Abstract
Background
Androgen deprivation therapy (ADT) can decrease the physical performance (PP) of older men with prostate cancer (PC).
Methods
We conducted a three-arm randomized pilot study (n = 19) comparing a home-based walking and resistance intervention (EXCAP) and a technology-mediated walking and resistance intervention using Wii-Fit to a usual-care-arm in men ≥ 70 years with PC receiving ADT. The intervention lasted for 6-weeks, with follow-up at 12-weeks. The primary pre-specified outcome was change in Short Physical Performance Battery (SPPB) score. Mixed effects regression models were used to assess change in outcomes over time.
Results
Mean participant age was 70 years (range: 67-93). Eight patients were randomized to the Wii-Fit-arm, 6 to EXCAP-arm, and 5 to usual-care-arm. SPPB scores remained nearly constant in the usual-care-arm (β=−0.12; p=0.79), while individuals in the EXCAP-arm had on average a 1.2 point increase at each follow-up (β=1.20; 95% CI: 0.36, 2.06). The Wii-fit-arm had a non-significant increase in SPPB score over time relative to usual-care (β=0.32; 95% CI −0.43,1.06; p=0.46).
Individuals in the EXCAP-arm had an increase in steps per day over time compared to the usual-care-arm (p-value = 0.006); the EXCAP-arm had an increase of 2720 steps (95% CI: 1313, 4128) while the usual-care-arm had an increase of 97 steps (95% CI: −1140, 1333). Participants in the Wii-Fit-arm had an increase of 1020 steps (95% CI: −474, 1238, p=0.710). Other outcomes (i.e., handgrip strength, lean muscle mass, and chest press repetitions) were not statistically significant.
Conclusions
A home-based aerobic and resistance exercise program, EXCAP, shows promise for improving PP in older men with PC on ADT.
Keywords: Androgen deprivation therapy, elderly, older adults, prostate cancer, Wii-Fit, home-based exercise interventions
INTRODUCTION
Prostate cancer (PC) is the most common non-cutaneous malignancy in men (1). Over two thirds of PC is diagnosed in men aged 70 and older.(1) The use of androgen deprivation therapy (ADT) is first line therapy for patients with PC recurrence, including men with biochemical recurrence (BCR) or those with metastatic disease.(2) ADT is accomplished by orchiectomy or gonadotrophin-releasing hormone (GnRH) agonists, lowering testosterone levels to castrate levels (7-30 ng/dL). Once initiated, ADT is typically continued life-long. The use of ADT is increasing in various clinical settings, such as primary therapy,(3) adjuvant therapy after radical prostatectomy,(4) and BCR.(5) Since older men are more likely to be diagnosed with indolent cancers and live with PC as a chronic disease, they are exposed to long-term effects of ADT including cardiovascular complications, osteoporosis, fatigue, weakness, and obesity.(6, 7) The effects of ADT on muscle strength, muscle mass, and physical performance (PP) are well-known, especially in older patients.(8, 9) ADT increases lean muscle loss and decreases muscle strength in older men with PC, which can lead to a decrease in PP and functional abilities in older men.(10, 11)
The Short Physical Performance Battery (SPPB) is an objective test that assesses timed gait speed, balance, and chair stands (a marker of muscle strength), and it is an important measure of PP in older adults.(12) In a pilot study, over 50% of older patients aged ≥ 70 on ADT with asymptomatic PC exhibited impaired SPPB scores of ≤ 9(10). Gait speed was significantly worse in older men on ADT compared to a usual-care group.(9, 13)
The benefits of exercise in improving the PP of older adults have been the focus of recent research.(14, 15) Exercise interventions in an older patient with PC on ADT should include a multi-component exercise program focusing on improving PP, reducing loss of muscle strength and mass, and improving aerobic capacity.(16) Few randomized studies to date have evaluated the role of home-based combined resistance and aerobic exercise interventions in men on ADT.(16-19) The home-based aerobic and resistance program (EXCAP) combines resistance exercises using resistance bands, with an individually tailored walking-prescription.(20) It has demonstrated an improvement in PP in patients with breast cancer and PC receiving radiation therapy.(20, 21) Technology-based interventions such as the Wii-Fit as a home-based exercise modality (22) is considered safe for use at home (23) and has been reported as enjoyable for balance training in unimpaired older adults (24) and impaired older adults without cancer.(25) The potential benefits of Wii-Fit and EXCAP (improved balance, PP and fall reduction) in older men with PC on ADT have not been studied to date.
In accordance with other literature describing the purpose for conducting pilot studies (e.g., deriving preliminary efficacy estimates to design larger trials, assessing feasibility, fidelity or safety),(26) we conducted a randomized-three arm pilot trial designed to assess feasibility (as measured by number of steps per 7 days) and preliminary data on possible impact of EXCAP and a comparable program using Wii-Fit on PP (as measured by SPPB) in older men on ADT.
MATERIALS AND METHODS
Sample
Eligibility criteria were chosen to identify older PC patients on ADT who were at high risk of PP problems. The sample consisted of men aged ≥ 65 years who were receiving ADT for at least three months for histologically-confirmed PC. Patients had to have stable disease on ADT (stable or declining PSA, no progressive clinical symptoms, and no new metastatic lesions for one month prior to study entry). Subjects with >5 errors on the Short Portable Mental Status Questionnaire (SPMSQ) (27), signifying possible cognitive impairment, were excluded from the study. Although men needed to be sedentary, those with significant PP impairment (i.e. unable to walk 4 meters as part of SPPB measured walk) were excluded because they would be unable to perform the interventions. Sedentary was defined by patients not performing activity in a systemic, organized manner for the purpose of improving physical fitness or those who were not exercising regularly more than once weekly. Further, patients who were in active or maintenance physical activity, i.e. men planning to change their exercise behavior as documented by a standard screening questionnaire, the Stages of Exercise Stage Questionnaire, were excluded.(28)
Other inclusion criteria included the ability to read and understand English and receipt of care at the University of Rochester or the University of Chicago Genito-urinary Medical Oncology clinics. Both sites received IRB approval from their respective institutions for conducting the study.
Study Design
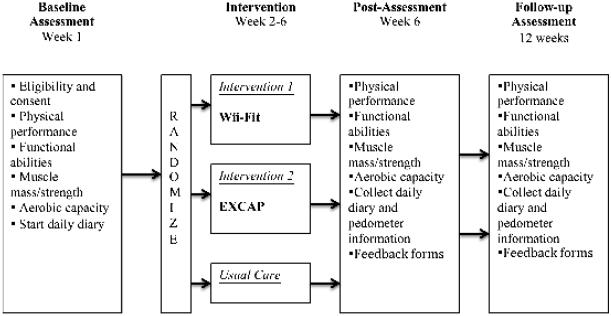
In this three-arm, randomized pilot study, patients were randomized to a tailored, multi-component, home-based aerobic and progressive resistance exercise program (EXCAP), a tailored, multi-component technology-mediated exercise program utilizing the Wii-Fit technology (Wii-Fit), or usual-care. An American College of Sports Medicine (ACSM)-trained exercise physiologist, designated at each site, performed the objective tests and explained the home-based exercise programs to participants. Weekly reminders were conducted by phone to facilitate completion of the diary and pedometer assessments for all groups, until the 6-week post-intervention visit. Patients continued exercise interventions on their own between 6-weeks and 12-weeks of follow-up. Assessments were conducted at baseline, 6-weeks (post-intervention) and 12-weeks (follow-up). (Figure 1). The investigators, study statistician, and data managers remained blinded at all times.
Figure 1.
Study Schema
Recruitment and Baseline Assessment
After providing written informed consent, demographic, comorbidity and exercise history were obtained and the SPMSQ was administered.(27) Eligible patients performed the SPPB,(12) a 6-minute walk test,(29) a handgrip dynamometry test(30) and a chest-press maximum repetition test (31) (see Measurements.) Patients were instructed to wear pedometers during waking hours and complete a daily diary for one week. At the end of the week, patients who completed at least 70% of their daily diary entries were considered sufficiently adherent with study procedures (i.e. completed 5 of 7 days of the diary) to continue. A dual-energy X-ray absorptiometry (DEXA) scan was completed on day 7. When the baseline assessments were completed, patients were randomly assigned to one of the three study arms.
Measurements
Except for clinical data, all measures were obtained at each of the time points.
a) Clinical Data
A questionnaire recorded demographic, comorbidity, diagnostic, treatment information including time on ADT and body mass index. PSA was collected from the medical record. Subjects were screened with Exercise Stages of Change Form (28) to confirm they were not already actively exercising.
b) Functional and Aerobic Measures
i) SPPB
The primary outcome, objective physical performance, was assessed with the SPPB. The SPPB, an objective, clinically-relevant measure of physical performance was the pre-specified primary outcome of the pilot study.(32) A score less than 10 (out of a possible 12) indicates an increased risk of incident disability and mortality.(12)
ii) 6-minute walk test (33)
Participants walked at their normal speed for a total of 6 minutes and covered as much distance as they could during this time. The total distance walked in meters was recorded and used to calculate an estimate of aerobic capacity.
c) Skeletal muscle strength and muscular mass measure
iii) Handgrip dynamometer (30)
Trials were performed in an alternating bilateral sequence for a total of six attempts (three with each arm), and a rest period of 30 seconds was required between each trial. An average of the measures from the three trials was recorded.
iv) Chest press repetition maximum test: The standard chest press repetition maximum test was used to estimate patients’ repetition maximums for bench press (pectoralis and deltoid muscles).(31)
iv) DEXA Scan: DEXA can provide measures of body composition, specifically fat mass and lean mass, from which estimates of skeletal muscle mass can be derived.(34)
Interventions
Each intervention was designed to provide similar modes, intensities, and durations of the exercise components. Exercises were designed by the Physical Exercise Activity and Kinesiology Core (PEAK) Human Performance Clinical Research Core Lab at the University of Rochester and were designed to adhere to the ACSM guidelines for exercise testing. Patients randomized to the exercise arms received a single, 45-minute, instructional session with an ACSM-trained exercise physiologist.
Prior to study initiation, video-conferences were held between principal investigators, co-investigators, exercise physiologists and research coordinators at both sites. Research coordinators at both sites underwent training to standardize use of equipment including SPPB, handgrip dynamometers , use of Wii-Fit, the 6-minute walk test and chest press repetition maximum test. A standard operating procedure checklist was implemented to ensure procedural consistency at both sites.
i) EXCAP
Patients received an “exercise kit” which contained instructions and materials for use of the pedometer and resistance bands. EXCAP-arm consists of two components, aerobic and resistance, which are outlined below. (20, 21)
The first component was a moderately intense aerobic walking exercise program meeting 60%–70% of heart rate reserve and a 3–5 exercise rating of perceived exertion on the ACSM revised rating scale (35) at least 5 days a week. Patients were instructed to increase their total steps walked each day by 5% to 20% each week and were encouraged to reach the ACSM-suggested 10,000 steps a day. A pedometer calculated the average number of steps walked daily. Participants wore their pedometer during waking hours and noted their daily steps in a diary. Steps per day were calculated by adding the total number of steps walked over seven days and dividing the total number by seven.
The second component of the exercise program was a tailored therapeutic resistance band exercise prescription designed to provide low to moderate intensity progressive resistance exercises (3–5 exercise rating of perceived exertion on the ACSM revised rating scale) at least 5 days a week. Patients were given a set of three color-coded therapeutic resistance bands, each representing different resistance levels. Patients were instructed to begin with an individually determined number of sets (1 set = 8–15 repetitions) for each of the exercises for a minimum of 5 days a week. Patients were instructed to increase the intensity by switching from low to moderate resistance bands, or by shortening the initial length of the band. They were instructed to progressively increase from their baseline sets and repetitions to a maximum of 4 sets of 15 repetitions for each exercise daily.
ii) Technology Mediated Home-Based Interventions (Wii-Fit)
This exercise program was individually tailored and designed to deliver a similar mode, intensity, and duration of exercise as EXCAP with the addition of a balance component. The patient was provided with an individual “exercise kit”. The kit contained instructions and materials (Wii-Fit technology, instruction manual and pedometer).
A pedometer was used to calculate the average number of steps walked daily. Wii-Fit technology delivers the exercise program using different exercises modules. The exercise physiologist tailored the exercises to each subject during the baseline assessment. Exercise programs of increasing intensity were unlocked as the patients demonstrated increase in physical performance.
ANALYTICAL METHODS
A change in SPPB score of 1.0 to 1.5 is considered clinically meaningful.(32) In order to provide an 80% power and a type 1 error rate of 0.05 for detecting a difference for a mean change score in SPPB of 1.2 between the three arms, an expected standard deviation of 0.5, a goal sample size of 18 subjects was needed. However, due a larger standard deviation for SPPB (2-3.4 depending on arm) and baseline differences in SPPB, a linear mixed-effects model was conducted, still using the pre-specified primary outcome of SPPB change scores. The potential mediators of the exercise interventions included changes in muscle mass (as measured by DEXA), muscle strength (as measured by handgrip dynamometer and chest press repetition maximum test), and aerobic capacity (as measured by 6-minute walk). These secondary analyses were exploratory, and due to the statistical power limitations of the study, findings were considered to be hypothesis-generating.
Statistical Analysis
The distribution of covariates by study group were compared, using ANOVA and Pearson chi-square tests as appropriate, to determine whether there are significant differences in demographic and clinical covariates at baseline. Next, the change in the pre-specified primary outcome, the SPPB, for each participant who completed at least one follow-up visit was compared with changes in outcomes from baseline to each follow up visit for each study group. Mixed-effects linear regression models with a maximum-likelihood estimation method were used to assess the rate of change in the primary (SPPB) and each of the four secondary outcomes (steps per day, handgrip strength, lean muscle mass, chest press repetition maximum) over the three study time points. These models assess the overall rate of change in study outcome over time, while accounting for variability in individual trajectories. These models do not require complete follow-up data, and thus allow the inclusion of individuals who have only one follow-up visit (36). In the first set of models, we included covariates for the assigned study group, each time point, and a (time x study group) interaction term. The interaction term tests whether the rate of change for each intervention group was significantly different from the rate of change in the usual-care-arm. We included age, race, and education in the models to account for differences in baseline demographics and socioeconomic status, and we included PSA in the models because of concern about baseline differences between groups (p=0.09) which may reflect an increased disease burden. Model coefficients were presented along with 95% confidence intervals and p-values. All statistical analysis was conducted using Stata 12.1.
RESULTS
Thirty-one patients were initially pre-screened and consented for the study, of which 19 completed the baseline assessment, 19 completed the 6-week assessment, and 13 completed the 12-week assessment. Our final analysis is based on 19 fully-evaluable patients with at least 2 time points (11 from University of Rochester, 8 from University of Chicago). Following recruitment, 12 patients did not fulfill minimum eligibility criteria of completing their daily diary 5 out of 7 days of the screening period and therefore were not randomized. Among the 6 drop-outs between 6-week and 12-week assessment, 3 were in the Wii-Fit-arm, 1 in the EXCAP-arm and 2 in the usual-care-arm. Among the three dropouts in the Wii-Fit-arm, one patient lost his Wii-Fit, while two reported loss of interest in the exercises. The dropout in the EXCAP-arm stated exercises were tedious. The two dropouts in the usual-care-arm found filling daily diaries to be cumbersome.
Table 1 presents the baseline sample characteristics. Patients in Wii-Fit-arm had non-significantly higher baseline SPPB scores (9.1) compared to usual-care-arm (7.8) (p=0.69). Handgrip strength was non-significantly higher in usual-care-arm compared to EXCAP-arm (handgrip strength: 29.1 kg in Wii-fit-arm, 33.8 kg in EXCAP-arm, 34.7 kg in usual-care-arm, p=0.69). Baseline differences existed in lean muscle mass between the three groups (lean muscle mass: 49 kg in Wii-Fit-arm, 56.3 kg in EXCAP-arm, 59.4 kg in usual-care-arm, p=0.04).
Table 1.
Sample baseline characteristics
| Variables |
Wii
(n=8) N (%) or Mean (SD) |
EXCAP
(n=6) N (%) or Mean (SD) |
Control
(n=5) N (%) or Mean (SD) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 77.5 (6.7) | 75.7 (9.5) | 71.8 (5) | 0.41 |
| Range | 70-87 | 67-93 | 67-80 | |
| Marital Status | ||||
| Married | 6 (75.0) | 3 (50.0) | 4 (80.0) | 0.49 |
| Unmarried | 2 (25.0) | 3 (50.0) | 1 (20.0) | |
| Education | ||||
| HS/Some | ||||
| college | 6 (75.0) | 3 (50.0) | 2 (40.0) | 0.41 |
| Bachelors+ | 2 (25.0) | 3 (50.0) | 3 (60.0) | |
| Work | ||||
| Working | 3 (37.5) | 4 (66.7) | 1 (20.0) | 0.28 |
| Not working | 5 (62.5) | 2 (33.3) | 4 (80.0) | |
| Race | ||||
| White | 7 (87.5) | 4 (66.7) | 3 (60.0) | 0.49 |
| African | ||||
| American | 1 (12.5) | 2 (33.3) | 2 (40.0) | |
|
| ||||
|
Clinical
characteristics |
||||
| BMI | 29.5 (4.2) | 28.5 (3.5) | 31.8 (4.7) | 0.43 |
| IADL | 11.1 (1.7) | 11.8 (0.4) | 11.4 (0.5) | 0.56 |
| KPS | 87.5 (8.9) | 88.3 (13.3) | 88 (16.4) | 0.99 |
| Time on ADT (months) | 67.4 (67.9) | 58.7 (56.3) | 60 (66.6) | 0.96 |
| Gleason Score | 7.1 (1.6) | 8.0 (1) | 7.6 (1.3) | 0.54 |
| PSA | 1.9 (2.9) | 2.4 (3.1) | 21.9 (30.2) | 0.09 |
| Comorbidity | ||||
| 0 to 1 | 6 (75.0) | 4 (66.7) | 3 (60.0) | 0.96 |
| 2 | 2 (25.0) | 2 (33.3) | 2 (40.0) | |
| Outcomes | ||||
| SPPB | 9.1 (2.0) | 8.7 (2.8) | 7.8 (3.4) | 0.69 |
| Handgrip | 29.1 (6.2) | 33.8 (11.0) | 34.7 (6.8) | 0.41 |
| Steps per day | 2999.9 (2627.9) | 3593.9 (2777.1) | 2625.7 (2286.1) | 0.82 |
| Muscle Mass | 49 (6.2) | 56.3 (6.8) | 59.4 (8.2) | 0.04 |
| Chest press repetition max |
59.1 (12.2) | 78.8 (29.1) | 72 (19.2) | 0.22 |
Abbreviations: BMI – Body Mass Index, IADL – instrumental activities of daily living, KPS – Karnofsky Performance Status, ADT – Androgen deprivation therapy, PSA – Prostate-specific antigen, SPPB –Short physical performance battery P-values represent comparisons of covariates across study arms using ANOVA or Pearson Chi-Square tests
* Two individuals with high PSA were in the control arm with values of 44.8 and 63.5
In terms of feasibility, individuals in the EXCAP-arm had a higher rate of change in steps per day compared to the usual-care (p<0.01); the usual-care-arm had an increase in number of steps by 97 steps (95% CI: - 1140,1333) while the EXCAP-arm had an increase of 2720 steps at each follow-up (95% CI: 1313,4128). Patients in the Wii-Fit-arm reported a non-significant increase of 382 steps at each follow-up (95% CI: - 473,1238; p=0.71).
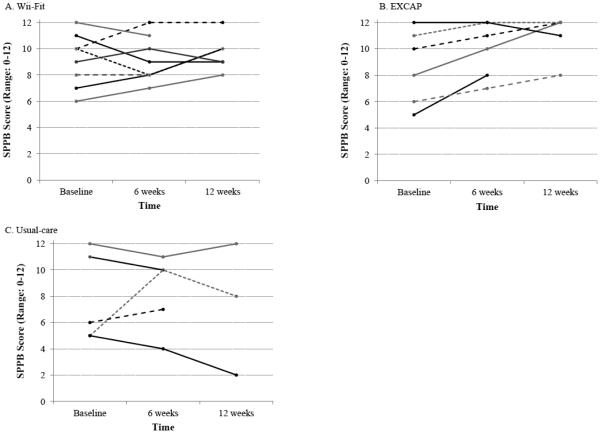
Table 2 presents the mean SPPB score and other secondary outcomes at each time point for each group. Overall, the EXCAP-arm had a 2.3 point change in SPPB score after 12 weeks, compared to 0.6 point in Wii-Fit arm and −0.5 points in the usual-care arm. Figure 2 illustrates the change in SPPB score for each participant with at least one follow-up visit.
Table 2.
Study outcomes at each time point (n=18)
| Wii (n=8) | EXCAP (n=6) | Control (n=5) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variables (N) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) |
| SPPB | ||||||
| Baseline (18) | 9.1 | (7.7, 10.5) | 8.7 | (6.4, 10.9) | 7.8 | (4.8, 10.8) |
| 6 weeks (18) | 9.1 | (7.9, 10.3) | 10.0 | (8.3, 11.7) | 8.4 | (5.9, 10.9) |
| 12 weeks (13) | 9.7 | (8.6, 10.8) | 11.0 | (9.5, 12.5) | 7.3 | (1.6, 13) |
| Handgrip* | ||||||
| Baseline (18) | 29.1 | (24.7, 33.4) | 33.8 | (25, 42.5) | 34.7 | (28.7, 40.7) |
| 6 weeks (18) | 30.7 | (25.2, 36.2) | 35.5 | (25.2, 45.8) | 34.2 | (30.5, 37.9) |
| 12 weeks (13) | 28.0 | (22.7, 33.2) | 39.5 | (34.6, 44.5) | 32.7 | (27.6, 37.9) |
| Steps per day | ||||||
| Baseline (18) | 2999.9 | (1178.9, 4821) | 3593.9 | (1371.8, 5816.1) | 2625.7 | (621.9, 4629.5) |
| 6 weeks (18) | 4223.7 | (2110.6, 6336.7) | 5544.3 | (2308.5, 8780.2) (3482.2, 14718.8) |
2242.3 | (315.3, 4169.3) |
| 12 weeks (13) | 4019.1 | (880.3, 7157.9) | 9100.5 | 2655.0 | (1130.1, 4179.9) | |
|
Lean muscle
mass** |
||||||
| Baseline (18) | 49.0 | (44.7, 53.3) | 56.3 | (50.9, 61.7) | 59.4 | (52.2, 66.6) |
| 6 weeks (18) | 49.8 | (44.6, 55.1) | 56.0 | (50.7, 61.2) | 57.8 | (49, 66.6) |
| 12 weeks (13) | 47.4 | (41.3, 53.4) | 53.2 | (45.8, 60.7) | 66.9 | (59.1, 74.6) |
|
Chest press
reps*** |
||||||
| Baseline (18) | 59.1 | (50.6, 67.6) | 78.8 | (55.5, 102) | 72.0 | (55.1, 88.9) |
| 6 weeks (18) | 63.8 | (55.9, 71.6) | 82.9 | (59.2, 106.7) | 73.5 | (57.3, 89.7) |
| 12 weeks (13) | 59.2 | (44.7, 73.6) | 89.0 | (66.1, 111.9) | 70.8 | (49.6, 92.1) |
Abbreviations: SPPB –Short physical performance battery; Chest press reps –Chest press repetition maximum
Hand grip was measured in kilograms
Lean muscle mass was measured in kilograms
Chest press reps were measured in kilograms
Figure 2.
Change in SPPB Score for each participant by study group
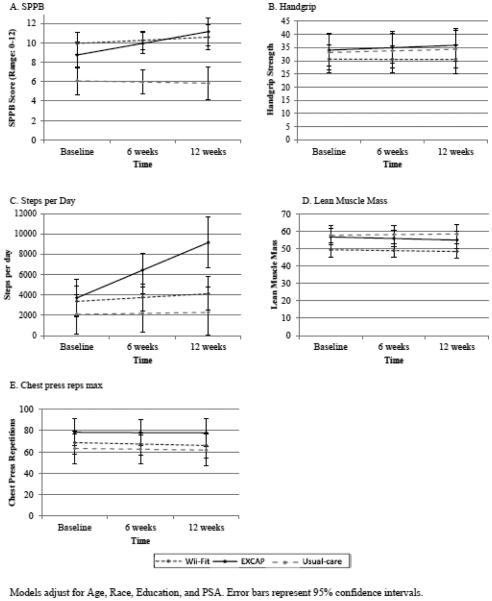
Results from the mixed-effects linear regressions are presented in Table 3, and the adjusted model-based estimates of each outcome over time for each study arm are illustrated in Figure 3. Individuals in the EXCAP- arm had a greater change in SPPB score over time compared to the usual-care (p=0.04); SPPB scores remained nearly constant in the usual-care-arm (β=−0.12; p=0.79), while individuals in the EXCAP-arm had on average a 1.2 point increase at each follow-up (β=1.20; 95% CI: 0.36, 2.06). The Wii-fit-arm had a non-significant increase in SPPB score over time relative to usual-care (β=0.32; 95% CI −0.43,1.06; p=0.46).
Table 3.
Mixed effects linear regression comparing rate of change in study outcomes by study group (n=18)
| Coefficient | 95% CI | p-value | |
|---|---|---|---|
| SPPB | |||
| Wii | 3.41 | (0.67,6.14) | 0.015 |
| EXCAP | 1.33 | (−1.57,4.23) | 0.369 |
| Time | −0.12 | (−1.04,0.79) | 0.790 |
| Time × Wii | 0.44 | (−0.74,1.62) | 0.461 |
| Time × EXCAP | 1.33 | (0.08,2.58) | 0.038 |
|
| |||
| Handgrip | |||
| Wii | −2.01 | (−11.96,7.95) | 0.693 |
| EXCAP | 0.53 | (−9.82,10.89) | 0.919 |
| Time | 0.57 | (−1.28,2.42) | 0.543 |
| Time × Wii | −0.59 | (−2.95,1.78) | 0.628 |
| Time × EXCAP | 0.34 | (−2.16,2.84) | 0.791 |
|
| |||
| Steps per day | |||
| Wii | 987.37 | (− 2499,68,4474.42) |
0.579 |
| EXCAP | −1002.50 | (− 4939.64,2934.64) |
0.618 |
| Time | 96.58 | (− 1140.05,1333.21) |
0.878 |
| Time × Wii | 285.48 | (− 1221.68,1792.64) |
0.710 |
| Time × EXCAP | 2623.83 | (756.18,4491.48) | 0.006 |
|
| |||
| Lean muscle mass | |||
| Wii | −7.54 | (−14.9,−0.16) | 0.045 |
| EXCAP | 0.30 | (−7.36,7.96) | 0.939 |
| Time | 0.37 | (−0.8,1.54) | 0.540 |
| Time × Wii | −0.83 | (−2.26,0.59) | 0.252 |
| Time × EXCAP | −1.26 | (−2.74,0.22) | 0.095 |
|
| |||
| Chest press reps | |||
| Wii | 6.12 | (−15.50,27.74) | 0.579 |
| EXCAP | 14.73 | (−7.86,37.32) | 0.201 |
| Time | −0.73 | (−5.82,4.35) | 0.777 |
| Time × Wii | −0.63 | (−7.14,5.88) | 0.850 |
| Time × EXCAP | 0.46 | (−6.42,7.33) | 0.897 |
Model is adjusted controls for Age, Race, Education, and PSA.
Change in outcomes data shown include patients who completed the study1
Figure 3.
Adjusted estimates of the change in outcomes over time by study groups
Among the secondary outcomes, we observed an increase in grip strength and chest press repetitions in EXCAP-arm compared to usual care arm. Grip strength increased from 33.8 kg (95% CI: 25, 42.5) to 39.5 kg (95% CI: 34.6, 44.5) at 12 weeks; chest press repetitions had an increase from 78.8 kg (95% CI: 50.6, 67.6) to 89.0 kg (95% CI: 66.1, 111.9) at 12 weeks. These observed changes were not significant. No differences over time were noted with lean muscle mass.
DISCUSSION
The combination of a novel home-based aerobic and resistance exercise program (EXCAP) improved PP in older PC patients on ADT. Improvement in PP was shown by improved SPPB compared to usual-care in the EXCAP arm; total number of steps was also higher in the EXCAP arm. The EXCAP program was well tolerated with no adverse effects. These findings are consistent with prior research showing improvements in quality of life and physical function in patients with breast cancer and PC receiving radiation therapy.(21, 37, 38) The Wii-Fit intervention showed a non-significant improvement in SPPB scores compared with usual-care.
Older men on ADT are a vulnerable population. Previous studies have demonstrated a loss of lean muscle mass on the order of 2-5% within weeks of initiating ADT.(17, 39, 40) Loss of lean body mass and muscular strength (i.e. sarcopenia) due to ADT are associated with weakness, physical decline, and falls in older men.(10) Our study demonstrates that a tailored exercise program for older men with PC on ADT may improve PP. These results are consistent with several studies that support the benefits of exercise interventions in men on ADT with improvements in physical well-being and quality of life.(17-19, 41) Although using different measures of strength, others have found similar improvements in lean mass, muscle strength, PP, and balance using combined resistance and aerobic programs. In a larger study, 155 men were randomized to a supervised resistance exercise program three times per week for 12-weeks or to a usual-care group.(17) Men assigned to resistance exercise had higher levels of muscular fitness (p<.001), less fatigue (p =.002), and higher quality of life (p =.001) than men in the usual-care group. (35) Limitations to prior studies were lack of a tailored or multi-component design, and lack of PP outcomes, such as SPPB. These measures are of importance since they correlate with improved function and quality of life (QoL) and can predict adverse outcomes in the elderly.(42) EXCAP demonstrated more impact on PP possibly through improved adherence. Number of steps increased by 2.5 fold in EXCAP-arm. An increase in steps may allow patients to increase their daily activities and function. (43, 44)
Technology has potential for increasing adherence to physical activity.(45, 46) In 2008, Nintendo launched Wii-Fit, a computerized exercise program that included strength training, aerobics, and balance activities. (47, 48) A Wii-Fit based intervention improved mobility, walking endurance, and decreased fear of falling among older residents in assisted living facilities. (49-51) The Wii-Fit program requires minimal space and equipment at home.(52) Elders who participated in Wii-Fit exercises at least 3 times per week over 4-12-weeks showed similar improvements in balance and strength as other exercise programs.(51) However, our study was unable to replicate these findings in older men with PC on ADT. In our patient population, the Wii-Fit program showed a trend towards improvement in PP and steps, but these findings were not significant. EXCAP was more effective at improving PP in comparison to Wii-Fit. This is likely due to a robust resistance exercise component utilizing resistance exercise bands. Wii-fit and EXCAP had similar aerobic exercise components and therefore we do not suspect that these aerobic exercises contribute to statistically significant improvement in PP. These findings are consistent with prior literature establishing that resistance training provides substantial increase in muscle strength and endurance, while smaller improvements are noted with aerobic training.(53) With respect to future trials, Wii-Fit would not satisfy the requirements for adequate resistance to improve PP in our patient population. Future trials would utilize EXCAP given statistically significant improvement in PP.
The limitations of this study include, men in both exercise arms were older and with higher SPPB scores compared with usual-care-arms. Baseline differences in lean muscle mass were noted between the three arms. However, our study compared muscle mass within each arm. The small sample size may explain why handgrip strength and chest press repetition maximum did not achieve statistical significance, and limits the capture of adverse events. Our interpretation of outcomes was limited with 6 dropouts from a total of 19 patients and cannot be addressed with imputation-based calculations given small sample size. We attempted to select a group of patients who were motivated. Our results may not be generalizable to cognitively impaired older adults or adults who are already physically active. Sedentary was defined with a qualitative definition rather than using quantitative methodology (time and METS), which may be a limitation to how patients were selected.
Despite these limitations, the results of this pilot study provides evidence that home-based aerobic resistance exercise could improve PP of older men on ADT.
CONCLUSIONS
Strengthening muscles through resistance exercises and aerobic exercises may improve PP among older men with PC on ADT. Large phase III randomized-controlled trials designed to study a home-based resistance exercise program using EXCAP are needed to validate and expand these findings.
Acknowledgments
Funding Source
University of Chicago Clinical and Translational Science Award – Short-term studies by Post-Doctoral Clinician-Scientists, University of Chicago (to Saleha Sajid). University of Rochester Clinical and Translational Science pilot award R03 AG042342 from the National Institute on Aging: and R25 CA102618, UG1 CA18961, and R01 CA177592 from the National Cancer Institute (to Supriya Mohile).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in an Oral Presentation at International Society of Geriatric Oncology (SIOG) in September 2012, Manchester, U.K.
Disclosures and Conflict of Interest Statements
The authors have no conflicts of interest to disclose.
Author Contributions
Study Concepts: S Mohile, K Mustian, S Sajid, W Dale Study Design: S Mohile, K Mustian, W Dale
Data Acquisition: S Sajid, W Dale, K Mustian, A Kotwal, C Heckler, M Porto, C Fung, S Mohile Quality Control of Data and Algorithms: S Sajid, W Dale, K Mustian, A Kotwal, C Heckler, M Porto, C Fung, S Mohile
Data Analysis and Interpretation: A Kotwal, C Heckler, S Sajid, W Dale, S Mohile Statistical Analysis: S Sajid, W Dale, K Mustian, A Kotwal, C Heckler, M Porto, C Fung, S Mohile Manuscript Preparation: S Sajid, W Dale, K Mustian, A Kotwal, C Heckler, M Porto, C Fung, S Mohile Manuscript Editing: S Sajid, W Dale, K Mustian, A Kotwal, C Heckler, M Porto, C Fung, S Mohile Manuscript Review: S Sajid, W Dale, K Mustian, A Kotwal, C Heckler, M Porto, C Fung, S Mohile
Conflicts of interest: None
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW. Prostate specific antigen only progression of prostate cancer. The Journal of urology. 2000;163(6):1632–42. [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103(8):1615–24. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. Journal of the National Cancer Institute. 2003;95(13):981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen EK, Wickstrom JE, Vahlberg T, Duchesne GM. Trends in the use of androgen deprivation in prostate cancer. Acta oncologica. 2004;43(4):382–7. doi: 10.1080/02841860410029500. [DOI] [PubMed] [Google Scholar]
- 6.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(27):4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 7.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. The New England journal of medicine. 2005;352(2):154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 8.Mohile SG, Mustian K, Bylow K, Hall W, Dale W. Management of complications of androgen deprivation therapy in the older man. Critical reviews in oncology/hematology. 2009;70(3):235–55. doi: 10.1016/j.critrevonc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alibhai SM, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(34):5038–45. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 10.Bylow K, Dale W, Mustian K, Stadler WM, Rodin M, Hall W, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008;72(2):422–7. doi: 10.1016/j.urology.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109(4):802–10. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Clay CA, Perera S, Wagner JM, Miller ME, Nelson JB, Greenspan SL. Physical function in men with prostate cancer on androgen deprivation therapy. Physical therapy. 2007;87(10):1325–33. doi: 10.2522/ptj.20060302. [DOI] [PubMed] [Google Scholar]
- 14.Sprod LK, Mohile SG, Demark-Wahnefried W, Janelsins MC, Peppone LJ, Morrow GR, et al. Exercise and Cancer Treatment Symptoms in 408 Newly Diagnosed Older Cancer Patients. Journal of geriatric oncology. 2012;3(2):90–7. doi: 10.1016/j.jgo.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301(18):1883–91. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culos-Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18(5):591–9. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 17.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(9):1653–9. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 18.Buffart LM, Galvao DA, Chinapaw MJ, Brug J, Taaffe DR, Spry N, et al. Mediators of the resistance and aerobic exercise intervention effect on physical and general health in men undergoing androgen deprivation therapy for prostate cancer. Cancer. 2014;120(2):294–301. doi: 10.1002/cncr.28396. [DOI] [PubMed] [Google Scholar]
- 19.Winters-Stone KM, Dobek JC, Bennett JA, Dieckmann NF, Maddalozzo GF, Ryan CW, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Archives of physical medicine and rehabilitation. 2015;96(1):7–14. doi: 10.1016/j.apmr.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustian KM, Sprod Lisa, Janelsins Michelle Christine, Peppone Luke Joseph, Mohile Supriya Gupta, Trevino Lara Anne, Gewandter Jennifer S., Chandwani Kavita Dayal, Heckler Charles E., Morrow Gary R. EXCAP exercise to improve fatigue, cardiopulmonary function, and strength: A phase II RCT among older prostate cancer patients receiving radiation and androgen deprivation therapy. Journal of Clinical Oncology. 2012;30(15) [Google Scholar]
- 21.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. The journal of supportive oncology. 2009;7(5):158–67. [PMC free article] [PubMed] [Google Scholar]
- 22.Clark RA, Bryant AL, Pua Y, McCrory P, Bennell K, Hunt M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait & posture. 2010;31(3):307–10. doi: 10.1016/j.gaitpost.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Pluchino A, Lee SY, Asfour S, Roos BA, Signorile JF. Pilot study comparing changes in postural control after training using a video game balance board program and 2 standard activity-based balance intervention programs. Archives of physical medicine and rehabilitation. 2012;93(7):1138–46. doi: 10.1016/j.apmr.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson VP, McKean M, Lowe J, Fawcett C, Burkett B. Six weeks of unsupervised Nintendo Wii Fit gaming is effective at improving balance in independent older adults. Journal of aging and physical activity. 2015;23(1):153–8. doi: 10.1123/japa.2013-0148. [DOI] [PubMed] [Google Scholar]
- 25.Barry G, Galna B, Rochester L. The role of exergaming in Parkinson's disease rehabilitation: a systematic review of the evidence. Journal of neuroengineering and rehabilitation. 2014;11:33. doi: 10.1186/1743-0003-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of psychiatric research. 2011;45(5):626–9. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 28.Dannecker EA, Hausenblas HA, Connaughton DP, Lovins TR. Validation of a stages of exercise change questionnaire. Research quarterly for exercise and sport. 2003;74(3):236–47. doi: 10.1080/02701367.2003.10609088. [DOI] [PubMed] [Google Scholar]
- 29.Enright PL. The six-minute walk test. Respiratory care. 2003;48(8):783–5. [PubMed] [Google Scholar]
- 30.Budziareck MB, Pureza Duarte RR, Barbosa-Silva MC. Reference values and determinants for handgrip strength in healthy subjects. Clinical nutrition. 2008;27(3):357–62. doi: 10.1016/j.clnu.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Rydwik E, Karlsson C, Frandin K, Akner G. Muscle strength testing with one repetition maximum in the arm/shoulder for people aged 75 + - test-retest reliability. Clinical rehabilitation. 2007;21(3):258–65. doi: 10.1177/0269215506072088. [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 33.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–98. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 34.Trutschnigg B, Kilgour RD, Reinglas J, Rosenthall L, Hornby L, Morais JA, et al. Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual-energy X-ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008;33(6):1232–9. doi: 10.1139/H08-122. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons R, HD, Du Toit S. Advances in Analysis of Longitudinal Data. Annual review of clinical psychology. 2010;(6):79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncology nursing forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- 38.Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psycho-oncology. 2005;14(6):464–77. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 39.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63(4):742–5. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 40.Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU international. 2008;102(1):44–7. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 41.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(2):340–7. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 42.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) The journal of nutrition, health & aging. 2009;13(6):538–44. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmeli E, Kessel S, Coleman R, Ayalon M. Effects of a treadmill walking program on muscle strength and balance in elderly people with Down syndrome. The journals of gerontology Series A, Biological sciences and medical sciences. 2002;57(2):M106–10. doi: 10.1093/gerona/57.2.m106. [DOI] [PubMed] [Google Scholar]
- 44.Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. Journal of the American Geriatrics Society. 2003;51(3):387–92. doi: 10.1046/j.1532-5415.2003.51113.x. [DOI] [PubMed] [Google Scholar]
- 45.Graves LE, Ridgers ND, Williams K, Stratton G, Atkinson G, Cable NT. The physiological cost and enjoyment of Wii Fit in adolescents, young adults, and older adults. Journal of physical activity & health. 2010;7(3):393–401. doi: 10.1123/jpah.7.3.393. [DOI] [PubMed] [Google Scholar]
- 46.Studenski S, Perera S, Hile E, Keller V, Spadola-Bogard J, Garcia J. Interactive video dance games for healthy older adults. The journal of nutrition, health & aging. 2010;14(10):850–2. doi: 10.1007/s12603-010-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugarman HW-EA, Burstin A. Use of the Wii Fit system for the treatment of balance problems in the elderly: A feasibility study; Virtual Rehabilitation International Conference.2009. pp. 111–6. [Google Scholar]
- 48.Nitz JC, Kuys S, Isles R, Fu S. Is the Wii Fit a new-generation tool for improving balance, health and well-being? A pilot study. Climacteric : the journal of the International Menopause Society. 2010;13(5):487–91. doi: 10.3109/13697130903395193. [DOI] [PubMed] [Google Scholar]
- 49.Bateni H. Changes in balance in older adults based on use of physical therapy vs the Wii Fit gaming system: a preliminary study. Physiotherapy. 2012;98(3):211–6. doi: 10.1016/j.physio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Chao YY, Scherer YK, Wu YW, Lucke KT, Montgomery CA. The feasibility of an intervention combining self-efficacy theory and Wii Fit exergames in assisted living residents: A pilot study. Geriatric nursing. 2013;34(5):377–82. doi: 10.1016/j.gerinurse.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Jorgensen MG, Laessoe U, Hendriksen C, Nielsen OB, Aagaard P. Efficacy of Nintendo Wii training on mechanical leg muscle function and postural balance in community-dwelling older adults: a randomized controlled trial. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(7):845–52. doi: 10.1093/gerona/gls222. [DOI] [PubMed] [Google Scholar]
- 52.Bieryla KA, Dold NM. Feasibility of Wii Fit training to improve clinical measures of balance in older adults. Clinical interventions in aging. 2013;8:775–81. doi: 10.2147/CIA.S46164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(4):335–46. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]