Abstract
Background
The U.S. National Healthcare Safety Network (NHSN) has provided a definition of mucosal barrier injury–associated, laboratory-confirmed bloodstream infection (MBI-LCBI) to improve infection surveillance. To date there is little information about its impact in pediatric oncology centers in low- to middle-income countries.
Objectives
To determine the impact of the definition on the rate of central line-associated bloodstream infection (CLABSI) and compare the clinical characteristics of MBI vs. non-MBI LCBI cases.
Methods
We retrospectively applied the NHSN definition to all CLABSIs recorded at a pediatric oncology center in Tijuana, Mexico, from January 2011 through December 2014. CLABSI events were re-classified according to the MBI-LCBI definition. Clinical characteristics and outcomes of MBI and non-MBI CLABSIs were compared.
Results
Of 55 CLABSI events, 44% (24/55) qualified as MBI-LCBIs; all were MBI-LCBI subcategory 1 (intestinal flora pathogens). After the number of MBI-LCBI cases was removed from the numerator, the CLABSI rate during the study period decreased from 5.72 to 3.22 infections per 1,000 central line days. Patients with MBI-LCBI were significantly younger than non-MBI-LCBI patients (P=0.029) and had a significantly greater frequency of neutropenia (100% vs. 39%, P=0.001) and chemotherapy exposure (87% vs. 58%, P=0.020) and significantly longer median hospitalization (34 vs. 23 days, P=0.008).
Conclusion
A substantial proportion of CLABSI events at our pediatric cancer center met the MBI-LCBI criteria. Our results support separate monitoring and reporting of MBI and non-MBI– -LCBIs in low- to middle-income countries to allow accurate detection and tracking of preventable (non-MBI) bloodstream infections.
Keywords: Bacteremia, Bloodstream infection, Central venous access device, Mucosal barrier injury, Pediatric cancer, Length of stay, Low income
Introduction
Benchmarking of healthcare-associated infections in low- to middle-income countries (LMICs) is a work in progress. Although data are available about device-associated infection rates in pediatric intensive care units in LMICs, 1,2 little is known about rates of infection, particularly central line-associated bloodstream infection (CLABSI), at pediatric cancer centers in LMICs.
Bloodstream infections, especially CLABSIs, are a major category of healthcare-associated infection among pediatric cancer patients.3,4 In the U.S., data reported to the Centers for Disease Control and Prevention (CDC)'s National Healthcare Safety Network (NHSN) are used to assess infection rates, outbreaks, and trends and design quality improvement initiatives for U.S. healthcare facilities.5-10 However, CLABSI data are likely to differ in oncology and non-oncology facilities.11,12 Higher CLABSI rates at cancer healthcare facilities can result from more frequent use of central lines and from a population at higher risk of infection.3
In 2013, the CDC/NHSN introduced a new surveillance definition of mucosal barrier injury-associated laboratory-confirmed bloodstream infection (MBI-LCBI) to prevent misclassification of bloodstream infections caused by oral/intestinal microbiota in cancer patients and improve the comparability of CLABSI rates at cancer and non-cancer centers.13 The MBI-LCBI surveillance definition was revised and improved after testing at 94 cancer centers in the U.S.14 Recently, an oncology center reported using the MBI-LCBI surveillance protocol to separately track MBI and non-MBI events to measure the impact of a CLABSI quality improvement initiative.15 Because MBI-LCBI cases define a specific at-risk population, the CDC will continue requiring their tracking and reporting. However, MBI-LCBI data may be removed from the main bloodstream infection events tracking metrics by 2016, in which case they will not be included in CLABSI data publicly reported to the U.S. Centers for Medicare and Medicaid Services.16
Here we retrospectively reviewed data in the prospective infection surveillance database of a pediatric cancer center housed at the Hospital General de Tijuana (Hospital Tijuana, Tijuana, Mexico). We applied the MBI-LCBI definition to (1) determine the impact of separate reporting of MBI-LCBI cases on CLABSI rates and (2) compare the clinical characteristics and outcomes of MBI- vs. non-MBI bloodstream infections at our pediatric cancer center during the past four years.
Methods
Study setting
The Hospital Tijuana is the largest public hospital in Northwestern Mexico. It has 197 beds with a bed occupancy rate of 124% and a 12-day mean length of stay. The pediatric cancer center comprises a separate 10-bed oncology ward and an ambulatory infusion center containing 10 beds and 5 reclining chairs.
In 2007, stakeholders at St. Jude Children's Research Hospital (St. Jude, Memphis, TN, USA), Rady Children's Hospital (San Diego, CA, USA) and the Hospital Tijuana implemented a partnership to establish a pediatric oncology program17 aimed at improving clinical outcomes and survival of children with cancer in the Mexican State of Baja California. Several initiatives have been added since the start of the project, including a robust infection prevention and control program. In 2009, a dedicated infection prevention and control team, which included a general pediatrician, a dedicated infection preventionist nurse, and a microbiologist, was added to the pediatric oncology program. A pediatric infectious disease physician joined the team in 2014.
Since 2011, the infection prevention and control team at the pediatric cancer center has used the CDC/NHSN surveillance definitions for its internal reporting and prospective infection surveillance. During monthly mentoring meetings, the team uses the St. Jude www.cure4kids.org teleconference virtual rooms to report and discuss aggregate infection data with the St. Jude Infectious Disease - International Outreach Program (ID – IOP) team. These reports include aggregate patient days, central-line days, discharges, etc. Outcome surveillance includes rates of healthcare-associated infections per 1000 patient days; CLABSIs per 1000 central-line days; CLABSIs per 1000 central-line days stratified by type of device; and mortality rates due to infection.
In 2014, 274 inpatient discharges, 41 new cancer diagnoses, and 11 deaths were reported at the pediatric cancer center. The most common cancer diagnosis was acute leukemia, followed by lymphoma and central nervous system tumors. Patients were younger than 18 years of age at diagnosis of cancer. Infection was the main cause of treatment-related morbidity and mortality. This study was approved by the Institutional Review Board of the Hospital Tijuana. The requirement for informed consent was waived.
Study design
Included in this retrospective cohort study were all CLABSI events documented in the pediatric cancer center's prospective infection surveillance database between January 1, 2011 and December 31, 2014. CLABSI events were re-classified on the basis of the CDC/NHSN 2013 definition of MBI-LCBI,18 and associated clinical information was obtained from patients' medical records.
Training and data collection
All members of the Hospital Tijuana's infection prevention and control team completed the 2010 version of the St. Jude Infection Control Course before this study began.19,20 The course was offered by the ID – IOP and included training on CDC surveillance methodology.18,21
The local team retrospectively applied the revised 2013 NHSN CLABSI definition to all study cases to minimize bias due to changes in the CLABSI definitions during the study years.9 An adapted version of the NHSN primary bloodstream infection case report form22 was used to classify CLABSI cases as MBI or non-MBI. Variables collected included age at admission, sex, cancer diagnosis, cancer treatment phase, venous access device removal due to CLABSI, and death. Data were entered into an Epi Info 7 database (the study database) by a local team member (DT). Encrypted and de-identified data were forwarded to collaborating St Jude ID-IOP investigators for analysis.
Definition and analysis of outcome variables
Classification as a CLABSI required that a central venous access device had been in place for more than 2 calendar days or, in the case of a permanent central line, more than 2 days had elapsed after first device access on the date of event (date of positive blood culture). If a central line was removed, only a positive blood culture on the day of removal or the next day could be defined as a CLABSI event.18 Primary bloodstream infection and MBI-LCBI required meeting the revised CDC definition criteria.16,18
We calculated the annual rate of CLABSIs and device utilization ratio (DUR) by using the standardized CDC criteria.18,21 Patient days were defined as the total number of inpatient days in the oncology unit during a specified time period. Length of stay (LOS) was defined as the number of days from admission to discharge or death. History of chemotherapy was defined as having received chemotherapy during the same admission or within the previous 2 weeks.
Validation of data
The adapted NHSN primary bloodstream infection case report form included microbiological (type of pathogen) and clinical (signs, symptoms, and underlying conditions such as neutropenia) information.22 We added the absolute neutrophil counts during the 3 calendar days before, the day of, and the 3 calendar days after the event. Information obtained from the center's prospective infection surveillance database was corroborated by review of the medical record and documented in the primary bloodstream infection case report form by the local team. This information was entered into the study database (Epi Info 7 database) developed to collect all study variables by the St. Jude ID-IOP team. CLABSIs entered into the study database were reviewed by a member of the St Jude ID-IOP team (MG) to ensure that the CDC/NHSN criteria were met.
Statistical analysis
Analysis was performed by using SPSS version 19.0 software. The Fisher exact test was used to compare binary variables, the Pearson chi-square test was used to compare underlying malignancy type, and the Mann Whitney test was used to compare continuous variables. Two-sample test of proportions was used to compare rates between adjacent years. All comparisons were two-sided, and P values ≤ 0.05 were considered statistically significant.
Results
Patients and events
Forty-nine children experienced 61 CLABSI events during the study period. Six of these events were excluded, as they did not meet the 2013 CLABSI criteria. Therefore, 55 CLABSI events in 49 patients were included in this study. Table 1 lists the annual totals and CLABSI rates. Overall CLABSI rates were significantly reduced (P=<0.001) due largely to the significant reduction of non-MBI-LCBI rates (P=<0.001). This reduction was observed solely between 2011 and 2012. Reduction in MBI-LCBI rates were not found to reach statistical significance (P=0.079).
Table 1. Annual surveillance data and rates of central line-associated bloodstream infection.
| 2011 | 2012 | 2013 | 2014 | Total | |
|---|---|---|---|---|---|
| Annual totals (n) | |||||
| Discharges | 120 | 339 | 240 | 274 | 973 |
| Patient days | 1280 | 2633 | 2603 | 3326 | 9842 |
| Central line days | 1201 | 2566 | 2564 | 3294 | 9625 |
| Device utilization ratios | 0.94 | 0.97 | 0.99 | 0.99 | 0.98 |
| Annual CLABSI rates | |||||
| Overall CLABSI | 9.99 | 5.07* | 4.68 | 5.48 | 5.72 |
| MBI-LCBI rates (per 1000 CL days) | 4.16 | 2.73 | 2.34 | 1.83 | 2.50 |
| Non-MBI-LCBI rates (per 1000 CL days) | 5.83 | 2.34* | 2.34 | 3.65 | 3.22 |
CLABSI= central line-associated bloodstream infection, CL=central line, MBI= mucosal barrier injury, LCBI=laboratory-confirmed bloodstream infection.
These proportions changed significantly from the previous year (P<0.05).
Impact of the MBI-LCBI criteria on CLABSI rates
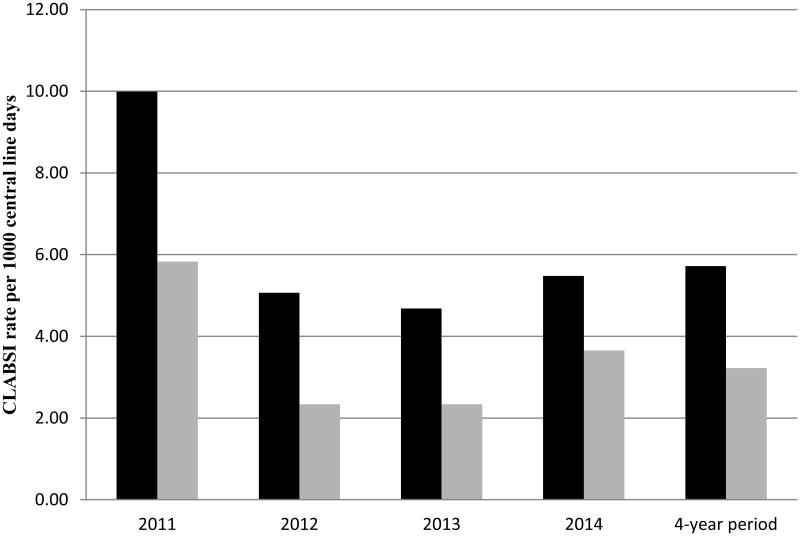
Forty-four percent (24/55) of CLABSI events qualified as MBI-LCBIs. All met the CDC criteria for MBI-LCBI subcategory 1 (intestinal flora). No cases of MBI-LCBI subcategory 2 (oral flora) or subcategory 3 (oral flora, patient ≤ 1 year of age) were observed. Neutropenia (absolute neutrophil count <500 cells/mm3) was the underlying condition in 100% of the MBI-LCBI cases. Removal of the number of MBI-LCBI events from the numerator reduced the overall CLABSI rate by nearly half (5.72 vs. 3.22 events per 1000 central line days). The effect on annual rates was similar (Figure 1).
Figure 1.
Annual rates of central line–associated bloodstream infection before (black) and after (grey) removal of number of cases of mucosal barrier injury–associated bloodstream infections from the total.
Comparison of clinical characteristics and outcomes of MBI- vs. non-MBI–LCBI cases
Despite some similarities, the MBI-LCBI and non-MBI-LCBI cases differed significantly in median age (patients with MBI-LCBI were younger, P=0.029), frequency of neutropenia (100% vs. 39%, respectively, P=0.001), and history of chemotherapy during the same admission or within the previous 2 weeks (87% vs. 58%, P=0.020). As a clinical outcome, median LOS was significantly greater in MBI-LCBI cases (34 days vs. 23 days, P=0.008). We found no significant difference between the groups in either the rate of mortality during the admission or the rate of removal of the central line (Table 2). The organisms most commonly isolated from blood were Klebsiella pneumoniae (29%), Escherichia coli (25%), and Enterococcus faecium (12.5 %) in patients with MBI-LCBI and Staphylococcus epidermidis (19%), Acinetobacter baumannii (13%), and Staphylococcus aureus (13%) in patients with non-MBI LCBI (Table 3).
Table 2. Characteristics and outcomes of mucosal barrier injury–associated vs. non-mucosal barrier injury–associated laboratory-confirmed bloodstream infections.
| MBI-LCBI (n=24) No. (%) | Non-MBI (n=31) No. (%) | P valuea | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Male sex | 10 (42) | 16 (52) | 0.588 |
| Age (years) | 11.5 (1-18) | 7 (0-18) | 0.029 |
| Primary disease type | 0.403 | ||
| Leukemia | 18 (75) | 20 (64) | |
| Lymphoma | 2 (8) | 1 (3) | |
| Solid Tumor | 4 (17) | 8 (26) | |
| Other | 0 | 2 (7) | |
| Neutropenia within 3 days before or after | 24 (100) | 12 (39) | <0.001 |
| CLABSI eventb | |||
| Mucositis | 6 (25) | 3(10) | 0.157 |
| Typhlitis | 5 (21) | 1 (3) | 0.075 |
| Chemotherapy during same admission | 21 (87) | 18 (58) | 0.020 |
| Clinical outcomes | |||
| Length of stay, median (range) | 34 (8-108) | 23(6-57) | 0.008 |
| Death during same hospitalization | 6 (25) | 1 (3) | 0.035 |
| Line removed owing to CLABSI | 16 (67) | 22(71) | 0.775 |
CLABSI= central line-associated bloodstream infection, MBI= mucosal barrier injury, LCBI=laboratory-confirmed bloodstream infection.
2-sided P values calculated by using Fisher exact test (binary variables), Pearson chi-square test (primary disease type), or Mann-Whitney test (continuous variables).
Absolute neutrophil count <500/mm3 within 3 days before or after event.
Table 3. Organisms isolated from blood in mucosal barrier injury–associated and non-mucosal barrier injury–associated, laboratory-confirmed bloodstream infections.
| Organism isolated | All CLABSI n=55 | MBI-LCBI* n=24 | Non-MBI-LCBI n=31 | |
|---|---|---|---|---|
| no. (%) | rank | no. (%) | no. (%) | |
| Klebsiella pneumoniae | 8 (14.5) | 1, tie | 7 (29) | 1 (3) |
| Escherichia coli | 8(14.5) | 1, tie | 6 (25) | 2 (6) |
| Candida species | 6(11) | 2, tie | 3 (12.5) | 3 (10) |
| Staphylococcus epidermidis | 6(11) | 2, tie | 0 | 6 (19) |
| Enterobacter cloacae | 5(9) | 3 | 2 (8) | 3 (3) |
| Acinetobacter baumannii | 4(7) | 4, tie | 0 | 4 (13) |
| Staphylococcus aureus | 4(7) | 4, tie | 0 | 4 (13) |
| Enterococcus faecium | 3(5) | 5, tie | 3 (12.5) | 0 |
| Pseudomonas aeruginosa | 3(5) | 5, tie | 0 | 3 (10) |
| Enterococcus faecalis | 1 (2) | 6, tie | 1 (4) | 0 |
| Enterococcus galllinarum | 1 (2) | 6, tie | 1 (4) | 0 |
| Klebsiella oxytoca | 1 (2) | 6, tie | 1 (4) | 0 |
| Streptococcus pneumoniae | 1 (2) | 6, tie | 0 | 1 (3) |
| Bacillus sp | 1 (2) | 6, tie | 0 | 1 (3) |
| Staphylococcus sp | 1 (2) | 6, tie | 0 | 1 (3) |
| Enterobacter aerogenes | 1 (2) | 6, tie | 0 | 1 (3) |
| Citrobacter freundii | 1 (2) | 6, tie | 0 | 1 (3) |
CLABSI= central line–associated bloodstream infection, MBI= mucosal barrier injury, LCBI=laboratory-confirmed bloodstream infection.
MBI-LCBI events met the criteria of the CDC's National Healthcare Safety Network.
Discussion
Here we have described the impact of the MBI-LCBI surveillance protocol on CLABSI rates at a pediatric cancer center in a general hospital in Tijuana, Mexico. Our results demonstrate the significance of applying these surveillance criteria in pediatric cancer centers in LMICs. A substantial proportion of CLABSI events in our patients met the criteria for association with mucosal barrier injury. Importantly, separate tracking of MBI and non-MBI cases revealed the number of preventable central line-associated bloodstream infections, i.e., those not associated with mucosal barrier injury. These cases would not have been differentiated by the previous bloodstream infection surveillance definition.23 Separate tracking also reduced the chance that the overall reduction of CLABSI rates between 2011 and 2012 (Table 1) would be misinterpreted. Because both MBI-LCBI and non-MBI-LCBI rates declined during this period, evidence-based strategies implemented in the pediatric cancer center in 2011 could have been misunderstood as effective against both types of infection. However, only the reduction of non-MBI-LCBI rates was statistically significant. This reduction of preventable LCBI rates was the result of a policy that improved line maintenance by limiting the use of less well trained “float” nurses in the pediatric oncology center, providing central line maintenance by trained nurses, and establishing mentoring and active surveillance of CLABSI. However, these strategies may not have reduced the translocation of intestinal microbiota. Finally, presenting the MBI and non-MBI rates separately helped to build the credibility of the local team and confirm the effectiveness of their efforts.
Benchmark rates of bloodstream infections based on the revised CDC surveillance protocol at pediatric cancer centers are not yet available. Our overall CLABSI rate, including MBI and non-MBI LCBI, was 5.72/1000 CL days, similar to the pooled rate of 6.07/1000 CL days in pediatric intensive care units in LMICs.2 However, annual rates of CLABSI differed after removal of MBI-LCBIs from the total (3.22/1,000 vs. 5.72/1,000 CL days). Moreover, our device use ratio (0.99) differed from the pooled mean reported in LMIC pediatric intensive care units (0.5),2 suggesting the likelihood of a greater number of CLABSI among our patients.21 Benchmarks for LMICs that categorize central venous access device utilization ratios for oncology by type of device should be derived by using CDC methods.24 Because access to temporary and permanent central lines may vary in developing countries depending on availability of supplies, the use of CDC methods would allow comparison of CLABSI rates among institutions with similar DURs by type of central line (temporary or permanent).
Neutropenia was the MBI underlying condition in all of our MBI-LCBI cases, as our center does not offer allogeneic hematopoietic stem cell transplantation. A previous study in adult hematology/oncology and stem cell transplant settings has also reported MBI-LCBI events to be associated mainly with neutropenia but without a significant difference in the frequency of neutropenia in MBI-LCBI vs. non-MBI–LCBI cases.15 However, we found a statistically significant difference in the frequency of neutropenia between MBI and non-MBI LCBI events in pediatric cancer patients. Additional studies in pediatric cancer centers in LMIC that offer allogeneic hematopoietic stem cell transplantation would help to determine whether the absence of transplantation at our center may explain this difference.
We found that patients with MBI-LCBI had a significantly greater median length of stay. This finding differs from those of a similar study in adult oncology patients in high-income countries.15 The difference may be attributable to other risk factors identified as statistically significant in our study but not in the similar adult study. We found that patients with MBI-LCBI had more often received chemotherapy during the same admission or during the previous 2 weeks; this factor may have contributed to the length of stay in our patients. Moreover, social factors such as illiteracy, poverty, and distance from the cancer center (longer travel time) may prevent early discharge among pediatric cancer patients in LMICs.
The types of microorganisms isolated in our MBI-LCBI cases differed from those reported in neutropenic pediatric oncology patients experiencing similar events in high-income countries.3 We found more gram-negative than gram-positive pathogens. While the U.S. Hematology-oncology CLABSI Collaborative Project (a network of more than 36 pediatric oncology centers) reported viridans streptococci in 14% of CLABSI events in neutropenic patients, 3 we observed none. This difference may be attributable to patient characteristics and the use of different chemotherapy regimens. Van der Velden et al. compared myeloablative vs. non-myeloablative conditioning regimens. Bacteremia due predominantly to oral viridans streptococci and coagulase-negative staphylcocci was found in as many as 85% of patients after intensive myeloablative versus none among patients receiving non-myeloablative conditioning regimens.25 Our study did not include patients undergoing transplantion, and information about specific chemotherapy regimens was not collected.
Importantly, most CLABSI events meeting the criteria for MBI-LCBI appeared to be related to chemoterapy, whose hematopoietic toxicity is not likely to be altered by CLABSI quality improvement interventions. Therefore, such interventions will affect mainly the rate of non-MBI cases. Future studies should seek to identify interventions that reduce the likelihood of MBI-LCBI events, which were significantly associated with a longer length of stay. Further, the relation between specific chemotherapy protocols and MBI-LCBI in LMICs should be studied. Implementation of a standardized CLABSI surveillance protocol that includes the MBI-LCBI criteria could expedite benchmarking efforts of pediatric oncology networks and identify additional quality improvement opportunities in LMICs.
Our study had several limitations. The sample size of our study group was relatively small, and because it was conducted at a single center it cannot represent pediatric cancer patients in all LMICs. Moreover, we were not able to control for confounding factors, such as severity of illness and LOS prior to CLABSI onset, which would have independently contributed to the post-CLABSI length of stay and risk of mortality. A future prospective study, with a larger sample size and multicenter participation, must be conducted to address this limitation.
In summary, we noted a decrease in overall CLABSI rates when MBI-LCBI cases were considered separately. Our findings not only identified significant differences between MBI and non-MBI LCBI cases in our pediatric cancer patients but will also encourage infection control professionals in LMICs to implement the MBI-LCBI surveillance protocol at pediatric cancer centers. Finally, our findings demonstrate the usefulness of implementing the MBI-LCBI surveillance protocol in LMICs, especially when a well-equipped microbiology laboratory and a dedicated infection prevention and control team are available. Twinning initiatives such as that between St. Jude, Rady Children's Hospital, and Hospital Tijuana help to establish infection control and prevention programs and prospective infection surveillance activities which are essential for quality healthcare delivery in pediatric cancer centers in LMICs. Sustaining such efforts in the long-term requires the buy-in and support of the institution hosting the pediatric cancer center.
Highlights.
Forty-four percent of CLABSI events met the NHSN criteria for MBI-LCBI
Removal of MBI-LCBI events from the numerator nearly halved the CLABSI rate.
Patients with MBI-LCBI were significantly younger than those with non-MBI-LCBI.
Patients with MBI-LCBI had significantly longer median hospitalization.
The CDC/NHSN MBI-LCBI surveillance protocol can be implemented in LMICs.
Acknowledgments
The authors thank Sharon Naron for editing the manuscript. We thank all oncology physicians and nurses who participated in or supported the infection control team to reduce CLABSIs in the pediatric oncology center at Hospital Tijuana. These doctors and nurses are: Angélica Martinez, MD, Rebeca Rivera, MD, Laura Nuno, MD, Mario Ornelas, MD, Martha Magdaleno, MD, Magdalena Pérez, MD, Daniel Valencia, RN, Braulia Soto, RN, Mitzy Romano, RN, Verónica Martinez, RN, Alicia Sánchez, RN, Amanda Acosta, RN, Miriam Armenta, RN and Martha Morones, RN.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenthal VD, Bijie H, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control. 2012 Jun;40(5):396–407. doi: 10.1016/j.ajic.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal VD, Maki DG, Mehta Y, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007-2012. Device-associated module. Am J Infect Control. 2014 Sep;42(9):942–956. doi: 10.1016/j.ajic.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Gaur AH, Bundy DG, Gao C, et al. Surveillance of hospital-acquired central line-associated bloodstream infections in pediatric hematology-oncology patients: lessons learned, challenges ahead. Infect Control Hosp Epidemiol. 2013 Mar;34(3):316–320. doi: 10.1086/669513. [DOI] [PubMed] [Google Scholar]
- 4.Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis. 2008;8:70. doi: 10.1186/1471-2334-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015 Mar;43(3):206–221. doi: 10.1016/j.ajic.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am J Infect Control. 2013 Apr;41(4):286–300. doi: 10.1016/j.ajic.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudeck MA, Weiner LM, Allen-Bridson K, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am J Infect Control. 2013 Dec;41(12):1148–1166. doi: 10.1016/j.ajic.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaur AH, Miller MR, Gao C, et al. Evaluating application of the National Healthcare Safety Network central line-associated bloodstream infection surveillance definition: a survey of pediatric intensive care and hematology/oncology units. Infect Control Hosp Epidemiol. 2013 Jul;34(7):663–670. doi: 10.1086/671005. [DOI] [PubMed] [Google Scholar]
- 9.Hazamy PA, Haley VB, Tserenpuntsag B, et al. Effect of 2013 National Healthcare Safety Network definition changes on central line bloodstream infection rates: Audit results from the New York State Department of Health. Am J Infect Control. 2015 Mar;43(3):280–282. doi: 10.1016/j.ajic.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 10.CDC. [Accessed 3/31/2015];The Targeted Assessment for Prevention Strategy. 2014 http://www.cdc.gov/hai/prevent/tap.html.
- 11.Fraser TG, Gordon SM. CLABSI rates in immunocompromised patients: a valuable patient centered outcome? Clin Infect Dis. 2011 Jun;52(12):1446–1450. doi: 10.1093/cid/cir200. [DOI] [PubMed] [Google Scholar]
- 12.Sexton DJ, Chen LF, Anderson DJ. Current definitions of central line-associated bloodstream infection: is the emperor wearing clothes? Infect Control Hosp Epidemiol. 2010 Dec;31(12):1286–1289. doi: 10.1086/657583. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg JP, Coffin SE. Improving the central line-associated bloodstream infection surveillance definition: a work in progress. Infect Control Hosp Epidemiol. 2013 Aug;34(8):777–779. doi: 10.1086/671369. [DOI] [PubMed] [Google Scholar]
- 14.See I, Iwamoto M, Allen-Bridson K, Horan T, Magill SS, Thompson ND. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol. 2013 Aug;34(8):769–776. doi: 10.1086/671281. [DOI] [PubMed] [Google Scholar]
- 15.Metzger KE, Rucker Y, Callaghan M, et al. The burden of mucosal barrier injury laboratory-confirmed bloodstream infection among hematology, oncology, and stem cell transplant patients. Infect Control Hosp Epidemiol. 2015 Feb;36(2):119–124. doi: 10.1017/ice.2014.38. [DOI] [PubMed] [Google Scholar]
- 16.CDC. [Accessed 5/5/2015];Surveillance for Central Line-associated Bloodstream Infections (CLABSI):New NHSN HAI CLABSI, Secondary BSI Slides Training. 2015 Feb; 2015; http://www.cdc.gov/nhsn/PDFs/training/2015/CLABSI-2015.pdf. 2015.
- 17.Aristizabal P, Fuller S, Rivera R, Beyda D, Ribeiro RC, Roberts W. Improving Pediatric Cancer Care Disparities Across the United States-Mexico Border: Lessons Learned from a Transcultural Partnership between San Diego and Tijuana. Front Public Health. 2015;3:159. doi: 10.3389/fpubh.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Website NHSN. [Accessed 4/13/2015];Central Line-Associated Bloodstream Infection (CLABSI) Event. http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf.
- 19.Gonzalez M, Maysam H, Relyea G, Caniza M. Interventions for enhancing an online infection control course for Latin American countries. Antimicrobial Resistance and Infection Control. 2015;4(Suppl 1):254. [Google Scholar]
- 20.Caniza MA, Maron G, McCullers J, et al. Planning and implementation of an infection control training program for healthcare providers in Latin America. Infect Control Hosp Epidemiol. 2007 Dec;28(12):1328–1333. doi: 10.1086/521655. [DOI] [PubMed] [Google Scholar]
- 21.System NNIS. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004 Dec;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 22.CDC. [Accessed 3/31/2015];Primary Bloodstream Infection. 2014 http://www.cdc.gov/nhsn/forms/57.108_PrimaryBSI_BLANK.pdf.
- 23.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008 Jun;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.CDC. [Accessed 3/31/2015];Surveillance for Central Line-associated Bloodstream Infections (CLABSI) 2014 http://www.cdc.gov/nhsn/forms/57.117_DenominatorSCA_BLANK.pdf.
- 25.van der Velden WJ, Herbers AH, Feuth T, Schaap NP, Donnelly JP, Blijlevens NM. Intestinal damage determines the inflammatory response and early complications in patients receiving conditioning for a stem cell transplantation. PLoS One. 2010;5(12):e15156. doi: 10.1371/journal.pone.0015156. [DOI] [PMC free article] [PubMed] [Google Scholar]