Abstract
Purpose
Surgical resection is considered as a curative treatment modality for hepatocellular carcinoma (HCC); however, the incidence of postoperative tumor recurrence is high, leading to worse patient survival. Persistent hepatitis (inflammation) is one of risk factors of tumor recurrence after surgical resection. The aim of this study is to investigate the underlying mechanisms linking liver inflammation to HCC progression.
Experimental Design
In this study, we used a cytokine array to identify important cytokines whose levels are increased in liver microenvironment with severe hepatitis. We evaluated the morphological changes, migration and invasion ability, and signal transduction in HCC cells with or without inflammatory cytokine in vitro. Finally, we analyzed the NF-κB signal pathway in tumor specimens from 232 patients with HCC by immunohistochemical staining.
Results
The pro-inflammatory cytokine TNF-α was increased in the peritumoral microenvironment and contributed to tumor recurrence and metastasis. Specifically, TNF-α promoted HCC cancer cell migration, invasion, and epithelial–mesenchymal transition (EMT) by upregulating the transcriptional regulator, Snail. We identified Snail as a direct target gene downstream of the TNF-α-mediated canonical NF-κB activation. In addition, tumor recurrence-free survival of HCC patients correlated negatively with high p65 and Snail expression and positively with high E-cadherin expression.
Conclusion
Our results establish a signaling axis that explains how inflammatory tumor microenvironment promotes HCC recurrence and metastasis. These findings suggest that controlling liver inflammation and/or targeting NF-κB–mediated Snail expression may be a potential therapeutic strategy to prevent HCC recurrence after hepatectomy.
Keywords: Hepatocellular carcinoma, epithelial–mesenchymal transition, TNF-α, NF-κB, Snail
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer type worldwide, which accounts for nearly 5.6% of all cancers, and a leading cause of cancer-related deaths (1, 2). More than 90% patients with HCC have preexisting chronic liver disease that is caused most commonly by chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, and/or alcohol consumption (3, 4). Indeed, cumulative evidence indicates that chronic liver inflammation by long-term exposure to infectious agents (hepatotropic viruses) or toxins (ethanol) results in liver cirrhosis and hepatocarcinogenesis (5). While the molecular mechanisms linking chronic inflammation to HCC are not well defined, there is growing evidence to suggest that the crosstalk between tumor cells and the surrounding stroma in the tumor microenvironment serves as a key modulator in hepatocarcinogenesis and HCC progression. In the tumor stroma, hepatic stellate cells, fibroblasts, inflammatory cells, and vascular endothelia cells have been shown to secrete extracellular matrix (ECM) proteins, proteolytic enzymes, growth factors, and inflammatory cytokines that alter cancer signaling pathways to promote tumor cell initiation, invasion, and metastasis (6).
The microenvironment of inflamed liver turns on the nuclear factor κB (NF-κB) pathway to promote proliferation of hepatocytes, rendering them resistant to growth arrest (7). The inhibitor κB kinases (IKKs) complex, which consists of three subunits, two catalytic kinases (IKKα and IKKβ) and a regulatory scaffold partner (IKKγ)(8), plays a key role in the NF-κB signaling pathway that is known to induce inflammation-associated cancers (9). IKKβ-dependent NF-κB activation has been shown to promote hepatocyte survival in both developing and adult liver (10). In a study using a Mdr2-knockout mouse model, which spontaneously develops cholestatic hepatitis followed by HCC, Pikarsky et al. demonstrated that the inflammatory process triggers NF-κB activation in hepatocytes through upregulation of tumor-necrosis factor-alpha (TNF-α) in adjacent endothelial and inflammatory cells and that inhibition of NF-κB by anti-TNF-α treatment or induction of IκB super-repressor in the later stages of tumor development results in apoptosis of transformed hepatocytes, which prevents progression to HCC (11). In addition, our previous study indicated that non-canonical NF-κB activation is also important for tumor initiation. Specifically, IKKα activated by TNF-α interacts with and phosphorylates FOXA2 at S107/S111, thereby suppressing FOXA2 transactivation activity that leads to decreased NUMB expression and further activating the downstream NOTCH pathway to promote HCC proliferation and tumorigenesis (12).
The long-term prognosis after surgical resection of HCC remains unsatisfactory due to high incidence of recurrence associated with HCC (13) that ranges from 50% to 70% five years after first curative hepatectomy (14). Several risk factors have been reported to associate with HCC recurrence, including tumor size, multifocal lesions, and vascular invasion, which could predict patient survival after surgical resection. In addition, investigation into the role of HBV infection in HCC recurrence following tumor resection by multivariate analysis showed that elevated hepatic inflammatory activity and HBV DNA levels as well as multinodular tumors are significantly associated with late HCC recurrence after operation (15). The severity of hepatitis may also influence the survival outcome of patients after surgery such that sustained chronic hepatitis is associated with worse clinical outcome in HCC patients. However, the mechanisms of tumor progression in chronic hepatitis have not yet been explored. In this study, we investigate how chronic hepatitis or liver inflammation may be involved in HCC progression, in particular tumor recurrence and metastasis, after curative hepatectomy in the context of chronic inflammation in the liver microenvironment.
Materials and Methods
Cell migration and invasion assay, Western blot analysis, real time PCR, chromatin immunoprecipitation (ChIP) assay, and luciferase reporter assay have previously been described (16). The antibodies used for immunoblotting, immunofluorescence, and immunohistochemical (IHC) staining are listed in Supplementary Table S1.
Cell culture
Human HCC cell lines Hep3B, Huh7, Tong/HCC, PLC/PRF/5, HepG2, HA22T/VGH, HA59T/VGH, Malhavu, and SK-HEP-1 were obtained from Center for Molecular Medicine, China Medical University (Taichung, Taiwan). The Hep3B, Huh7, and HepG2 cell lines were validated by STR DNA fingerprinting using the AmpFlSTR Identifiler kit according to manufacturer instructions (Applied Biosystems). The STR profiles were compared to known ATCC fingerprints (ATCC.org), to the Cell Line Integrated Molecular Authentication database (CLIMA) version 0.1.200808 (Nucleic Acids Research 37:D925-D932 PMCID: PMC2686526) and to the MD Anderson fingerprint database. The STR profiles matched known DNA fingerprints or were unique. All cell lines were maintained in Dulbecoo’s modified Eagle’s medium (DMEM)/F12 medium supplemented with 10% fetal bovine serum and antibiotics. IKKα−/−, IKKβ−/−, and wild-type MEFs were maintained as previous described (16). For analysis of ligand-dependent Snail expression, cells were serum-starved overnight and harvested directly at indicated the time points. Human TNF-α (10 ng/ml) used for ligand stimulation was purchased from Roche (Indianapolis, IN). Details of various inhibitors used in this study are shown in Supplementary Table S2.
Primers, shRNAs, DNA plasmids
Supplementary Table S3 lists the details of the primers used in this study. The sequences of the short hairpin RNA (shRNA) used to knock down the expression of IKKβ and p65 have been previously described (16). The negative control vector expressing scrambled short hairpin RNA was obtained from Addgene (#1864). The plasmid constructs used for over-expressing IKKs were described in our published paper (17). Snail-pGL2 Snail promoter luciferase reporter plasmid was purchased from Addgene (#31694) (18). For ectopic overexpression of Snail, Snail expressing plasmid (PCDH-Snail) was constructed by cloning the full-length Snail ORF into the pCDH-CMV-MCS-EF1-Puromycin vector (CD510B-1; System Biosciences, Mountain View, CA) between EcoRI and BamH1 site. PCR primers for cloning are as follows:
5′–ATACTGAATTCATGGACTACAAAGACGATGACGACAAGATGCCGCGCTCTTTCCTCGTCAGG–3′ (forward)
5′– ATGATGGATCCTCAGCGGGGACATCCTGAGCA–3′ (reverse)
Patients and IHC staining of human tumor tissue samples
Between September 2004 and August 2008, 232 HCC patients undergoing hepatectomy in Chang Gung Memorial Hospital Linko medical center were reviewed. Patients who were considered eligible for hepatectomy at our hospital had acceptable liver reservoir function (i.e., acceptable indocyanine green (ICG) retention rate at the 15-minute and CHILD A status), lacked multifocal tumors at bilateral lobe of liver, and showed no presence of extra-hepatic lesion pre-operatively. Patients in our cohort did not receive antiviral therapy. All patients received adequate resection margin (> 1 cm). Curative resection considered by pathology proved section margin was free of malignance. The study was approved by the institutional review board at Chang Gung Memorial Hospital (Protocol No. 100-4467B). After surgery, follow-ups were conducted every 2 to 3 months at the outpatient clinic. Tumor recurrence was suspected if progressive elevation of serum AFP levels is present and/or if a new hepatic lesion is detected by ultrasonography. Dynamic CT scan or MRI is routinely arranged if recurrence was suspected. Intra-hepatic recurrence was diagnosed by images showing contrast enhancement during arterial phase and wash-out in venous phase. Extra-hepatic recurrence was diagnosed depending on its location according to the images taken by CT, MRI, Tc-99m methylene diphosphonate (Tc-99m MDP) bone scan, or 2-[18F]-fluoro-2-deoxy-D-gluocose ([18F]FDG) PET scan. Image-guided biopsy was performed only when considered necessary. Diagnosis of recurrent HCC was confirmed in accordance with the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases guidelines. The last follow-up date in this study was December 31, 2011. The duration from date of operation to the date of first confirmation of recurrence (time-to-recurrence) was recorded as the primary endpoint (shown as tumor-recurrence-free survival).
Surgical specimens from tumorous and non-tumorours sections of liver were randomly sampled for histopathological analyses using hematoxylin and eosin (H&E) staining. The ISHAK score was used to grade the severity of hepatitis (19) by a pathologist blinded to the patients’ clinical and biochemical information. The ISHAK score contains five parameters: (A) periportal or periseptial interface hepatitis, score 0–4; (B) confluent necrosis, score 0–6; (C) focal (spotty) lytic necrosis, apoptosis and focal inflammation, score 0–4; (D) portal inflammation, score 0–4; (E) architectural changes, fibrosis and cirrhosis, score 0–6. (19) In this study, severe hepatitis is defined as the sum of total ISHAK scores > 6 (15).
IHC staining of p65 (Milipore), Snail (Abcam) and E-cadherin (Leica Biosystems) were performed on formalin-fixed paraffin-embedded tissue. A single representative block from each tissue was sectioned at 3 µm onto positively charged slides. Slides were then stained using the Bond-Max autostainer (Leica Microsystems) according to the manufacturer’s protocol. Slides were dewaxed in Bond Dewax Solution (Leica Microsystems) and hydrated in Bond Wash Solution (Leica Microsystems). Antigen retrieval was performed at acidic pH using Epitope Retrieval 1 solution (Leica Microsystems) for 20 min at 100 °C. Slides were then incubated with the primary antibody at sutible concentration for 15 min at room temperature. Antibody detection was performed using the biotin-free Bond Polymer Refined Detection System (Leica Microsystems). Finally, slides were counterstained with hematoxylin. The percentage of chromogen containing cells were estimated in the ranges of 0%, <5%, 5%–25%, 26%–50%, >51% and semiquantitively scored as 0 to 4. The expression intensity was determined from at least 4 fields per slide.
Statistical analyses
Median expression ratio from cytokine array and the expression level from ELISA validation were analyzed by Mann-Whitney U test for statistical significance. All data for histologic parameters and in vitro assays are expressed as mean ±SD. Data with continuous variables were analyzed by Student’s t test. Pearson’s chi-square test or Fisher’s exact test was used to determine statistical significance on category variables. The correlation between two parameters was analyzed by Spearman’s correlation test. Kaplan–Meier survival curve with log-rank test and Cox regression model were used for survival analysis. All statistical analyses were performed using the SPSS software program (version 17; SPSS).
Results
Severe hepatitis is associated with tumor recurrence and extra-hepatic recurrence after hepatectomy
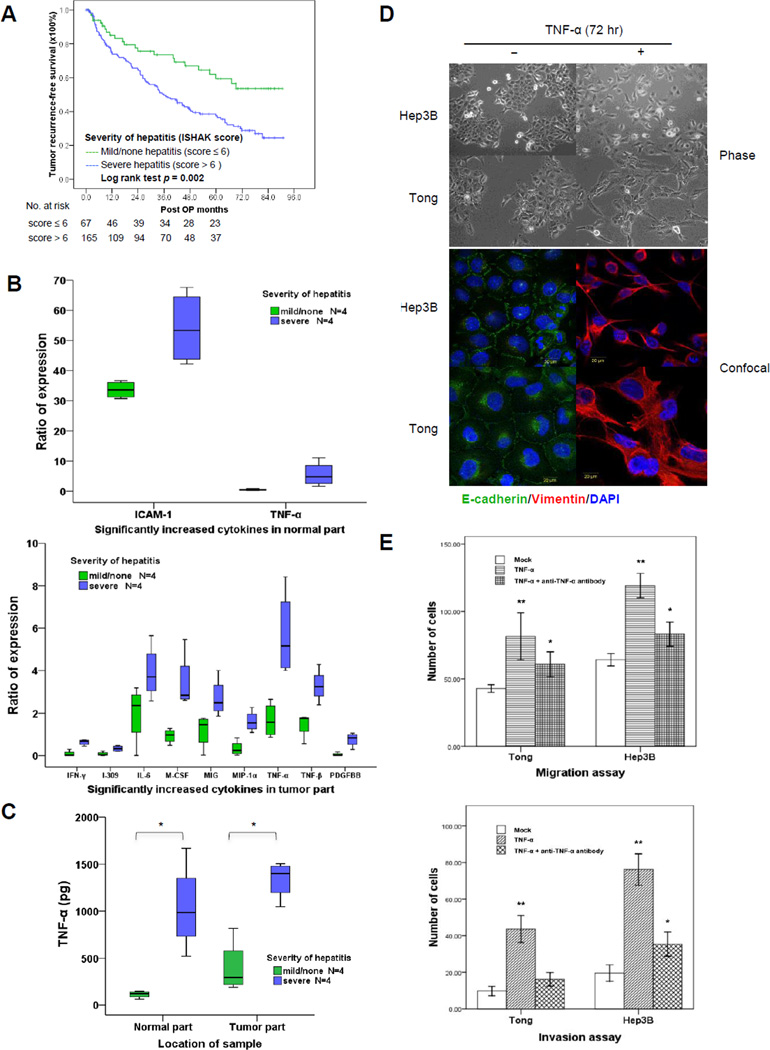
To determine whether the severity of liver inflammation affects tumor recurrence or metastasis after curative hepatectomy, we reviewed 232 HCC cases in which patients underwent curative resection from 2004 to 2008 at Chung Gung Memorial Hospital (Linko. Taiwan). The clinical features and tumor characteristics of these patients are summarized in Supplementary Table S4. We used ISHAK score (19) to grade the pathological severity of chronic hepatitis from the patients’ liver tissue specimens. Among these 232 HCC patients, 165 and 67 had tumors stemming from severe hepatitis (ISHAK score > 6) and mild/none hepatitis background (ISHAK score ≤ 6), respectively. Viral hepatitis was significantly associated with more severe liver inflammation and higher ISHAK score (Supplementary Table S5). At the median 52.8-month follow-up, 123 patients suffered from disease recurrence. The recurrence rate was significantly higher (p < 0.0001) in patients with severe hepatitis (100 of 165 patients, 60.6%) than those with none/mild hepatitis (23 of 67 patients, 34.3%). Moreover, among the 123 patients with disease recurrence, the rate of extra-hepatic metastasis was also significantly higher in those who had severe hepatitis (61 of 100 patients, 61.0%) than those who had none/mild hepatitis (3 of 23 patients, 13.0%; p < 0.0001) (Supplementary Table S6). There were 66 (53.6%) patients who suffered from early tumor recurrence within 2 years after hepatectomy, and 57 (46.3%) who had late disease recurrence over 2 years after resection. Interestingly, patients with severer hepatic inflammation, not necrosis or cirrhosis, in the liver microenvironment had higher incidence of both early and late tumor recurrence (Supplementary Table S7). The mean recurrence-free survival in patients who had none/mild hepatitis (total ISHAK score ≤ 6) was longer than those who had severe hepatitis (total ISHAK score > 6) [62.9 ± 4.6 years (95% CI, 53.9 to 71.9) vs. 44.9 ± 2.8 years (95% CI, 39.4 to 50.3); p = 0.002] (Fig. 1A). Univariate and multivariate analyses identified severe hepatitis (total ISHAK score > 6) as an important predictor independently associated with poor HCC tumor recurrence-free survival after hepatectomy (Table 1).
Figure 1.
Inflammatory cytokine TNF-α promotes EMT in HCC cells. A, Kaplan–Meier survival curve with log rank test showed the correlation between recurrence-free survival and the severity of hepatitis (by ISHAK score) in HCC patients received curative hepatectomy. The number of patients at risk is shown below the survival curves. B, differential expression of inflammatory cytokines from patient samples by cytokine array analysis. Values only showed the ratio of cytokine expression significantly increased in normal or tumor part respectively in severe hepatitis in compare to none/mild hepatitis. C, ELSA analysis showed the expression of TNF-α in normal and tumor parts from HCC patients with or without severe hepatitis. *p < 0.05. D, morphological changes and expression of EMT markers (phase and confocal microscopy) in cells treated with TNF-α (10 ng/ml) for 72 hours. E, invasion and migration properties of TNF-α-treated HCC cells. Cells were treated with TNF-α (10 ng/ml) alone or with human TNF-α neutralizing (D1B4) rabbit mAb (10 ng/ml). For migration assay cells were incubated 48 hours after treatment. For invasion assay, cells were incubated 72 hours after treatment. *p < 0.05 and **p < 0.001 compared with mock.
Table 1.
Univariate and multivariate analyses of predictors of tumor-recurrence free survival in 232 patients after resection for HCC.
| Risk factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | p | Hazard ratio | 95% confidence interval | p# | |
| Large tumor (maximal tumor diameter > 5cm) | 1.046 | 0.711 – 1.538 | 0.821 | - | ||
| Multiple tumors (tumor number ≥ 2) | 1.061 | 0.627 – 1.795 | 0.825 | - | ||
| Tumor rupture | 1.622 | 0.707 – 3.720 | 0.254 | - | ||
| Portal vein tumor thrombosis | 4.327 | 2.285 – 8.194 | <0.0001 | 2.475 | 1.237 – 4.951 | 0.010 |
| Tumor with satellite nodules | 1.739 | 1.211 – 1.498 | 0.003 | - | ||
| Tumor with capsule | 0.871 | 0.507 – 1.495 | 0.616 | - | ||
| Higher histologic grade (G4 > G3 > G2 > G1)* | 1.410 | 1.065 – 1.866 | 0.016 | - | ||
| Tumor with microvascular invasion | 2.210 | 1.528 – 3.195 | <0.0001 | 1.824 | 1.219 – 2.730 | 0.003 |
| Severe hepatitis (total ISHAK score > 6) | 2.022 | 1.283 – 3.184 | 0.002 | 1.710 | 1.079 – 2.709 | 0.022 |
| AFP > 400 (ng/ml) | 1.130 | 0.749 – 1.706 | 0.561 | - | ||
Edmondson-Steiner grading system
The statistical significance of p value was analyzed by Cox proportional hazard regression model with forward stepwise selection.
TNF-α is a major cytokine in the inflammatory microenvironment of liver and promotes EMT in HCC
Several inflammatory cytokines in tumor microenvironment are associated with cancer progression, invasion, and metastasis (6). To determine which cytokines are elevated in the liver tissues of HCC patients with severe hepatitis, we screened 8 pairs of human tissue specimens (normal and tumor) via a cytokine antibody arrays (RayBio® Human Inflammation Antibody Array, Cat# AAH-INF-G3-4) and measured the expression level of 40 different inflammatory cytokines (Supplementary Table S8). Interestingly, only TNF-α was consistently elevated in both normal and tumor tissues with severe hepatitis compared to mild/none hepatitis (Fig. 1B). In addition, ELSA examination further validated TNF-α as the major cytokine that was significantly elevated in the microenvironment of both tumorous and non-tumorous tissues with severe hepatitis (Fig. 1C).
To investigate the biological effects of TNF-α on HCC, two epithelial-type HCC cell lines, Hep3B and Tong, were stimulated by TNF-α for 72 hours, which induced morphological changes, including the loss of cell-cell adhesion, spindle shape transformation, cellular process elongation, and loss of cell polarity (Fig. 1D, top). These changes suggest that EMT may have occurred. Indeed, Western blot analysis also demonstrated elevated levels of mesenchymal markers, N-cadherin, and vimentin, and decreased levels of epithelial markers, E-cadherin and plakoglobin, post TNF-α treatment for 72 h in these cells (Supplementary Fig. S1A). The increase in vimentin and decrease in E-cadherin after TNF-α stimulation were also visualized by confocal microscopy (Fig. 1D, bottom). In addition, TNF-α-treated cells demonstrated increased cell migration and invasion (Fig. 1E), which was abrogated by the addition of a TNF-α neutralizing antibody. Together, these results indicate that TNF-α is able to induce EMT in HCC cells in culture and suggest that the increased levels of TNF-α in the microenvironment of severe hepatitis may stimulate EMT in HCC cells.
EMT regulator Snail is required for TNF-α-mediated EMT
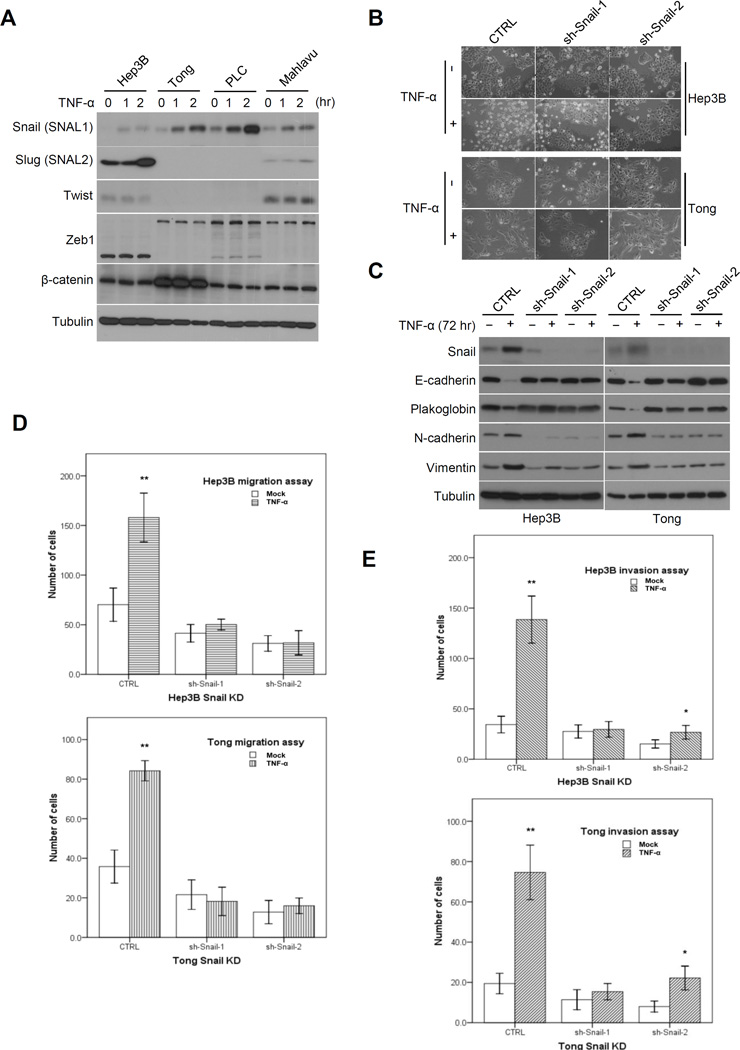
Several EMT regulators, including of Twist (20, 21), Snail (21, 22), Slug (23, 24), Zeb1/2 (25, 26), β-catenin (27), FoxC1/2 (28) and Sox4 (29) are known to induce EMT in HCC cells, and their expression levels also correlate with patient survival. To further delineate the importance of these EMT regulators in HCC, we profiled their gene expression using the CCLE database and found that Twist, Snail, Slug, Zeb1/2 and β-catenin were upregulated in mesenchymal-type but downregulated in epithelial-type HCC cells (Supplementary Fig. S1B). We examined the protein expression level of these EMT regulators in a panel of TNF-α-treated HCC cells to identify the potential regulator(s) responsible for TNF-α-mediated EMT, As shown in Figure 2A, only expression of Snail was consistently induced upon TNF-α treatment. We then ectopically overexpressed Snail in two epithelial-type HCC cells (Hep3B and Tong) to validate its role in EMT in HCC. Confocal microscopy analysis revealed morphological changes associated with EMT when Snail was overexpressed in Tong cells (Supplementary Fig. S2A). In addition, overexpression of Snail increased expression of N-cadherin and vimentin and decreased the expression E-cadherin and plakoglobin in Hepa3B and Tong cells as indicated by Western blot analysis (Supplementary Fig. S2B). The migration and invasion ability of these cells also increased when Snail was overexpressed (Supplementary Fig. S2C). To determine whether Snail is required for TNF-α-mediated EMT, we knocked down Snail using short hairpin RNA (shRNA) in Tong and Hep3B cells. TNF-α stimulation did not induce morphological changes (Fig. 2B) or alter the expression levels of the EMT markers (Fig. 2C) in the absence of Snail. Furthermore, TNF-α-induced cell migration (Fig. 2D) and invasion (Fig. 2E) ability was significantly reduced in these Snail knockdown cells. Collectively, these results indicate that Snail is sufficient to induce EMT in epithelial-type HCC cells and plays an essential role in TNF-α-induced EMT.
Figure 2.
EMT regulator Snail is required for TNF-α-mediated EMT in HCC cells. A, Western blot analysis of several EMT regulators in HCC cells treated with TNF-α (10 ng/ml) at different time points. B, morphological changes of Hep3B and Tong Snail knockdown stable cells with or without TNF-α stimulation (10 ng/ml for 72 hours). C, Western blot analysis of the Snail and several EMT markers from treated cells as described in (B). D, migration analysis of Hep3B and Tong Snail knockdown stable cells after TNF-α (10 ng/ml) stimulation for 48 hours. *p < 0.05. **p < 0.001 compared with mock. E, invasion analysis of Hep3B and Tong Snail knockdown stable cells after TNF-α (10 ng/ml) stimulation for 72 hours. *p < 0.05. **p < 0.001 compared with mock.
TNF-α upregulates Snail expression through canonical NF-κB activation
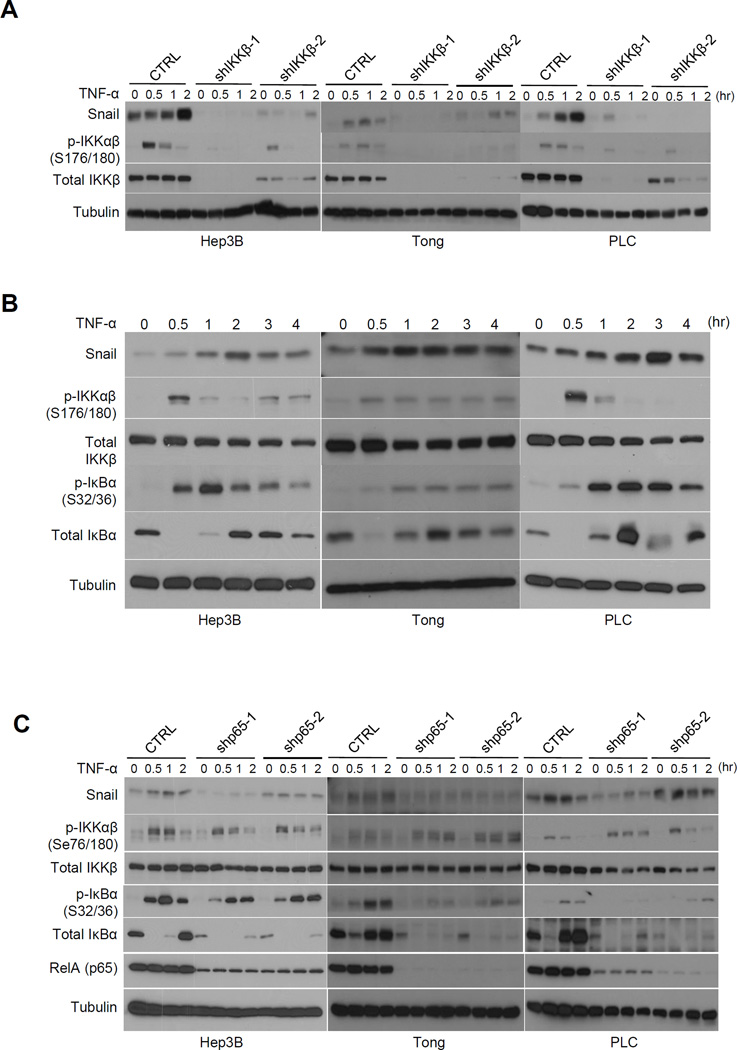
Because TNF-α activates various signaling pathways, we next explored which signaling cascade is responsible for TNF-α-mediated upregulation of Snail expression in HCC cells. To this end, Hep3B and PLC cells were serum starved overnight and pretreated with various inhibitors prior to TNF-α stimulation. As shown in Supplementary Figure S3A, upregulation of Snail by TNF-α was abolished only in the presence of the NF-κB inhibitor, Bay11-7082, but not by inhibitors against MAPK, p38, JNK kinase, PI3K/mTOR, or Akt. The NF-κB pathway can be activated by IKKα (non-canonical) and IKKβ (canonical) kinase. To determine whether IKKα or IKKβ is involved in TNF-α-mediated Snail upregulation, we transiently overexpressed IKKα or IKKβ in 293T cells and examined Snail expression. We found that ectopically expressed IKKβ but not IKKα increased the basal level of Snail (Supplementary Fig. S3B). Conversely, a kinase-dead mutant of IKKβ (nIKKβ) failed to do so. Furthermore, we knocked down IKKβ by shRNA in three HCC cell lines (Hep3B, Tong, and PLC) and examined their Snail expression upon TNF-α treatment. In the absence of IKKβ, TNF-α failed to induce Snail expression (Fig. 3A). In a parallel experiment using IKKα or IKKβ knockout (KO) mouse embryonic fibroblasts (MEFs), we showed that TNF-α induced Snail expression in wild-type and IKKα-deficient (IKKα−/−) MEFs but not IKKβ-deficient (IKKβ−/−) MEFs (Supplementary Fig. S4A). Re-expression of wild-type IKKβ but not kinase dead (KD) IKKβ mutant in IKKβ-deficient MEFs restored TNF-α-induced Snail expression (Supplementary Fig. S4B). These results support IKKβ as a major downstream kinase mediating TNF-α-induced Snail expression.
Figure 3.
TNF-α rapidly upregulates Snail expression through canonical NF-κB activation. A, Western blot analysis of Snail expression in HCC cells with a stable knockdown of IKKβ compared to scrambled control. TNF-α (10 ng/ml) was added at the indicated time points. B, activity of canonical NF-κB pathway at different time points after TNF-α (10 ng/ml) stimulation in Hep3B, Tong, and PLC cells. C, Western blot analysis of Snail and canonical NF-κB activation in cells with a stable knockdown of p65 compared with scrambled control. TNF-α (10 ng/ml) was added at the indicated time points. Tubulin was used as loading control.
Next, we examined the time-dependent expression of Snail and found it increased significantly along with the canonical NF-κB cascade in which the signal initiated from IKKβ phosphorylation, followed by biphasic IκBα phosphorylation and degradation after 1 hour of TNF-α stimulation (Fig. 3B). Similar results were observed in 8 out of 9 liver cancer cell lines (Supplementary Fig. S5A). Snail expression reached a maximum at 2 to 3 hours post TNF-α treatment (Fig. 3B) which was attenuated by pretreatment with Bay 11-7082 (Supplementary Fig. S5B). Collectively, these data suggest that TNF-α upregulates Snail expression through the canonical NF-κB pathway via IKKβ.
RelA (p65) is required for TNF-α-mediated NF-κB activation and EMT
RelA (p65) is a key factor that mediates transcriptional program in NF-κB signaling. Thus, we asked whether p65 plays a role in TNF-α-induced EMT by knocking down p65 in Hep3B, Tong, and PLC cells. First, we showed that TNF-α-induced Snail expression was attenuated in p65 knockdown cells (Fig. 3C). Overexpression of p65 in Tong and Hep3B cells was sufficient to drive EMT as indicated by decreased expression of E-cadherin and plakoglobin and increased expression of in N-cadherin and vimentin (Supplementary Fig. S6A). Nuclear translocation of p65 is known to correlate with its activity (8). Thus, to further validate the association between upregulated Snail and p65 activity, we examined the p65 nuclear localization in Tong and PLC cells treated with TNF-α at different time points by subjecting nuclear and cytoplasmic fractions to Western blot analysis (Supplementary Fig. S6B). TNF-α-induced nuclear translocation of p65 at 30 min led to a corresponding increase in the nuclear expression of Snail at 1 hour after treatment (Supplementary Fig. S6C). These results suggest that TNF-α-mediated upregulation of Snail and subsequent EMT requires p65 in HCC cells.
TNF-α signaling transcriptionally upregulates Snail expression
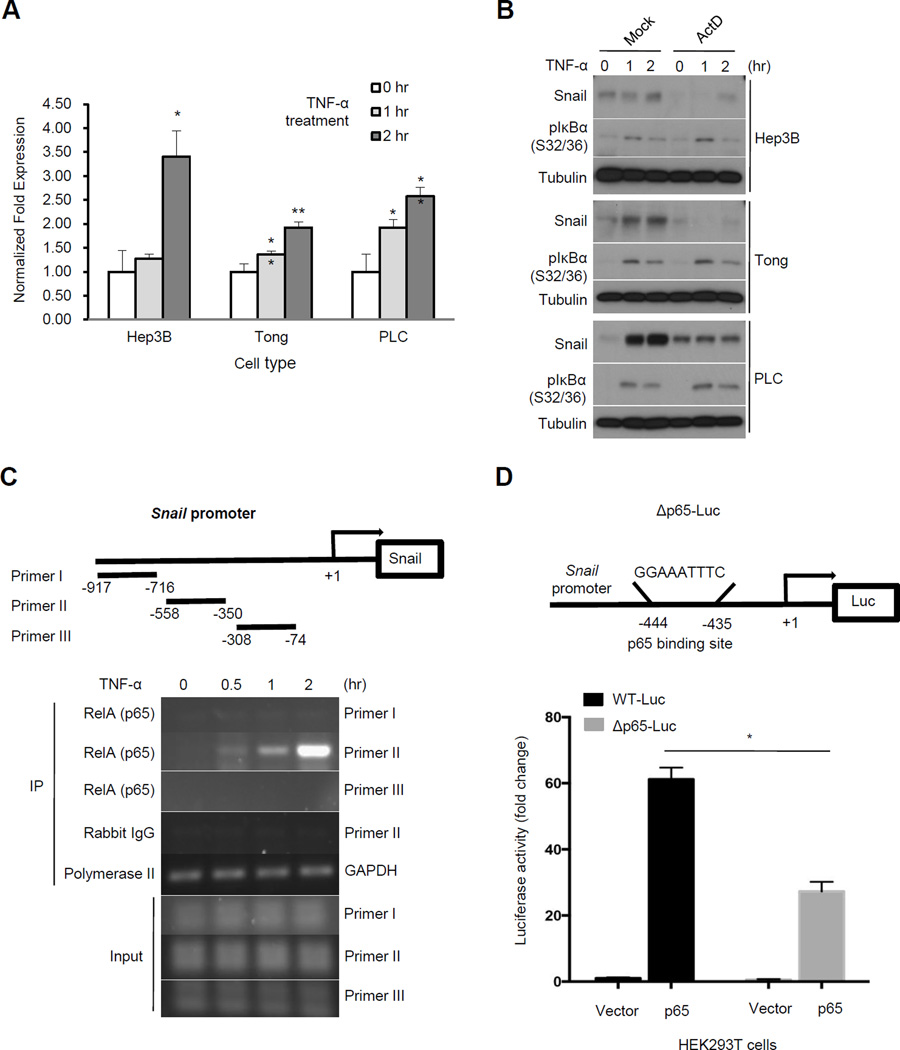
Because TNF-α-induced Snail expression requires p65, we asked whether its targets Snail transcriptionally. To this end, we analyzed the mRNA expression level of Snail with TNF-α in Hep3B, Tong, and PLC HCC cells. Snail mRNA expression was elevated by TNF-α treatment in a time-dependent manner (Fig. 4A). TNF-α-induced Snail expression was attenuated in cells pretreated with actinomycin D, a transcriptional inhibitor (Fig. 4B). Next, we performed a chromatin immunoprecipitation of p65 in cells treated with TNF-α and showed that p65 occupied the promoter region (−558 to −350) of Snail (Figure 4C), indicating that Snail is specifically targeted by p65. Moreover, results from luciferase reporter assay also showed that p65 activated the Snail promoter. To further identify the site(s) within the Snail promoter (−558 to −350) targeted by p65, we scanned this region using the TFSEARCH program to predict potential p65-binding sequences. We identified only one putative site (−435 to −444) that shared high similarity with the canonical p65-binding site. Deletion of this region within the Snail promoter reduced its responsiveness to p65 (Fig. 4D). These results indicate that Snail is a p65 target gene downstream of TNF-α signaling.
Figure 4.
TNF-α transcriptionally upregulates Snail expression. A, quantitative RT-PCR analysis of Snail mRNA isolated from TNF-α-treated HCC cells normalized to GAPDH. *p < 0.05. **p < 0.001 compared with the zero time point. B, Western blot analysis of Snail protein in HCC cells pretreated with actinomycin D (ActD; 500 ng/ml) for 1 hour followed by TNF-α (10 ng/ml) stimulation for 2 hours with the indicated antibodies. C, chromatin immunoprecipitation (ChIP) analysis of p65 occupancy on Snail promoter in response to TNF-α treatment. Primer sets used for RT-PCR detection are shown. D, luciferase reporter assay of Snail promoter in response to p65 in HEK 293T cells. Predicted p65 binding site was deleted from the promoter (Δp65-Luc). Relative luciferase activity is presented as means ±SE from three independent experiments.
Clinical correlation of p65/Snail/EMT axis in HCC patients
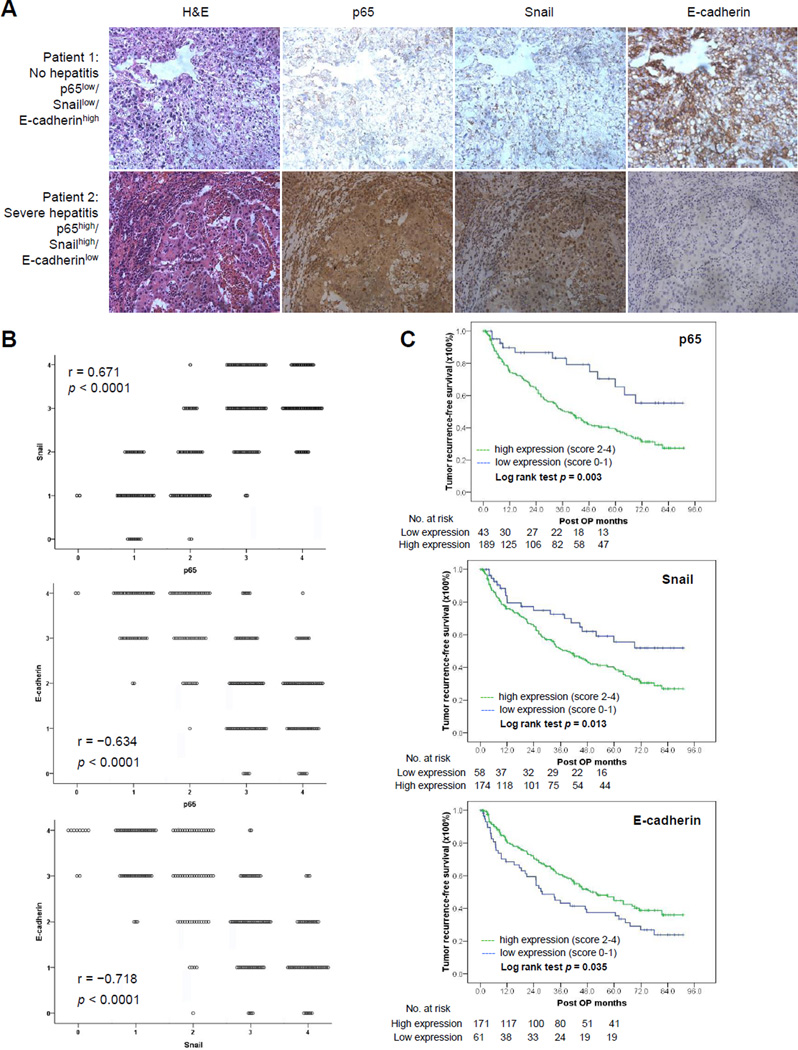
To further examine the possible clinical relevance of the aforementioned inflammation-related p65/Snail axis, we analyzed surgical specimens from 232 HCC patients who received curative hepatectomy by H&E and IHC staining. As shown in the Figure 5A, tumors from patients with severe hepatitis background had more infiltration of inflammatory cells in the peritumoral area than those from clean portohepatic areas without hepatitis. In tumors with a severe hepatitis background, we detected high expression of p65 and Snail and low expression of membranous E-cadherin. In contrast, tumors from patient without hepatitis had no expression of p65 or Snail but had high levels of membranous E-cadherin (Fig. 5A). The severity of hepatitis was significantly correlated with the intensity of p65, Snail and E-cadherin expression (Supplementary Table S9). Meanwhile, Snail expression correlated positively with p65 (N = 232; p < 0.0001), and E-cadherin expression correlated negatively with p65 (p < 0.0001) and Snail (p < 0.0001) (Fig. 5B). Furthermore, patients with higher expression of p65 and Snail had shorter tumor recurrence-free survival, while patients with higher expression of E-cadherin had longer tumor recurrence-free survival (Fig. 5C). These data suggest the inflammation-induced HCC tumor progression is highly associated with upregulation of p65 and Snail and downregulation of E-cadherin.
Figure 5.
Association of chronic hepatitis and p65/Snail/E-cadherin axis in human samples and its clinical significance. A, representative images from H&E and IHC staining of p65, Snail, and E-cadherin in tumors from HCC patients with or without hepatitis. B, correlations between the expression level of p65, Snail, and E-cadherin in 232 HCC tumors quantitated from IHC staining. p values, Spearman’s rank test. C, Kaplan-Meier recurrence-free survival curves according to the expression of p65, Snail, and E-cadherin in 232 patients with HCC after curative hepatectomy. P values were statistic by log-rank test. The number of patients at risk is shown below the survival curves.
Discussion
Chronic or severe hepatitis is linked to increased risk of HCC development and tumor progression, such as invasion and metastasis, as well as worsened clinical outcome (30). However, the underlying mechanism governing the metastatic nature by inflammation in HCC has not been clearly defined. In the present study, we identified an important signaling axis in HCC that links inflammatory cytokine TNF-α and EMT. Infiltration of inflammatory cells at the tumor site and surrounding liver (Figure 5A) secret TNF-α to promote EMT in HCC tumor cells and facilitate their migration and invasion ability. Specifically, we proposed a model in which elevated levels of TNF-α in the tumor microenvironment from chronic hepatitis upregulates the canonical NF-κB signaling via activation of IKKβ but not IKKα; the liberated cytoplasmic p65 then translocates into the nucleus, binds to the Snail promoter, and rapidly turns on Snail expression which promotes tumor metastasis through EMT. Several EMT regulators have been reported to initiate EMT in HCC and correlate to patient survival in clinic; however, in our system, we found that Snail is the major regulator of EMT downstream of TNF-α signaling. Of note, our earlier study showed that TNF-α induces expression of Twist but not Snail in breast cancers to promote EMT (16), suggesting an intricate nature of cancer type-specific EMT program that governs inflammation-induced cancer metastasis.
NF-κB provides a mechanistic link between inflammation and cancer and is a major factor that controls the ability of both pre-neoplastic and malignant cells to resist apoptosis, regulates tumor angiogenesis, and promotes invasiveness (9). Upregulation of EMT regulator Snail by the NF-κB pathway in cancer cells may be through a transcriptional-dependent or -independent manner. For example, Akt and MAPK kinase can activate NF-κB-mediated Snail mRNA upregulation in squamous cell carcinoma (31) and peritoneal mesothelial cells (32), respectively. Moreover, NF-κB can stabilize Snail protein via upregulation of COP9 which subsequently blocks ubiquitination of Snail protein (33). Results from this study and from others (31, 32) have demonstrated that Snail expression is transcriptionally regulated. Interestingly, in SW480 colon cancer cells, the minimal p65 responsive promoter region of Snail was identified at −194 to −78 (34); however, in HCC cells, p65 did not occupy this region (Fig. 4C). Instead, deletion of the predicted p65 binding site in the Snail promoter at −435 to −444 substantially inactivated its responsiveness to p65 (Fig. 4C, D). These observations are consistent with previously reported complexity and cell-specific regulation of the Snail promoter by NF-κB (31).
In the present study, we found that HCC patients with more severe hepatitis had a higher tendency toward more intrahepatic recurrence and extrahepatic metastasis after curative hepatectomy. The significance of the severity of hepatitis on the clinical outcome for HCC patients after surgical resection of primary tumor may be explained by the activation of the TNF-α/NF-κB/Snail pathway. First, it may be that there are more cancer cells with EMT potential in the microenvironment of HCC tumors from patients with severe hepatitis. These mesenchymal-type HCC cells within primary tumors may have already undergone micrometastases prior to operation. In addition, conventional liver resection may induce the release of cancer cells from the liver into the peripheral blood circulation, especially when liver is mobilized during hepatectomy (35). These procedure-related disseminations of cancer cells have been shown as a predicator of postsurgical recurrence of HCC (36), suggesting that the microenvironment surrounding HCC tumors from patients with severe hepatitis may shed cancer cells more easily into the systemic circulation during hepatectomy than those with mild hepatitis. Moreover, microscopic tumor cells in multifocal lesions may gain EMT potential and promote intrahepatic recurrence and distant metastasis after operation if hepatitis is sustained after resection. Thus, recurrent or metastatic HCC induced by hepatic inflammation may lead to adverse clinical outcome after surgical resection, and targeting the TNF-α/NF-κB/Snail pathway or controlling hepatitis may improve patient survival after hepatectomy. Our results are in agreement with a previous a large-scale study, which profiled gene expression and survival of HCC patients, that the inflammatory signatures, TNF-α and NF-κB signaling, in the surrounding liver tissue are correlated with poor survival (37).
It has become clear that dysregulation of NF-κB and the signaling pathways that control its activity are involved in cancer development and progression (38) and that targeting NF-κB may provide therapeutic benefit. One successful example is the treatment of multiple myeloma by bortezomib (Velcade®; Millenium Pharmaceuticals), a reversible 26S proteasome inhibitor that effectively inhibits NF-κB activity (39). This drug also effectively induces apoptosis and inhibits growth of HCC cell in pre-clinical studies (40, 41). However, subsequent clinical studies of bortezomib as monotherapy for patients with unresectable HCC failed to bring survival benefit (42). By contrast, direct inhibition of NF-κB has been shown to reduce liver inflammation and attenuate liver fibrosis/cirrhosis (43). Several inhibitors of NF-κB, such as caffeic acid, captopril, curcumin, pyrrolidine dithiocarbamate, resveratrol, silymarin, and thalidomide, have demonstrated antinecrotic, anticholestatis, antifibrotic, and anticancer activities in the liver (43), but large prospective and randomized control clinical trials are still required to demonstrate their efficacy in treating HCC. Given that HCC with severe hepatitis background has more frequent vascular invasion and poorer patient survival (44), the results from our study suggest that HCC may be managed by controlling hepatitis in addition to NF-κB inhibition. As viral infection is a major cause of hepatitis, antiviral therapies may also attenuate virus-induced inflammation in the peritumor microenvironment and prevent HCC recurrence. Indeed, postoperative adjuvant antiviral therapy improved survival of HCC patients with viral infection and hepatitis (45–50).
Our observations also raised the intriguing questions of whether other cytokines are responsible for HCC recurrence and whether late recurrent HCC is a consequence of cancer metastasis. Recognizing the limited size of clinical samples due to scope of the current study, we certainly do not exclude the potential importance of cytokines other than TNF-α although the results from our cytokine array analysis suggest that TNF-α is correlated with the degree of hepatitis. Meanwhile, even though we observed a correlation between inflammation, EMT, and early and late tumor recurrence (two years as the cutoff point; Supplementary Table S7), it should be noted that only early reappearance of tumors that happen within the first year are generally accepted as recurrences from the original tumors. However, late recurrences (more than two years) can occur as a result of de novo cancer formation in the microenvironment of sustained hepatitis. The possibility that parts of late recurrence may have originated from an early metastatic event of primary tumors after a long dormancy should not be fully excluded until a systematic genomics analysis is performed to compare the evolution of primary and late recurrent tumors.
In summary, identification of Snail as a downstream target of IKK-β/p65 links the inflammatory cytokine TNF-α-mediated NF-κB activation and EMT in HCC. Moreover, the findings in this study suggest that chronic liver inflammation leads to tumor metastasis and decreases patient survival after curative resection of primary HCC. Inhibition of NF-κB activation or diminishment of hepatitis after surgery, therefore, has important clinical implications for the treatment or prevention of HCC recurrence and metastasis.
Supplementary Material
Translational Relevance.
Chronic liver inflammation caused by viral infection or alcohol consumption is associated with hepatocarcinogenesis. In this study, we show that severe hepatitis is significantly associated with recurrence and metastasis of hepatocellular carcinoma (HCC) after surgical resection of the primary tumor. Results from cytokine array analysis indicate that elevated levels of inflammatory cytokine TNF-α in the peritumoral microenvironment with severe hepatitis stimulate the initiation of epithelial-mesenchymal transition (EMT) in HCC cells. Moreover, TNF-α activates its downstream canonical NF-κB signaling pathway through IKK-β and p65 to transcriptionally upregulate the expression of EMT regulator Snail. This p65/Snail/E-cadherin axis is inversely correlated with tumor-recurrence free survival after operation. These findings identify a major inflammation-mediated pathway involved in HCC recurrence and metastasis, providing a potential therapeutic strategy to prevent tumor recurrence after hepatectomy by controlling liver inflammation and/or targeting NF-κB-mediated Snail expression.
Acknowledgments
We thank Dr. Jennifer L. Hsu for her assistance with manuscript preparation. We also thank the Liver Research Center at Linkou Chang Gung Memorial Hospital for providing access to the equipment to perform immunohistochemical staining.
Grant Support
This study was funded in part by the following: National Science Council (grant NMRP 101-2314-B-182A-032-MY2; to T.-J.W.); National Institutes of Health (CA109311; to M.-C.H.); Ministry of Science and Technology, International Research-intensive Centers of Excellence in Taiwan (I-RiCE; MOST 104-2911-I-002-302); Ministry of Health and Welfare, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002); and Center for Biological Pathways.
Abbreviations
- HCC
Hepatocellular carcinoma
- EMT
epithelial–mesenchymal transition
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ECM
extracellular matrix
- NF-κB
nuclear factor κB
- IKKs
inhibitor κB kinases
- TNF-α
tumor-necrosis factor-alpha
- ChIP
chromatin immunoprecipitation
- KO
knockout
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Authors’ Contributions
Conception and design: T.-J. Wu and M.-C. Hung.
Development of methodology: T.-J. Wu, S.-S. Chang, C.-W. Li, and M.-C. Hung.
Acquisition of data (provision of animals and facilities, enrollment and management of patients): T.-J. Wu, S.-S. Chang, Y.-H. Hsu, T.-C. Chen, W.-C. Lee, and C.-T. Yeh.
Analysis and interpretation of data (statistical analysis, biostatistics, computational analysis): T.-J. Wu, S.-S. Chang, and M.-C. Hung.
Writing, review, and/or revision of the manuscript: T.-J. Wu, S.-S. Chang and M.-C. Hung.
Administrative, technical, or material support (reporting or organizing data, constructing databases): T.-J. Wu, S.-S. Chang, Y.-H. Hsu, and C.-W. Li.
Study supervision: T.-C. Chen, W.-C. Lee, and C.-T. Yeh and M.-C. Hung.
References
- 1.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Hirohata T, Takeshita S, Hirohata I, Koga S, Sugimachi K, et al. Hepatitis B virus, cigarette smoking and alcohol consumption in the development of hepatocellular carcinoma: a case-control study in Fukuoka, Japan. Int J Cancer. 1992;51:509–514. doi: 10.1002/ijc.2910510402. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53. [PubMed] [Google Scholar]
- 6.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 10.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Lee DF, Chen CT, Yen CJ, Li LY, Lee HJ, et al. IKKalpha activation of NOTCH links tumorigenesis via FOXA2 suppression. Mol Cell. 2012;45:171–184. doi: 10.1016/j.molcel.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahbari NN, Mehrabi A, Mollberg NM, Muller SA, Koch M, Buchler MW, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 15.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 19.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 21.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 22.Sugimachi K, Tanaka S, Kameyama T, Taguchi K, Aishima S, Shimada M, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003;9:2657–2664. [PubMed] [Google Scholar]
- 23.Sun D, Sun B, Liu T, Zhao X, Che N, Gu Q, et al. Slug promoted vasculogenic mimicry in hepatocellular carcinoma. J Cell Mol Med. 2013;17:1038–1047. doi: 10.1111/jcmm.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Song GD, Sun N, Chen JQ, Yang SS. Slug overexpression induces stemness and promotes hepatocellular carcinoma cell invasion and metastasis. Oncol Lett. 2014;7:1936–1940. doi: 10.3892/ol.2014.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou YM, Cao L, Li B, Zhang RX, Sui CJ, Yin ZF, et al. Clinicopathological Significance of ZEB1 Protein in Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2011;19:1700–1706. doi: 10.1245/s10434-011-1772-6. [DOI] [PubMed] [Google Scholar]
- 26.Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, et al. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208:875–883. doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, et al. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450–456. [PubMed] [Google Scholar]
- 28.Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z, et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–624. doi: 10.1002/hep.26029. [DOI] [PubMed] [Google Scholar]
- 29.Liao YL, Sun YM, Chau GY, Chau YP, Lai TC, Wang JL, et al. Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008;27:5578–5589. doi: 10.1038/onc.2008.168. [DOI] [PubMed] [Google Scholar]
- 30.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 31.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, et al. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 32.Strippoli R, Benedicto I, Perez Lozano ML, Cerezo A, Lopez-Cabrera M, del Pozo MA. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Model Mech. 2008;1:264–274. doi: 10.1242/dmm.001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 35.Louha M, Nicolet J, Zylberberg H, Sabile A, Vons C, Vona G, et al. Liver resection and needle liver biopsy cause hematogenous dissemination of liver cells. Hepatology. 1999;29:879–882. doi: 10.1002/hep.510290348. [DOI] [PubMed] [Google Scholar]
- 36.Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: a prospective study. Hepatology. 2002;35:853–860. doi: 10.1053/jhep.2002.32100. [DOI] [PubMed] [Google Scholar]
- 37.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 40.Chen KF, Yeh PY, Yeh KH, Lu YS, Huang SY, Cheng AL. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008;68:6698–6707. doi: 10.1158/0008-5472.CAN-08-0257. [DOI] [PubMed] [Google Scholar]
- 41.Saeki I, Terai S, Fujisawa K, Takami T, Yamamoto N, Matsumoto T, et al. Bortezomib induces tumor-specific cell death and growth inhibition in hepatocellular carcinoma and improves liver fibrosis. J Gastroenterol. 2013;48:738–750. doi: 10.1007/s00535-012-0675-z. [DOI] [PubMed] [Google Scholar]
- 42.Kim GP, Mahoney MR, Szydlo D, Mok TS, Marshke R, Holen K, et al. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30:387–394. doi: 10.1007/s10637-010-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muriel P. NF-kappaB in liver diseases: a target for drug therapy. J Appl Toxicol. 2009;29:91–100. doi: 10.1002/jat.1393. [DOI] [PubMed] [Google Scholar]
- 44.Ng IO, Poon RT, Shek TW, Fan ST. Clinicopathologic and prognostic significance of the histologic activity of noncancerous liver tissue in hepatitis B virus-associated hepatocellular carcinoma. Am J Clin Pathol. 2002;117:411–418. doi: 10.1309/4231-RCVB-WK8X-R1JK. [DOI] [PubMed] [Google Scholar]
- 45.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 46.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–1112. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 48.Hsu YC, Ho HJ, Wu MS, Lin JT, Wu CY. Postoperative peg-interferon plus ribavirin is associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma. Hepatology. 2013;58:150–157. doi: 10.1002/hep.26300. [DOI] [PubMed] [Google Scholar]
- 49.Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, Zhao Y, et al. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010;16:2931–2942. doi: 10.3748/wjg.v16.i23.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287–292. doi: 10.1111/j.1365-2893.2009.01181.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.