiss1 expression is affected by obesity.
One of the inflammatory molecules that plays a role in the human ovulatory process and relates to both aging and obesity [14–19] is monocyte chemoattractant protein-1 (MCP-1) [20]. MCP-1, a polypeptide composed of 76 amino acids, is produced by various cell types including endothelial cells, fibroblasts, tumor cells, monocytes, and macrophages [20, 21]. It regulates the migration and infiltration of monocytes, basophils, T lymphocytes, and natural killer cells into various tissues, including the ovaries [22]. In humans, physiologic levels of MCP-1 are important for the ovulatory process [20]. As far as aging and obesity, data have shown that systemic MCP-1 levels are increased in aging animals [14–16] and that there is an increased abundance of this pro-inflammatory chemokine in both white adipose tissue and plasma in obese animals [17–19]. Additionally, we have recently demonstrated in women undergoing in vitro fertilization (IVF) that serum and follicular fluid MCP-1 are positively correlated with body mass index (BMI) (Obehi et al, manuscript under review). Taken together, data to date suggest that the pro-inflammatory MCP-1 might play a role in age- and obesity-related ovarian dysfunction hypothetically by attracting large number of monocytes into the ovaries. Despite these findings, data pertaining to the effect of this age- and obesity-related chemokine on ovarian kiss1 expression and other genes important in follicular development (such as anti-Mullerian hormone [AMH] and AMH receptor [AMHR-II]) are lacking. Because kisspeptin and MCP-1 share a similar intracellular pathway, a relationship between these two molecules could potentially exist. At the hypothalamic level, kisspeptin signals via phosphorylation of MAP kinases, such as ERK1/2 and p38 [23, 24]. Similarly, MCP-1 signals via the same MAP kinases, ERK1/2 and p38, in various tissues [25, 26].
Using a high-fat (HF) diet-induced mouse model of obesity and comparing reproductive-aged mice to older mice, we aimed to evaluate the influence of aging and obesity on the expression of ovarian kiss1/kiss1r system. We also used an MCP-1 knockout mouse model in order to determine whether MCP-1 affects kiss1/kiss1r and AMH/AMHR-II. We thus compared ovarian kiss1, kiss1r, AMH, and AMHR-II gene expression between age-matched wild-type and MCP-1 knockout mice.
In order to determine whether alterations in the kisspeptinergic system are occurring at the level of the granulosa cells (GCs), we evaluated in the current studies kiss1/kiss1r expression in human luteinized GCs obtained from women who underwent IVF. We and others have used human luteinized GCs as a long-standing effective model to characterize ovarian physiology in humans [27–31]. Given the new role of kiss1 in ovarian function, it is important to evaluate whether changes in kiss1/kiss1r expression occur in human GCs with aging and obesity.
Materials and methods
Experimental animals and diets
All protocols were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine and University of Vermont College of Medicine.
Old and reproductive-aged mice on different diets
C57BL/6J wild-type female mice were obtained from Jackson Laboratory (Bar Harbor, ME) at 5 weeks of age. Mice were housed five to a cage and maintained on a 14-h light, 10-h dark cycle with ad libitum access to standard mouse chow and water. After 1 week of acclimation, mice (n = 12) were assigned to receive a normal chow diet (PicoLab® Mouse Diet #5058, chemical composition = 9 % fat, 20 % protein, 53 % carbohydrate, 3.59 kcal/g; LabDiet, St. Louis, MO, USA), while another group of mice (n = 12) received high-fat (HF) diet (Diet #F3282, chemical composition = 36 % fat, 20.5 % protein, 35.7 % carbohydrate, 5.49 kcal/g; Bioserve, Frenchtown, NJ, USA). All mice were weighed weekly in order to ensure that mice given HF diet became obese.
Six mice on normal chow and six mice on HF diet were sacrificed at 20 weeks (reproductive-aged group). The other six mice in the normal chow group continued normal chow while the remaining six mice on HF diet were converted to normal chow and both groups were sacrificed at 32 weeks (older group) by cervical dislocation. At the time of sacrifice, oophorectomy was performed and the ovary was snap frozen in liquid nitrogen and stored in −80 °C to be used for gene expression analysis.
Young MCP-1 knockout mice versus young control mice
Young MCP-1 knockout mice (B6.129S4-Ccl2tm1Rol/J) (5–6 weeks old; n = 6) and young C57BL/6J wild-type control female mice (5–6 weeks old; n = 5) were housed five to a cage and bred in house under specific pathogen-free conditions and normal light dark cycles (14-h light, 10-h dark cycle) with ad libitum access to standard mouse chow (ProLab® Isopro®, chemical composition = 5 % fat, 22 % protein, 59 % carbohydrate, 3.46 kcal/g; PMI Nutrition International, Brentwood, MO, USA) and water. All mice were sacrificed at 6 weeks of age by cervical dislocation. At the time of sacrifice, oophorectomy was performed and ovaries were snap frozen in liquid nitrogen and stored in −80 °C to be used for gene expression analysis.
Subjects
Fourteen infertile women undergoing fresh IVF and/or intracytoplasmic sperm injection (ICSI) cycles utilizing autologous oocytes at the University of Vermont College of Medicine (UVM COM) in July and August 2013 were prospectively enrolled. Inclusion criteria consisted of women with normal ovarian reserve defined as day 3 follicle-stimulating hormone (FSH) <10 mIU/mL and day 3 estradiol (E2) <80 pg/mL. Reasons for infertility were male, tubal, unexplained, and uterine factors. Women with polycystic ovary syndrome (PCOS) as defined by the Rotterdam criteria [32] were excluded from the study. All patients gave informed consent and the study was approved by the Institutional Review Board of UVM COM (M13-062).
Collection of GCs
Follicular aspirates included mural GCs and oocytes surrounded by cumulus GCs. The follicular fluid from the first aspirated follicle was not used for gene expression evaluation because of possible contamination with vaginal mucosal cells. After removal of the cumulus–oocyte complex by the embryologist, fluid was pooled from each participant in order to isolate mural GCs.
Mural GCs were isolated and concentrated as we previously described [27]. Briefly, mural GCs were added to 40 % PureCeption gradient solution (Cooper Surgical, Trumbull, CT) then centrifuged to remove red and white blood cells. Cells were then washed with phosphate-buffered saline (PBS) and incubated with CD 45+ tagged magnetic beads (Invitrogen, Carlsbad, CA) for 20 min at +4 °C to remove the remaining white blood cells. The beads were then separated, and the remaining fluid was centrifuged for 5 min at 600×g to collect the mural GCs.
After identification of the cumulus–oocyte complex in the aspirate, cumulus GCs were collected by mechanically cutting the cumulus cell layer from each oocyte then washed with PBS. Cumulus GCs collected from each participant were also pooled. The collected mural and cumulus GCs were added on the same day to a tube containing TRIzol reagent (Invitrogen, Carlsbad, CA) then stored at −20 °C to be used for gene expression analysis.
RNA extraction, reverse transcription, and real-time polymerase chain reaction from mural and cumulus GCs as well as mice ovaries
Mice ovaries were lysed and homogenized using a homogenizer. For mice ovaries and for GCs, RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) and chloroform extraction using QIAGEN’s RNeasy mini kit according to the manufacturer’s instructions. RNA quality analysis was assessed by a Nanodrop spectrophotometer and Agilent Bioanalyzer (Santa Clara, CA). Only samples with a minimum concentration of 10 ng/μL and with an optical density (OD) 260:280 ratio of 1.8 to 2.0 were used for real-time polymerase chain reaction (RT-PCR). The RNA quality was additionally confirmed using RNA electrophoresis. mRNA expression levels were measured by RT-PCR kinetics using the SYBR Green I chemistry (Roche, Indianapolis, IN) as described elsewhere [27]. The primers used (Table 1) were synthesized by Fisher (Pittsburgh, PA). For human GCs, kiss1 and kiss1r were evaluated. For mouse ovaries, kiss1 and kiss1r, as well as AMH and its receptor AMHR-II (these two genes play a role in ovarian folliculogenesis) were evaluated. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used as a loading control and the levels of mRNA for each gene relative to GAPDH were calculated using the 2−ΔΔCT method [33].
Table 1.
Primers used for RT-PCR in mouse ovaries and human granulosa cells
| Gene | Sequence primers (5′–3′) |
|---|---|
| Mice | |
| Kiss1 | Forward: ATGATCTCAATGGCTTCTTGG Reverse: CCAGGCATTAACGAGTTCCT |
| Kiss1r | Forward: GCACATGCAGACAGTTACCAA Reverse: CACGCAGCACAGTAGGAAAGT |
| AMH | Forward: CGTCACCGCAGCCAGCACA Reverse: CCCGCAGAGCACGAACCAAG |
| AMHR-II | Forward: CCACAGACCACCACCTTTCC Reverse: GTCTGCGTCCCAGCAATCTT |
| Humans | |
| Kiss1 | Forward: TGGGGAATTCTAGACCCACAGG Reverse: GTAGCAGCTGGCTTCCTCTC |
| Forward: GGACCTGCCTCTTCTCACCAA Reverse: TGCTGGCCTGTGGGTCTAGA | |
| Kiss1r | Forward: GGGAACTCGCTGGTCATCTA Reverse: GTTGACGAACTTGCACATGAA |
Kiss1 kisspeptin, Kiss1r kisspeptin receptor, AMH anti-Mullerian hormone, AMHR-II AMH receptor
Statistics
Data were expressed as mean ± SEM if normally distributed and median (range) if not normally distributed. RT-PCR results were expressed as relative number of copies ± SEM. Mann-Whitney U test or t test was used as appropriate according to the data distribution. Because the data were not normally distributed, Spearman correlation was used to evaluate the association between kiss1r and clinical parameters in humans. Because data on kiss1r mRNA was not normally distributed, log transformation was performed, followed by multivariate linear regression to test the correlation between age and logkiss1r in cumulus GCs after adjusting for BMI, total days of ovarian stimulation, and total dose of gonadotropins (IUs). All statistical procedures were run on STATA software (StataCorp). p ≤ 0.05 was considered statistically significant.
Results
Effect of age- and diet-induced obesity on kiss1 and kiss1r mRNA in mice
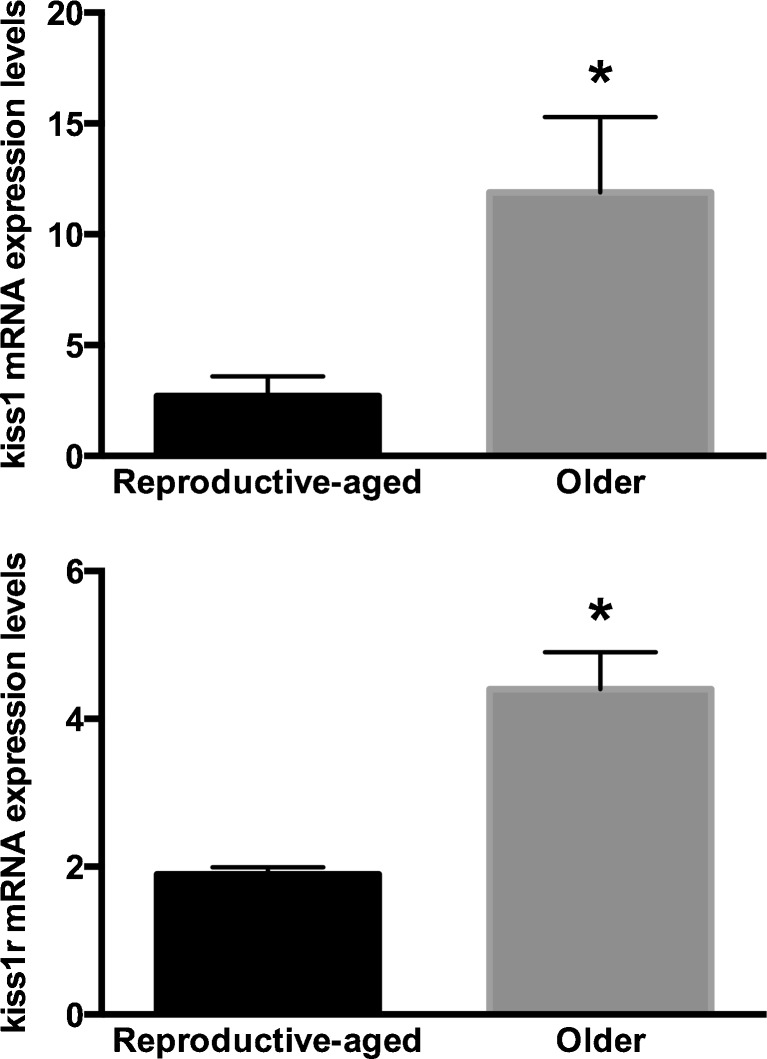
Mice on HF diet gained (from 15.7 ± 0.2 g at baseline to 25.8 ± 1.4 g) significantly more weight than mice on normal chow diet (from 15.1 ± 0.2 g at baseline to 18.4 ± 0.3 g; p = 0.0001) after a 12-week feeding period (Fig. 1). Regardless of diet and weight, mRNA expression levels of kiss1 and kiss1r were significantly higher in older compared to reproductive-aged mice (Fig. 2). There was no difference in kiss1 mRNA expression between reproductive-aged mice on normal chow (n = 4) compared with HF diet (n = 4) (2.7 ± 0.9 vs. 3.7 ± 0.2, respectively; p = 0.25) or between older mice on normal chow (n = 5) compared with older mice on HF converted to normal chow diet (n = 5) (11.9 ± 3.4 vs. 19.1 ± 8.4, respectively; p = 0.9). Similarly, there was no difference in kiss1r mRNA expression between reproductive-aged mice on normal chow compared with HF diet (1.9 ± 0.1 vs. 2.3 ± 0.5; p = 0.77) or between older mice on normal chow compared with older mice on HF converted to normal chow diet (4.4 ± 0.5 vs. 7.5 ± 1.5; p = 0.1).
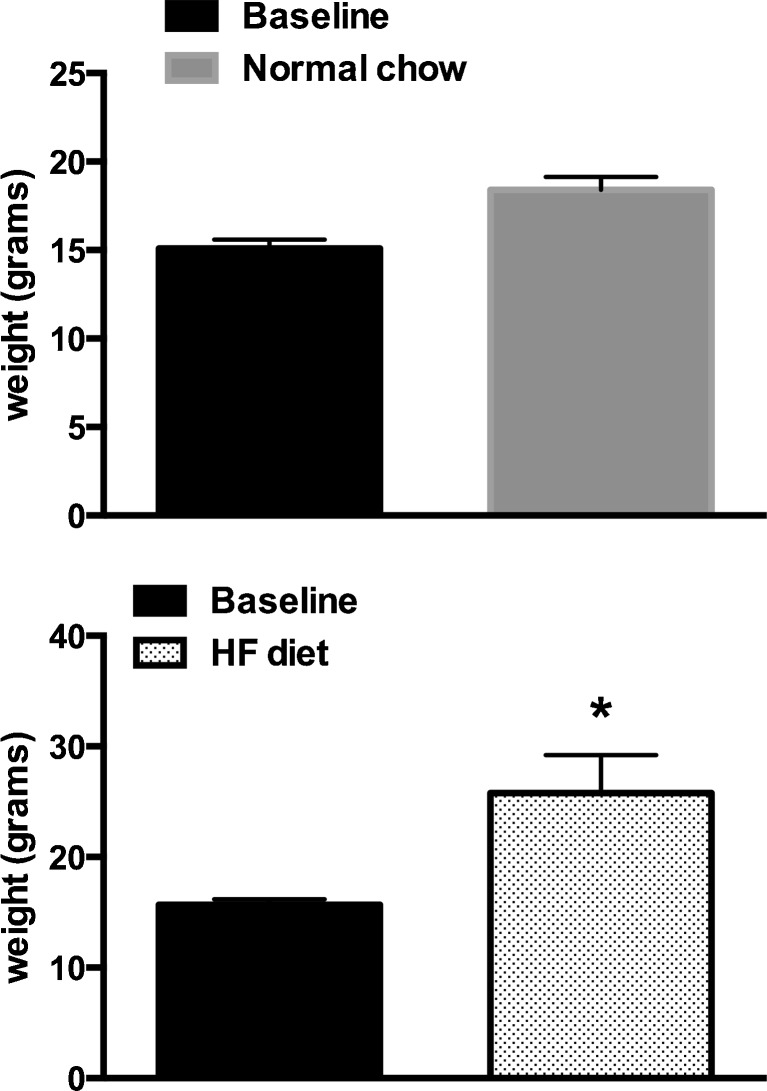
Fig. 1.
Weight gain following high-fat (HF) diet. Mice on HF diet (n = 6) gained significantly more weight than mice on normal chow diet (n = 6) after a 12-week feeding period. Baseline represents 6-week-old mice. *p < 0.05
Fig. 2.
Ovarian kiss1 and kiss1r expression in relatively reproductive-aged and older mice. Six mice on normal chow or six mice on high-fat diet had oophorectomy at 20 weeks (reproductive-aged group). The remaining six mice in the NC group continued NC while the remaining six on HF diet were converted to NC and both groups had oophorectomy at 32 weeks (older group) of age. Kiss1 and kiss1r mRNA expression levels were significantly higher in older (n = 10) compared to reproductive-aged mice (n = 8). Diet-induced weight gain did not affect kiss1 or kiss1r mRNA expression levels. *p < 0.05
Effect of MCP-1 knockout on kiss, kiss1r, AMH, and AMHR-II mRNA in mice
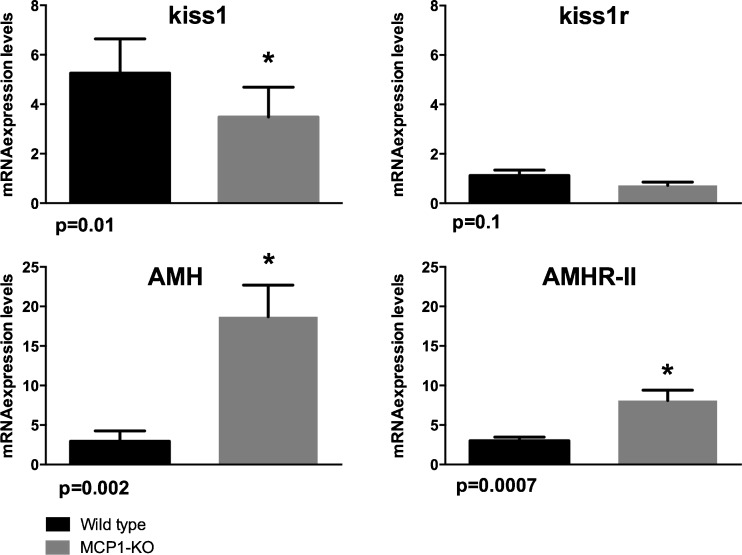
Young MCP-1 knockout mice (n = 6) on normal chow diet had similar weights compared to young wild-type mice controls (n = 5) on normal chow diet (p > 0.05). Compared to control mice, MCP-1 knockout mice had significantly lower ovarian kiss1 mRNA expression levels (p = 0.01) and had a tendency for lower kiss1r mRNA expression levels (p = 0.1) (Fig. 3). On the other hand, MCP-1 knockout mice had significantly higher AMH (p = 0.02) and AMHR-II (p = 0.0007) compared to control mice (Fig. 3).
Fig. 3.
Ovarian kiss1, kiss1r, AMH, and AMHR-II mRNA expression levels in young MCP-1 knockout and young wild-type mice. Young MCP-1 knockout mice and young C57BL/6J wild-type female mice had oophorectomy at 6 weeks of age. Compared to wild-type mice (n = 5), MCP-1 KO mice (n = 6) had significantly lower ovarian kiss1 mRNA expression levels (p = 0.01) but significantly higher AMH (p = 0.02) and AMHR-II mRNA expression levels (p = 0.0007). *p < 0.05
Humans
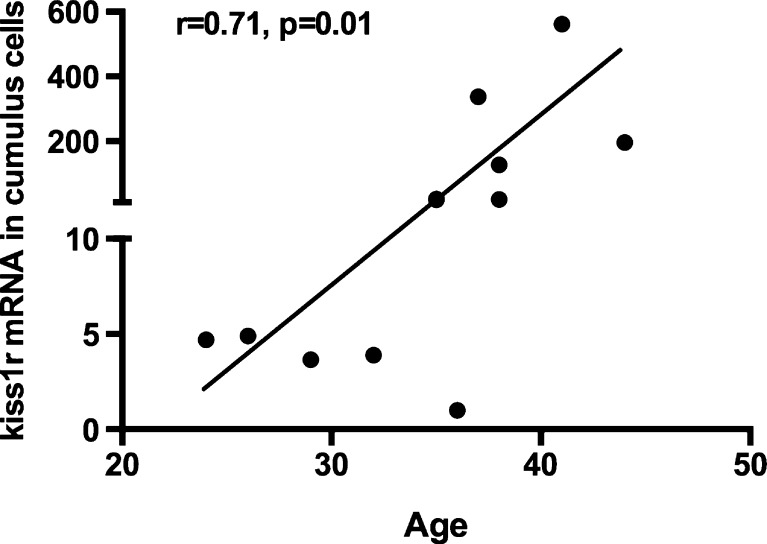
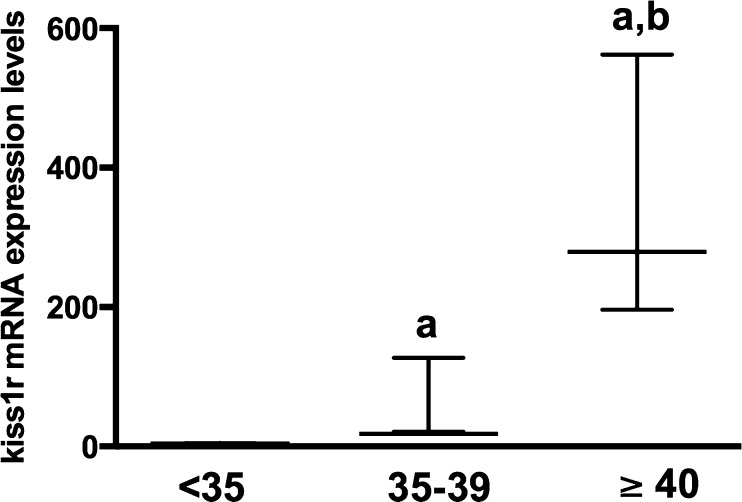
Table 2 summarizes the demographics and clinical characteristics of the participants. The age of the participants ranged from 19 to 42. There was no amplification of kiss1 mRNA in our samples despite the use of several types of primers (placental tissue was used as a positive control) and despite previous data indicating its expression in non-luteinized GCs [34]. The kiss1r mRNA levels in cumulus, not mural, GCs were positively correlated with age (r = 0.71, p = 0.01; n = 11 mRNA samples) (Fig. 4). In a multivariate linear regression model adjusting for BMI, total days of ovarian stimulation, and total dose of gonadotropins (IUs), we measured age as a predictor and logkiss1r mRNA in cumulus GCs as the outcome variable of interest. The higher the age, the higher the kiss1r mRNA levels in cumulus GCs mRNA levels (p = 0.047). In cumulus GCs, women aged less than 35 (n = 4) had significantly lower kiss1r mRNA expression level compared to women aged between 35 and 39 (n = 6) who, in turn, had significantly lower kiss1r mRNA expression levels compared to women aged 40 or more (n = 2) (4.3 [range = 3.8–4.8], vs. 18 [range = 21–127] vs. 379 [range = 196–562], respectively; p = 0.004) (Fig. 5).
Table 2.
Demographics and clinical characteristics of the participants
| All participants (n = 14) | Mean ± SEM | Correlation with mural kiss1r | p value | Correlation with cumulus kiss1r | p value |
|---|---|---|---|---|---|
| Age (years) | 34.6 ± 1.5 | −0.4 | 0.3 | 0.7 | 0.01* |
| BMI (kg/m2) | 28.5 ± 1.9 | −0.3 | 0.4 | −0.1 | 0.7 |
| Day 3 FSH (mIU/mL) | 4.7 ± 0.5 | −0.1 | 0.9 | 0.1 | 0.8 |
| Day 3 E2 (pg/mL) | 48.2 ± 9.1 | 0.8 | 0.1 | −0.3 | 0.6 |
| Total days of ovarian stimulation | 11.4 ± 0.5 | 0.3 | 0.5 | −0.3 | 0.3 |
| Peak E2 on day of HCG trigger (pg/mL) | 1854.0 ± 193.5 | 0.2 | 0.7 | 0.06 | 0.8 |
| Total gonadotropins dose, IUs | 2590.0 ± 304.8 | −0.5 | 0.2 | 0.3 | 0.3 |
| Total number of oocytes retrieved | 16.0 ± 2.3 | −0.4 | 0.3 | −0.1 | 0.8 |
BMI body mass index, E2 estradiol, FSH follicle-stimulating hormone, HCG human chorionic gonadotropin, IU international unit, Kiss1r kisspeptin receptor
*p = 0.047 in a multivariate linear regression model adjusting for BMI, total days of ovarian stimulation, and total dose of gonadotropins (IUs)
Fig. 4.
Correlation between age and kiss1r mRNA expression levels in cumulus granulosa cells (GCs) collected from women who underwent in vitro fertilization. Cumulus GCs were collected by mechanically cutting the cumulus cell layer from each oocyte following oocyte retrieval. Kiss1r mRNA levels were positively correlated with age
Fig. 5.
Stratification by age of kiss1r mRNA expression levels in cumulus granulosa cells (GCs) collected from women who underwent in vitro fertilization. Cumulus GCs were collected by mechanically cutting the cumulus cell layer from each oocyte following oocyte retrieval. Women aged 35 (n = 4) had significantly lower kiss1r mRNA expression level compared to women aged between 35 and 39 (n = 6) who had significantly lower kiss1r mRNA expression levels compared to women aged ≥ 40 (n = 2). Data are described as median (range). a p < 0.05 compared to <35; b p < 0.05 compared to 35–39
In both cumulus and mural GCs, there was no correlation between kiss1r mRNA levels and BMI, baseline day 3 FSH and E2 levels, the total number of days of ovarian stimulation, peak E2 level on the day of HCG maturation trigger, the total dose of gonadotropins administered during the cycle, or the total number of oocytes retrieved (Table 2).
Discussion
The present study evaluated the influence of age and diet-induced obesity on kiss1 and kiss1r mRNA expression levels in mouse ovaries. It also assessed the effect of knocking down MCP-1 gene on ovarian kiss1/kiss1r and AMH/AMHR-II mRNA levels. In order to determine whether alterations in the kisspeptinergic system occur at the level of the GCs, the relationship between age and BMI on kiss1r expression in human cumulus and mural GCs was evaluated. First, the results indicated that kiss1 and kiss1r expression levels in mice were markedly elevated with aging, but were not affected by high-fat, diet-induced obesity. Second, kiss1r mRNA expression levels in human cumulus GCs were positively correlated with age, but were not correlated with BMI. These results support the hypothesis that, in addition to its role in the central nervous system, the ovarian kisspeptinergic system may affect ovarian function in older women. Third, MCP-1, a chemokine important for ovulatory function (when present at normal physiologic levels) and abnormally elevated with aging and obesity, is involved in the regulation of ovarian kiss1 and ovarian AMH/AMHR-II.
Cumulus GCs play a vital role in regulating oocyte maturation, and the health of the cumulus cell determines oocyte quality [35, 36]. Although not fully defined yet, cumulus GCs express characteristics distinct from mural GCs, which are steroidogenically more active [37, 38]. Cumulus cells communicate with each other and with the oocyte through specialized gap junctions that allow metabolic exchange and transport of signaling molecules for appropriate follicular development [35, 36, 39]. Interestingly, cumulus and mural GCs have been shown to reflect the characteristics of the oocyte thus providing a noninvasive means to assess oocyte quality [35]. Our current findings suggest that in an infertile population, age-related upregulation in kiss1r expression may alter kisspeptin sensitivity in human cumulus GCs indicating a possible age-related physiologic role, similar to that observed in the hypothalamus [7, 8], for the kisspeptin system in human follicular dynamics. These findings were supported by current data from mice where old mice had significantly higher kiss1 and kiss1r in their ovaries compared to younger reproductive-aged mice. Interestingly, kisspeptin has been shown to decrease the recruitment of follicles by decreasing their sensitivity to FSH (via downregulating FSHR) and thus participates in the reduction of developing follicles observed during aging [40]. As of today, data are still controversial as to which compartment in the ovaries kiss1 is mainly expressed. Although a study by Zhou et al. [41] showed that the major site of kiss1 expression is the theca and interstitial stroma cells, a recent study by Laoharatchatathanin et al. [42] demonstrated that kiss1 is mainly expressed in granulosa cells. Although in our study, kiss1 was not detected in granulosa cells, this could be due to the fact that we have studied luteinized granulosa cells that might behave differently than those that are not luteinized. Taking into account this limitation, further studies are needed to localize the increase in kiss1/kiss1r expression in mammalian ovaries during aging.
We have previously shown in humans that obesity has a negative impact on ovarian function, in particular on genes important in follicular development and ovarian reserve [27, 43–48]. In the current study, we did not find an association between BMI and kisspeptin expression in human GCs and we did not find that diet-induced obesity affected ovarian kiss1/kiss1r expression in mice. This is in contrast to a study by Zhou et al. [41] who demonstrated that HF diet-induced obesity caused a marked suppression of ovarian kiss1 mRNA levels in mice at postnatal days 42 and 70 compared with normal-weight mice on normal chow diet. One explanation for this discrepancy is the fact that we studied mice aged 20 and 32 weeks whose ovaries might be more resistant to insult by HF diet-induced obesity. Interestingly, our findings demonstrated that young MCP-1 knockout mice had suppression in kiss1 mRNA levels, suggesting that MCP-1 gene could be necessary for ovarian kiss1 gene expression which is important in ovulation, further supporting the physiologic role of MCP-1 in the ovulatory process [20].
AMH and AMHR-II genes are important in follicular development and upregulation in these two genes are usually observed in women with ovulatory dysfunction, particularly those with PCOS [30]. Our findings demonstrated that young MCP-1 knockout mice had significantly higher ovarian AMH and AMHR-II mRNA expression levels compared to young wild-type mice. These findings further suggest that MCP-1 may play a role in normal follicular development and ovulation. Understanding the role of MCP-1 in ovarian function may further our understanding of the pathophysiology of ovulatory dysfunction observed in cases where there is upregulation of AMH and/or AMHR-II, such as in PCOS [30].
This study has several limitations. Although our results showed that diet-induced obesity did not affect ovarian kiss 1 or kiss1r mRNA expression levels, the sample size could have been small to detect a difference, if any existed. The luteinized GCs were collected from women who were hyperstimulated with gonadotropins. Although this model may not be the best for studying the ovarian kisspeptinergic system, we [27] and others [30] have shown that GCs could represent a reliable model for studying ovarian physiology and that GCs are responsive to kisspeptin treatment in vitro [34]. Another limitation is that we studied kiss1 and kiss1r expression in the whole mouse ovary, which represents a mixture of different types of cells including granulosa, theca, and stromal cells. In order to bypass this limitation, future experimental work in animals should evaluate separate ovarian compartments. Although MCP-1 knockout mice were used, future experiments should evaluate ovary-specific MCP-1 knockout model to better evaluate the role of this pro-inflammatory marker in ovarian function. Finally, only mRNA expression was evaluated, which might not represent the corresponding protein expression.
In conclusion, alterations in kisspeptin sensitivity in human GCs might provide more insight in understanding the function of ovarian kisspeptin signaling and its implications in ovarian biology. Additionally, the data from the mouse model call for further investigation into the role of the ovarian kisspeptinergic system and ovarian MCP-1 in folliculogenesis, steroidogenesis and ovulation. Given that ovarian kiss1r expression increases with age, future directions should evaluate the response of this receptor to the administration of kisspeptin peptide antagonist (such as peptide 234) in vitro and in vivo and whether this antagonist could reverse some of the features of ovarian aging. It is possible that the rise in adrenergic activity in the ovary that occurs naturally with aging provokes the increase in ovarian kisspeptin [4, 9]. One would expect that the upregulation of kiss1r with age might be due to a decline is systemic plasma kisspeptin levels. However, plasma kisspeptin levels have been shown to be constant in pre- and post-menopausal women [49, 50], thus refuting this hypothesis. Therefore, with age and similar to what happens in the hypothalamus, kiss1r expression changes significantly in the ovary and the sensitivity of the receptors to kisspeptin peptide might unveil some of the mechanisms in reproductive aging. Finally, data pertaining to the role of kisspeptin in assisted reproductive technology are rising. Indeed, the administration of kisspeptin-54 peptide has been used to effectively and safely trigger oocyte maturation in women undergoing IVF treatment [51]. Additionally, kisspeptin and kiss1r might be involved in the fertilization process in the female reproductive tract since the rate of IVF success in animals significantly decreased after treatment of sperm with the kisspeptin antagonist peptide 234 [52]. The pharmacologic manipulation of kisspeptin receptor could represent a potential therapeutic aid in human reproduction.
Compliance with ethical standards
Funding
Grant from Ferring Pharmaceuticals Inc. to Z.M. and the American Diabetes Association to M.J.C.
Conflict of interest
None
Footnotes
Capsule
These data suggest a possible age-related physiologic role for the kisspeptinergic system in ovarian physiology.
References
- 1.Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12:631–9. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- 2.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009. [DOI] [PMC free article] [PubMed]
- 3.Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M, Castellano JM, Dissen GA, et al. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure in spite of preserved gonadotropin secretion. Endocrinology. 2014;en20141110. [DOI] [PMC free article] [PubMed]
- 4.Ricu MA, Ramirez VD, Paredes AH, Lara HE. Evidence for a celiac ganglion-ovarian kisspeptin neural network in the rat: intraovarian anti-kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology. 2012;153:4966–77. doi: 10.1210/en.2012-1279. [DOI] [PubMed] [Google Scholar]
- 5.Dorfman MD, Garcia-Rudaz C, Alderman Z, Kerr B, Lomniczi A, Dissen GA, et al. Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology. 2014;en20141111. [DOI] [PMC free article] [PubMed]
- 6.Gaytan F, Gaytan M, Castellano JM, Romero M, Roa J, Aparicio B, et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009;296:E520–31. doi: 10.1152/ajpendo.90895.2008. [DOI] [PubMed] [Google Scholar]
- 7.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, et al. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–20. doi: 10.1016/j.neuropharm.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heider U, Pedal I, Spanel-Borowski K. Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertil Steril. 2001;75:1141–7. doi: 10.1016/S0015-0282(01)01805-2. [DOI] [PubMed] [Google Scholar]
- 10.Wahab F, Shahab M, Behr R. The involvement of gonadotropin inhibitory hormone and kisspeptin in the metabolic regulation of reproduction. J Endocrinol. 2015;225:R49–66. doi: 10.1530/JOE-14-0688. [DOI] [PubMed] [Google Scholar]
- 11.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–25. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki T, Iwasa T, Kinouchi R, Yoshida S, Murakami M, Gereltsetseg G, et al. Fasting reduces the kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr J. 2011;58:1003–12. doi: 10.1507/endocrj.K11E-131. [DOI] [PubMed] [Google Scholar]
- 13.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–50. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slimani H, Zhai Y, Yousif NG, Ao L, Zeng Q, Fullerton DA, et al. Enhanced monocyte chemoattractant protein-1 production in aging mice exaggerates cardiac depression during endotoxemia. Crit Care. 2014;18:527. doi: 10.1186/s13054-014-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–9. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R, Kapila R, Haq MR, Salingati V, Kapasiya M, Kapila S. Age-associated aberrations in mouse cellular and humoral immune responses. Aging Clin Exp Res. 2014;26:353–62. doi: 10.1007/s40520-013-0190-y. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 18.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JP, Leung BP, Ding YY, Tay L, Ismail NH, Yeo A, et al. Monocyte chemoattractant protein-1: a proinflammatory cytokine elevated in sarcopenic obesity. Clin Interv Aging. 2015;10:605–9. doi: 10.2147/CIA.S78901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahm-Kahler P, Ghahremani M, Lind AK, Sundfeldt K, Brannstrom M. Monocyte chemotactic protein-1 (MCP-1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil Steril. 2009;91:231–9. doi: 10.1016/j.fertnstert.2007.07.1330. [DOI] [PubMed] [Google Scholar]
- 21.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. doi: 10.1016/S0065-2776(08)60509-X. [DOI] [PubMed] [Google Scholar]
- 22.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytokine Res: Off J Int Soc Interf Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terasaka T, Otsuka F, Tsukamoto N, Nakamura E, Inagaki K, Toma K, et al. Mutual interaction of kisspeptin, estrogen and bone morphogenetic protein-4 activity in GnRH regulation by GT1-7 cells. Mol Cell Endocrinol. 2013;381:8–15. doi: 10.1016/j.mce.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castano JP, Martinez-Fuentes AJ, Gutierrez-Pascual E, Vaudry H, Tena-Sempere M, Malagon MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides. 2009;30:10–5. doi: 10.1016/j.peptides.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Yang CQ, Li W, Li SQ, Li J, Li YW, Kong SX, et al. MCP-1 stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol. 2014;34:266–76. doi: 10.1159/000362997. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Zheng Y, Li T, Wang Q, Qian J, Lu Y, et al. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget. 2015;6:24218–29. doi: 10.18632/oncotarget.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S, Jr, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28:1661–9. doi: 10.1093/humrep/det072. [DOI] [PubMed] [Google Scholar]
- 28.Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;jc20134161. [DOI] [PMC free article] [PubMed]
- 29.Merhi Z, Irani M, Doswell AD, Ambroggio J. Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): a potential indicator of ovarian reserve. J Clin Endocrinol Metab. 2014;99:E226–33. doi: 10.1210/jc.2013-3839. [DOI] [PubMed] [Google Scholar]
- 30.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–61. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 31.Pierre A, Peigne M, Grynberg M, Arouche N, Taieb J, Hesters L, et al. Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod. 2013;28:762–9. doi: 10.1093/humrep/des460. [DOI] [PubMed] [Google Scholar]
- 32.Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91:786–9. doi: 10.1210/jc.2005-2501. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Ortega J, Pinto FM, Fernandez-Sanchez M, Prados N, Cejudo-Roman A, Almeida TA, et al. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum Reprod. 2014;29:2736–46. doi: 10.1093/humrep/deu247. [DOI] [PubMed] [Google Scholar]
- 35.Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99:979–97. doi: 10.1016/j.fertnstert.2013.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–16. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Vanderhyden BC, Tonary AM. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol Reprod. 1995;53:1243–50. doi: 10.1095/biolreprod53.6.1243. [DOI] [PubMed] [Google Scholar]
- 38.Eppig JJ, Chesnel F, Hirao Y, O’Brien MJ, Pendola FL, Watanabe S, et al. Oocyte control of granulosa cell development: how and why. Hum Reprod. 1997;12:127–32. [PubMed] [Google Scholar]
- 39.Simerman AA, Hill DL, Grogan TR, Elashoff D, Clarke NJ, Goldstein EH, et al. Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2015;103:249–57. doi: 10.1016/j.fertnstert.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandois DD, Na EK, Cuevas FC, Cruz G, Lara H, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2015. [DOI] [PubMed]
- 41.Zhou Q, Chen H, Yang S, Li Y, Wang B, Chen Y, et al. High-fat diet decreases the expression of Kiss1 mRNA and kisspeptin in the ovary, and increases ovulatory dysfunction in postpubertal female rats. Reprod Biol Endocrinol. 2014;12:127. doi: 10.1186/1477-7827-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod. 2015;93:15. doi: 10.1095/biolreprod.115.127902. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JJ, Feret M, Chang L, Yang M, Merhi Z. Obesity adversely impacts the number and maturity of oocytes in conventional IVF not in minimal stimulation IVF. Gynecolo Endocrinol: Off J Int Soc Gynecol Endocrinol. 2015;31:409–13. doi: 10.3109/09513590.2015.1014785. [DOI] [PubMed] [Google Scholar]
- 44.Merhi ZO. Impact of bariatric surgery on female reproduction. Fertil Steril. 2009;92:1501–8. doi: 10.1016/j.fertnstert.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 45.Merhi ZO. Weight loss by bariatric surgery and subsequent fertility. Fertil Steril. 2007;87:430–2. doi: 10.1016/j.fertnstert.2006.07.1499. [DOI] [PubMed] [Google Scholar]
- 46.Merhi Z, Polotsky AJ, Bradford AP, Buyuk E, Chosich J, Phang T, et al. Adiposity alters genes important in inflammation and cell cycle division in human cumulus granulosa cell. Reprod Sci. 2015;22:1220–8. doi: 10.1177/1933719115572484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merhi Z, McGee EA, Buyuk E. Role of advanced glycation end-products in obesity-related ovarian dysfunction. Minerva Endocrinol. 2014;39:167–74. [PubMed] [Google Scholar]
- 48.Moy V, Jindal S, Lieman H, Buyuk E. Obesity adversely affects serum anti-mullerian hormone (AMH) levels in Caucasian women. J Assist Reprod Genet. 2015. [DOI] [PMC free article] [PubMed]
- 49.Kanasaki H, Purwana IN, Oride A, Mijiddorj T, Sukhbaatar U, Miyazaki K. Circulating kisspeptin and pituitary adenylate cyclase-activating polypeptide (PACAP) do not correlate with gonadotropin serum levels. Gynecolo Endocrinol: Off J Int Soc Gynecol Endocrinol. 2013;29:583–7. doi: 10.3109/09513590.2013.788624. [DOI] [PubMed] [Google Scholar]
- 50.Peng J, Xu H, Yang B, Hu J, Zhang BP, Zou L, et al. Plasma levels of kisspeptins in postmenopausal Chinese women do not show substantial elevation. Peptides. 2010;31:2255–8. doi: 10.1016/j.peptides.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Abbara A, Jayasena CN, Christopoulos G, Narayanaswamy S, Izzi-Engbeaya C, Nijher GM, et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab. 2015;100:3322–31. doi: 10.1210/jc.2015-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu MC, Wang JY, Lee YJ, Jong DS, Tsui KH, Chiu CH. Kisspeptin modulates fertilization capacity of mouse spermatozoa. Reproduction (Camb Engl) 2014;147:835–45. doi: 10.1530/REP-13-0368. [DOI] [PubMed] [Google Scholar]