Abstract
Cardiovascular responses to smoking cessation may differ in adolescents compared to adults. We administered nicotine by osmotic minipump infusion for 17 days to adolescent and adult rats (30 and 90 days of age, respectively) and examined cardiac norepinephrine levels during treatment, after withdrawal, and for months after cessation. In adults, nicotine evoked a significant elevation of cardiac norepinephrine and a distinct spike upon withdrawal, after which the levels returned to normal; the effect was specific to males. In contrast, adolescents did not show significant changes during nicotine treatment or in the immediate post-withdrawal period. However, beginning in young adulthood, males exposed to adolescent nicotine showed sustained elevations of cardiac norepinephrine, followed by later-emerging deficits that persisted through six months of age. We then conducted adolescent exposure using twice-daily injections, a regimen that augments stress associated with inter-dose withdrawal episodes. With the injection route, adolescents showed an enhanced cardiac norepinephrine response, reinforcing the relationship between withdrawal stress and a surge in cardiac norepinephrine levels. The relative resistance of adolescents to the acute nicotine withdrawal response is likely to make episodic nicotine exposure less stressful or aversive than in adults. Equally important, the long-term changes after adolescent nicotine exposure resemble those known to be associated with risk of hypertension in young adulthood (elevated norepinephrine) or subsequent congestive heart disease (norepinephrine deficits). Our findings reinforce the unique responses and consequences of nicotine exposure in adolescence, the period in which most smokers commence tobacco use.
Keywords: Adolescence, Heart, Nicotine, Norepinephrine, Sex differences, Withdrawal stress
INTRODUCTION
It is increasingly clear that the heightened vulnerability of the adolescent to nicotine addiction entails underlying biological differences in nicotine responsiveness, with the adolescent brain showing exquisite sensitivity to nicotine (Dwyer et al., 2009; Lydon et al., 2014; Slotkin, 2008). Much less attention has been paid to the potential role of differential responses to nicotine withdrawal between adolescents and adults. Adolescents are resistant to activation or inhibition of reward pathways during withdrawal (Natividad et al., 2012) and tend to show lesser withdrawal signs, affective problems or anxiety compared to adults (O’Dell et al., 2006; O’Dell et al., 2007; Wilmouth and Spear, 2006). Although the mechanisms underlying these differences remain to be elucidated, there is increasing evidence that differences in neurotransmitter responses to withdrawal underlie the behavioral disparities (Carcoba et al., 2014; Slotkin, 2002).
The current study focuses on peripheral autonomic components that may distinguish nicotine administration and withdrawal in the adolescent vs. the adult. Withdrawal from smoking in adults triggers autonomic stress responses, notably sympathetic activation, that are thought to contribute to difficulties in smoking cessation (Kotlyar et al., 2006; Sofuoglu et al., 2003); these include exacerbation of cardiac and vascular responses to acute stress (al’Absi et al., 2002; Vanderkaay and Patterson, 2006). In contrast, smoking cessation in adolescents does not trigger heart rate increases (Rubinstein et al., 2009) and may actually reduce heart rate (Killen et al., 2001). There is little known about potential central or peripheral neuronal mechanisms that may underlie these differences, nor is there any information as to whether nicotine exposure and/or withdrawal in adolescence might evoke persistent changes in autonomic function. Adolescent nicotine uniquely elicits long-term alterations in the function of central monoamine systems (Slotkin et al., 2007a; Slotkin and Seidler, 2009; Trauth et al., 2001), raising the possibility that lasting effects might also occur in peripheral sympathetic activity. Here, we compared the effects of nicotine administration and withdrawal in adolescent and adult rats, assessing cardiac norepinephrine levels during treatment, during withdrawal, and for months after the termination of exposure. We also evaluated the specific role of withdrawal stress in the effects on cardiac norepinephrine by comparing continuously-administered nicotine (osmotic minipump infusions) to repeated nicotine injections, which elicit inter-dose withdrawal episodes (Abreu-Villaça et al., 2003; Slotkin, 1998, 2002). Our studies provide some of the first evidence for immediate and persistent biological differences in the autonomic effects of nicotine between adolescents and adults.
MATERIALS AND METHODS
Animal treatments
Although the determinations presented here are entirely new, the tissue samples were archived from earlier studies (Abreu-Villaça et al., 2003; Abreu-Villaça et al., 2004; Slotkin et al., 2008; Slotkin and Seidler, 2009) and were maintained at −45° C, so that no additional animals were actually used. Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) were shipped by climate-controlled truck (transit time <1 hr) at least one week prior to commencing drug treatments. Animals were allowed free access to food and water and were housed individually to avoid potential problems with the surgical site that could occur with multiple animals per cage. Adolescent nicotine treatment commenced at 30 days of age. For treatments by minipump infusion, each rat was quickly anesthetized with ether, a 2 × 2 cm area on the back was shaved, and an incision was made to permit subcutaneous insertion of an Alzet osmotic minipump (Durect Corp., Cupertino, CA), either type 1002 (two week infusion) or 1007D (one week infusion). Pumps were prepared with nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) dissolved in bacteriostatic water (Abbott Laboratories, N. Chicago, IL), to deliver an initial dose rate of 6 mg/kg of nicotine (calculated as free base) per day. The incision was closed with wound clips and the animals were permitted to recover in their home cages. Control animals were implanted with minipumps containing only the water and an equivalent concentration of sodium bitartrate. It should be noted that the pumps actually take 17.5 (type 1002 pump) or 8 days (type 1007D) to be exhausted completely (information supplied by the manufacturer) and thus the nicotine infusions terminated at 47 days of age and 38 days of age, respectively; we previously showed that exhaustion of the pumps occurs at the predicted time by direct measurement of plasma nicotine and cotinine levels (Trauth et al., 2000). Plasma nicotine levels achieved with this administration model resemble those seen in typical smokers (25 ng/ml) as characterized previously (Trauth et al., 2000). For nicotine infusions in adults, type 2ML2 pumps were implanted at 90 days of age as already described, again set to deliver either vehicle or nicotine at an initial dose rate of 6 mg/kg per day, with the infusion terminating at 107 days of age, thus producing a exposure parallel to the longer infusion period in adolescents.
Experiments in adolescents utilizing subcutaneous nicotine injections were designed to deliver the same daily dose of nicotine in a bolus, thus providing a maximum pharmacokinetic contrast to the continuous infusion model. We administered nicotine for 7 days beginning at 30 days of age, delivered as two injections of 3 mg/kg 12 hours apart, the maximum tolerated dose (Abreu-Villaça et al., 2003). Control animals received injections of bacteriostatic water containing an equivalent concentration of sodium bitartrate.
Studies were conducted at time points encompassing the period of nicotine administration, the posttreatment withdrawal period and long-term changes out to 6 months of age. Animals were decapitated and tissues were dissected, frozen in liquid nitrogen, and stored at −45°C until assayed.
Assays
The samples were thawed on ice and deproteinized by homogenization in 0.1 N perchloric acid containing 3,4-dihydroxybenzylamine (Sigma) as an internal standard. Homogenates were sedimented at 26,000 × g for 20 minutes, the supernatant solutions were decanted, and norepinephrine was then trace-enriched by alumina adsorption, separated by reverse-phase high performance liquid chromatography and quantitated by electrochemical detection (Seidler and Slotkin, 1981); values were corrected for recovery of the internal standard.
Data analysis
To avoid the increase in type 1 errors that could occur from multiple statistical tests on the same data, each set of determinations was first evaluated using multivariate ANOVA considering all relevant variables (treatment, sex, age) in a single test; data were log-transformed because of heterogeneous variance between males and females, and among the different ages. Interactions of treatment with the other factors triggered subdivisions, each of which was then tested with lower-order tests. As permitted by the interaction terms, individual differences from controls were identified post-hoc with Fisher’s Protected Least Significant Difference Test. However, where treatment effects were not interactive with other variables, we report only the main treatment effects without performing lower-order analyses of individual values. Significance was assumed at the level of p < 0.05.
Data were compiled as means and standard errors. For ready visualization of treatment effects across the different studies and ages, the results are given as the percent change from control values; Table 1 provides the corresponding control values, normalized across all studies. Statistical procedures were always conducted on the original data, with log transforms because of heterogeneous variance as noted above, and with treatment comparisons made only against the contemporaneous control group.
Table 1.
Control Norefpinephrine Concentration (ng/g tissue)
| Age (days) | Heart | Cerebellum | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 37 | 581 ± 33 | 624 ± 33 | 195 ± 12 | 182 ± 10 |
| 45 | 548 ± 11 | 639 ± 18 | 190 ± 9 | 230 ± 10 |
| 50 | 586 ± 23 | 616 ± 29 | — | — |
| 60 | 502 ± 25 | 684 ± 34 | — | — |
| 65 | 562 ± 17 | 696 ± 24 | 235 ± 6 | 230 ± 8 |
| 75 | 497 ± 32 | 748 ± 50 | — | — |
| 105 | 670 ± 31 | 976 ± 53 | — | — |
| 110 | 602 ± 25 | 970 ± 51 | — | — |
| 120 | 573 ± 25 | 843 ± 45 | — | — |
| 180 | 723 ± 24 | 1067 ± 45 | — | — |
RESULTS
Adolescent nicotine infusion
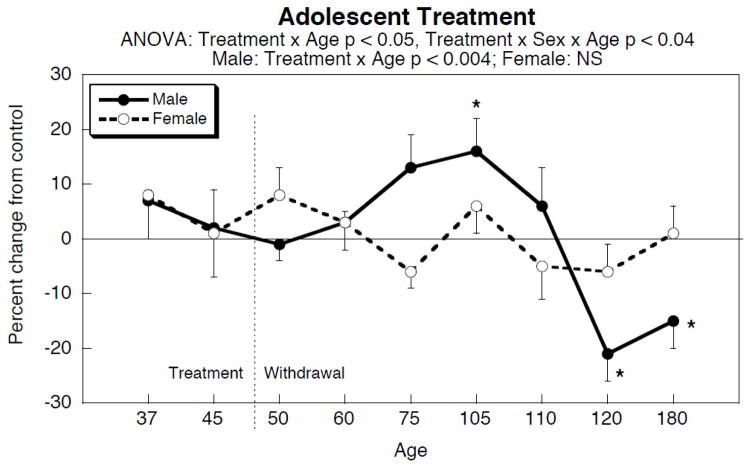
Nicotine infusions given to adolescents had effects on cardiac norepinephrine that were highly dependent on both age and sex (Figure 1). Neither males nor females showed any significant changes during the period of nicotine treatment, nor did withdrawal trigger a change. However, beginning nearly a month after the termination of nicotine exposure, males exhibited a significant increase in cardiac norepinephrine (p < 0.02 for the period from 75 to 105 days of age) that peaked a month later and then declined. Subsequently, a long-term deficit emerged that was still present at six months of age. In contrast, females showed no significant overall change in cardiac norepinephrine throughout this period.
Figure 1.
Effects of adolescent nicotine administration and withdrawal on cardiac norepinephrine levels. Treatment took place from 30 to 47 days of age via the infusion route. Data represent means and standard errors of 6–20 animals for each sex at each age. ANOVA appears at the top of the panel; asterisks denote age points at which the nicotine group differs significantly from the corresponding control. Abbreviation: NS, not significant.
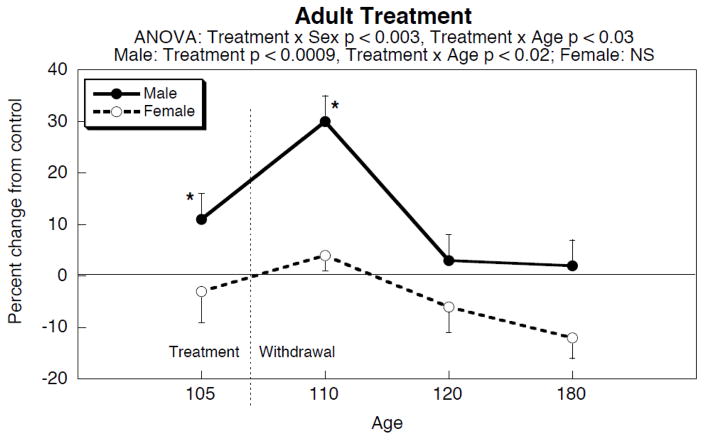
Adult nicotine infusion
A very different pattern was obtained in adults given nicotine (Figure 2). For males, there was a small, but significant elevation during the period of nicotine treatment, and then withdrawal elicited a sharp spike. Values declined to normal ten days later and remained within control limits throughout the ensuing two months, out to 180 days of age. At that point, the values for females were subnormal, but this effect should be interpreted with caution, since the overall ANOVA did not find a significant treatment effect or treatment × age interaction in females. We then conducted a statistical comparison of the effects of nicotine in adults to those in adolescents. For the corresponding withdrawal age points (50 days in adolescents, 110 days in adults), we found a significant difference in the sequelae of nicotine exposure between the two age groups for males (p < 0.0009) but not females. For the long-term effects (120 and 180 days of age for both adolescents and adults), the adolescent nicotine effect was again distinguishable from that in adult (p < 0.0008) in males but not females.
Figure 2.
Effects of adult nicotine administration and withdrawal on cardiac norepinephrine levels. Treatment took place from 90 to 107 days of age via the infusion route. Data represent means and standard errors of 12 animals for each sex at each age. ANOVA appears at the top of the panel; asterisks denote age points at which the nicotine group differs significantly from the corresponding control. Abbreviation: NS, not significant.
Adolescent nicotine infusions vs. injections
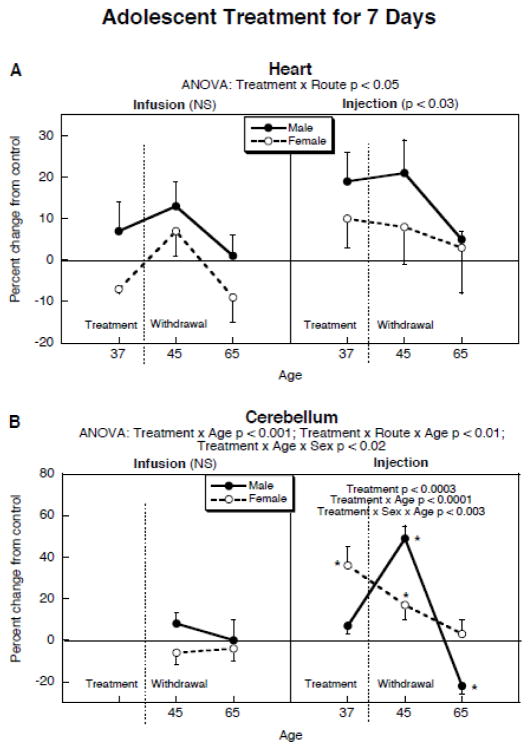
To evaluate different patterns of nicotine administration in adolescents (continuous vs. intermittent), we performed an additional study comparing the nicotine infusion model to the same total daily dose of nicotine delivered by twice-daily subcutaneous injections, carried out over a one week period beginning at 30 days of age. Initially, nicotine injection elicited a brief period (10–15) of respiratory stimulation, impaired motor activity and blanching of the skin indicative of vasoconstriction (Abreu-Villaça et al., 2003); however, tolerance developed to all these effects by the second day (third injection). Again, nicotine infusions did not have a statistically significant effect on cardiac norepinephrine during treatment or withdrawal, although there was a trend toward higher values in males than females (Figure 3A). Nicotine injections produced an exaggerated response, with significant overall elevations of norepinephrine levels. We did not find significant interactions of the nicotine injection effect with age or sex, but this may just reflect the lower number of subjects and fewer time points in this study, and the correspondingly lower statistical power for detecting interactions. It should be noted that we found a greater effect of injected nicotine as compared to infused nicotine despite the fact that the infusion model actually delivered the drug for one day longer (through 38 days of age) than did the injections.
Figure 3.
Comparison of nicotine delivered to adolescents by infusion vs. injection: (A) heart norepinephrine levels, (B) cerebellum norepinephrine levels (note different y-axis scales). Treatment took place beginning at 30 days of age and lasted for one week. Data represent means and standard errors of 6 animals for each sex at each age. ANOVA appears at the top of the panels. For the heart, individual age points were not compared because of the absence of a treatment × age interaction. For the cerebellum, lower-order ANOVAs were significant for both males (treatment × age, p < 0.0001) and females (treatment, p < 0.0005; treatment × age, p < 0.04), with the interaction terms enabling determinations of significance for individual age points (asterisks). Abbreviation: NS, not significant.
We also evaluated whether nicotine injections in adolescents would produce a heightened response for central noradrenergic pathways (Figure 3B). Just as in the heart, nicotine infusions did not significantly affect cerebellar norepinephrine levels. In contrast, nicotine injections had a profound effect that again, was highly sex-dependent, although in this case, both males and females showed significant effects. In males, withdrawal initiated a large increase in cerebellar norepinephrine; three weeks later, they showed significant deficits of about the same magnitude as the long-term effects of nicotine infusions on heart norepinephrine. Females displayed a significant increase in cerebellar norepinephrine during treatment with nicotine injections, followed by a decline during withdrawal, falling to normal levels by three weeks after cessation.
Finally, to resolve whether the effects of nicotine injections on cerebellar norepinephrine reflected acute intoxication episodes vs. episodic withdrawal stress, we examined a group receiving a lower dose of nicotine (1 mg/kg s.c. twice daily), which was devoid of overt toxicity. Males still showed a withdrawal spike (treatment × age interaction, p < 0.004), although the magnitude was smaller: 6 ± 6% increase during treatment at 37 days of age, rising to 16 ± 4% increase upon withdrawal at 45 days. More importantly, though, the lower dose of nicotine still elicited a long-term deficit of about the same magnitude as seen with the 3 mg/kg treatment (17 ± 3% decrease at 65 days of age, p < 0.002). Females receiving the lower dose also showed a pattern similar to that of the higher dose, with a smaller, but significant overall increase in cerebellar norepinephrine that declined after cessation of treatment: 16 ± 5% above control at 37 days of age, 10 ±4% at 45 days, 3 ± 4% at 65 days (p < 0.02 for the main effect of nicotine).
DISCUSSION
Our results indicate that adolescents differ substantially from adults in the cardiac sympathetic response to nicotine treatment and withdrawal, both in the immediate impact and for long-term effects. In adults, nicotine evoked an elevation in cardiac norepinephrine that spiked upon withdrawal, an effect that was restricted to males. In contrast, no such effects were seen in adolescents given an equivalent nicotine regimen. These findings provide a neural mechanism underpinning the cardiovascular differences seen in adolescent and adult smokers (al’Absi et al., 2002; Killen et al., 2001; Kotlyar et al., 2006; Rubinstein et al., 2009; Sofuoglu et al., 2003; Vanderkaay and Patterson, 2006). Since autonomic effects contribute to withdrawal distress, our findings point to a lessened impact in adolescents, potentially fostering the ability to maintain sporadic or episodic smoking with lessened aversive consequences.
More importantly, though, adolescent males uniquely showed long-term effects on cardiac norepinephrine, characterized by a sustained period of hyperactivity in early adulthood, followed by deficits that emerged later and persisted into the equivalent of middle age (six months). Thus, adolescent nicotine exposure changes the trajectory of cardiac sympathetic innervation, such that differences emerge and persist long after the immediate effects of treatment or withdrawal. These autonomic effects echo the delayed appearance of abnormalities in central noradrenergic projections evoked by adolescent nicotine (Trauth et al., 2001), indicating a more general targeting of norepinephrine neurons regardless of their location. Future studies are needed to address the functional consequences of the synaptic abnormalities, particularly with regard to blood pressure control or other aspects of cardiovascular function. Notably, cardiac sympathetic overdrive in young adulthood contributes to hypertension risk (Grassi et al., 2015), whereas later-life deficits are a hallmark of congestive heart disease (Chidsey and Braunwald, 1966). The magnitude of the long-term deficits in cardiac norepinephrine seen here, are about one-third the magnitude of those seen in terminal stages of congestive heart failure (Chidsey and Braunwald, 1966).
We also evaluated two other factors that contribute to age-related differences in nicotine responses: sex and route of administration. The fact that males showed much greater susceptibility than females reinforces the importance of sex differences in the effects of adolescent nicotine exposure. It is increasingly clear that males and females differ substantially in their patterns of acquisition of nicotine dependence and of their response to withdrawal, both in terms of symptomatology and underlying neural mechanisms (DiFranza, 2008; DiFranza et al., 2002; Jacobsen et al., 2007; Kandel et al., 1994; Panday et al., 2007; Roberts et al., 2005; Slotkin, 2002). Sex differences in nicotine response appear to be common to multiple neurotransmitter pathways (Slotkin et al., 2007b; Slotkin and Seidler, 2009) and thus represent a fundamental difference in the addiction process. Clearly, this can have important consequences for smoking cessation strategies, which will need to take into account the different neural substrates of addiction and withdrawal in males compared to females.
Our studies in adolescents, comparing repeated nicotine injections to nicotine infusions, were designed to exploit the short half-life of nicotine in rats; the injection route emphasizes inter-dose withdrawal stress and acute nicotine effects, both of which are minimized with infusions (Abreu-Villaça et al., 2003; Slotkin, 1998, 2002). The effects on cardiac norepinephrine were exaggerated with the nicotine injection route, so that adolescents began to show an elevation akin to that seen with adult infusions, again with a trend toward greater effects in males. This exacerbation was even more pronounced for central noradrenergic pathways. For males, withdrawal produced a large increase in cerebellar norepinephrine, followed by decrements three weeks later, akin to the long-term deficits in cardiac norepinephrine seen with the longer-term infusions. In females, nicotine injections elevated cerebellar norepinephrine during treatment, with a gradual return to normal after cessation. Importantly, we obtained the same patterns when the nicotine dose was reduced to 1 mg/kg, which did not elicit any acute signs of intoxication. This reinforces the interpretation that the effects of nicotine treatment and withdrawal on noradrenergic systems, regardless of central or peripheral location, are enhanced by stress-related components, including repeated, episodic withdrawal.
In conclusion, cardiac noradrenergic responses to nicotine treatment and withdrawal are fundamentally different in adolescents as compared to adults. Adolescents do not show the spike of activity triggered by withdrawal, but instead show late-emerging changes that do not occur with adult treatment. The latter comprise two phases, a sustained period of elevation in young adulthood, followed by deficits persisting into middle age. The lack of acute withdrawal responses is likely to make episodic nicotine exposure less stressful or aversive in adolescents, whereas the long-term changes resemble those known to be associated with risk of hypertension in young adulthood or subsequent congestive heart disease. Our findings reinforce the unique responses and consequences of nicotine exposure in adolescence, the period in which most smokers commence tobacco use.
Highlights.
Nicotine increases heart norepinephrine in adult rats, and spikes upon withdrawal
This response is absent in adolescents given nicotine
Adolescents instead show later-emerging, persistent changes in heart norepinephrine
Adolescent nicotine may produce less aversive autonomic responses to withdrawal
Persistent effects are consistent with elevated risk of hypertension and CHD
Acknowledgments
Research was supported by NIH ES022831 and EPA 83543701. EPA support does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Abbreviations
- ANOVA
analysis of variance
Footnotes
Conflict of interest statement
TAS has received consultant income in the past three years from the following firms: Acorda Therapeutics (Ardsley NY), Carter Law (Peoria IL), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX), Taylor, Reams, Tilson & Harrison (Morristown, NJ) and Chaperone Therapeutics (Research Triangle Park, NC).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaça Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacol Biochem Behav. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- Carcoba LM, Orfila JE, Natividad LA, Torres OV, Pipkin JA, Ferree PL, Castaneda E, Moss DE, O’Dell LE. Cholinergic transmission during nicotine withdrawal is influenced by age and pre-exposure to nicotine: implications for teenage smoking. Dev Neurosci. 2014;36:347–355. doi: 10.1159/000360133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidsey CA, Braunwald E. Sympathetic activity and neurotransmitter depletion in congestive heart failure. Pharmacol Rev. 1966;18:685–700. [PubMed] [Google Scholar]
- DiFranza JR. Hooked from the first cigarette. Sci Am. 2008;298(5):82–87. doi: 10.1038/scientificamerican0508-82. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30-month follow-up data from the DANDY study. Tobacco Control. 2002;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Exp Clin Psychopharmacol. 2001;9:176–182. doi: 10.1037//1064-1297.9.2.176. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Brauer LH, al’Absi M, Adson D, Robiner W, Thuras P, et al. Effect of bupropion on physiological measures of stress in smokers during nicotine withdrawal. Pharmacol Biochem Behav. 2006;83:370–379. doi: 10.1016/j.pbb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Lydon DM, Wilson SJ, Child A, Geier CF. Adolescent brain maturation and smoking: what we know and where we’re headed. Neurosci Biobehav Rev. 2014;45:323–342. doi: 10.1016/j.neubiorev.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Parsons LH, Torres OV, O’Dell LE. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. J Neurochem. 2012;123:578–588. doi: 10.1111/j.1471-4159.2012.07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panday S, Reddy SP, Ruiter RAC, Bergstrom E, de Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolescent Health. 2007;40:144–150. doi: 10.1016/j.jadohealth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Roberts KH, Munafò MR, Rodriguez D, Drury M, Murphy MFG, Neale RE, Nettle D. Longitudinal analysis of the effect of prenatal nicotine exposure on subsequent smoking behavior of offspring. Nicotine Tobacco Res. 2005;7:801–808. doi: 10.1080/14622200500262840. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. Withdrawal in adolescent light smokers following 24-hour abstinence. Nicotine Tobacco Res. 2009;11:185–189. doi: 10.1093/ntr/ntn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Development of central control of norepinephrine turnover and release in the rat heart: responses to tyramine, 2-deoxyglucose and hydralazine. Neuroscience. 1981;6:2081–2086. doi: 10.1016/0306-4522(81)90047-6. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res Bull. 2008;76:152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at six months of age. Neuropsychopharmacology. 2007a;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Tate CA, Seidler FJ. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain Res Bull. 2007b;73:259–272. doi: 10.1016/j.brainresbull.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Nicotine exposure in adolescence alters the response of serotonin systems to nicotine administered subsequently in adulthood. Dev Neurosci. 2009;31:58–70. doi: 10.1159/000207494. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb D, Hatsukami DK. Labetalol treatment enhances the attenuation of tobacco withdrawal symptoms by nicotine in abstinent smokers. Nicotine Tobacco Res. 2003;5:947–953. doi: 10.1080/14622200310001615312. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Vanderkaay MM, Patterson SM. Nicotine and acute stress: effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biol Psychiat. 2006;71:191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2006;85:648–657. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]