Abstract
Magnetic resonance elastography (MRE), an advanced MR-based imaging technique, and acoustic radiation force impulse (ARFI), an ultrasound-based imaging technique, have been shown to be accurate for diagnosing nonalcoholic fatty liver disease (NAFLD) fibrosis. However, no head-to-head comparisons between MRE and ARFI for diagnosing NAFLD fibrosis have been performed. We aimed to compare MRE versus ARFI head-to-head for diagnosing fibrosis in well-characterized patients with biopsy-proven NAFLD.
Methods
This cross-sectional analysis of a prospective cohort involved 125 patients (54.4% female) who underwent MRE, ARFI, and contemporaneous liver biopsies scored using the Nonalcoholic Steatohepatitis Clinical Research Network histological scoring system. MRE versus ARFI’s performances for diagnosing fibrosis were evaluated using area under receiver operating characteristic curves (AUROCs).
Results
The mean (±SD) age and BMI were 48.9 (±15.4) years and 31.8 (±7.0) kg/m2, respectively. For diagnosing any fibrosis (≥ stage 1), MRE’s AUROC was 0.799 (95% CI, 0.723–0.875), significantly (p=0.012) higher than ARFI’s AUROC of 0.664 (95% CI, 0.568–0.760). In stratified analysis by presence/absence of obesity, MRE was superior to ARFI for diagnosing any fibrosis in obese patients (p<0.001) but not in non-obese patients (p=0.722). MRE’s AUROCs for diagnosing ≥ stages 2, 3, and 4 fibrosis were 0.885 (95% CI, 0.816–0.953), 0.934 (95% CI, 0.863–1.000), and 0.882 (95% CI, 0.729–1.000), and ARFI’s AUROCs were 0.848 (95% CI, 0.776–0.921), 0.896 (95% CI, 0.824–0.968), and 0.862 (95% CI, 0.721–1.000). MRE had higher AUROCs than ARFI for discriminating dichotomized fibrosis stages at all dichotomization cut-points, but the AUROC differences decreased as the cut-points (fibrosis stages) increased.
Conclusions
MRE is more accurate than ARFI for diagnosing any fibrosis in all NAFLD patients and obese NAFLD patients, although not in non-obese NAFLD patients.
Keywords: MRE, ARFI, NAFLD, fibrosis, biopsy
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver diseases characterized by hepatic steatosis in patients with no significant alcohol use or secondary causes of hepatic steatosis.(1,2) NAFLD is associated with features of the metabolic syndrome, including obesity, type 2 diabetes mellitus, and dyslipidemia, (1,3–6) and is now a major cause of liver disease worldwide affecting up to one hundred million people in the United States alone.(7) Nonalcoholic steatohepatitis (NASH) is a more severe form of NAFLD characterized by hepatocellular ballooning and inflammation.(8) NASH patients with hepatic fibrosis are at greater risk for disease progression to cirrhosis and its associated complications, including hepatocellular carcinoma, and these patients require greater monitoring and therapy.(9,10) Biopsy is currently the gold standard for diagnosing fibrosis in NAFLD patients, but is invasive, has high inter-observer variability, and is associated with various complications, including death.(11) There is a need for noninvasive tests that can diagnose fibrosis in NAFLD patients.
There are no noninvasive tests currently approved to diagnose fibrosis in NAFLD patients. Magnetic resonance elastography (MRE) is a magnetic resonance-based imaging technique shown to accurately diagnose fibrosis in NAFLD patients, although it is associated with high costs and is currently not widely available.(12–15) Ultrasound-based imaging techniques, including transient elastography (FibroScan) (16) and acoustic radiation force impulse (ARFI) elastography, (17) are cheaper and more widely available than MRE. However, ultrasound-based imaging techniques are reported to be operator-dependent and their accuracy may be less than that of MRE, especially in obese patients.(18,19) While ARFI has been shown to be accurate for diagnosing fibrosis in a variety of liver diseases, including NAFLD, (20–22) no head-to-head comparisons between MRE and ARFI have been performed to evaluate their accuracy for diagnosing fibrosis in a prospective cohort of patients with biopsy-proven NAFLD.
Using a well-characterized, prospective cohort of patients with biopsy-proven NAFLD, we performed comparisons of the accuracy of MRE and ARFI for diagnosing fibrosis in NAFLD patients. Additionally, we compared the accuracy of MRE and ARFI for diagnosing NAFLD fibrosis in sub-groups of obese and non-obese patients.
METHODS
Study Design
This was a cross-sectional analysis of a prospectively-recruited cohort of 125 patients with biopsy-proven NAFLD who underwent MRE and ARFI examinations. All patients had a clinical indication for a liver biopsy and were enrolled in the study after a written informed consent was obtained. Patients were carefully screened and excluded for other causes of liver diseases and secondary causes of hepatic steatosis. Enrolled patients were invited for a clinical research visit and they underwent standardized history, anthropometric exam, physical exam, and biochemical testing at the University of California at San Diego (UCSD) NAFLD Translational Research Unit (23–29) and MRE and ARFI exams at the UCSD MR3T Research Laboratory. Written informed consent was obtained from all patients prior to the study, and the study was approved by the UCSD Institutional Review Board and the UCSD Clinical and Translational Research Institute.
Inclusion/Exclusion Criteria
Patients were included if they were adults ≥18 years old with biopsy-proven NAFLD and provided written informed consent. Patients were excluded if they had regular or excessive alcohol use within two years of recruitment (≥14 drinks/week for men or ≥7 drinks/week for women); use of hepatotoxic drugs or drugs known to cause hepatic steatosis; clinical or laboratory evidence of secondary causes of NAFLD, including nutritional and iatrogenic gastrointestinal disorders and human immunodeficiency virus infection; clinical or laboratory evidence of liver diseases other than NAFLD, including hepatitis B (if positive serum hepatitis B surface antigen), hepatitis C (if positive hepatitis C viral RNA), autoimmune hepatitis, alpha-1 antitrypsin deficiency, Wilson’s disease, hemochromatosis, glycogen storage diseases, and cholestatic or vascular liver diseases; clinical or biochemical evidence of decompensated liver disease (Child-Pugh ≥7 points); active substance abuse; significant systemic illnesses; contraindication(s) to magnetic resonance imaging, including claustrophobia and the presence of metal implants; pregnant status or trying to become pregnant; or any conditions which, in the opinion of the principal investigator, may affect the patient’s competence, compliance, or study participation.
Histological Assessment
Definition of NAFLD: All patients were confirmed to have biopsy-proven NAFLD as defined by the NAFLD practice guidelines. Other causes of secondary NAFLD were excluded as explained in the inclusion and exclusion criteria. All patients underwent a liver biopsy, which was scored using the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) histological scoring system (30) by an experienced hepatopathologist blinded to the patients’ clinical and radiologic data. The histological scoring system is further described in the Supporting Information.
Outcome Measures
The primary outcome was fibrosis (stage 1–4 fibrosis vs. stage 0 fibrosis). Secondary outcomes included dichotomized stages of fibrosis: stage 2–4 vs. stage 0–1 fibrosis, stage 3–4 (advanced fibrosis) vs. stage 0–2 fibrosis, and stage 4 (cirrhosis) vs. stage 0–3 fibrosis.
Magnetic Resonance Elastography
MRE was performed with commercially available software and hardware (Resoundant Inc., Rochester, MN) using previously described techniques.(12,13,31–33) Briefly, shear waves at 60 Hz were generated in the liver by an acoustic passive driver attached to the body wall anterior to the liver and coupled to an acoustic active driver outside of the MRI room. The shear waves were imaged using a 2D gradient-recalled-echo MRE pulse sequence with oscillating motion-sensitizing gradients synchronized to the shear wave frequency. Four noncontiguous axial slices (10 mm thick with 10 mm interslice gap) were acquired, each in a separate 16-second breath hold, through the widest transverse dimension of the liver with the following acquisition parameters: repetition time (TR), 50 ms; echo time (TE), 20.2 ms; flip angle (FA), 30 degrees; matrix, 256 × 64; field of view (FOV), 48 × 48 cm; one-signal average; receiver bandwidth (BW) ± kHz (confirm); and parallel imaging accelerating factor, 2. The total acquisition time was approximately two minutes for the four breath-holds with short recovery times in between.
The wave images generated at each slice location were automatically processed on the scanner computer using specialized software and algorithm (called an inversion algorithm) to generate quantitative cross-sectional maps (called elastograms) depicting tissue stiffness. The elastograms were transferred offline for analysis (34,35) by a trained imaging analyst (at least six months of experience with MRE) in the MR3T research laboratory. The imaging analyst manually drew regions of interest on the elastograms using a custom software package in portions of the liver where the corresponding wave images showed clearly observable wave propagation, and liver edges, large blood vessels, and artifacts were avoided. The mean liver stiffness, calculated by averaging the per-pixel stiffness values across regions of interests at the four slice locations, were automatically outputted to an electronic spreadsheet.
Acoustic Radiation Force Impulse
ARFI (36,37) was performed using the Siemens Acuson S3000 scanner (Siemens AG, Mountain View, CA) by a research physician (E.H.) blinded to clinical and histology data, using the 4C1 curved vector array transducer (1- 4.5 MHz). Subjects were asked to fast 4 hours prior to their ultrasound examinations. Scanning was done in dorsal decubitus position with the right arm fully abducted, through a right intercostal approach with the transducer placed at ~90° to the skin surface and the patient instructed to suspend breathing. The region of interest (ROI) was placed in a homogenous area, away from major vasculature in the right lobe of the liver 2–5 cm deep from the proximal liver capsule. 11 valid shear wave speed (SWS) acquisitions were acquired for each subject, with one acquisition per breath hold. For each acquisition, if the proscribed measurement quality threshold is not achieved by the ultrasound scanner, it shows an error message, and no SWS value is displayed and the acquisition is considered invalid. ARFI was considered successful if at least seven measurements were obtained from a patient, and subjects with fewer than seven successful measurements were considered to have failed ARFI exams. A monitor screen shot of the ROI location, depth, and SWS value was saved for each acquisition (Supplementary Figure 1). SWS values and ROIs depth were manually recorded to our database for all subjects, and the mean SWS was then calculated for each subject.
Duration between MRE and Liver Biopsy
The median time interval between MRE and biopsy was 46.5 (interquartile range: 62) days.
Duration between ARFI and Liver Biopsy
The median time interval between ARFI and biopsy was 47.5 (interquartile range: 87) days.
Duration between MRE and ARFI
The median time interval between MRE and ARFI was 0 (interquartile range: 1.5) days with the majority of MRE and ARFI done on the same day.
Clinical Research Assessment
All patients underwent a clinical research assessment at the UCSD NAFLD Translational Research Unit, which included standardized history, anthropometric exam including body mass index (BMI), physical exam, and biochemical testing (see Supporting Information for details).
Statistical Analyses
All statistical analyses was performed using SPSS version 22.0 and “R” statistical computing software version 3.1.1 (Vienna, Austria).(38) Patients’ demographic, laboratory, histological, and imaging data were summarized with mean and standard deviation for continuous variables and numbers and percentages for categorical variables. A two-tailed p-value ≤ 0.05 was considered statistically significant. Based on previously published results for MRE (13,14) and ARFI, (21) we anticipated a difference of about 0.19 between the AUROCs of MRE and ARFI for diagnosing fibrosis. A sample of at least 118 patients with MRE and ARFI was needed to achieve a power of 90% with an alpha value of 0.05.
Main Analysis
Receiver operating characteristic (ROC) curve analysis was performed for both MRE and ARFI as classifiers of fibrosis (stage 1–4 fibrosis) using biopsy as the gold standard. The overall performance of MRE and ARFI for diagnosing fibrosis was evaluated using the area under ROC curve (AUROC). AUROCs of MRE and ARFI for diagnosing fibrosis were also separately calculated for obese (BMI ≥ 30.0) and non-obese patients. 95% confidence intervals were calculated for each AUROC using its standard error. The optimal thresholds of MRE and ARFI for diagnosing fibrosis was selected using the Youden Index, (39) and the following performance parameters were calculated: sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The DeLong test (40) was used to perform head-to-head comparisons of accuracy of MRE vs. ARFI for diagnosing biopsy-proven fibrosis.
Secondary Analyses
Additional ROC analyses were performed for the overall cohort and separately in obese and non-obese patients and their corresponding AUROCs, diagnostic thresholds, and performance parameters were calculated for the accuracy of MRE and ARFI for diagnosing other dichotomized stages of fibrosis (stage 2–4 vs. stage 0–1, stage 3–4 vs. stage 0–2, and stage 4 vs. stage 0–3). The DeLong test was used to perform head-to-head comparisons between MRE and ARFI for diagnosing each clinical outcome.
RESULTS
Baseline Characteristics
125 consecutive patients (54.4% female) with biopsy-proven NAFLD, MRE, and ARFI were prospectively enrolled between December 2013 and March 2015. The mean (±SD) age and BMI were 48.9 (±15.4) and 31.8 (±7.0), respectively. 71 patients were obese (BMI ≥30.0) and 54 patients were not obese (BMI <30.0). Patients’ baseline demographic, biochemical, histological, and imaging data are summarized in Table 1. A total of 184 patients with biopsy-proven NAFLD were initially evaluated at the UCSD NAFLD Translational Research Unit, although 16 patients did not undergo ARFI due to patient schedule, 11 patients did not undergo MRE due to patient schedule, 28 patients did not undergo both ARFI and MRE due to patient schedule, three patients failed ARFI, and one patient failed MRE (Supplementary Figure 2).
Table 1.
Demographic, biochemical, and histological characteristics of subjects with biopsy-proven NAFLD
| Patients with biopsy, 2D-MRE, and ARFI (n = 125) | |
|---|---|
| Demographic | |
| Female patients (%) | 68 (54.4%) |
| Age (years) at biopsy (SD) | 48.9 (15.4) |
| Height (cm) mean (SD) | 167.7 (9.5) |
| Weight (kg) mean (SD) | 89.8 (24.0) |
| BMI (kg/m2) mean (SD) | 31.8 (7.0) |
| Ethnic origin: | |
| White (%) | 61 (48.8%) |
| Black (%) | 0 (0%) |
| Asian (%) | 22 (17.6%) |
| Hispanic (%) | 37 (29.6%) |
| Multi-racial (%) | 1 (0.8%) |
| Other (%) | 1 (0.8%) |
| Refused to disclose | 3 (2.4%) |
| Diabetes (%) | 32 (26.0%) |
| Metabolic syndrome (%) | 31 (24.8%) |
| Biochemical Profile | |
| AST U/L mean (SD) | 41.9 (35.0) |
| ALT U/L mean (SD) | 56.4 (51.8) |
| AST/ALT ratio mean (SD) | 0.87 (0.63) |
| Alk Phos U/L mean (SD) | 75.1 (32.0) |
| GGT U/L mean (SD) | 62.1 (70.3) |
| Total Bilirubin mg/dL mean (SD) | 0.56 (0.40) |
| Direct Bilirubin mg/dL mean (SD) | 0.14 (0.08) |
| Albumin g/dL mean (SD) | 4.8 (3.5) |
| Glucose mg/dL mean (SD) | 106.4 (31.6) |
| Hgb A1c mean (SD) | 6.1 (0.9) |
| Triglycerides mg/dL mean (SD) | 157.2 (80.8) |
| Total Cholesterol mg/dL mean (SD) | 185.0 (38.9) |
| HDL mg/dL mean (SD) | 50.1 (19.4) |
| LDL mg/dL mean (SD) | 106.0 (32.6) |
| Platelet count 109/L mean (SD) | 250.1 (70.4) |
| Protime mean (SD) | 10.7 (0.9) |
| INR mean (SD) | 1.0 (0.1) |
| Histology | |
| Steatosis | |
| 0 | 4 (3.3%) |
| 1 | 41 (33.3%) |
| 2 | 39 (31.7%) |
| 3 | 39 (31.7%) |
| Lobular Inflammation | |
| 0 | 4 (3.3%) |
| 1 | 57 (46.7%) |
| 2 | 60 (49.2%) |
| 3 | 1 (0.8%) |
| Ballooning | |
| 0 | 32 (26.7%) |
| 1 | 70 (58.3%) |
| 2 | 18 (15.0%) |
| Fibrosis | |
| 0 | 53 (42.4%) |
| 1 | 39 (31.2%) |
| 2 | 12 (9.6%) |
| 3 | 12 (9.6%) |
| 4 | 9 (7.2%) |
| NAS mean (SD) | 4.29 (1.54) |
| Imaging | |
| 2D-MRE and biopsy date interval median (IQR) | 46.5 (62) |
| ARFI and biopsy date interval median (IQR) | 47.5 (87) |
| 2D-MRE mean (SD) | 3.13 (1.27) |
| ARFI mean (SD) | 1.48 (0.68) |
Metabolic syndrome was calculated using the International Diabetes Federation criteria, which included central obesity and at least two of the following: elevated triglycerides, reduced HDL-cholesterol, elevated blood pressure, and elevated fasting plasma glucose.
Abbreviations: BMI: body mass index, AST: aspartate aminotransferase, ALT alanine aminotransferase, Alk phos: alkaline phosphatase, GGT: gamma-glutamyl transpeptidase, Hgb A1c: hemoglobin A1c, HDL: high-density lipoprotein, LDL: low-density lipoprotein, INR: international normalized ratio, NAS: nonalcoholic fatty liver disease activity score, ARFI: acoustic radiation force impulse, 2D-MRE: 2-dimensional magnetic resonance elastography, ARFI: acoustic radiation force impulse
Distribution of Fibrosis Stages
53, 39, 12, 12, and 9 patients had stage 0, 1, 2, 3, and 4 fibrosis, respectively.
Accuracy of MRE vs. ARFI for the Diagnosis of Fibrosis
MRE had an AUROC of 0.799 (95% CI: 0.723–0.875) for diagnosing fibrosis (Figure 1). At a threshold of 2.99 kPa (Figure 2A), MRE had sensitivity 0.583, specificity 0.906, PPV 0.894, and NPV 0.615 for diagnosing fibrosis. ARFI had an AUROC of 0.664 (95% CI: 0.568–0.760) for diagnosing fibrosis. At a threshold of 1.29 meters/second (Figure 2B), ARFI had sensitivity 0.542, specificity 0.774, PPV 0.765, and NPV 0.554 for diagnosing fibrosis. In a head-to-head comparison using the DeLong test, MRE had significantly higher accuracy than ARFI (p=0.012) for diagnosing fibrosis (Table 2).
Figure 1.

MRE had an AUROC of 0.799 for diagnosing fibrosis and ARFI had an AUROC of 0.664 for diagnosing fibrosis.
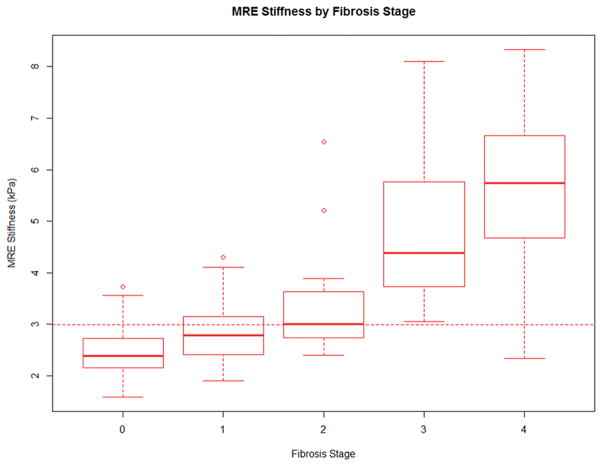
Figure 2.
Figure 2A–2B: Distribution of MRE (Figure 2A) and ARFI (Figure 2B) readings by fibrosis stage. The dichotomization cut points for any fibrosis was 2.99 for MRE and 1.29 for ARFI.
Table 2.
Diagnostic test characteristics of 2D-MRE and ARFI for the diagnosis of fibrosis
| N Positive | N Negative | AUROC (95% Confidence Interval) | Cutoff | Sensitivity | Specificity | PPV | NPV | MRE vs. ARFI (p-value) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary Analysis | ||||||||||
| Stage 1–4 vs. Stage 0 | MRE | 72 | 53 | 0.799 (0.723, 0.875) | 2.99 | 0.583 | 0.906 | 0.894 | 0.615 | 0.012 |
| ARFI | 0.664 (0.568, 0.760) | 1.29 | 0.542 | 0.774 | 0.765 | 0.554 | ||||
| Secondary Analyses | ||||||||||
| Stage 2–4 vs. Stage 0–1 | MRE | 33 | 92 | 0.885 (0.816, 0.953) | 3.62 | 0.667 | 0.957 | 0.846 | 0.889 | 0.343 |
| ARFI | 0.848 (0.776, 0.921) | 1.34 | 0.818 | 0.783 | 0.574 | 0.923 | ||||
| Stage 3–4 vs. Stage 0–2 | MRE | 21 | 104 | 0.934 (0.863, 1.000) | 3.62 | 0.905 | 0.933 | 0.731 | 0.980 | 0.113 |
| ARFI | 0.896 (0.824, 0.968) | 1.34 | 0.952 | 0.740 | 0.426 | 0.987 | ||||
| Stage 4 vs. Stage 0–3 | MRE | 9 | 116 | 0.882 (0.729, 1.000) | 4.15 | 0.889 | 0.914 | 0.444 | 0.991 | 0.418 |
| ARFI | 0.862 (0.721, 1.000) | 2.48 | 0.778 | 0.931 | 0.467 | 0.982 | ||||
p-value: AUROC of MRE vs. ARFI → DeLong test
Abbreviations: ARFI: acoustic radiation force impulse, MRE: magnetic resonance elastography, AUROC: area under receiver operating characteristic curve
For MRE, the AUROC for diagnosing fibrosis in obese (n=71) patients was 0.850 (95% CI: 0.761–0.939) and in non-obese patients (n=54) was 0.729 (95% CI: 0.586–0.872). For ARFI, the AUROC for diagnosing fibrosis in obese patients was 0.603 (95% CI: 0.457–0.749) and in non-obese patients was 0.694 (95% CI: 0.537–0.850). MRE had significantly higher accuracy than ARFI for diagnosing fibrosis in obese patients (p<0.001) but did not have significantly higher accuracy than ARFI for diagnosing fibrosis in non-obese patients (p=0.722) (Table 3).
Table 3.
AUROCs and AUROC comparisons in obese and non-obese patients
| Obese (n=71) | Non-Obese (n=54) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N Positive | N Negative | AUROC (95% Confidence Interval) | MRE vs. ARFI (p-value) | N Positive | N Negative | AUROC (95% Confidence Interval) | MRE vs. ARFI (p-value) | ||
| Primary Analysis | |||||||||
| Stage 1–4 vs. Stage 0 | MRE | 50 | 21 | 0.850 (0.761, 0.939) | <0.001 | 22 | 32 | 0.729 (0.586, 0.872) | 0.722 |
| ARFI | 0.603 (0.457, 0.749) | 0.694 (0.537, 0.850) | |||||||
| Secondary Analyses | |||||||||
| Stage 2–4 vs. Stage 0–1 | MRE | 24 | 47 | 0.885 (0.796, 0.974) | 0.274 | 9 | 45 | 0.867 (0.753, 0.980) | 1.000 |
| ARFI | 0.829 (0.730, 0.928) | 0.867 (0.762, 0.971) | |||||||
| Stage 3–4 vs. Stage 0–2 | MRE | 17 | 54 | 0.917 (0.823, 1.000) | 0.235 | 4 | 50 | 0.955 (0.871, 1.000) | 0.607 |
| ARFI | 0.869 (0.772, 0.966) | 0.935 (0.861, 1.000) | |||||||
| Stage 4 vs. Stage 0–3 | MRE | 6 | 65 | 0.821 (0.582, 1.000) | 1.000 | 3 | 51 | 1.000 (1.000, 1.000) | 0.185 |
| ARFI | 0.821 (0.612, 1.000) | 0.961 (0.905, 1.000) | |||||||
p-value: AUROC of MRE vs. ARFI → DeLong test
Abbreviations: ARFI: acoustic radiation force impulse, MRE: magnetic resonance elastography, AUROC: area under receiver operating characteristic curve
27 patients in our cohort had class II obesity (BMI 35.0–39.9 kg/m2), with 20 patients in the class II obesity group having fibrosis and 7 patients having no fibrosis. For diagnosing any fibrosis in patients with class II obesity, the AUROC of MRE remained robust at 0.914 (95% CI: 0.805–1.000), whereas the AUROC of ARFI declined further to 0.536 (95% CI: 0.262–0.810). MRE had significantly higher accuracy than ARFI for diagnosing fibrosis in patients with class II obesity (p=0.007).
Accuracy of MRE vs. ARFI for the Diagnosis of other Stages of Fibrosis
The AUROCs of MRE and ARFI for diagnosing stage 2–4 vs. stage 0–1 fibrosis, stage 3–4 vs. stage 0–2 fibrosis, and stage 4 vs. stage 0–3 are shown in Table 2. MRE had higher AUROCs than ARFI at each dichotomization cutpoint, although the differences in AUROCs decreased as the dichotomization cutpoint (i.e., the stage of fibrosis) increased. Additionally, no significant difference existed between MRE and ARFI between obese and non-obese patients for diagnosing other stages of fibrosis (Table 3).
DISCUSSION
Main Findings
Utilizing a prospective cohort of patients with biopsy-proven NAFLD, our study showed that MRE was significantly more accurate than ARFI for diagnosing fibrosis in NAFLD patients. The difference in diagnostic accuracy between MRE and ARFI decreased as the dichotomization cutpoint increased, and MRE and ARFI were both highly accurate for diagnosing advanced fibrosis (stage 3–4 fibrosis) and cirrhosis (stage 4 fibrosis). Apart from any fibrosis (stage 1–4 fibrosis), there were no significant differences between MRE and ARFI for diagnosing other stages of fibrosis at higher dichotomization cutpoints. Additionally, MRE was significantly more accurate than ARFI for diagnosing fibrosis in obese, but not non-obese, patients. In obese individuals, MRE should be considered for diagnosis of fibrosis in patients with NAFLD. However, in non-obese individuals ARFI measurements may provide adequate diagnostic accuracy. These findings may have important clinical implications in non-invasive assessment of NAFLD and developing an optimal strategy for resource utilization. Cost-effectiveness studies are needed to determine the optimal strategy but we believe that an approach using an MRE in those with obesity and ARFI in non-obese may be applicable if these findings are confirmed in studies published by independent investigators or in a multicenter setting. Additionally, MRE may be preferable to ARFI if diagnosing the earliest stages of fibrosis is needed, although ARFI may be sufficient in patients with higher risks of advanced fibrosis, even if obese.
In Context of Published Literature
This is the first study to make head-to-head comparisons between the accuracy of MRE vs. ARFI for diagnosing liver fibrosis in a large, prospective cohort of NAFLD patients with strict inclusion and exclusion criteria and liver biopsies within one year of ARFI.(41,42) The results of our study are consistent with those of previous studies showing MRE to be highly accurate for diagnosing fibrosis in NAFLD patients.(12–14) Our study is also consistent with previous studies showing ARFI to be accurate for diagnosing fibrosis, especially at higher dichotomization cut-points.(17,20–22) The accuracy of ARFI becomes less for diagnosing fibrosis at lower dichotomization cut-points.(21)
In a recent study by Angulo et al of NAFLD patients, any fibrosis (stage 1–4 fibrosis) was shown to be a significant, independent predictor of increased mortality or liver transplantation compared to no fibrosis.(43,44) Additionally, any fibrosis was associated with increased mortality or liver transplantation compared to no fibrosis even when individuals with more severe stage 3–4 fibrosis were removed from the analysis.(43) Therefore, early detection and screening of any fibrosis before its progression into more severe stage 3–4 fibrosis may be beneficial in NAFLD patients. These patients may require increased monitoring and management, and may also be candidates for treatment trials targeting NAFLD patients. Our study shows that MRE is superior to ARFI for identifying these NAFLD patients with any fibrosis who are at increased risk for mortality and liver transplantation.
While ultrasound-based imaging techniques for detecting hepatic fibrosis may be less accurate in obese patients, earlier studies have suggested that ARFI may still be feasible in obese NAFLD patients with good accuracy.(45) Our study shows that MRE was significantly more accurate than ARFI for diagnosing fibrosis in obese patients, but not in non-obese patients. Our study also shows that for patients with class II obesity (BMI 35.0–39.9 kg/m2), the AUROC of MRE remained robust at 0.914 whereas the AUROC of ARFI declined further to 0.536, compared to 0.603 for ARFI in the overall cohort of obese patients (BMI ≥ 30.0). As the population becomes more obese, the difference between the AUROCs of MRE versus ARFI for diagnosing fibrosis widens, with a relative increase in the diagnostic accuracy of MRE and a relative decrease in the diagnostic accuracy of ARFI. Therefore, obesity and body habitus may negatively affect the accuracy of ARFI for diagnosing fibrosis in NAFLD patients. Additionally, three patients in our study, all of whom were obese, failed their ARFI examinations due to difficulty in obtaining accurate reads, and obesity may have played a role in the failure to obtain accurate readings in these patients. Only one patient in our study failed MRE due to poor wave progression from an iron-overloaded liver. Obesity appears to have less of an effect on the accuracy of MRE than on the accuracy of ARFI.
Strengths and Limitations
The strength of this study lies in its use of a well-characterized, prospective cohort of patients with biopsy-proven NAFLD in which all patients had a clinical indication for a liver biopsy and underwent a paired MRE and ARFI evaluation. Liver biopsy assessments were done using the NASH CRN Histologic Scoring System. All patients underwent a dedicated research visit at the UCSD NAFLD Translational Research Unit, and other causes of liver diseases were systematically ruled out before enrollment into the study. The study was performed by an experienced group of investigators at a center that is highly specialized for NAFLD research, including imaging with MRE and ARFI. The median time interval between MRE and biopsy was only 46.5 days and that between ARFI and biopsy was only 47.5 days.
However, there are also a few limitations to this study. It was performed at a center highly specialized for both clinical and radiological NAFLD research, and the generalizability of its results to other settings requires validation in a multicenter setting. The study is cross-sectional, and the utility of MRE and ARFI for monitoring longitudinal changes in fibrosis progression remains unknown. The diagnostic accuracy of ARFI is reported to be operator dependent and may be subject to inter- and intra-operator variability, although at our center all ARFI was performed by a single experienced investigator. MRI-based techniques, including MRE, may be expensive to perform, even though at our center the price of an MRE is less than that of a liver biopsy without the morbidity associated with liver biopsies. Although MRE is more accurate than ARFI, it is more expensive to perform and has limited availability compared to ARFI. Further research is needed to evaluate the cost-effectiveness of MRE vs. ARFI for diagnosing NAFLD-associated fibrosis before utilizing these competing strategies for the non-invasive assessment of NAFLD in routine clinical practice.
Implication for Future Research
This study provides a prospective, head-to-head comparison of MRE vs. ARFI for diagnosing fibrosis in a cohort of patients with biopsy-proven NAFLD. We show MRE to have significantly higher accuracy than ARFI for diagnosing fibrosis in NAFLD patients, and this difference exists in obese patients but not in non-obese patients. Therefore, while both MRE and ARFI may be accurate for diagnosing fibrosis in non-obese NAFLD patients, MRE may be more preferable than ARFI for diagnosing fibrosis in obese NAFLD patients. Future studies will be needed to assess the utility of MRE and ARFI for diagnosing fibrosis in multicenter, longitudinal settings, the cost-effectiveness of MRE vs. ARFI for diagnosing fibrosis in both obese and non-obese NAFLD patients, and the cost-effectiveness of using MRE to diagnosis early stages of fibrosis and ARFI to diagnose later stages of fibrosis, even in obese patients. Additional studies are also needed to evaluate the diagnostic utility of more novel imaging techniques, including three-dimensional MRE (3D-MRE), for diagnosing NAFLD-associated fibrosis.
Supplementary Material
Supplementary Figure 1: ARFI screen save showing a B-mode image with region of interest (ROI) placed in the right lobe of the liver. The shear wave speed was 1.26 meters/second at a depth of 5.0 cm.
Supplementary Figure 2: Derivation of cohort.
Acknowledgments
Funding Support: The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. CS and RL serve as co-PIs on the grant R01-DK106419. JC is supported by NIH T32 training grant 5TL1TR000098. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- MRE
magnetic resonance elastography
- ARFI
acoustic radiation force impulse
- UCSD
University of California at San Diego
- CRN
Clinical Research Network
- TR
repetition time
- TE
echo time
- FA
flip angle
- FOV
field of view
- BW
bandwidth
- ROI
region of interest
- SWS
shear wave speed
- BMI
body mass index
- ROC
receiver operating characteristic
- AUROC
area under receiver operating characteristic
- PPV
positive predictive value
- NPV
negative predictive value
Footnotes
Role of study sponsor: The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript. All authors report that no conflicts of interest exist.
Conflict of interests: Dr. Sirlin consults, advises, and is on the speakers’ bureau for Bayer. He received grants from GE Healthcare. The authors report no other conflict of interests.
Author contributions:
Jeffrey Cui: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission.
Elhamy Heba: study concept and design, data collection, imaging analysis, drafting of the manuscript, critical revision of the manuscript, approved final submission.
Carolyn Hernandez: patient visits, data collection, critical revision of the manuscript, approved final submission.
William Haufe: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Jonathan Hooker: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Michael P. Andre: study concept and design, critical revision of the manuscript, approved final submission
Mark A. Valasek: interpreted biopsies, critical revision of the manuscript, approved final submission
Hamed Aryafar: performed biopsies, critical revision of the manuscript, approved final submission
Claude B. Sirlin: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Rohit Loomba: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission.
All authors approved the final version of this article.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012 Jun;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013 Nov;10(11):686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010 Sep 30;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011 Jan;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012 Sep;56(3):943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arulanandan A, Ang B, Bettencourt R, Hooker J, Behling C, Lin GY, et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2015 Aug;13(8):1513–1520. e1. doi: 10.1016/j.cgh.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004 Dec;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 8.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999 Jun;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 9.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005 Jul;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006 Oct;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 11.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005 Jun;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013 Aug;268(2):411–419. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014 Dec;60(6):1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015 Jun;41(12):1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2015 Sep 15; doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012 Jan;55(1):199–208. doi: 10.1002/hep.24624. [DOI] [PubMed] [Google Scholar]
- 17.Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011 Sep;55(3):666–672. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Ledinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R) J Hepatol. 2012 Apr;56(4):833–839. doi: 10.1016/j.jhep.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012 Dec;107(12):1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 20.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010 Aug;256(2):640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012 Mar;81(3):e325–31. doi: 10.1016/j.ejrad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka K, Hashimoto S, Kawabe N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol Res. 2015 Jan;45(2):142–151. doi: 10.1111/hepr.12388. [DOI] [PubMed] [Google Scholar]
- 23.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012 Sep;56(3):922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel NS, Peterson MR, Brenner DA, Heba E, Sirlin C, Loomba R. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013 Mar;37(6):630–639. doi: 10.1111/apt.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015 Mar 14;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015 Apr;61(4):1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015 Jul;13(7):1337–1345. e6. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarrinpar A, Gupta S, Maurya MR, Subramaniam S, Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. 2015 May 22; doi: 10.1136/gutjnl-2015-309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015 Aug 20; doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007 Oct;5(10):1207–1213. e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011 Jun;259(3):749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013 Mar;37(3):544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012 Jul;36(1):22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013 Dec;58(6):1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haque M, Robinson C, Owen D, Yoshida EM, Harris A. Comparison of acoustic radiation force impulse imaging (ARFI) to liver biopsy histologic scores in the evaluation of chronic liver disease: A pilot study. Ann Hepatol. 2010 Jul-Sep;9(3):289–293. [PubMed] [Google Scholar]
- 37.Rifai K, Cornberg J, Mederacke I, Bahr MJ, Wedemeyer H, Malinski P, et al. Clinical feasibility of liver elastography by acoustic radiation force impulse imaging (ARFI) Dig Liver Dis. 2011 Jun;43(6):491–497. doi: 10.1016/j.dld.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011 Mar 17;12:77-2105-12-77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youden WJ. Index for rating diagnostic tests. Cancer. 1950 Jan;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–845. [PubMed] [Google Scholar]
- 41.Yoon JH, Lee JM, Woo HS, Yu MH, Joo I, Lee ES, et al. Staging of hepatic fibrosis: comparison of magnetic resonance elastography and shear wave elastography in the same individuals. Korean J Radiol. 2013 Mar-Apr;14(2):202–212. doi: 10.3348/kjr.2013.14.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H, Chen J, Meixner DD, Xie H, Shamdasani V, Zhou S, et al. Noninvasive assessment of liver fibrosis using ultrasound-based shear wave measurement and comparison to magnetic resonance elastography. J Ultrasound Med. 2014 Sep;33(9):1597–1604. doi: 10.7863/ultra.33.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015 Aug;149(2):389–397. e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loomba R, Chalasani N. The Hierarchical Model of NAFLD: Prognostic Significance of Histologic Features in NASH. Gastroenterology. 2015 Aug;149(2):278–281. doi: 10.1053/j.gastro.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Guzman-Aroca F, Frutos-Bernal MD, Bas A, Lujan-Mompean JA, Reus M, de Berna-Serna JD, et al. Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging. Eur Radiol. 2012 Nov;22(11):2525–2532. doi: 10.1007/s00330-012-2505-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: ARFI screen save showing a B-mode image with region of interest (ROI) placed in the right lobe of the liver. The shear wave speed was 1.26 meters/second at a depth of 5.0 cm.
Supplementary Figure 2: Derivation of cohort.