Abstract
Patients with posttraumatic stress disorder (PTSD) show hypo-active ventromedial prefrontal cortices (vmPFC) that correlate with their impaired ability to discriminate between safe and dangerous contexts and cues. Previously, we found that auditory fear conditioning depresses the excitability of neurons populating the homologous structure in rodents, the infralimbic cortex (IL). However, it is undetermined if IL depression was mediated by the cued or contextual information. The objective of this study was to examine whether contextual information was sufficient to depress IL neuronal excitability. After exposing rats to context-alone, pseudoconditioning, or contextual fear conditioning, we used whole-cell current-clamp recordings to examine the excitability of IL neurons in prefrontal brain slices. We found that contextual fear conditioning reduced IL neuronal firing in response to depolarizing current steps. In addition, neurons from contextual fear conditioned animals showed increased slow afterhyperpolarization potentials (sAHPs). Moreover, the observed changes in IL excitability correlated with contextual fear expression, suggesting that IL depression may contribute to the encoding of contextual fear.
INTRODUCTION
The increased fear responses in patients with posttraumatic stress disorder (PTSD) are associated with reduced ventromedial prefrontal cortex (vmPFC) activity (Milad et al., 2009; Rougemont-Bücking et al., 2011). However, it is unclear if this vmPFC hypo-activity is caused by the traumatic experience or is present prior to the traumatic experience. Either mechanism could lead to the development of PTSD, since low vmPFC activity is associated with decreased inhibition of the amygdala resulting in hyperactivation of the amygdala and subsequent increased fearful behavior (Milad et al., 2009; Rougemont-Bücking et al., 2011).
Studies done in the rodent homologue to the human vmPFC, the infralimbic cortex (IL) (Milad et al., 2006; Koenigs and Grafman, 2009; Milad and Quirk, 2012), found that auditory fear conditioning depresses the excitability of IL neurons (Santini et al., 2008; Cruz et al., 2014). This mechanism mimics the depressed vmPFC observed in patients with PTSD and demonstrates that aversive learning can depress vmPFC neurons. Interestingly, fear conditioning does not induce synaptic depression in IL (Pattwell et al., 2012; Sepulveda-Orengo et al., 2013) indicating that intrinsic rather than synaptic plasticity is the key determinate of IL excitability after aversive learning. Furthermore, pharmacological manipulation of IL intrinsic excitability is sufficient to reduce conditioned-fear expression (Santini and Porter, 2010; Santini et al., 2012) indicating that the depression is functionally important.
Since our previous studies used auditory fear conditioning (Santini et al., 2008; Cruz et al., 2014), we could not determine whether contextual or cued information was depressing IL excitability. Although IL is more known for its role in the extinction of fear memory (Milad and Quirk, 2002; Vidal-Gonzalez et al., 2006; Burgos-Robles et al., 2007), a recent study suggests that IL contributes to the contextual discrimination of fear conditioning memory (Zelikowsky et al., 2013). The depression of IL excitability after fear conditioning could convey contextual information which is key to determining which cues signal danger (Bouton and Bolles, 1979; Bouton, 2004). To examine this possibility, we investigated whether contextual information alone could depress IL excitability by combining a contextual fear conditioning paradigm with whole-cell patch-clamp recordings of IL neurons.
METHODS
Contextual fear conditioning
Male Sprague Dawley rats (postnatal day 30 to P45) were group housed on a 12 h light/dark schedule with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee of the Ponce Health Sciences University. On day 1, the contextual fear conditioned group (COND) was exposed to contextual fear conditioning consisting of a three minute exploration phase followed by three 0.7 mA scrambled footshocks (0.5 s in duration) with two minutes between shocks. A control group of rats (EXPOSURE) received the same contextual exposure time as the COND group but without shocks. An additional control group, the pseudoconditioned group (PSEUDO), received three consecutive shocks and was immediately removed from the conditioning context. On day 2, all groups of rats were placed in the conditioning context for two minutes and tested for contextual fear memory.
Patch-clamp recordings in prefrontal slices
Animals were sacrificed immediately after the test on day 2 and whole-cell recordings of IL neurons in prefrontal slices were done as previously described (Santini et al., 2008). Prefrontal slices were maintained at room temperature (21–23°C) in artificial cerebrospinal fluid (ACSF) at least 1 h before experiments. The composition of the incubating and recording ACSF was the following: 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 26 mM NaHCO3, 20 mM glucose, and 2 mM CaCl2 and bubbled with 95% O2 and 5% CO2. Whole-cell recordings of layer V pyramidal neurons were done blind with respect to group assignment using KMeSO4-based internal solution: 150 mM KMeSO4, 10 mM KCl, 0.1 mM EGTA, 10 mM HEPES, 0.3 mM GTP, and 0.2 mM ATP (pH 7.3, 291 mOsm). Neuronal responses to depolarizing current pulses were measured from a holding potential of −70 mV and were not corrected for the junction potential of 9 mV. Responses were filtered at 4 kHz, digitized at 10 kHz, and saved using pCLAMP9 (MultiClamp 700A, Axon Instruments, Union City, CA). As shown in Table 1, all groups had similar series resistance (Ra) and input resistance (Rin), which was measured from a 5 mV, 50 ms depolarizing pulse in voltage-clamp mode at a holding of −60 mV. The excitability of IL neurons was determined from responses to 800 ms depolarizing current pulses ranging from −40 to 350 pA at 10 pA increments with an intertrial interval of 5 s. The number of action potentials evoked by each current step was counted from individual responses. Fast afterhyperpolarizing potentials (fAHPs), medium afterhyperpolarizing potentials (mAHPs), and slow afterhyperpolarizing potentials (sAHPs) were measured as previously described (Santini et al., 2008). The amplitude of the fAHPs was measured in the second and third current evoked spikes within the 800 ms pulse and was assessed by subtracting the voltage at the peak of the fAHP from the threshold potential for spike initiation. The mAHPs and sAHPs were measured after the end of the 800 ms current pulse. The mAHP was measured as the peak of the AHP, and the sAHP was measured as the average potential during a 50 ms period beginning 280 ms after the end of the 800 ms depolarizing pulse (Sah and Louise Faber, 2002) in traces with the same number of spikes (2 spikes) (Santini et al., 2008). The first interspike interval (ISI), threshold, and fAHP were measured from the traces that showed the maximum number of evoked spikes. All recorded neurons were filled with biocytin and post hoc confirmed to be IL pyramidal neurons.
Table 1.
Electrophysiological Properties of IL Neurons.
| PSEUDO | EXPOSURE | COND | |
|---|---|---|---|
| E rest (mV) | −61 ± 1 * | −56 ± 1 | −55 ± 1 |
| Threshold (mV)a | −39 ± 0.7 * | −36 ± 1 | −35 ± 1 |
| Rin (MΩ) | 196 ± 17 | 176 ± 10 | 191 ± 13 |
| Ra (MΩ) | 14 ± 1 | 13 ± 0.6 | 14 ± 0.6 |
| Rheobase (pA) | 135 ± 18 | 136 ± 14 | 164 ± 13 |
| mAHP (mV)b | −3.5 ± 0.3 | −4.5 ± 0.4 | −4.8 ± 0.4 |
| fAHP (mV)a | −12.9 ± 1.1 | −12.9 ± 0.8 | −11.4 ± 0.9 |
| ISI (ms)a | 43 ± 7 | 52 ± 10 | 107 ± 34 |
One-way ANOVA showed a main effect of group in E rest (F(2,55) = 7.15, p = 0.0017) and threshold (F(2,55) = 4.23, p = 0.02). Post-hoc comparisons indicated that the PSEUDO group had a more negative E rest than the EXPOSURE (p = 0.016) and COND (p = 0.0015) groups, and a more negative threshold than the COND (p = 0.015) group.
Measured in the trace that showed the maximum number of spikes.
In all groups, the mAHP was measured in traces that showed 2 spikes.
Statistical analysis
Context conditioned fear was measured as the percent of time spent freezing during one-minute intervals after each shock during training and after placing the rat into the conditioning context on day 2 (FreezeScan, Clever Systems). Behavioral data were compared with repeated measures ANOVA (STATISTICA, Statsoft, Tulsa, OK) followed by Tukey HSD post hoc test. The electrophysiological data were analyzed using Clampfit (Axon Instruments, Union City, CA) and were compared with one-way ANOVA or Kruskal-Wallis test. Following a significant main effect with a one-way ANOVA or Kruskal-Wallis test, post-hoc tests were performed with Tukey HSD test or Dunn test (sAHPs), respectively. Nonparametric Kruskal-Wallis test was selected for analyzing sAHPs since data showed skewness in its distribution. Chi-square test was utilized to compare the cumulative percentage of cells versus the maximum number of evoked spikes or the magnitude of the sAHP in each group. Values are reported as the mean ± the standard error of the mean (S.E.M.).
RESULTS
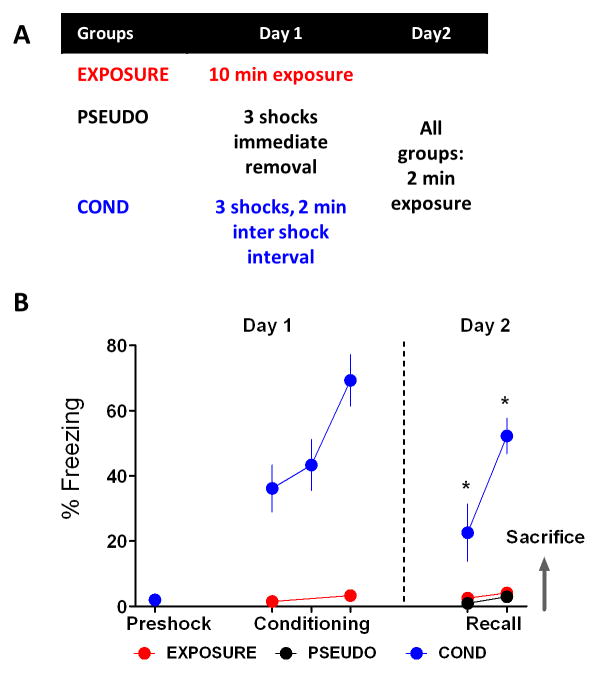
Three experimental groups were designed to test whether contextual fear conditioning affects IL intrinsic excitability (Fig. 1A). On day 1 the COND group (n = 5) received contextual fear conditioning, the EXPOSURE group (n = 7) received contextual exposure with no shock presentations, and the PSEUDO group (n = 3) received 3 consecutive shocks and was immediately removed from the conditioning chamber. All animals were tested for contextual fear on day 2 and immediately sacrificed. As expected (Fig. 1B), a repeated measures ANOVA showed a significant main effect (F(2,12) = 40.63, p < 0.001) and post hoc analysis confirmed that rats from the COND group had significant higher levels of freezing to the conditioning context on day 2 compared to rats from the EXPOSURE and PSEUDO groups (p < 0.05). The difference in fear expression among groups indicates that only the COND group had acquired fear to the context.
Figure 1.
Behavioral experiments design. A, Three experimental groups were studied to assess the effect of contextual fear conditioning on IL intrinsic excitability. In the exposure control group (EXPOSURE; n = 7), rats received contextual exposure on day 1 and were tested for contextual fear on day 2. The pseudoconditioned control group (PSEUDO; n = 3) received 3 consecutive shocks and were removed immediately from the conditioning chamber on day 1. PSEUDO rats were tested for contextual fear on day 2. On day 1, the contextual fear conditioned group (COND; n = 5) received three shocks within 2 min intervals. On day 2, COND rats were tested for contextual fear. B, Average percent freezing of all the experimental groups during the two days of behavioral procedures. As expected, COND rats showed significant higher freezing levels to the conditioning context when compared to EXPOSURE and COND rats. *p < 0.05
Contextual fear conditioning depresses the intrinsic excitability of IL neurons
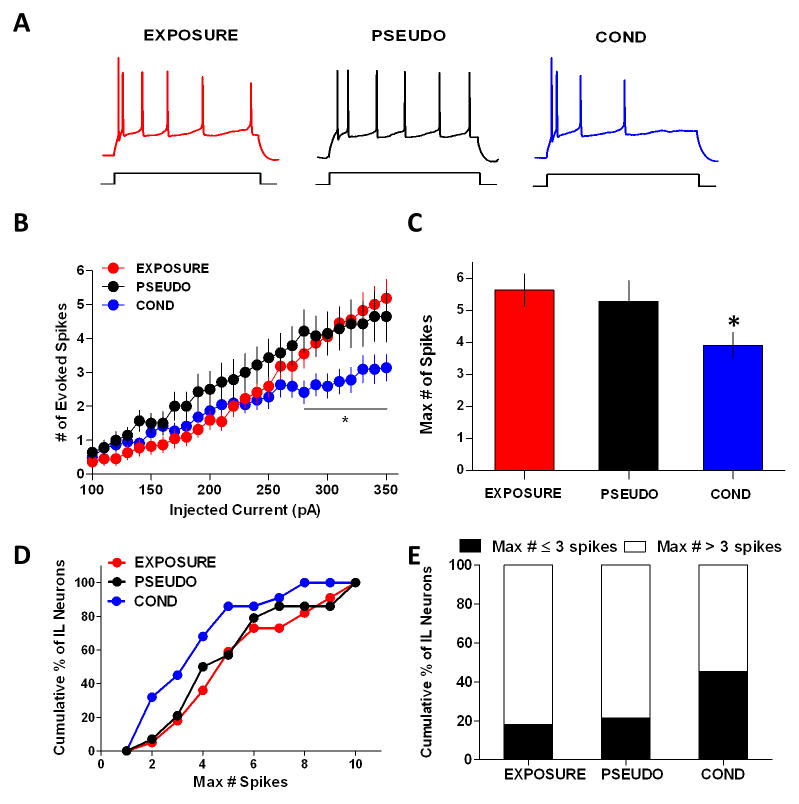
After the test for recall of contextual fear on day 2, we sacrificed the rats and assessed the intrinsic excitability of IL pyramidal neurons using whole-cell current-clamp recordings in prefrontal brain slices. Consistent with the representative responses to a 310 pA depolarizing pulse in single neurons from each group (Fig. 2A), neurons from the COND group (n = 22) fired significantly fewer spikes in response to depolarizing current steps compared to neurons from the EXPOSURE (n = 22) and PSEUDO (n = 14) groups (Fig. 2B). One-way ANOVAs revealed a main group effect at each step from 280 to 350 pA (280 pA: F(2,55) = 4.12, p = 0.021; 290 pA: F(2,55) = 3.39, p = 0.041; 300 pA: F(2,55) = 3.68, p = 0.032; 310 pA: F(2,55) = 4.30, p = 0.018; 320 pA: F(2,55) = 4.06, p = 0.023; 330 pA: F(2,55) = 3.38, p = 0.041; 340 pA: F(2,55) = 3.91, p = 0.026; 350 pA: F(2,55) = 4.26, p = 0.019). Post hoc analysis indicated that neurons of the COND group fired fewer action potentials than neurons from the PSEUDO (intensities between 280 pA and 320 pA) and EXPOSURE (intensities between 290 pA and 350 pA) groups (p < 0.05 for all comparisons). In addition, when we compared the averages of the maximum number of evoked spikes at any current step (EXPOSURE: 5.6 spikes; PSEUDO: 5.3 spikes; COND: 3.9 spikes), we found that IL neurons from the COND group had fewer spikes than neurons from the EXPOSURE and PSEUDO groups (Fig. 2C). One-way ANOVA revealed a main group effect in the maximum number of evoked spikes (F(2,55) = 3.60, p = 0.034) and post-hoc analysis indicated that the average maximum number of spikes of the COND group was smaller than the EXPOSURE group (p = 0.033) but not the PSEUDO group (p = 0.18). Moreover, we found that a greater percentage of cells (Chi-square = 21.73, df = 2, p < 0.0001) in the COND group (45%) fired 3 or less spikes than cells from the EXPOSURE (18%) or PSEUDO (21%) groups (Fig. 2D–E). These results indicate that contextual fear conditioning depresses IL pyramidal neurons.
Figure 2.
Contextual fear conditioning depresses the intrinsic excitability of IL pyramidal neurons. A, Representative traces of the response to a 310 pA current pulse in a single neuron from each experimental group. The neuron in the conditioned group fired fewer spikes. B, Input-output curve shows that depolarizing steps evoked fewer spikes in neurons from the COND group. C, The maximum number of evoked spikes at any current step was significantly reduced in the COND group. D, Cumulative histogram of the maximum number of spikes fired by neurons from each group. E, The COND group had more cells with a maximum of ≤ 3 spikes compared to cells in the EXPOSURE and PSEUDO groups. * p < 0.05
Contextual fear conditioning increases the slow afterhyperpolarization potential (sAHP) in IL
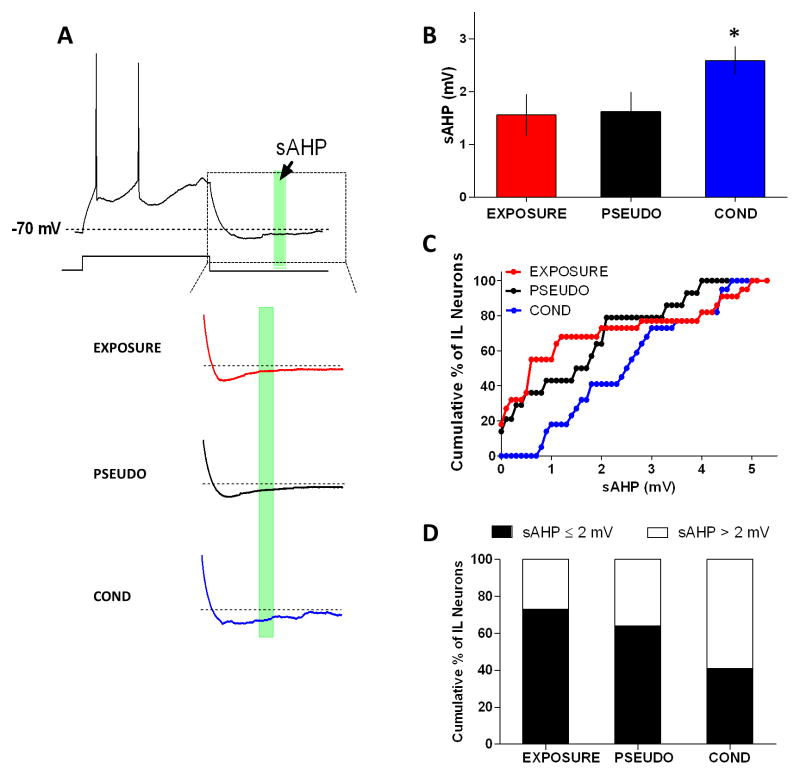
We previously found that the depressed IL intrinsic excitability observed after auditory fear conditioning correlated with an increase in the sAHP (Santini et al., 2008). Therefore, we compared the sAHP to elucidate if contextual fear conditioning depressed IL excitability by increasing the sAHP. As shown in Figure 3A–B, the sAHPs from the COND group were larger. Kruskall-Wallis test revealed a main group effect in the sAHPs (H(2,58) = 7.62, p = 0.022) and post-hoc comparisons indicated that sAHPs of the COND group were larger than those of the EXPOSURE group (p = 0.026) but not the PSEUDO group (p = 0.16). In addition, we found that a greater percentage of neurons (Chi-square = 22.57, df = 2, p < 0.0001) in the COND group (59%) had sAHPs larger than 2 mV compared to neurons of the EXPOSURE (27%) and PSEUDO (36%) groups (Fig. 3C–D). Consistent with the results of auditory fear conditioning (Santini et al., 2008), the medium (mAHP) and fast (fAHP) afterhyperpolarizing potentials were similar in all groups (Table 1).
Figure 3.
Contextual fear conditioning depressed IL excitability by increasing the sAHP. A, Sample traces showing the time interval where sAHPs were measured (green box; 50 ms interval starting 280 ms after the end of the 800 ms depolarizing pulse). B, Neurons from the COND group had larger sAHPs compared to neurons from the EXPOSURE and PSEUDO groups. C, Cumulative histogram showing that neurons from the COND group had larger sAHPs. D, The COND group had more cells with a sAHP > 2 mV. *p < 0.05
IL depression correlates with contextual fear
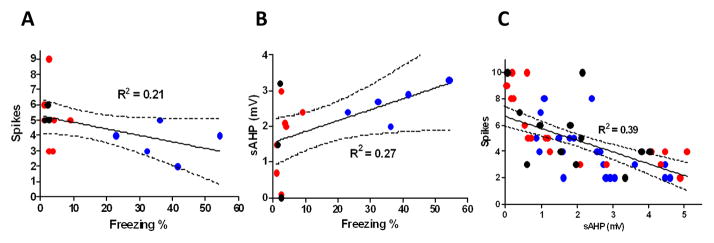
We further examined whether the observed reduction in spikes and increase in sAHP correlated with the freezing behavior of the rats. We found a strong negative correlation between the average number of fired action potentials and the freezing levels of the individual rats (R2 = 0.21; Fig. 4A). Furthermore, the magnitude of the sAHP showed a strong positive correlation with the freezing levels of the rats (R2 = 0.27; Fig. 4B). In addition, we found a strong negative correlation between the number of spikes and the size of the sAHP in individual neurons (R2 = 0.39; Fig. 4C). Taken together, these results suggest that contextual fear conditioning depressed IL excitability by increasing the sAHP.
Figure 4.
IL intrinsic excitability inversely correlates with contextual fear. A, Higher contextual fear correlates with fewer spikes in IL pyramidal neurons. B, Higher contextual fear correlates with larger sAHPs in IL pyramidal neurons. C, Larger sAHPs are associated with fewer spikes in IL pyramidal neurons.
DISCUSSION
The main finding presented in this paper is that contextual fear conditioning depresses the intrinsic excitability of IL pyramidal neurons. The significant decrease in the number of action potentials and increase in sAHP suggest a similar mechanism of IL depression as that observed after auditory fear conditioning (Santini et al., 2008; Cruz et al., 2014). Furthermore, both parameters, number of spikes and sAHP, strongly correlated with the freezing behavior of the animals suggesting that IL depression may contribute to the encoding of contextual fear. These findings demonstrate that aversive contextual information alone is sufficient to depress IL excitability and suggest a role of the hippocampus in mediating such depression by conveying contextual information to IL.
Relatively brief stimulation of neurons in brain slices or in vivo is sufficient to induce a long-lasting plasticity of intrinsic excitability (Daoudal et al., 2002; Mahon and Charpier, 2012). Both the dorsal (dHPC) and ventral (vHPC) hippocampus innervate IL and are critical for contextual fear (Cenquizca and Swanson, 2007; Hoover and Vertes, 2007; Hunsaker and Kesner, 2008; Fanselow and Dong, 2010; Liu et al., 2012; Maren et al., 2013). However, the vHPC sends more dense projections to IL than the dHPC. Although not all IL neurons receive direct inputs from the vHPC, vHPC activation is likely to activate a larger number of IL neurons through polysynaptic activity initiated by the initial monosynaptic vHPC input. Our data suggest that during contextual fear conditioning the association between the contextual information conveyed by vHPC CA1 projections and shock information carried by BLA projections (Rozeske et al., 2015) depress IL excitability. However, many indirect pathways could also contribute to the depression of IL neurons including recruitment of neuromodulatory inputs. Further investigations are needed to identify the precise projections and cellular pathways that depress IL excitability to encode contextual information during aversive learning.
Our findings are in agreement with the current growing literature that points to the importance of intrinsic plasticity in modulating learning, memory formation, and behavior (for reviews see Beck & Yaari, 2008; Kourrich, Calu, & Bonci, 2015; Sehgal, Song, Ehlers, & Moyer, 2013). Furthermore, contextual and cued fear learning alter intrinsic neuronal excitability in important structures of the fear circuit such as the hippocampus and the amygdala (McKay et al., 2009, 2013; Motanis et al., 2014; Sehgal et al., 2014). In this study as in our previous, the observed depression of IL excitability was caused by the increase in sAHP suggesting a cellular mechanism in which K+ conductance is enhanced, possibly through KCNQ channels, which are involved in sAHP modulation (Larsson, 2013).
In contrast to our findings, a recent study found that trace fear conditioning increases the excitability of IL layer V neurons projecting to the amygdala (Song et al., 2015). There are several differences between the studies that could contribute to the discrepancy. Fear acquisition could increase the excitability of IL neurons projecting to the amygdala while depressing the excitability of IL projections to other structures. It is also possible that delay and trace fear conditioning could involve different cellular mechanisms and circuits. Finally, since the importance of IL for fear inhibition appears to differ between rat strains (Chang and Maren, 2010; Chang et al., 2010), the difference in rat strain could contribute to the discrepancy.
Fear conditioning-induced depression of IL is consistent with the evidence suggesting that IL inhibits amygdala activation through its projections to GABAergic neurons (Likhtik et al., 2008; Amano et al., 2010; Cho et al., 2013). Thus, aversive learning could depress IL excitability to reduce inhibition of the amygdala and produce appropriate behavioral responses to dangerous contexts. While this simple model is convenient, the actual neuronal circuits that allow IL activity to modulate fear expression are unlikely to be so anatomically simple. IL pyramidal neurons project to many different structures and relatively few IL neurons project to the amygdala (Gabbott et al., 2005). It is possible that the IL projections to the amygdala do not mediate the reduced freezing seen after IL activation. In fact, a recent study found that optogenetic activation of IL terminals in BLA did not induce an immediate reduction in conditioned freezing (Bukalo et al., 2015). Therefore, it is quite possible that depression of IL excitability could reduce freezing via outputs to other structures such as the ventrolateral periaqueductal gray matter (vlPAG), which plays a crucial role in fear expression (McNally et al., 2011; Ferreira et al., 2015).
A similar mechanism may also occur in humans, since a functional imaging study done in healthy human subjects demonstrated that aversive learning decreases vmPFC activity (Milad et al., 2007). In addition, studies of patients with PTSD suggest that their persistent fearful responses are due to reduced activation of the vmPFC which could be produced by depressed excitability. Furthermore, patients with PTSD show impaired discrimination between threatening and unthreatening contexts (Rougemont-Bücking et al., 2011), suggesting that vmPFC activation is important for appropriate contextual discrimination.
Contextual fear conditioning was sufficient to depress IL excitability.
The depression correlated with increases in the slow afterhyperpolarizing potential.
IL depression correlated with contextual fear expression suggesting that IL depression may partake in encoding contextual fear.
Acknowledgments
Special thanks to Ana López and María Colón for their technical assistance. This work was supported by R36 1R36MH102080-01 to EC, MBRS-RISE 1R25 GM 082046 to OSC and MCM, NIMHHD G12MD007579-27 and R15 MH101700 to JTP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- Bukalo O, Pinard CR, Silverstein S, Brehm C, Hartley ND, Whittle N, Colacicco G, Busch E, Patel S, Singewald N, Holmes A. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci Adv. 2015;1:e1500251–e1500251. doi: 10.1126/sciadv.1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cenquizca La, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One. 2010;5:e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Maren S. Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci. 2010;124:391–397. doi: 10.1037/a0019479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J-H, Deisseroth K, Bolshakov VY. Synaptic Encoding of Fear Extinction in mPFC-amygdala Circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz E, López AV, Porter JT. Spontaneous Recovery of Fear Reverses Extinction-Induced Excitability of Infralimbic Neurons. PLoS One. 2014;9:e103596. doi: 10.1371/journal.pone.0103596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoudal G, Hanada Y, Debanne D. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99:14512–14517. doi: 10.1073/pnas.222546399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AN, Yousuf H, Dalton S, Sheets PL. Highly differentiated cellular and circuit properties of infralimbic pyramidal neurons projecting to the periaqueductal gray and amygdala. Front Cell Neurosci. 2015;9:161. doi: 10.3389/fncel.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16:173–184. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- Larsson HP. What determines the kinetics of the slow afterhyperpolarization (sAHP) in neurons? Biophys J. 2013;104:281–283. doi: 10.1016/j.bpj.2012.11.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Charpier S. Bidirectional plasticity of intrinsic excitability controls sensory inputs efficiency in layer 5 barrel cortex neurons in vivo. J Neurosci. 2012;32:11377–11389. doi: 10.1523/JNEUROSCI.0415-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol. 2009;102:2763–2770. doi: 10.1152/jn.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Disterhoft JF. Learning increases intrinsic excitability of hippocampal interneurons. J Neurosci. 2013;33:5499–5506. doi: 10.1523/JNEUROSCI.4068-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends Neurosci. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E. Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cereb Cortex. 2014;24:1075–1087. doi: 10.1093/cercor/bhs394. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley Ca, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci Ther. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Valerio S, Chaudun F, Herry C. Prefrontal neuronal circuits of contextual fear conditioning. Genes, Brain Behav. 2015;14:22–36. doi: 10.1111/gbb.12181. [DOI] [PubMed] [Google Scholar]
- Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30:12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic Receptors Modulate the Intrinsic Excitability of Infralimbic Neurons and Consolidation of Fear Extinction. Neuropsychopharmacology. 2012;37:2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Ehlers VL, Moyer JR. Learning enhances intrinsic excitability in a subset of lateral amygdala neurons. Learn Mem. 2014;21:161–170. doi: 10.1101/lm.032730.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, Moyer JR. Learning to learn - Intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem. 2013;105:186–199. doi: 10.1016/j.nlm.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeño O, Porter JT. Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci. 2013;33:7184–7193. doi: 10.1523/JNEUROSCI.5198-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Ehlers VL, Moyer JR. Trace Fear Conditioning Differentially Modulates Intrinsic Excitability of Medial Prefrontal Cortex-Basolateral Complex of Amygdala Projection Neurons in Infralimbic and Prelimbic Cortices. J Neurosci. 2015;35:13511–13524. doi: 10.1523/JNEUROSCI.2329-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hast Ta, Bennett RZ, Merjanian M, Nocera Na, Ponnusamy R, Fanselow MS. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry. 2013;73:345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]